Peer-review started: November 10, 2017
First decision: December 13, 2017
Revised: December 25, 2017
Accepted: January 23, 2018
Article in press: January 23, 2018
Published online: March 6, 2018
Processing time: 116 Days and 14.9 Hours
Diabetic muscle infarction (DMI) refers to spontaneous ischemic necrosis of skeletal muscle among people with diabetes mellitus, unrelated to arterial occlusion. People with DMI may have coexisting end-stage renal disease (ESRD) but little is known about its epidemiology and clinical outcomes in this setting. This scoping review seeks to investigate the characteristics, clinical features, diagnostic evaluation, management and outcomes of DMI among people with ESRD. Electronic database (PubMed/MEDLINE, CINAHL, SCOPUS and EMBASE) searches were conducted for (“diabetic muscle infarction” or “diabetic myonecrosis”) and (“chronic kidney disease” or “renal impairment” or “dialysis” or “renal replacement therapy” or “kidney transplant”) from January 1980 to June 2017. Relevant cases from reviewed bibliographies in reports retrieved were also included. Data were extracted in a standardized form. A total of 24 publications with 41 patients who have ESRD were included. The mean age at the time of presentation with DMI was 44.2 years. Type 2 diabetes was present in 53.7% of patients while type 1 in 41.5%. In this cohort, 60.1% were receiving hemodialysis, 21% on peritoneal dialysis and 12.2% had kidney transplantation. The proximal lower limb musculature was the most commonly affected site. Muscle pain and swelling were the most frequent manifestation on presentation. Magnetic resonance imaging (MRI) provided the most specific findings for DMI. Laboratory investigation findings are usually non-specific. Non-surgical therapy is usually used in the management of DMI. Short-term prognosis of DMI is good but recurrence occurred in 43.9%. DMI is an uncommon complication in patients with diabetes mellitus, including those affected by ESRD. In comparison with unselected patients with DMI, the characteristics and outcomes of those with ESRD are generally similar. DMI may also occur in kidney transplant recipients, including pancreas-kidney transplantation. MRI is the most useful diagnostic investigation. Non-surgical treatment involving analgesia, optimization of glycemic control and initial bed rest can help to improve recovery rate. However, recurrence of DMI is relatively frequent.
Core tip: Diabetic muscle infarction (DMI) is an uncommon complication in patients with end-stage renal disease, including kidney transplant recipients. Early recognition of DMI is vital to initiation of prompt treatment. Magnetic resonance imaging is the investigation of choice for diagnosing DMI. Non-surgical treatment involving analgesia, optimization of glycemic control and initial bed rest appears to improve recovery rate. However, recurrence of DMI is relatively common.
- Citation: Yong TY, Khow KSF. Diabetic muscle infarction in end-stage renal disease: A scoping review on epidemiology, diagnosis and treatment. World J Nephrol 2018; 7(2): 58-64
- URL: https://www.wjgnet.com/2220-6124/full/v7/i2/58.htm
- DOI: https://dx.doi.org/10.5527/wjn.v7.i2.58
Diabetic muscle infarction (DMI) refers to spontaneous ischemic necrosis of skeletal muscle among people with diabetes mellitus, unrelated to atheroembolism or occlusion of major arteries. DMI is also referred to as spontaneous diabetic myonecrosis. Angervall and Sterner initially described DMI in 1965 as “tumoriform focal muscular degeneration”[1]. Although diabetes mellitus is a relatively common condition, DMI is a comparatively infrequent complication.
Diabetic nephropathy or other forms of chronic kidney disease are commonly seen in patients with DMI, affecting as many as 71%[2]. Of the diabetes-related microvascular complications, diabetic nephropathy is the most common one to be present among patients with DMI. Therefore, a proportion of DMI occurs in patients with end-stage renal disease (ESRD). However, little is known about the manifestation, diagnosis, management and outcomes of DMI in patients with ESRD.
This scoping review seeks to answer several questions about DMI regarding patient characteristics, clinical features, diagnostic evaluation, management and outcomes when this condition occurs in people with ESRD.
The literature search was performed in July 2017 in four electronic databases. We searched MEDLINE/PubMed, CINAHL, SCOPUS and EMBASE with key words: (“Diabetic muscle infarction” OR “Diabetic myonecrosis”), AND (“Chronic kidney disease” OR “Renal impairment” OR “Dialysis” OR “Renal replacement therapy” OR “Renal transplant”). The search was done on publications between January 1980 and June 2017. Searches were limited to English language publications. Relevant references from articles identified from the search were also reviewed for comprehensive identification.
This scoping review included cohort studies, case series and case reports with DMI in the setting of ESRD. Inclusion criteria include patients with ESRD requiring renal replacement therapy (RRT), clinical presentation of cases were adequately described, sufficient data on investigations, treatment and clinical outcome. The authors excluded studies that had not sufficiently eliminated trauma, inflammatory myositis, infection, neoplasm and deep vein thrombosis as possible aetiologies.
The selection process consisted of three stages. In the first stage, two reviewers (KK and TY) independently screened articles based on the title. In the case of doubt or disagreement, the articles were included in the abstract review stage. In the second stage, the abstracts of all articles are selected from the initial stage were reviewed and assessed by two reviewers (KK and TY). Any disagreement was resolved by discussion between the two reviewers. In the final stage, the remaining articles were fully reviewed using pre-determined inclusion criteria and assessed by two independent reviewers (KK and TY). Any disagreement between reviewers was solved by the two reviewers.
A standardized template was used to extract data from the included studies using the following heading: general information such as title of article, main author and publication year, patient or cohort characteristics, type of diabetes mellitus, type of RRT (hemodialysis, peritoneal dialysis and kidney transplant), microvascular complications (retinopathy and peripheral neuropathy), macrovascular complications (coronary artery disease, ischaemic strokes and peripheral arterial disease) and pattern of muscle involvement. Laboratory and radiological investigation findings during initial evaluation or during recurrence were also obtained. Clinical outcomes to be considered in this review include time to recovery from DMI, recurrence rate and death during the follow-up period.
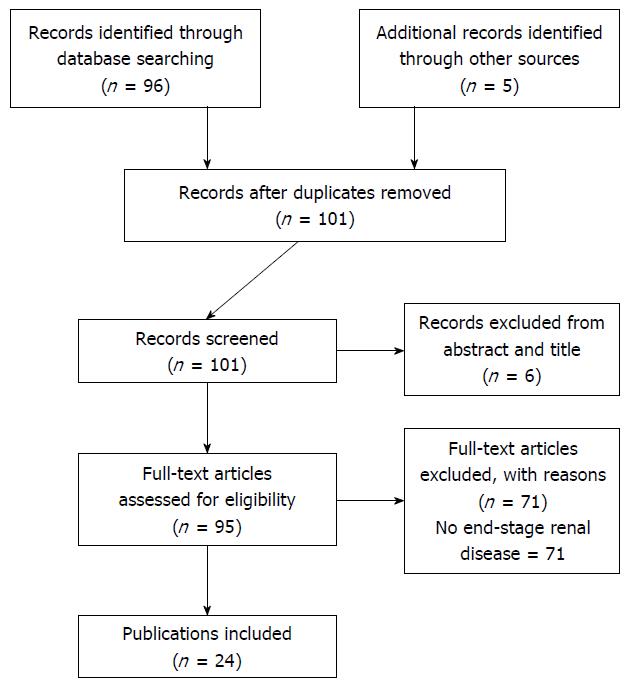
We identified 101 articles from our initial search. Six were excluded because these were published in non-English languages (Figure 1). Twenty-four publications containing 41 cases with DMI in the setting of ESRD were eligible to be included in this review[3-26].
Of the 41 patients with DMI in the setting of ESRD, 22 occurred in women (53.7%). The mean age of presentation of all cases was 44.2 years (range: 19.0-67.0 years). Of these, 17 (41.5%) had type 1 diabetes, 22 (53.7%) had type 2 diabetes and 2 (4.9%) had cystic fibrosis related diabetes (Table 1). One report did not provide information about the type of diabetes that the patient had. The two patients with cystic fibrosis also had lung transplantation. The mean duration of diabetes mellitus at the time of DMI diagnosis was 17.3 (range: 10.0-30.0) years for type 1 diabetes and 15.7 (range: 3.0-24.0) years for type 2 diabetes. Glycohemoglobin (HbA1c) values at the time of DMI diagnosis were reported in 18 cases and the average value was 7.2% (range: 5.0%-12.4%). Eleven out of 18 (61.1%) had HbA1c above 7.0%.
| Current review (n = 41) | Findings from Horton et al[2] (n = 126) | |
| Mean age (range), yr | 44.2 (19.0-67.0) | 44.6 (20.0-67.0) |
| Female/male, n (%) | 22 (53.7)/19 (46.3) | 68 (54.0)/58 (46.0) |
| Type of diabetes | ||
| Type 1, n (%) | 17 (41.5) | 54/108 (50.0) |
| Type 2, n (%) | 22 (53.7) | 45/108 (41.7) |
| Cystic-fibrosis-related, n (%) | 2 (4.9) | 2/108 (1.9) |
| Concurrent diabetes-related complications | ||
| Retinopathy, n (%) | 24 (58.5) | 83 (65.8) |
| Neuropathy, n (%) | 22 (56.1) | - |
| Coronary artery disease, n (%) | 6 (14.6) | - |
| Peripheral arterial disease, n (%) | 3 (7.3) | - |
| Type of renal replacement therapy | ||
| Hemodialysis, n (%) | 25 (60.1) | - |
| Peritoneal dialysis, n (%) | 9 (21.9) | - |
| Pancreas-kidney transplantation, n (%) | 4 (9.8) | - |
| Kidney transplantation only, n (%) | 1 (2.4) | - |
| Pattern of muscle involvement | ||
| Lower limbs | 36 (66.7) | - |
| Proximal lower limbs (above knee) | 24 (58.5) | 90 (71.2) |
| Distal lower limbs (below knee) | 6 (14.6) | 19 (15.3) |
| Both proximal and distal lower limbs | 6 (14.6) | - |
| Upper limbs | 5 (12.2) | 7 (5.4) |
| Unilateral limb | 33 (80.5) | - |
All patients in this cohort had diabetic nephropathy. The RRT modalities included hemodialysis (25 patients; 59%), peritoneal dialysis (9; 22%), combined kidney-pancreas transplantation (4; 16%), kidney transplantation (1; 3%). For eight patients in whom information was provided, the mean duration of receiving dialysis was 2.1 years (range: 2 mo to 6 years). Twenty-four patients had concurrent retinopathy (58.5%) and 23 had peripheral neuropathy (56.1%). In terms of macrovascular diseases, six were reported to have coronary artery disease and three had peripheral arterial disease.
The most frequently affected muscle groups were in the lower limbs (36; 87.8%), in comparison with the upper limbs which were reported only in five cases[11,22]. In the lower limb, DMI more commonly affected the proximal leg (24/36; 66.7% of those with lower limb involvement) than distal leg, i.e., below knee (6/36; 16.7%). Six patients (16.7%) had both proximal and distal leg muscles involved. Most had unilateral (80.5%) involvement on initial presentation, while eight cases had bilateral involvement. Sixteen (39.0%) involved multiple muscle groups on initial presentation.
The most common clinical presentation of DMI was abrupt onset of pain in the affected muscle group (100%), accompanied by local swelling (97.6%). Fever was reported in six patients (14.6%). In the current analysis, compartment syndrome has been reported in one case[23].
Results of laboratory investigations are usually non-specific for DMI. White cell count (WCC) values were reported in 35 cases but only 31.4% were elevated (Table 2). One patient was found to be leucopenic[15]. Inflammatory markers are commonly increased in DMI. C-reactive protein (CRP) was reported in 11 cases and was elevated in nine (81.8%) (Table 2). The mean CRP in these cases was 153.6 mg/L (range: 5.0-361.0 mg/L). Erythrocyte sedimentation rate (ESR) in nine cases and was increased in eight (88.9%) (Table 2). The mean ESR was 82.9 mm/h (range: 3.0-137.0 mm/h).
| Present review | Findings from Horton et al[2] | ||
| Number of patients who had the investigation | n (%) | n/n (%) | |
| Leucocytosis (WCC > 11.0 × 109 cells/L) | 35 | 11 (31.4) | 48/112 (42.5) |
| Leucopenia (WCC < 4.0 × 109 cells/L) | 35 | 1 (2.9) | - |
| Elevated CRP (> 10 mg/L) | 11 | 9 (81.8) | 27/30 (90.0) |
| Elevated ESR (> 20 mm/h) | 8 | 9 (88.9) | 50/60 (83.3) |
| Elevated creatine kinase (> 150 IU/L for females; > 250 IU/L for males) | 34 | 17 (50.0) | 31/98 (31.6) |
| HbA1c > 7.0%, n (%) | 18 | 11 (61.1) | - |
| MRI findings | 35 (85.4) | ||
| Muscle enlargement | 35 | 33 (94.2) | - |
| Muscle edema | 35 | 30 (85.7) | 76.8 |
| Subcutaneous edema | 35 | 17 (43.6) | - |
| Muscle biopsy findings | 16 (39.0) | ||
| Muscle necrosis | 16 | 16 (100) | - |
| Inflammatory cell infiltration | 16 | 14 (87.5) | - |
| Muscle fibre regeneration | 16 | 7 (43.8) | - |
Creatine kinase (CK) values were reported in 34 cases but was elevated in 50.0% (considered as greater than 150.0 IU/L for females or 250.0 IU/L for males) (Table 2). When measured on initial presentation, the mean absolute CK was 292.9 IU/L (range: 23.0-1066.0 IU/L).
Magnetic resonance imaging (MRI) was performed in most patients (35/41; 85.4%). The common abnormalities observed on MRI is muscle enlargement (33/35; 94.2%) and muscle oedema with hyperintense T2 signal (30/35; 85.7%) (Table 2). Other findings include subcutaneous edema (17/35; 48.6%).
Ultrasound was performed in 28 (68.3%) patients but all were done to exclude deep vein thrombosis. Subcutaneous or muscle edema on ultrasound was reported in six cases (21.4%). Computed tomography (CT) was performed less frequently among cases included in this review (12/41; 29.3%).
Muscle biopsy was performed in 16 cases (39.0%). Biopsies usually demonstrate muscle necrosis (100%) and inflammatory cell infiltration (87.5%) (Table 2). In some cases, muscle fibre regeneration (43.8%) was also present.
Treatment details were provided in 36 cases. Most patients (31/36; 86%) were managed with analgesia. Four patients received aspirin therapy. There was no clear description in most cases as to whether patients rested in bed or received physiotherapy. Five patients (13.8%) required surgical intervention, which involved debridement of the affected muscle.
In a small number of patients, intensification of glycaemic control (four patients) and dialysis (one patient) were reported. Seven patients received other forms of treatment which included antibiotics, prednisolone and erythropoietin therapy. However, the effectiveness of these treatments could not be ascertained in this review due to the limited numbers.
As the management approaches to DMI vary considerably in between patients, the efficacy of each treatment modality or the comparisons of outcomes could not be evaluated in this small sample size. In 15 patients, the time interval between diagnosis and resolution of the initial presentation of DMI was 1.4 mo (range: 2 wk to 4 mo).
Recurrent muscle infarctions were reported in 43.9% of cases. None of the pancreas-kidney transplant recipients experienced any recurrence of DMI. Of those with recurrence of DMI, the mean time interval between the initial episode and the first recurrence was 5.3 mo (range: one week to 18 mo). Most of the recurrences in this case series occurred in a different muscle group to the initial episode (9/11; 81.8%).
In the cases reviewed, seven patients died during the follow-up period after developing DMI. Five of these patients were receiving haemodialysis and two were on peritoneal dialysis. No death was reported among kidney transplant recipients with DMI.
DMI is a rare disorder encountered in patients with longstanding diabetes mellitus and end-organ complications. Among patients with ESRD, the manifestation of DMI, investigation findings, management approach and rate of recurrence are similar to the other patients without ESRD who develop DMI. However, this study was not designed to estimate the incidence of DMI among patients with ESRD.
In a review by Trujillo-Santos et al[27] of 166 episodes of DMI in 115 patients, the mean age at the time of presentation was 42.6 years. The mean duration between diagnosis of diabetes mellitus and first episode of DMI was 14.4 years. In the present review, those with DMI in the setting of ESRD also have a similar age at the time of presentation and duration of diagnosis with diabetes mellitus. As this was a review of case report and series, it is not possible to determine if there is any difference in incidence of DMI between type 1 and type 2 diabetes. Similarly, any difference in incidence between patients who receive hemodialysis and peritoneal dialysis cannot be established in this review. Nonetheless, this review indicates that DMI can occur in patients after kidney transplantations including combined pancreas and kidney transplantations. Unsurprisingly, most patients have other concurrent diabetes-related microvascular complications such as retinopathy and peripheral neuropathy. In relation to patient characteristics, the current cohort of patients with ESRD and DMI was relatively similar to an unselected group of patients with DMI[2].
There is a propensity for DMI to occur among patients with longstanding diabetes mellitus whose glycaemic control has deteriorated over time. More than 60% of ESRD patients who develop DMI have HbA1c of greater than 7.0%, which indicated inadequate glycaemic control. DMI has been reported in a number of cases with poor glycaemic control[28]. The pathophysiology for DMI is still unknown but several hypotheses have been suggested. Atherosclerotic changes, diabetes-related microangiopathy, vasculitis with associated thrombosis, and ischemia-reperfusion injury have been postulated as possible mechanism for DMI[29]. The thromboembolic mechanism is thought to involve endothelial damage resulting in tissue ischemia which leads to muscle injury and ischaemic necrosis. Reperfusion of ischaemic muscles lead to increase in oxygen radicals and reduced nitric oxide, which in turn releases inflammatory mediators. Alterations in the coagulation-fibrinolysis system resulting in damage of vascular endothelium and hypercoagulability have also been suggested as part of the pathophysiology of DMI[30].
The diagnosis of DMI remains challenging and can lead to underdiagnosis[31]. At present, the diagnosis of DMI involves the combination of clinical assessment, MRI and muscle biopsy with atypical presentations. DMI is characterized by localized muscular pain, swelling and tenderness and there is a more frequent involvement of the lower limb musculature, especially the thigh. Rare cases of upper limb muscle involvement have been reported[11,22]. The reason for this predilection for lower limb muscles is uncertain.
Although MRI findings are not pathognomonic for DMI, this modality is still the most sensitive and specific for diagnosis of this condition[2]. Characteristic features on MRI include muscle enlargement, oedema within affected muscles, subcutaneous and interfascial oedema[32]. On MRI examination, the differential diagnoses include intramuscular abscess, inflammatory myositis and necrotising fasciitis[27]. Sometimes it can be difficult to distinguish these entities and muscle biopsy may be required. Other imaging modalities such as ultrasound or CT scans may assist in excluding other disorders but may not enable the correct diagnosis of DMI. In patients with ESRD, calciphylaxis or calcific uremic arteriolopathy (CUA) also needs to be considered in the differential diagnosis. CUA is characterized by vascular and other soft tissue calcification, intimal hypertrophy, and thrombosis of small vessels that results in painful tissue necrosis, including skeletal muscle[33]. The ischaemic injury in DMI is more confined to the muscle whereas in CUA, cutaneous and other soft tissues can be affected. A histopathological feature of CUA will often reveal extensive calcification, microthrombosis and endovascular fibrosis of small subcutaneous arteries leading to cutaneous ischemia whereas the calcification is not a prominent feature of DMI[33].
Muscle biopsy can lead to a conclusive diagnosis but this is not currently recommended because of the risk from procedure-related complications[34]. It can be used as a diagnostic tool in atypical presentations of DMI. Histopathological changes in DMI can vary depending on the timing of biopsy. In general, DMI presents as a pale nonhemorrhagic muscle. Initially, light microscopy would usually show muscle necrosis and phagocytosis of necrotic muscle fibres. At a later stage, the necrotic muscles are replaced by fibrous tissue, lymphocyte infiltration and muscle regeneration.
Blood investigations are generally non-specific for DMI. Surprisingly, a rise in CK has only been reported in half of the patients with DMI and ESRD. It is possible that the lack of rise in CK may be related to the delay in some patients seeking attention and measurement is performed after this marker has peaked. This is relatively consistent with other systematic review of unselected patients with DMI. CRP and ESR were commonly elevated in DMI but these markers were not specific for this condition.
Early recognition of DMI is important for prompt initiation of treatment. Among patients with DMI and ESRD, supportive care consisting of analgesia was the main management approach. Generally, patients with DMI also receive intensive glycaemic control. Some clinicians have recommended avoiding physical therapy during the acute phase of DMI because it can prolong recovery but others have not observed this[4,35]. Surgical intervention is required only in selected cases but this is not first-line option because of association with a more prolonged recovery[14]. Other treatment modalities such as glucocorticoid, antibiotics and erythropoietin have been tried but their efficacy in DMI is uncertain.
The short-term prognosis of DMI is good and most patients recover within 6 mo but recurrence is frequent among patients with ESRD. Almost 50% will experience recurrence of DMI, mostly in another muscle group, within 6 mo after the initial episode. In comparison to unselected patients with DMI, the recurrence rate was reported at 29.2%[2]. It was interesting to observe that no recurrence has been reported among transplant recipients. Further research is needed to confirm this observation. The long-term survival of patients with DMI can be variable. Death within a year by an episode of DMI are often related to other end-organ complications present in these patients.
The present review is limited by the use of case reports or series with the largest one having four patients. Not all patients had the same type of investigations for DMI. There are also limited reports about functional impairment experienced by patients during episodes of DMI. Furthermore, follow-up of patients in the included reports varied considerably.
More research is needed to better understand the pathogenesis of DMI, strategies for effective treatment and prevention of recurrence. More specifically, in the setting of ESRD and RRT, more investigation is needed to ascertain if adequacy of the RRT may influence outcomes. It will also be useful to better understand the natural history of DMI among patients who have had renal transplantation or those who are candidates for transplantation.
DMI is an uncommon complication of diabetes mellitus that can also affect patients with ESRD who are receiving dialysis or recipients of kidney transplantation. Clinical findings which supported by MRI examination is currently the best approach to confirm the diagnosis of DMI. Muscle biopsy is not recommended but may be required in atypical presentations. The best management approach for DMI is currently unclear and present practice is still largely based on expert opinion because of limited evidence. Nonetheless, nonsurgical treatment appears to be a better option than surgical intervention. The short-term prognosis of DMI is good but recurrence is frequent.
Manuscript source: Invited manuscript
Specialty type: Urology and Nephrology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Kute VBB, Lai S, Stavroulopoulos A, Sureshkumar KK S- Editor: Cui LJ L- Editor: A E- Editor: Li RF
| 1. | Angervall L, Stener B. Tumoriform focal muscular degeneration in two diabetic patients. Diabetologia. 1965;1:39-42. [DOI] [Full Text] |
| 2. | Horton WB, Taylor JS, Ragland TJ, Subauste AR. Diabetic muscle infarction: a systematic review. BMJ Open Diabetes Res Care. 2015;3:e000082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Reich S, Wiener SN, Chester S, Ruff R. Clinical and radiologic features of spontaneous muscle infarction in the diabetic. Clin Nucl Med. 1985;10:876-879. [PubMed] |
| 4. | Chester CS, Banker BQ. Focal infarction of muscle in diabetics. Diabetes Care. 1986;9:623-630. [PubMed] |
| 5. | Umpierrez GE, Stiles RG, Kleinbart J, Krendel DA, Watts NB. Diabetic muscle infarction. Am J Med. 1996;101:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Bingham C, Hilton DA, Nicholls AJ. Diabetic muscle infarction: an unusual cause of leg swelling in a diabetic on continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 1998;13:2377-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Madhan KK, Symmans P, Te Strake L, van Der Merwe W. Diabetic muscle infarction in patients on dialysis. Am J Kidney Dis. 2000;35:1212-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Grigoriadis E, Fam AG, Starok M, Ang LC. Skeletal muscle infarction in diabetes mellitus. J Rheumatol. 2000;27:1063-1068. [PubMed] |
| 9. | Pedicelli A, Belli P, Fratino M, Cina A, Di Gregorio F, Rollo M. Diabetic muscle infarction. Am J Med. 2001;111:671-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Silberstein L, Britton KE, Marsh FP, Raftery MJ, D’Cruz D. An unexpected cause of muscle pain in diabetes. Ann Rheum Dis. 2001;60:310-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Delis S, Ciancio G, Casillas J, Figueiro J, Garcia A, Miller J, Burke GW. Diabetic muscle infarction after simultaneous pancreas-kidney transplant. Clin Transplant. 2002;16:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Chow KM, Szeto CC, Griffith JF, Wong TY, Li PK. Unusual muscle pain in two patients with diabetic renal failure. Hong Kong Med J. 2002;8:368-371. [PubMed] |
| 13. | Melikian N, Bingham J, Goldsmith DJ. Diabetic muscle infarction: an unusual cause of acute limb swelling in patients on hemodialysis. Am J Kidney Dis. 2003;41:1322-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Kapur S, Brunet JA, McKendry RJ. Diabetic muscle infarction: case report and review. J Rheumatol. 2004;31:190-194. [PubMed] |
| 15. | Theodoropoulou E, Chelioti E, Revenas K, Katsilambros N, Kostakis A, Boletis JN. Diabetic muscle infarction after kidney and pancreas transplantation: case report and literature review. Transplant Proc. 2006;38:3147-3150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Lentine KL, Guest SS. Diabetic muscle infarction in end-stage renal disease. Nephrol Dial Transplant. 2004;19:664-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Joshi R, Vargas R. Diabetic muscle infarction in renal transplantation. Transplantation. 2004;77:321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Sahin I, Taskapan C, Taskapan H, Baysal T, Bentli R, Tekes S, Kosar F, Gurses I. Diabetic muscle infarction: an unusual cause of muscle pain in a diabetic patient on hemodialysis. Int Urol Nephrol. 2005;37:629-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Reyes-Balaguer J, Solaz-Moreno E, Morata-Aldea C, Elorza-Montesinos P. Spontaneous diabetic myonecrosis. Diabetes Care. 2005;28:980-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Macgregor JL, Chan P, Schneiderman PI, Grossman ME. Diabetic muscle infarction. Arch Dermatol. 2007;143:1456-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Glauser SR, Glauser J, Hatem SF. Diabetic muscle infarction: a rare complication of advanced diabetes mellitus. Emerg Radiol. 2008;15:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Joshi R, Reen B, Sheehan H. Upper extremity diabetic muscle infarction in three patients with end-stage renal disease: a case series and review. J Clin Rheumatol. 2009;15:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | De Vlieger G, Bammens B, Claus F, Vos R, Claes K. Diabetic muscle infarction: a rare cause of acute limb pain in dialysis patients. Case Rep Nephrol. 2013;2013:931523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Cabrera SO, Michel R, Lu A, Dimitrov V. Diabetic muscle infarction with complications. Int J Case Rep Images. 2013;4:303-307. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Mukherjee S, Aggarwal A, Rastogi A, Bhansali A, Prakash M, Vaiphei K, Dutta P. Spontaneous diabetic myonecrosis: report of four cases from a tertiary care institute. Endocrinol Diabetes Metab Case Rep. 2015;2015:150003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Janga KC, Sinha A, Wengrofsky P, Oo P, Greenberg S, Tarkovsky R, Sharma K. Diabetic Muscle Infarction Masquerading as Necrotizing Fasciitis. Case Rep Nephrol. 2017;2017:7240156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Trujillo-Santos AJ. Diabetic muscle infarction: an underdiagnosed complication of long-standing diabetes. Diabetes Care. 2003;26:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Chebbi W, Jerbi S, Klii R, Alaya W, Mestiri S, Zantour B, Sfar MH. Multifocal diabetic muscle infarction: a rare complication of poorly controlled diabetes mellitus. Intern Med. 2014;53:2091-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Habib GS, Nashashibi M, Saliba W, Haj S. Diabetic muscular infarction: emphasis on pathogenesis. Clin Rheumatol. 2003;22:450-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Bjornskov EK, Carry MR, Katz FH, Lefkowitz J, Ringel SP. Diabetic muscle infarction: a new perspective on pathogenesis and management. Neuromuscul Disord. 1995;5:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Madhuvan HS, Krishnamurthy A, Prakash P, Shariff S. Diabetic Muscle Infarction (Myonecrosis): Underdiagnosed or Underreported? J Assoc Physicians India. 2015;63:71-73. [PubMed] |
| 32. | Bajaj G, Nicholas R, Pandey T, Montgomery C, Jambhekar K, Ram R. MR imaging findings in diabetic muscle infarction. J Ark Med Soc. 2014;111:91-93. [PubMed] |
| 33. | Nigwekar SU, Kroshinsky D, Nazarian RM, Goverman J, Malhotra R, Jackson VA, Kamdar MM, Steele DJ, Thadhani RI. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 291] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 34. | Morcuende JA, Dobbs MB, Crawford H, Buckwalter JA. Diabetic muscle infarction. Iowa Orthop J. 2000;20:65-74. [PubMed] |
| 35. | Bodner RA, Younger DS, Rosoklija G. Diabetic muscle infarction. Muscle Nerve. 1994;17:949-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |