Peer-review started: September 28, 2018
First decision: October 26, 2018
Revised: November 15, 2018
Accepted: December 10, 2018
Article in press: December 10, 2018
Published online: January 21, 2019
Processing time: 115 Days and 23.8 Hours
Insulin is an important hormone that affects various metabolic processes, including kidney function. Impairment in insulin’s action leads to insulin resistance in the target tissue. Besides defects in post-receptor insulin signaling, impairment at the receptor level could significantly affect insulin sensitivity of the target tissue. The kidney is a known target of insulin; however, whether the kidney develops “insulin resistance” is debatable. Regulation of the insulin receptor (IR) expression and its function is very well studied in major metabolic tissues like liver, skeletal muscles, and adipose tissue. The physiological relevance of IRs in the kidney has recently begun to be clarified. The credit goes to studies that showed a wide distribution of IR throughout the nephron segments and their reduced expression in the insulin resistance state. Moreover, altered renal and systemic metabolism observed in mice with targeted deletion of the IR from various epithelial cells of the kidney has strengthened this proposition. In this review, we recapitulate the crucial findings from literature that have expanded our knowledge regarding the significance of the renal IR in normal- and insulin-resistance states.
Core tip: Dysregulation of the renal insulin receptor (IR) not only affects local renal metabolism, but also disturbs the systemic glucose homeostasis and blood pressure, leading to metabolic abnormalities. The objective of this review is to highlight the pathophysiological stature of renal IRs in the kidney function, as well as, overall metabolism.
- Citation: Singh S, Sharma R, Kumari M, Tiwari S. Insulin receptors in the kidneys in health and disease. World J Nephrol 2019; 8(1): 11-22
- URL: https://www.wjgnet.com/2220-6124/full/v8/i1/11.htm
- DOI: https://dx.doi.org/10.5527/wjn.v8.i1.11
The incidence of insulin resistance is increasing worldwide in parallel with the rate of obesity. Insulin resistance, per se, is often subclinical, and defined by inefficient insulin receptor (IR) signaling in major metabolic tissues, including liver, muscle, and adipose, resulting in impaired cellular glucose uptake. The function and role of reduced IR signaling has been extensively studied in these metabolic tissues. In addition to downstream signaling, alterations in the expression, binding, and phosphorylation of the IR itself may affect target cell sensitivity to insulin[1-3]. The kidney expresses IRs[4,5]; however, it is still debatable whether kidney develops classic “resistance” in the same manner as the liver, muscle, and adipose tissues. Reduced expression of IR and its phosphorylated form, the first step in IR signaling, have been demonstrated in renal epithelial cells of diabetic and insulin-resistant rat models[6,7]. Nevertheless, presence of these receptors throughout the nephron segments suggests an important role in renal metabolism. Insulin could undoubtedly regulate several vital kidney functions through its receptors. However, it has been a mere decade since the role of renal epithelial IR in kidney physiology and pathology began to be illuminated. In this review, we bring together the findings from published literatures that have contributed to our understanding in the area. For easy reading we will use the phrase “renal IR” in place of “IR in renal epithelial cells”.
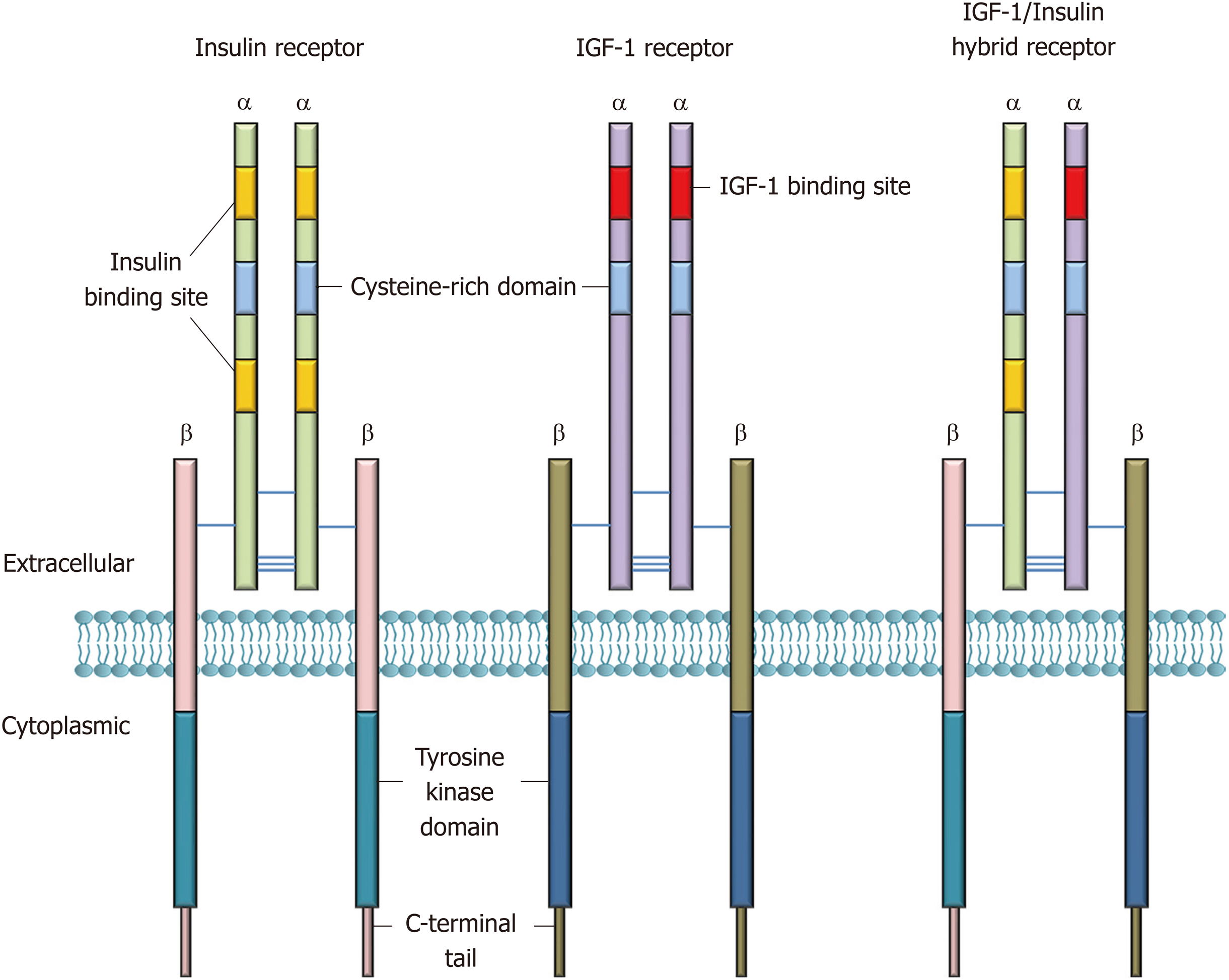
Insulin, secreted by pancreatic β-cells, is a peptide hormone with pleiotropic actions and plays an indispensable role in human metabolism. Biological effects of insulin are exerted by binding to IRs. IRs belong to the receptor tyrosine kinases and the IR subfamily, which consists of the IR, the insulin-like growth factor (IGF-I/-II) receptors, and the IR-related receptor[8]. The IR is a transmembrane protein that is composed of two α- and two β-subunits forming a heterotetramer α2β2 (Figure 1), with disulfide bonds between the α-subunits and between the α- and β-subunits[9]. The human IR cDNA was isolated and cloned in the 1980s[10,11]. These studies demonstrated that the α- and β-subunits are derived from proteolytic cleavage of a common precursor. Later, Seino et al[12] reported that the IR gene (INSR) is encoded by 22 exons and 21 introns. Alternative splicing of exon 11 results in two isoforms, A and B with differential insulin affinity, with isoform B having higher affinity.
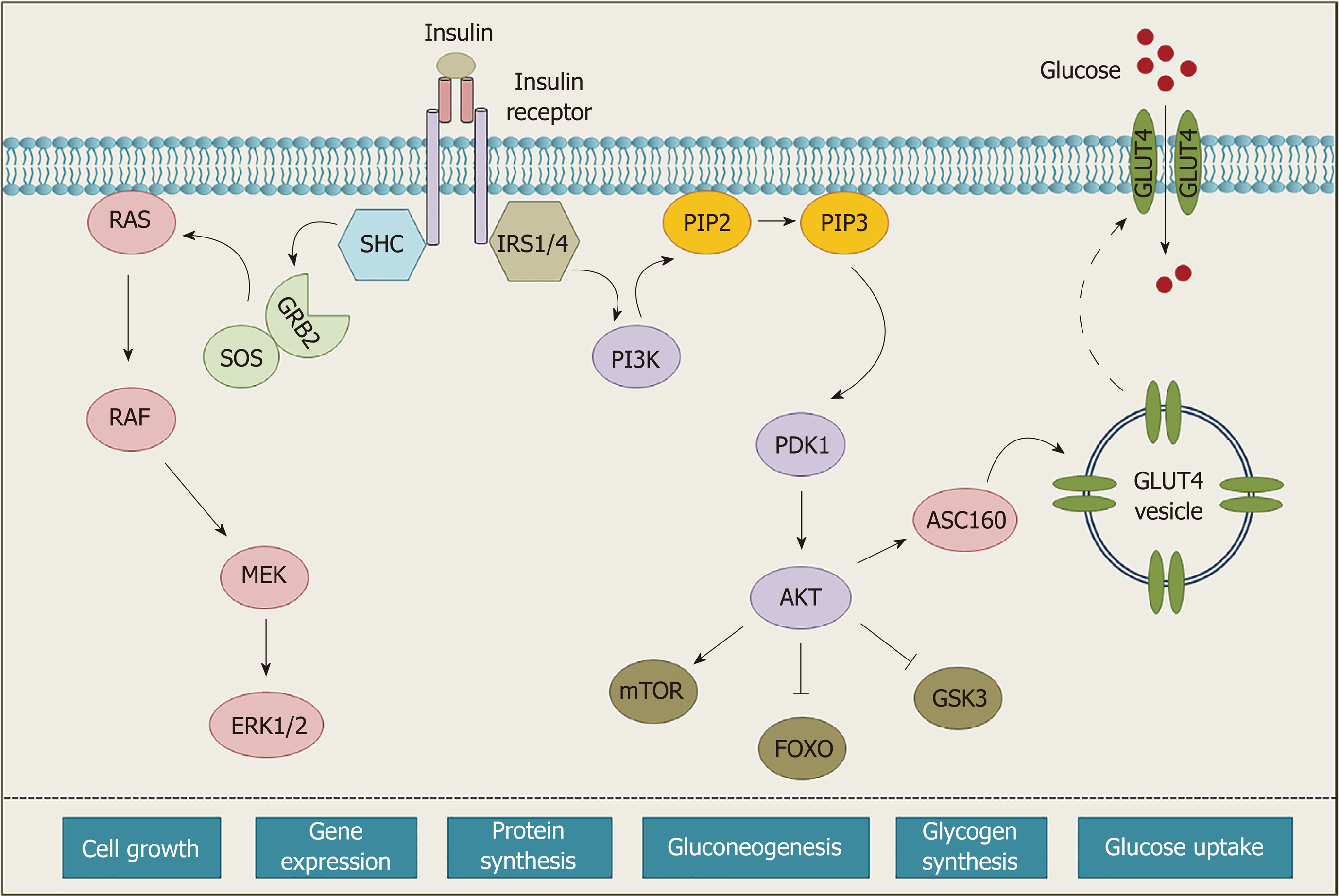
Insulin binding to extracellular α-subunits confers conformational changes within the molecule, leading to autophosphorylation of specific tyrosine residues in intracellular domains[13]. Upon activation, various adaptors and signaling proteins (IRS, SHC, GRB, etc.) are recruited to the receptor to initiate the intracellular signaling cascade and regulate different biological functions[8,13] (Figure 2).
The attempts to examine the expression pattern of IRs in the kidney had started about four decades ago; however, their physiological role in the kidney has recently come to light[4,14-16]. Renal localization of IRs was first studied by 125I-labeled insulin binding in microdissected rat glomeruli and tubules in 1988. The results showed high affinity binding sites in the proximal and distal convoluted tubules (PCT and DCT), and to a lesser extent in the cortical and outer medullary collecting duct (CD) and thick ascending limb (TAL)[15]. Later, Sechi et al[4] exploited an in situ autoradiographic technique to observe insulin binding in glomeruli, renal cortex, outer and inner renal medulla. Findings from their studies revealed the highest IR density in the inner portion of the medulla, which also exhibits the maximal insulin activity in the renal tubule. The localization of IR in the proximal tubule (PT), TAL, DCT, and CD have also been shown by immunofluorescence using polyclonal antibodies against the α- and β-subunits of IR[16]. This approach illustrated an exclusive localization pattern of IR as these antibodies did not overlap with IGF-1 receptor and the IR-related receptor in kidney[17,18]. The significance of IR expression in different segments of the nephron was later confirmed by targeted deletion of IR from these segments[19,20].
Renal regulation of sodium reabsorption is crucial for maintaining homeostasis, fluid balance, and systemic blood pressure. Excessive intake of dietary sodium and/or impaired salt excretion augments the incidences of hypertension[21]. There is substantial evidence suggesting restriction of dietary sodium could decrease cardiovascular risk and reduce blood pressure in normotensive and hypertensive individuals[22,23]. In kidney, sodium reabsorption occurs throughout the tubular segments of nephron including the PT, TAL, DT, and CD[24-26].
Insulin is reported to have antinatriuretic properties and has been shown to increase sodium absorption by regulating the activities of different renal sodium channels including the Na+/H+ exchanger type 3, the sodium-bicarbonate cotransporter, and the Na-K-ATPase in PT, the sodium-potassium-chloride cotransporter type 2 and the Na-K-ATPase in TAL, and the sodium-chloride cotransporter and the epithelial sodium channel in DCT and CD[27].
To elucidate the sodium-insulin interaction in the kidney, Sechi et al[28], examined renal IR binding and mRNA levels of IRs in rats fed on different salt concentration. They reported an inverse relationship between dietary salt (NaCl) intake and renal IR density. In concordance with this study, Catena et al[29] also reported a decrement in IR number and mRNA levels in control rats fed on a high-salt diet. However, IR densities were reported comparable in fructose-fed rats maintained on high- or low-salt diet. Further, a reduced antinatriuretic effect of insulin in high-salt-fed control rats was not observed in fructose-fed rats, implying that the fructose-fed animals lacked the feedback mechanism that limits insulin-induced sodium retention during high salt intake, which may contribute to fructose-induced hypertension[29].
Nevertheless, the expression pattern of IR in the PT, TAL, and CD implies the involvement of IRs in insulin-mediated renal sodium retention[15,30-32]. Therefore, investigating the correlation between IRs and renal sodium reabsorption has been a major focus of researchers to understand the connection between insulin resistance and hypertension. Hypertension is one of the most common cardiovascular complications worldwide. High blood pressure and associated complications lay a grave burden on patients. Among various determinants of hypertension, insulin resistance is considered to be a major determinant. Although the precise role of insulin resistance is debatable in the development of hypertension, activation of the sympathetic nervous system, insulin-regulated sodium retention, and activation of the renin-angiotensin system (RAS) are considered as plausible mechanisms[33-35]. The interrelation between insulin resistance and hypertension could either be a non-causal association (two independent processes) or a cause-and-effect relationship, where insulin resistance acts as a cause of hypertension[36].
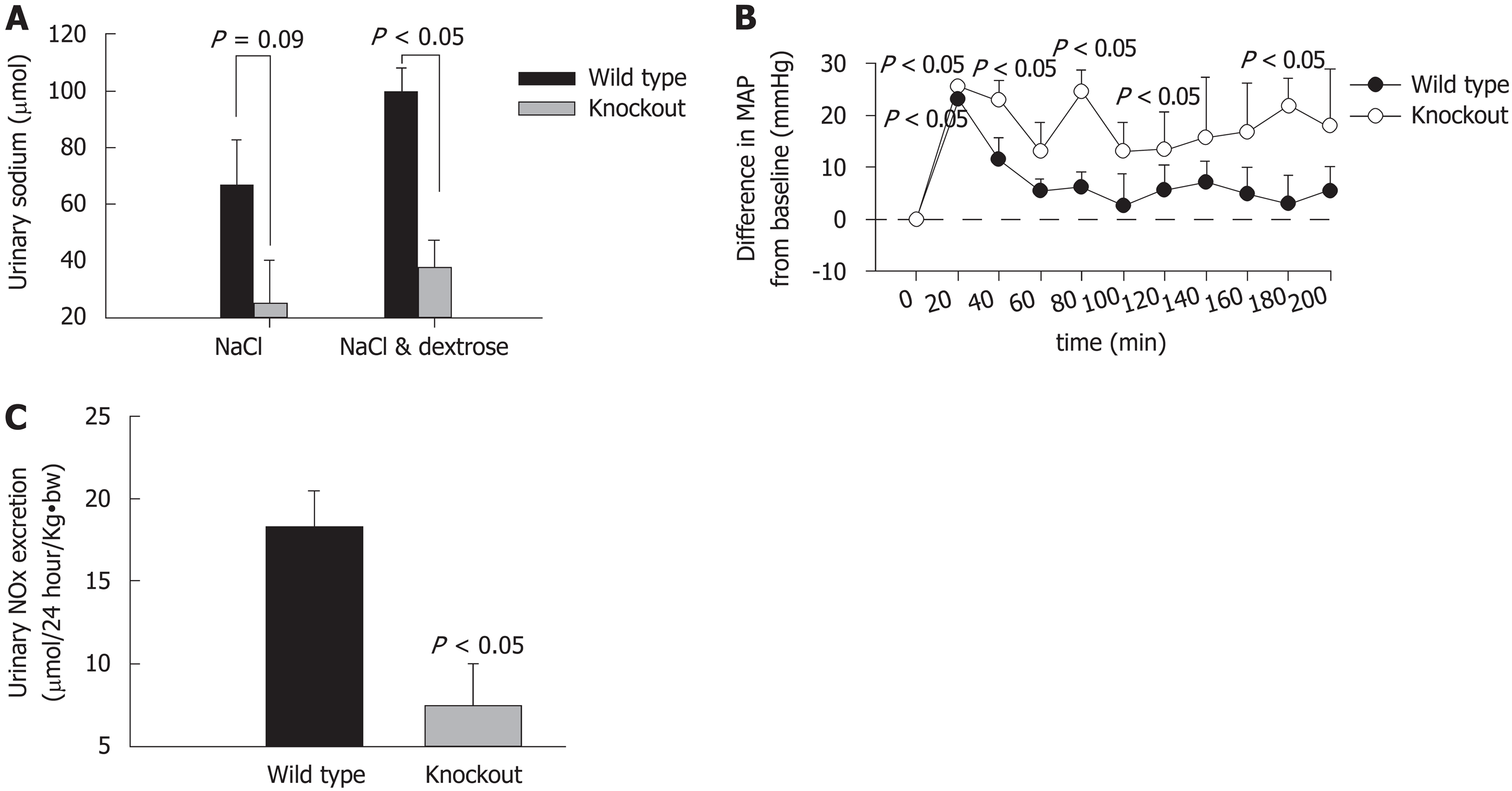
Interestingly, we observed that specific knockout of renal epithelial cell IR caused elevated systolic blood pressure in mice. Our study has shown that targeted deletion of IRs from renal epithelial cells significantly increased systolic blood pressure and impaired sodium excretion in response to saline load as compared to wild-type (WT). Moreover, intraperitoneal administration of insulin caused a significant drop in blood pressure in WT, but not in IR-knockout (KO) mice. Urinary excretion of nitrates and nitrites (UNOx) was also reduced in KO mice relative to WT mice (Figure 3). These observations suggested that renal IRs could play a key part in the maintenance of normal blood pressure and volume-expansion-associated natriuresis[19]. A study from Bhalla’s lab also has shown that renal tubule-specific knockout of IR decreased NCC-mediated sodium reabsorption in high fat-fed mice[37]. However, further investigation is required to comprehensively understand the IR-dependent regulation of sodium retention and associated hypertension during insulin resistance.
Insulin has a complex role in the maintenance of blood pressure. On one hand, insulin-induced sodium retention and increased sympathetic activity is a root cause of hypertension, at the same time, insulin itself has a vasodilatory effect, which is associated with nitric oxide (NO) production[38]. In kidney, hyperinsulinemia affects renal blood flow in a NO-dependent manner[39] and insulin resistance impedes this effect[40,41]. Moreover, experimental diabetes in rats has resulted in reduced renal NO production[42]. Local renal production of NO production has also been implicated in impaired renal blood flow during congestive heart failure[43]. Apart from its vasodilatory effects, NO is also reported to reduce sodium reabsorption in renal tubules[44,45]. Specific deletion of IR from renal epithelial cells has been reported to impair sodium and NO excretion and elevate systemic blood pressure in mice, suggesting a possible role of impaired renal NO production in blood pressure regulation[19]. Moreover, reduced renal expression of the IR in TAL has been linked to salt sensitivity of blood pressure via blunted production of NO[46]. These IR-knockout mice also exhibited low protein levels of nitric oxide synthase isoform, NOS1, which is expressed in macula densa cells, TAL, and in CD[46]. Together, these studies support a crucial role of renal NO in blood pressure regulation by its autocrine and paracrine actions, particularly in the medullary TAL and CD[47-49].
A fairly recent report from our group showed for the first time that insulin induces eNOS activation and NO generation in the renal inner medullary collecting duct (IMCD) cells[50]. We observed a time- and dose-dependent increase in NO and its metabolite NOx in insulin-stimulated mouse IMCD cells. Moreover, chronic insulin infusion in C57BL/6J mice led to increased expression of endothelial NOS (eNOS) and elevated NO levels in the inner medulla. However, treatment of cells with wortmannin (PI3K inhibitor) and IR-knockdown abolished these effects of insulin in vitro, implying the involvement of the IR/PI3K pathway in insulin-stimulated NO generation. Further, targeted deletion of IR from renal tubule epithelial cells resulted in significant downregulation of eNOS in inner medulla with concomitant rise in blood pressure in KO mice. These observations implied that IR signaling in the IMCD could contribute to hypertension in the insulin-resistant state.
The renal RAS is another imperative pathway that regulates systemic blood pressure and maintains water and electrolyte homeostasis. Typically, angiotensin II (Ang II) produced in the RAS pathway interacts with angiotensin type 1 receptors (AT1R) to exert its biological effects in various tissues including the kidney, the heart, adipocytes, adrenal tissues[51], etc. The classical RAS pathway induces sodium reabsorption, vasoconstriction, and blood pressure. Moreover, Ang II has been established to inhibit insulin-mediated PI3K activation and is involved in the pathogenesis of insulin resistance[52]. An interrelation between insulin resistance and the RAS pathway has been reported in hypertensive patients. Although precise mechanism of insulin resistance and RAS is not well established, these two pathways interact at multiple levels to regulate cellular metabolism[53]. Previously, it has been demonstrated that Ang II induces phosphorylation of IRS1 (a key substrate of IR) at Ser616 and Ser312, which is responsible for its inactivation and inhibition of insulin/PI3K signaling cascade[54]. A direct link between renal IRs, RAS, and cardiovascular complications has not been reported and warrants further investigation.
IR signaling has been reported to maintain blood glucose levels in the liver and other metabolic tissues[55-57], however, there is limited knowledge regarding this action in the kidney. Recent studies have highlighted that renal IR signaling is an equally important contributor and regulator of systemic glucose levels[19,58-60]. The first evidence on kidney’s involvement in glucose metabolism came in 1938, where Bergman et al observed that removal of kidneys in hepatectomized rabbits doubled the sugar utilization rate as compared to the hepatectomized animals only[61]. Following which a number of studies substantiated the glucose production activity of the kidney[62,63] and provided evidence that kidney, in addition to acidosis or prolonged starvation, also releases considerable amounts of glucose in normal post-absorptive conditions[64]. Moreover, accumulating evidence predicts that the kidneys impart a critical role in regulating overall glucose homeostasis by various mechanisms, such as reabsorption of glucose from the glomerular ultrafiltrate specifically in the renal epithelial cells, glucose uptake and utilization for meeting the body’s energy demands, and gluconeogenesis, i.e., endogenous glucose production from non-carbohydrate sources[55,56].
Similar to liver tissue, renal gluconeogenesis and metabolism were found to be dysregulated in diabetes and the insulin-resistant state[6,7]. There are studies which suggest that renal epithelial cells double their glucose uptake in response to insulin stimulation via translocation of GLUT (GLUT1 and GLUT4) to the plasma membrane, which accentuates the effect of insulin on renal gluconeogenesis and on systemic blood glucose levels[65]. Moreover, hyperinsulinemia is reported to inhibit glucose production and stimulate glucose uptake by the renal epithelial cells[60,66]. Both experimental and clinical studies have documented the insulin-mediated regulation of uptake and release of glucose. A hyperinsulinemic clamp study in humans showed a 61% decrease in renal glucose output and approximately a 72% decrease in renal glutamine gluconeogenesis [much higher than liver (25%)] in subjects treated with insulin[67]. Insulin has also been reported to affect the transport of gluconeogenic substrates in the kidney[68]. These studies highlighted the significance and magnitude of renal glucose production, and also revealed higher sensitivity of renal glucose release towards insulin as compared to liver. Moreover, enhanced renal gluconeogenesis in the post-absorptive conditions has been suggested to contribute towards hyperglycemia in Type 2 diabetes in the insulin-resistant state. This is supported by the increased intrinsic gluconeogenesis with simultaneous decrease in IR levels reported in the kidney cortex of Zucker diabetic fatty rats[69]. In addition, a marked decrease in IR expression has been observed in the renal cortex of high-fat diet-fed rats as well as in Type 2 diabetic patients[6,7,69,70].
The role of insulin/IR signaling in regulation of gluconeogenesis transcriptional modulation of gluconeogenic genes, i.e., PCK1 and G6PC is well known in liver[71-73]. However, studies on the role of the IR in renal gluconeogenesis regulation are limited. In 2012, a study from the DeFronzo lab elucidated that insulin negatively regulates gluconeogenesis via downregulating the expression of key gluconeogenic genes in the kidney[74]. Around the same time, our group demonstrated that targeted deletion of the IR from the PT resulted in hyperglycemia despite normal whole body insulin sensitivity[20]. More so, an increased activity and elevated mRNA expression of glucose-6-phosphatase (G6Pase, a rate-limiting enzyme in gluconeogenesis) was observed in the PT-specific IRKO mice, signifying the involvement of the IR in regulating the expression of key gluconeogenic genes. Further, reduced IR expression and early IR signaling along with a significant increase in phosphoenolpyruvate carboxykinase (PEPCK) levels were found in kidney cortex of high-fat diet-fed mice[75], providing a clue to the possible mechanism of insulin involving transcriptional regulation of PEPCK, also a rate-limiting gene in gluconeogenesis. In liver, insulin has been shown to suppress the expression of gluconeogenic genes, G6Pase and PEPCK[76]. In vitro studies performed in primary PT cells from human kidney (hPT) showed an inhibitory role of insulin on cAMP/DEXA-induced gluconeogenesis, and silencing of IR attenuated this inhibitory effect of insulin on PT-gluconeogenesis in hPT[77]. All these findings clearly state that reduced IR expression/signaling might have a causal function in gluconeogenic gene upregulation and gluconeogenesis.
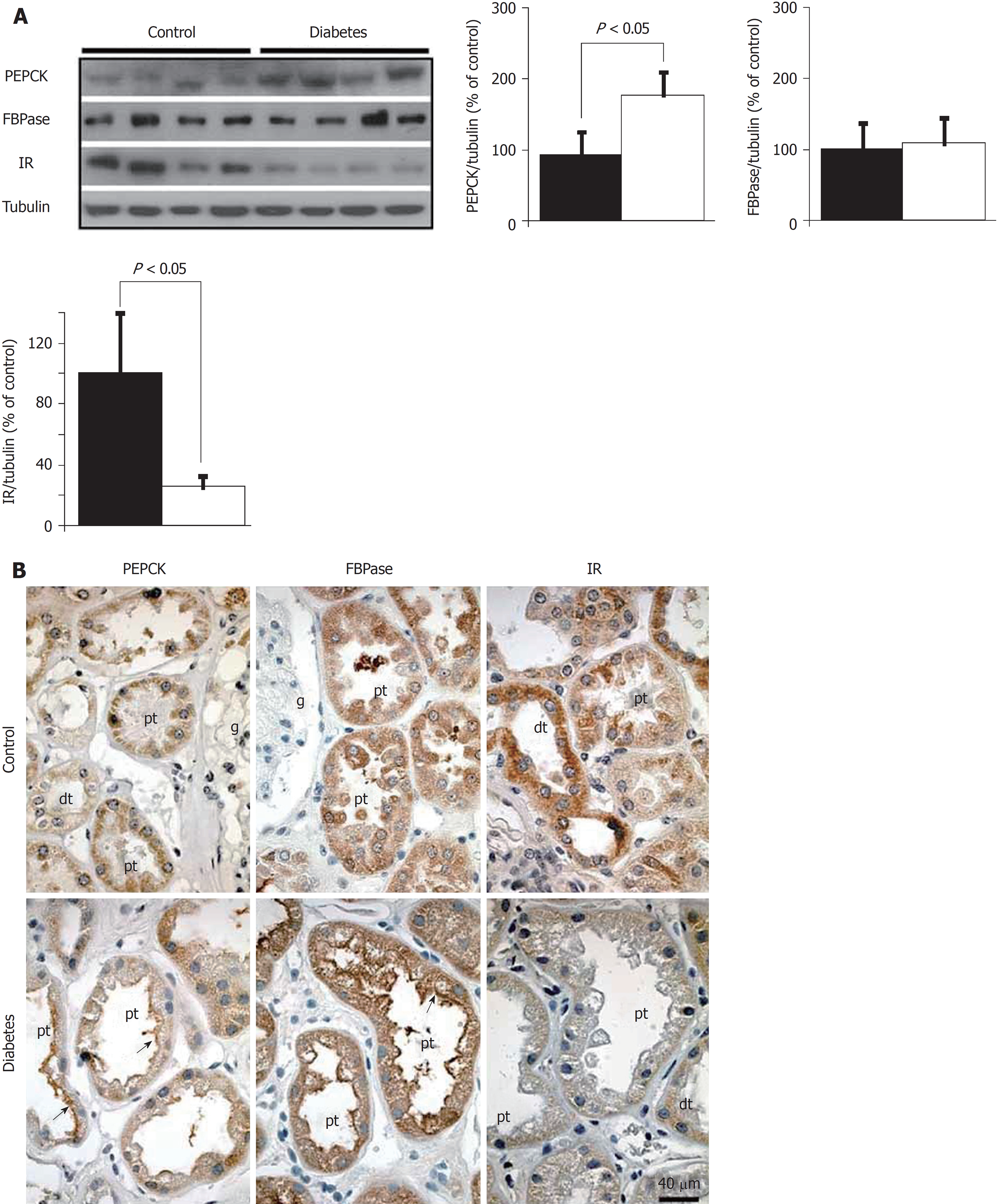
In vitro studies from our group has demonstrated that loss of IR in human proximal tubule cells attenuated the inhibitory effect of insulin on PEPCK expression in hPT cells[77]. These studies, suggest that impaired insulin sensitivity of PT may affect whole body glucose homeostasis by elevating gluconeogenesis via transcriptional induction of gluconeogenic enzymes in the kidney. However, the mechanism by which IR signaling targets gluconeogenic genes in PT needs to be further elucidated. A recent study from Yáñez lab demonstrated downregulation of IR levels, which was accompanied by increased expression and activity of PEPCK in the kidney of both Type 2 diabetic patients (Figure 4) and in a Type 1 diabetic rat model. Moreover, they also observed an apical redistribution of gluconeogenic genes in both the models, implying that insulin signaling may regulate gluconeogenesis through luminal substrate uptake[6]. Recently, Horita et al[78], put forward a concept of “selective insulin resistance” in kidney. The state of selective insulin resistance has been recognized in the case of liver, where inhibition of gluconeogenesis by the insulin receptor substrate (IRS) 2 is hindered, whereas IRS1-regulated lipogenesis is not altered. On the contrary, in kidney, IRS1-dependent inhibition of gluconeogenesis is impaired in the proximal tubule leading to hyperglycemia, while IRS2-dependent signaling is preserved[78-81]. Sasaki et al[82] have also reported the role of insulin signaling in maintaining systemic glucose homeostasis in IRS1/IRS2 double KO mice. This study emphasized dual regulation of gluconeogenesis by insulin signaling and glucose reabsorption. This is in consonance with previous studies suggesting impaired glucose levels in diabetic human PT because of enhanced glucose reabsorption and insulin-dependent inhibition of gluconeogenesis[74,83,84], ultimately leading to more glucose release by the kidney as compared to the liver[58]. In the light of these findings, regulation of renal gluconeogenesis is still a matter of debate because both suppression and elevation of gluconeogenic gene expression has been reported in experimental rodent models of diabetes[6,85]. These observations open a whole new avenue for investigating the role of IRs in renal glucose homeostasis.
Together, it can be implied that impaired renal insulin signaling (especially IR signaling) may increase gluconeogenesis, and in the setting of insulin resistance, these impairments can further contribute to other deleterious effects. Therefore, more conclusive studies are warranted to understand the pathophysiological association of renal insulin signaling and glucose metabolism.
The presence of proteins especially albumin in urine, aka proteinuria is an important hallmark of renal disease, including diabetic nephropathy. Although glomerular dysfunction is an established cause of proteinuria[86,87], impaired tubular function also contributes to albuminuria in diabetic nephropathy[88,89]. Normally, albumin is reabsorbed by the PT cells through receptor-mediated and fluid phase endocytosis[90]. In the proximal tubules, receptor-mediated reabsorption of albumin is executed by endocytic receptors, megalin and cubilin that are highly expressed in the apical membrane of the PT cells[91]. Existing evidences suggest that besides other factors, insulin could have a potential role in albumin uptake by the PT cells in diabetic and non-diabetic conditions. Retrieval of albumin from ultrafiltrate by the PT cells is crucial for kidney homeostasis. A cohort study on non-diabetic individuals (Relationship between Insulin Sensitivity and Cardiovascular Disease; RISC) proposed a causal relationship between insulin resistance and albuminuria[92]. The RISC study demonstrated that reduced insulin sensitivity, measured by a hyperinsulinemic-euglycemic clamp, is linked to increased risk of albuminuria in a healthy cohort.
Intriguingly, various studies have reported that insulin could also have a potential role in albumin uptake by the PT cells in diabetes. In the STZ-induced diabetic mice model, downregulation of pSer473-Akt expression in the proximal tubule epithelial cells was accompanied by decreased expression of megalin and cubilin establishing the link between insulin signaling and albumin uptake[93]. Recently, Zeng et al[94], showed that the ORAI (calcium release-activated calcium channels) are also accountable for the internalization of albumin in proximal tubular epithelial cells via clathrin-mediated endocytosis and expression of these channels is insulin-dependent. In concordance with previous studies, Mottl et al[95] showed that the urinary ACR was positively associated with insulin-resistant young Type 2 diabetic subjects. Moreover, insulin treatment under high-glucose conditions increased megalin expression and albumin internalization in OK cells[96]. Insulin treatment has attenuated urine albumin excretion in Akita mice also[97]. These reports establish a causal role of PT-specific insulin resistance in the pathogenesis of albuminuria; however, exact mechanism of insulin-dependent albumin uptake needs to be elucidated.
Recent data from our laboratory showed that targeted deletion of IRs from the proximal tubule impairs tubular albumin uptake and results in albuminuria in mice (unpublished data). We have also established circulating insulin levels as a determinant of tubular albumin uptake. Moreover, down-regulation of IR and early IR signaling in the kidney has been reported in Type 2 diabetes and models of insulin resistance[7] , which can contribute to elevated albuminuria. These recent findings support a direct physiological role of PT-specific insulin action on albumin uptake and albuminuria.
The physiological relevance of IRs in renal epithelial cells has gained more attention in recent years. Studies based on targeted deletion of IR have now provided sufficient evidence to suggest the significance of the renal IR in kidney physiology and pathology. In addition, these studies have enhanced our understanding surrounding the contribution of reduced renal IR observed in the insulin-resistance state. Overall, it can be suggested that modulation of insulin signaling at the receptor level could significantly affect kidney function, which thereby may result in systemic effects. However, more mechanistic studies are warranted to understand the causal role of reduced renal IR in the regulation of blood pressure, systemic glucose levels, and proteinuria.
We would like to thank Dr. Carolyn M Ecelbarger (Associate Professor, Division of Endocrinology and Metabolism, Georgetown University, Washington, DC, United States) for proofreading the manuscript.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: India
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Cheungpasitporn W, Taheri S, Trimarchi H, Stavroulopoulos A S- Editor: Ma YJ L- Editor: A E- Editor: Song H
| 1. | Gavin JR, Roth J, Neville DM Jr, de Meyts P, Buell DN. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci USA. 1974;71:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 612] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | Giorgino F, Almahfouz A, Goodyear LJ, Smith RJ. Glucocorticoid regulation of insulin receptor and substrate IRS-1 tyrosine phosphorylation in rat skeletal muscle in vivo. J Clin Invest. 1993;91:2020-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Grote CW, Groover AL, Ryals JM, Geiger PC, Feldman EL, Wright DE. Peripheral nervous system insulin resistance in ob/ob mice. Acta Neuropathol Commun. 2013;1:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Sechi LA, De Carli S, Bartoli E. In situ characterization of renal insulin receptors in the rat. J Recept Res. 1994;14:347-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Nakamura R, Emmanouel DS, Katz AI. Insulin binding sites in various segments of the rabbit nephron. J Clin Invest. 1983;72:388-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Gatica R, Bertinat R, Silva P, Carpio D, Ramírez MJ, Slebe JC, San Martín R, Nualart F, Campistol JM, Caelles C, Yáñez AJ. Altered expression and localization of insulin receptor in proximal tubule cells from human and rat diabetic kidney. J Cell Biochem. 2013;114:639-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Tiwari S, Halagappa VK, Riazi S, Hu X, Ecelbarger CA. Reduced expression of insulin receptors in the kidneys of insulin-resistant rats. J Am Soc Nephrol. 2007;18:2661-2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | De Meyts P. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, editors. SourceEndotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-2016 Apr 27; . [PubMed] |
| 9. | Hale LJ, Coward RJ. Insulin signalling to the kidney in health and disease. Clin Sci (Lond). 2013;124:351-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Ullrich A, Bell JR, Chen EY, Herrera R, Petruzzelli LM, Dull TJ, Gray A, Coussens L, Liao YC, Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature. 1985;313:756-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1578] [Cited by in RCA: 1623] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 11. | Ebina Y, Ellis L, Jarnagin K, Edery M, Graf L, Clauser E, Ou JH, Masiarz F, Kan YW, Goldfine ID. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985;40:747-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1045] [Cited by in RCA: 1080] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 12. | Seino S, Seino M, Nishi S, Bell GI. Structure of the human insulin receptor gene and characterization of its promoter. Proc Natl Acad Sci USA. 1989;86:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 232] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Hubbard SR. Structure and Mechanism of the Insulin Receptor Tyrosine Kinase. Handbook of Cell Signaling (Second Edition): Elsevier 2009; 307-313. |
| 14. | Bisbis S, Derouet M, Simon J. Characterization of insulin receptors in chicken kidneys: effect of nutritional status. Gen Comp Endocrinol. 1994;96:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Butlen D, Vadrot S, Roseau S, Morel F. Insulin receptors along the rat nephron: [125I] insulin binding in microdissected glomeruli and tubules. Pflugers Arch. 1988;412:604-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Tiwari S, Wade JB, Ecelbarger CA. Insulin receptor localization and regulation in rat kidney (Abstract). FASEB J. 2006;20:A1169. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Bates CM, Merenmies JM, Kelly-Spratt KS, Parada LF. Insulin receptor-related receptor expression in non-A intercalated cells in the kidney. Kidney Int. 1997;52:674-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Ozaki K, Takada N, Tsujimoto K, Tsuji N, Kawamura T, Muso E, Ohta M, Itoh N. Localization of insulin receptor-related receptor in the rat kidney. Kidney Int. 1997;52:694-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Tiwari S, Sharma N, Gill PS, Igarashi P, Kahn CR, Wade JB, Ecelbarger CM. Impaired sodium excretion and increased blood pressure in mice with targeted deletion of renal epithelial insulin receptor. Proc Natl Acad Sci USA. 2008;105:6469-6474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Tiwari S, Singh RS, Li L, Tsukerman S, Godbole M, Pandey G, Ecelbarger CM. Deletion of the insulin receptor in the proximal tubule promotes hyperglycemia. J Am Soc Nephrol. 2013;24:1209-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Whelton PK, Appel LJ, Sacco RL, Anderson CA, Antman EM, Campbell N, Dunbar SB, Frohlich ED, Hall JE, Jessup M, Labarthe DR, MacGregor GA, Sacks FM, Stamler J, Vafiadis DK, Van Horn LV. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation. 2012;126:2880-2889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 325] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 22. | Lambers Heerspink HJ, Holtkamp FA, Parving HH, Navis GJ, Lewis JB, Ritz E, de Graeff PA, de Zeeuw D. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 2012;82:330-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 23. | Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2017;4:CD004022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 24. | Wang X, Armando I, Upadhyay K, Pascua A, Jose PA. The regulation of proximal tubular salt transport in hypertension: an update. Curr Opin Nephrol Hypertens. 2009;18:412-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Doris PA. Renal proximal tubule sodium transport and genetic mechanisms of essential hypertension. J Hypertens. 2000;18:509-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Capasso G, Rizzo M, Garavaglia ML, Trepiccione F, Zacchia M, Mugione A, Ferrari P, Paulmichl M, Lang F, Loffing J, Carrel M, Damiano S, Wagner CA, Bianchi G, Meyer G. Upregulation of apical sodium-chloride cotransporter and basolateral chloride channels is responsible for the maintenance of salt-sensitive hypertension. Am J Physiol Renal Physiol. 2008;295:F556-F567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Horita S, Seki G, Yamada H, Suzuki M, Koike K, Fujita T. Insulin resistance, obesity, hypertension, and renal sodium transport. Int J Hypertens. 2011;2011:391762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Sechi LA, Griffin CA, Schambelan M. Effect of dietary sodium chloride on insulin receptor number and mRNA levels in rat kidney. Am J Physiol. 1994;266:F31-F38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Catena C, Cavarape A, Novello M, Giacchetti G, Sechi LA. Insulin receptors and renal sodium handling in hypertensive fructose-fed rats. Kidney Int. 2003;64:2163-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Blazer-Yost BL, Esterman MA, Vlahos CJ. Insulin-stimulated trafficking of ENaC in renal cells requires PI 3-kinase activity. Am J Physiol Cell Physiol. 2003;284:C1645-C1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Féraille E, Carranza ML, Gonin S, Béguin P, Pedemonte C, Rousselot M, Caverzasio J, Geering K, Martin PY, Favre H. Insulin-induced stimulation of Na+,K(+)-ATPase activity in kidney proximal tubule cells depends on phosphorylation of the alpha-subunit at Tyr-10. Mol Biol Cell. 1999;10:2847-2859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Fuster DG, Bobulescu IA, Zhang J, Wade J, Moe OW. Characterization of the regulation of renal Na+/H+ exchanger NHE3 by insulin. Am J Physiol Renal Physiol. 2007;292:F577-F585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Salvetti A, Brogi G, Di Legge V, Bernini GP. The inter-relationship between insulin resistance and hypertension. Drugs. 1993;46 Suppl 2:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Hall JE. Mechanisms of abnormal renal sodium handling in obesity hypertension. Am J Hypertens. 1997;10:49S-55S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 131] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 819] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 36. | Soleimani M. Insulin resistance and hypertension: new insights. Kidney Int. 2015;87:497-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 37. | Nizar JM, Walczak EM, Dong W, Bankir L, Bhalla V. Inducible Renal Tubule-specific Insulin Receptor Knockout Mice Have Decreased NCC-mediated Sodium Reabsorption and Reduced Sensitivity to Mineralocorticoid-induced Hypertension in Obesity and Insulin Resistance (Abstract). FASEB J. 2016;30:968. |
| 38. | Hayashi K, Fujiwara K, Oka K, Nagahama T, Matsuda H, Saruta T. Effects of insulin on rat renal microvessels: studies in the isolated perfused hydronephrotic kidney. Kidney Int. 1997;51:1507-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Schmetterer L, Müller M, Fasching P, Diepolder C, Gallenkamp A, Zanaschka G, Findl O, Strenn K, Mensik C, Tschernko E, Eichler HG, Wolzt M. Renal and ocular hemodynamic effects of insulin. Diabetes. 1997;46:1868-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Ter Maaten JC, Bakker SJ, Serné EH, Moshage HJ, Donker AJ, Gans RO. Insulin-mediated increases in renal plasma flow are impaired in insulin-resistant normal subjects. Eur J Clin Invest. 2000;30:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Scherrer U, Sartori C. Insulin as a vascular and sympathoexcitatory hormone: implications for blood pressure regulation, insulin sensitivity, and cardiovascular morbidity. Circulation. 1997;96:4104-4113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 236] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 42. | Erdely A, Freshour G, Maddox DA, Olson JL, Samsell L, Baylis C. Renal disease in rats with type 2 diabetes is associated with decreased renal nitric oxide production. Diabetologia. 2004;47:1672-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Elkayam U, Cohen G, Gogia H, Mehra A, Johnson JV, Chandraratna PA. Renal vasodilatory effect of endothelial stimulation in patients with chronic congestive heart failure. J Am Coll Cardiol. 1996;28:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na(+)-K(+)-2Cl(-) cotransporter activity. Am J Physiol Renal Physiol. 2001;281:F819-F825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Stoos BA, Garcia NH, Garvin JL. Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J Am Soc Nephrol. 1995;6:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 46. | Li L, Garikepati RM, Tsukerman S, Tiwari S, Ecelbarger CM. Salt sensitivity of nitric oxide generation and blood pressure in mice with targeted knockout of the insulin receptor from the renal tubule. Am J Physiol Regul Integr Comp Physiol. 2012;303:R505-R512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Cowley AW Jr, Mattson DL, Lu S, Roman RJ. The renal medulla and hypertension. Hypertension. 1995;25:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 150] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Cowley AW Jr, Mori T, Mattson D, Zou AP. Role of renal NO production in the regulation of medullary blood flow. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1355-R1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 49. | Wu F, Park F, Cowley AW Jr, Mattson DL. Quantification of nitric oxide synthase activity in microdissected segments of the rat kidney. Am J Physiol. 1999;276:F874-F881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Pandey G, Makhija E, George N, Chakravarti B, Godbole MM, Ecelbarger CM, Tiwari S. Insulin regulates nitric oxide production in the kidney collecting duct cells. J Biol Chem. 2015;290:5582-5591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Underwood PC, Adler GK. The renin angiotensin aldosterone system and insulin resistance in humans. Curr Hypertens Rep. 2013;15:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 52. | Csibi A, Communi D, Müller N, Bottari SP. Angiotensin II inhibits insulin-stimulated GLUT4 translocation and Akt activation through tyrosine nitration-dependent mechanisms. PLoS One. 2010;5:e10070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 53. | Yavuz D, Koç M, Toprak A, Akpinar I, Velioğlu A, Deyneli O, Haklar G, Akalin S. Effects of ACE inhibition and AT1-receptor antagonism on endothelial function and insulin sensitivity in essential hypertensive patients. J Renin Angiotensin Aldosterone Syst. 2003;4:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Andreozzi F, Laratta E, Sciacqua A, Perticone F, Sesti G. Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circ Res. 2004;94:1211-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 55. | Stumvoll M, Meyer C, Mitrakou A, Nadkarni V, Gerich JE. Renal glucose production and utilization: new aspects in humans. Diabetologia. 1997;40:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 158] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 56. | Stumvoll M, Meyer C, Mitrakou A, Gerich JE. Important role of the kidney in human carbohydrate metabolism. Med Hypotheses. 1999;52:363-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Cano N. Bench-to-bedside review: glucose production from the kidney. Crit Care. 2002;6:317-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Meyer C, Stumvoll M, Nadkarni V, Dostou J, Mitrakou A, Gerich J. Abnormal renal and hepatic glucose metabolism in type 2 diabetes mellitus. J Clin Invest. 1998;102:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 187] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 59. | Stumvoll M, Meyer C, Kreider M, Perriello G, Gerich J. Effects of glucagon on renal and hepatic glutamine gluconeogenesis in normal postabsorptive humans. Metabolism. 1998;47:1227-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Cersosimo E, Judd RL, Miles JM. Insulin regulation of renal glucose metabolism in conscious dogs. J Clin Invest. 1994;93:2584-2589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | Bergman H, Drury DR. The relationship of kidney function to the glucose utilization of the extra abdominal tissues. Am J Physiol (legacy content). 1938;124:279-284. [RCA] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Wilding JP. The role of the kidneys in glucose homeostasis in type 2 diabetes: clinical implications and therapeutic significance through sodium glucose co-transporter 2 inhibitors. Metabolism. 2014;63:1228-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 63. | Krebs HA, Bennett DA, DE Gasquet P, Gasquet P, Gascoyne T, Yoshida T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963;86:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 410] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 64. | Peres LA, Bredt LC, Cipriani RF. Acute renal injury after partial hepatectomy. World J Hepatol. 2016;8:891-901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Coward RJ, Welsh GI, Yang J, Tasman C, Lennon R, Koziell A, Satchell S, Holman GD, Kerjaschki D, Tavaré JM, Mathieson PW, Saleem MA. The human glomerular podocyte is a novel target for insulin action. Diabetes. 2005;54:3095-3102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 228] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 66. | Cersosimo E, Garlick P, Ferretti J. Insulin regulation of renal glucose metabolism in humans. Am J Physiol. 1999;276:E78-E84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 67. | Meyer C, Dostou J, Nadkarni V, Gerich J. Effects of physiological hyperinsulinemia on systemic, renal, and hepatic substrate metabolism. Am J Physiol. 1998;275:F915-F921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | McGivan JD, Pastor-Anglada M. Regulatory and molecular aspects of mammalian amino acid transport. Biochem J. 1994;299:321-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 267] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 69. | Eid A, Bodin S, Ferrier B, Delage H, Boghossian M, Martin M, Baverel G, Conjard A. Intrinsic gluconeogenesis is enhanced in renal proximal tubules of Zucker diabetic fatty rats. J Am Soc Nephrol. 2006;17:398-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 70. | Tejada T, Catanuto P, Ijaz A, Santos JV, Xia X, Sanchez P, Sanabria N, Lenz O, Elliot SJ, Fornoni A. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int. 2008;73:1385-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 71. | Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab. 2003;285:E685-E692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 340] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 72. | Valenti L, Rametta R, Dongiovanni P, Maggioni M, Fracanzani AL, Zappa M, Lattuada E, Roviaro G, Fargion S. Increased expression and activity of the transcription factor FOXO1 in nonalcoholic steatohepatitis. Diabetes. 2008;57:1355-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 73. | Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 803] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 74. | DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012;14:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 357] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 75. | Winzell MS, Ahrén B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53 Suppl 3:S215-S219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 769] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 76. | Radziuk J, Pye S. Hepatic glucose uptake, gluconeogenesis and the regulation of glycogen synthesis. Diabetes Metab Res Rev. 2001;17:250-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 187] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 77. | Pandey G, Shankar K, Makhija E, Gaikwad A, Ecelbarger C, Mandhani A, Srivastava A, Tiwari S. Reduced Insulin Receptor Expression Enhances Proximal Tubule Gluconeogenesis. J Cell Biochem. 2017;118:276-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 78. | Horita S, Nakamura M, Suzuki M, Satoh N, Suzuki A, Seki G. Selective Insulin Resistance in the Kidney. Biomed Res Int. 2016;2016:5825170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 79. | Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Alemán JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, Cohen DE, King GL, Ginsberg HN, Kahn CR. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 363] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 80. | Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 742] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 81. | Laplante M, Sabatini DM. mTORC1 activates SREBP-1c and uncouples lipogenesis from gluconeogenesis. Proc Natl Acad Sci USA. 2010;107:3281-3282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 82. | Sasaki M, Sasako T, Kubota N, Sakurai Y, Takamoto I, Kubota T, Inagi R, Seki G, Goto M, Ueki K, Nangaku M, Jomori T, Kadowaki T. Dual Regulation of Gluconeogenesis by Insulin and Glucose in the Proximal Tubules of the Kidney. Diabetes. 2017;66:2339-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 83. | Yañez AJ, Ludwig HC, Bertinat R, Spichiger C, Gatica R, Berlien G, Leon O, Brito M, Concha II, Slebe JC. Different involvement for aldolase isoenzymes in kidney glucose metabolism: aldolase B but not aldolase A colocalizes and forms a complex with FBPase. J Cell Physiol. 2005;202:743-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Mitrakou A. Kidney: its impact on glucose homeostasis and hormonal regulation. Diabetes Res Clin Pract. 2011;93 Suppl 1:S66-S72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 85. | Wang Q, Guo T, Portas J, McPherron AC. A soluble activin receptor type IIB does not improve blood glucose in streptozotocin-treated mice. Int J Biol Sci. 2015;11:199-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 86. | Tesch GH. Review: Serum and urine biomarkers of kidney disease: A pathophysiological perspective. Nephrology (Carlton). 2010;15:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 87. | Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med. 1984;311:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1005] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 88. | Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol. 2009;20:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 89. | Tojo A, Onozato ML, Ha H, Kurihara H, Sakai T, Goto A, Fujita T, Endou H. Reduced albumin reabsorption in the proximal tubule of early-stage diabetic rats. Histochem Cell Biol. 2001;116:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 90. | Dickson LE, Wagner MC, Sandoval RM, Molitoris BA. The proximal tubule and albuminuria: really! J Am Soc Nephrol. 2014;25:443-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 202] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 91. | Christensen EI, Birn H. Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. Am J Physiol Renal Physiol. 2001;280:F562-F573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 250] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 92. | Pilz S, Rutters F, Nijpels G, Stehouwer CD, Højlund K, Nolan JJ, Balkau B, Dekker JM; RISC Investigators. Insulin sensitivity and albuminuria: the RISC study. Diabetes Care. 2014;37:1597-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 93. | Coffey S, Costacou T, Orchard T, Erkan E. Akt Links Insulin Signaling to Albumin Endocytosis in Proximal Tubule Epithelial Cells. PLoS One. 2015;10:e0140417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 94. | Zeng B, Chen GL, Garcia-Vaz E, Bhandari S, Daskoulidou N, Berglund LM, Jiang H, Hallett T, Zhou LP, Huang L, Xu ZH, Nair V, Nelson RG, Ju W, Kretzler M, Atkin SL, Gomez MF, Xu SZ. ORAI channels are critical for receptor-mediated endocytosis of albumin. Nat Commun. 2017;8:1920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 95. | Mottl AK, Divers J, Dabelea D, Maahs DM, Dolan L, Pettitt D, Marcovina S, Imperatore G, Pihoker C, Mauer M, Mayer-Davis EJ; SEARCH for Diabetes in Youth Study. The dose-response effect of insulin sensitivity on albuminuria in children according to diabetes type. Pediatr Nephrol. 2016;31:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 96. | Bryniarski MA, Yee BM, Jaffri I, Chaves LD, Yu JA, Guan X, Ghavam N, Yacoub R, Morris ME. Increased Megalin Expression in Early Type 2 Diabetes: Role of Insulin Signaling Pathways. Am J Physiol Renal Physiol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 97. | Salem ES, Grobe N, Elased KM. Insulin treatment attenuates renal ADAM17 and ACE2 shedding in diabetic Akita mice. Am J Physiol Renal Physiol. 2014;306:F629-F639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |