Published online May 6, 2017. doi: 10.5527/wjn.v6.i3.132
Peer-review started: May 11, 2016
First decision: June 14, 2016
Revised: October 17, 2016
Accepted: October 25, 2016
Article in press: October 27, 2016
Published online: May 6, 2017
Processing time: 359 Days and 13.5 Hours
To determine the impact of allopurinol non-adherence as a proxy for uncontrolled disease on primary prevention of end-stage renal disease (ESRD).
A cohort of 2752 patients with gout diagnosis was reconstructed using the Québec Régie de l’assurance maladie du Québec and MedEcho administrative databases. Eligible patients were new users of allopurinol, aged 45-85, with a diagnosis of hypertension, and treated with an antihypertensive drug between 1997 and 2007.
Major risk factor for ESRD onset was chronic kidney disease at stages 1 to 3 [rate ratio (RR) = 8.00; 95% confidence interval (CI): 3.16-22.3 and the severity of hypertension (≥ 3 vs < 3 antihypertensives)] was a trending risk factor as a crude estimate (RR = 1.94; 95%CI: 0.68-5.51). Of 341 patients, cases (n = 22) and controls (n = 319), high adherence level (≥ 80%) to allopurinol therapy, compared with lower adherence level (< 80%), was associated with a lower rate of ESRD onset (RR = 0.35; 95%CI: 0.13-0.91).
Gout control seem to be associated with a significant decreased risk of ESRD onset in hypertensive populations, further research should be conducted confirming this potential associated risk.
Core tip: The question of whether serum uric acid has a pathogenic role in the onset and progression of chronic kidney disease (CKD) remains unanswered. Hyperuricemia and gout is common in CKD, and treatment adherence in gout patients is suboptimal and therefore a therapeutic challenge. Our population-based study assessed the impact of gout disease control on the risk of end stage renal disease (ESRD). Our study suggest that adherence to allopurinol of ≥ 80% as a proxy for gout control is associated with a significant, 65% reduction in the risk of ESRD onset.
- Citation: Perreault S, Nuevo J, Baumgartner S, Morlock R. Any link of gout disease control among hypertensive patients and onset of end-stage renal disease? Results from a population-based study. World J Nephrol 2017; 6(3): 132-142
- URL: https://www.wjgnet.com/2220-6124/full/v6/i3/132.htm
- DOI: https://dx.doi.org/10.5527/wjn.v6.i3.132
Gout is a chronic disease and one of the most common inflammatory arthritis conditions among adults. Gout is caused primarily by inefficient renal excretion of uric acid, resulting in increased serum uric acid (sUA) levels (i.e., hyperuricemia), and ultimately, deposition of monosodium urate crystals within joints, connective tissue, kidneys, and other organs. The accumulation of crystal deposits lead to acute, painful inflammatory arthritis (i.e., flares) and tophi; if not appropriately treated, gout can cause permanent joint destruction, bone erosion, and kidney damage[1]. The prevalence estimates of gout from the 2007 to 2008 United States National Health and Nutrition Examination Survey are 5.6% among men and 2.0% among women[2].
The pathogenesis of gout is well established, and the role of sUA for acute and chronic conditions of gout is linked to the deposition of monosodium urate crystals. Reducing sUA levels prevents further crystal deposition and allows dissolution of existing deposits, effectively curing the disease. The American College of Rheumatology[3] and European League Against Rheumatism[4] have published guidelines for the use of urate-lowering therapies for gout management. Targeting sUA is important in the management of gout because high levels are linked to up to a 3-times risk of an acute gout flare[5] and a higher annual frequency of flares[6]. Moreover, studies report continued poor outcomes in gout patients[7], including recurrent flares, functional incapacity, and risk of additional joint damage, as well as the potential for renal and cardiovascular complications[8]. The potential relationship of elevated sUA and all-cause mortality has also been suggested by several population-based[9,10] cohort studies. Recent estimates have shown that overall aggregate annual costs for the medical care of gout patients exceeded $20 billion in the United States alone, with provisional estimates of annual direct and indirect costs of gout patient care totaling over $6 billion[11].
In a recent meta-analysis on adherence to allopurinol, it was clear that allopurinol adherence was suboptimal and therefore a therapeutic challenge in gout[12]. Among the systematic reviews provided in the meta-analysis, 1 study evaluated adherence rates among patients with 7 different medical conditions, including hypertension (72.3%), hypothyroidism (68.4%), diabetes mellitus (65.4%), seizure disorders (60.8%), hypercholesterolemia (54.6%), osteoporosis (51.2%), and gout (36.8%), suggesting that adherence was worst in gout[13].
These results highlight the need for more research on the impact of allopurinol non-adherence on major clinical outcomes, such as kidney disease. In fact, hyperuricemia is common in chronic kidney disease (CKD), and it is thought to develop secondary to a decrease in glomerular filtration rate or hyperinsulinemia in the metabolic syndrome[14]. It is generally accepted that hyperuricemia is prevalent among patients with several risk factors for cardiovascular disease (CVD)[15], and elevated sUA levels have been found to predict the risk of developing CVD or CKD in the general population[16,17]. However, the question of whether sUA has a pathogenic role in the onset and progression of CKD remains unanswered.
Several observational studies have investigated whether an elevated sUA level is an independent risk factor for the development and progression of CKD, but the results were inconclusive and conflicting[18]. A previous study reported that 20%-60% of patients with gout and hyperuricemia developed renal impairment that was accompanied by histological damage of glomerulosclerosis, interstitial fibrosis, and arteriosclerosis[19]. Again, elevated sUA levels have been reported to predict the development of renal insufficiency in individuals with normal renal function[20].
Recent data underscoring the growing problem of gout and its associated comorbid conditions have called for the need for more research of its impact on major outcomes. Evidence from large data sets has reported uric acid levels to be associated with the risk of end-stage renal disease (ESRD)[21-23]. Clinical evidence from randomized clinical trials is now emerging showing that allopurinol may slow the progression of CKD[24]. In a study using continued allopurinol treatment as a proxy for control, long-term treatment was suggestive of slowing the rate of kidney disease progression and reducing CV risk[25]. Thus, the present study assessed the impact of allopurinol non-adherence as a proxy for uncontrolled disease[25] on primary prevention of ESRD among patients newly treated with allopurinol for gout treatment. The objective was to assess the impact of gout disease control on the risk of ESRD among gout patients newly treated with allopurinol in a cohort of patients who initiated antihypertensive agents for essential hypertension.
This population-based study utilized the Régie de l’assurance maladie du Québec (RAMQ) and MedEcho databases, both of which are components of public health care insurance program management for Quebec, Canada. Specific types of health data comprise the RAMQ databases: (1) a demographic information file of all registrants, containing gender, age, postal code, and, if applicable, year of death; and (2) a medical services file, consisting of all inpatient/ambulatory medical services claims, that includes procedure/diagnostic code data, i.e., date, type, and site (office, emergency room, hospital). Diagnostic coding was performed according to the International Classification of Disease, Ninth Revision (ICD-9) and, from 2006 onward, ICD-10.
The Canadian Classification of Diagnostic, Therapeutic, and Surgical Procedures enclose and define the procedure codes. All drugs prescribed to patients who take RAMQ-insured medications are contained in the pharmaceutical file. The pharmaceutical file includes the name and dose of the drug, its quantity, date of treatment dispensation, and the quantity of days dispensed, as indicated by the pharmacist. Lastly, the MedEcho database file contains data on acute care hospitalizations, (e.g., admission date, length of stay, and primary/secondary diagnoses). Each of the database files also provided a unique identifier (individual health insurance number).
The first 2 RAMQ datasets contain information on all of those with provincial public health insurance plan coverage, comprising the total Quebec population. Prescription claims dataset (pharmaceutical file) data are from provincial public prescription drug insurance plan beneficiaries, representing approximately 43% of Quebec residents between the ages of 45 and 64, and about 94% of those aged ≥ 65 years. This dataset provides an accurate measure of identifying drugs that are prescribed to patients and is a validated research tool utilized in pharmacoepidemiologic studies. RAMQ database medical services claims have been also used as part of validity studies[26-30].
Subjects identified were aged 45 to 85 and newly treated with either angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, β-blockers, calcium channel blockers, diuretics, or a combination between January 1, 1999 and June 30, 2007; these subjects were also required to have an essential hypertension diagnosis (ICD-9 code: 401 and ICD-10 code: I10) for 2 years prior to the cohort entry date. The study cohort included 222582 individuals.
The exclusion criteria were as follows: (1) patients with hematologic cancer ICD-9 code 200-208 or ICD-10 code C77-C96 in the 5-year period prior to the cohort entry date; (2) other disorders of purine and pyrimidine metabolism ICD-9 code 277.2 or ICD-10 code E79.0, E79.1, E79.8, E79.9 in the 5-year period prior to the cohort entry date; (3) patients with tumor lysis syndrome ICD-9 code 277.88 or ICD-10 E88.3, E88.8, E88.9 in the 5-year period prior to the cohort entry date; (4) patients with familial Mediterranean fever ICD-9 code 277.31 or ICD-10 code E85.0 in the 5-year period prior to the cohort entry date; (5) patients with hemodialysis or peritoneal dialysis for more than 3 consecutive months in the 5-year period prior to the cohort entry date; (6) patients with autoimmune/congenital kidney diseases, as these conditions are highly associated with kidney diseases and, as a result, are also highly predictive of ESRD; (7) patients with kidney transplantation; and (8) patients with expected CKD of stage 4 and 5 using ICD-9 or ICD-10 with the presence of drug markers (in the 2 years prior to cohort entry date): Calcium carbonate ≥ 1200 mg/d or calcitriol (≥ 0.25 μg/d or ≥ 1.75 μg/wk or ≥ 7.5 μg/30 d) or doxercalciférol (≥ 2.5 μg/d or ≥ 17.5 μg/wk or ≥ 75 μg/30 d) or alfacalcidol ( ≥ 0.25 μg/d or ≥ 1.75 μg/wk or ≥ 7.5 μg/30 d).
We defined the patients as newly treated with allopurinol if they had not taken any allopurinol in 1 year during the follow-up. The date of the cohort entry is defined by the date of the first claim of allopurinol. In order to be eligible, patients needed to also have had a diagnosis of gout, defined as meeting all of the following criteria: (1) having a diagnosis of gout defined by ICD-9 code 712.0 (Gout arthritis) or ICD-9 code 274 (Gout) or ICD-10 code M10.0 (Idiopathic gout) or ICD-10 M10.9 (Non-specify gout) during the follow-up period; and (2) users of at least 2 claims of colchicine during the follow-up period (meaning any time during the follow-up prior and post inclusion in the cohort).
The study cohort included all new users of allopurinol who were followed from the date of first allopurinol claim issuance until the onset of ESRD or study period end (i.e., June 30, 2008). Individuals were censored when they lost RAMQ drug insurance coverage or died during follow-up. Subjects were followed for a period of 1 year to 9.5 years.
We conducted a nested case-control study to estimate rate ratios (RR) of ESRD associated with adherence to allopurinol for gout treatment. The definition of ESRD (case) comprised a composite endpoint that represented patients with a medical procedure lasting ≥ 3 mo’ for peritoneal dialysis, hemodialysis, or renal transplantation. The date of ESRD was defined as the index date. All cases were identified and up to 15 controls were randomly selected from the cohort based on the risk set of each case using density sampling. Sampling for each control was selected in proportion to time contribution to the person-time at risk in the source population (i.e., the whole cohort), giving an unbiased estimate of the RR. A hallmark of the risk set is that a cohort member who serves as control at one point in time may later become a case, and the same cohort member may be selected as a control for more than 1 case. Cases and controls were matched for age, sex, and entry into the cohort.
The primary outcome was ESRD described by the (case) composite endpoint case definition above. All patients were followed up to study end or primary outcome.
The definition of allopurinol adherence was described as the degree at which patients use allopurinol as prescribed. Adherence estimation was determined via “medication possession ratio” (MPR), which was calculated as the total number of days’ supply of medication dispensed divided by follow-up length. Evaluation of patient adherence was conducted from initiation of follow-up to index date (date of ESRD) or to time of non-cases’ selection. MPR was dichotomized, with an MPR threshold set to < 80% in order to identify those who were non-adherent, which is aligned to the medical literature. We also used categorical exposure. In many databases, serum urate is not captured and adherence to urate-lowering therapy may be a proxy for disease control[31,32].
Social assistance status and gender at the time of cohort entry was established using RAMQ database beneficiary file data. Risk factors for gout and associated comorbidities were considered using drug markers or ICD-9/ICD-10. Associated comorbidities were ischemic heart disease, heart failure, cardiac dysrhythmias, dyslipidemia, diabetes, CKD at grades 1 to 3, acute renal failure, cerebrovascular disease, peripheral vascular disease, pulmonary disease (chronic obstructive pulmonary disease or asthma), dementia, rheumatic disease, and gastrointestinal disease. Diagnosis and medical procedures were identified in the 5 years preceding cohort entry, while pharmacological treatment was identified in the 2 years preceding cohort entry date. Indirect measurement of hypertension severity was established by the number of antihypertensive medications (1, 2, ≥ 3) during the year prior to the index date.
Comparison of characteristics of controlled and uncontrolled patients was calculated using t-test for continuous variables and χ2 test for categorical variables. Through multivariate analysis, a conditional logistic regression model was constructed to evaluate the association between allopurinol adherence and ESRD. When studying a time-dependent exposure, as adherence to allopurinol in our study, an additional level of complexity is introduced based upon the need to account for a time-dependent exposure; this can be achieved via a cohort analysis using Cox regression that includes time-dependent covariates. Alternatively, the use of a nested case-control approach is appropriate, providing exposure and covariate information for controls and reflecting values corresponding to the selection time of their respective case. When studying time-dependent exposures, nested case-control analyses can yield similar results to that of Cox regression on the full cohort; the advantage being that there is superior computational efficiency with conditional logistic regression (given that only a sample of all possible controls are included in each case’s risk set). Aside from computational efficiency, the use of nested case-control studies offer additional advantages, including the possibility of matching controls to cases on the basis of possible fixed or time-dependent confounding covariates for which estimation of effect is not of interest.
To further weigh the strength of the findings, sensitivity analyses were performed to validate our approach. The validity of the approach was done by analyzing 2 drug variables (i.e., benzodiazepines, proton pump inhibitors) and examining varying usage patterns (adherence level < 80% and ≥ 80%). The goal was to determine whether adherence to non-renal-protective drugs was also associated with a reduced ESRD risk. By considering the potential influence of adherence to different classes of drugs, we were able to ensure that any impact on ESRD was due to allopurinol non-adherence, and not the potential influence from simultaneously discontinuing several other medications.
Unselected multivariable models were constructed to maximize adjustment for co-variables. Based on the number of cases, we included only CKD and severity of hypertension to obtain the adjusted estimators. RRs and 95% confidence intervals (CIs) were calculated for each independent variable in the multivariable models. Residuals from regression models were assessed for assumptions violations of multicollinearity or deviance. A precision threshold of 5% was utilized for all analyses and was executed utilizing SAS version 9.3 (SAS Institute, Cary, North Carolina, United States).
After inclusion/exclusion criteria were applied (Figure 1), the resulting cohort at entry comprised 2752 patients with a mean age of 67 years; more than 82% of them were male, 23% of advanced age (> 75 years), close to 50% had at least 1 CVD, 33.5% had dyslipidemia, 20.7% had diabetes, 12.7% had CKD, 21.1% were thiazides users, 34.3% were low acetylsalicylic acid users, and 42.8% were nonsteroidal anti-inflammatory drug (NSAID) users (Table 1).
| All patients (n) | 2752 |
| Mean (SD) age, yr1 | 67 (9) |
| Risk factors for gout, % (n) | |
| Advanced age (> 75 yr)1 | 23.0 (634) |
| Male1 | 82.8 (2278) |
| Obesity2 | 6.5 (179) |
| Drug use, % (n) | |
| Thiazides3 | 21.1 (581) |
| Low dose acetylsalicylic acid3 | 34.3 (945) |
| Niacin3 | 0.4 (11) |
| Alcoholic diagnosis3 | 1.3 (35) |
| Thyroid problems3 | 12.9 (356) |
| Associated comorbidities, % (n) | |
| Ischemic heart disease2 | 30.9 (849) |
| Heart failure2 | 10.5 (288) |
| Cardiac dysrhythmias2 | 12.5 (343) |
| Atrial fibrillation and flutter2 | 8.1 (224) |
| Peripheral vascular disease2 | 11.7 (321) |
| Cerebrovascular disease2 | 12.0 (330) |
| Dyslipidemia2 | 33.5 (921) |
| Diabetes2 | 20.7 (569) |
| Chronic kidney disease at grade 1 to 34 | 12.7 (350) |
| Acute renal failure2 | 4.3 (117) |
| Pulmonary disease (COPD/asthma)2 | 30.8 (847) |
| Dementia2 | 2.7 (73) |
| Rheumatic disease2 | 6.3 (174) |
| Gastrointestinal disease2 | 7.9 (218) |
| Drug use for gout treatment, % (n) | |
| NSAIDs3 | 42.8 (1178) |
| Colchicine3 | 29.3 (806) |
| Intra-articular corticosteroids3 | 5.9 (163) |
| Oral corticosteroids3 | 2.4 (65) |
| Narcotics3 | 12.8 (353) |
Table 2 provides a listing of patient demographic and clinical characteristics at cohort entry, stratified by adherence level within the cohort. No major clinically significant difference was observed between the groups. The median time of follow-up was 3.9 years. Over the time of follow-up, 22 ESRD cases o (0.80%, or 0.2 per 100 person-years) were identified. Over the course of follow-up for the entire cohort, the death rate was 6.7% (183 deaths, or 1.58 per 100 person-years). The allopurinol exposure at the baseline level and during follow-up is provided in Table 3.
| Variables | Adherent to allopurinol | Non-adherent to allopurinol | P-value |
| All patients (n) | 1392 | 1360 | |
| Mean (SD) age (yr)1 | 67 (9) | 67 (9) | 0.4457 |
| Risk factors for gout, % (n) | |||
| Advanced age (> 75 yr)1 | 22.2 (309) | 23.9 (325) | 0.2900 |
| Male1 | 84.2 (1172) | 81.3 (1106) | 0.0367 |
| Obesity2 | 6.4 (89) | 6.6 (90) | 0.8117 |
| Drug use | |||
| Thiazides3 | 20.0 (278) | 22.3 (303) | 0.1380 |
| Low dose acetylsalicylic acid3 | 35.8 (499) | 32.8 (446) | 0.0917 |
| Niacin3 | 0.4 (5) | 0.4 (6) | 0.7333 |
| Alcoholic diagnosis2 | 1.2 (16) | 1.4 (19) | 0.5622 |
| Thyroid problems2 | 13.1 (182) | 12.8 (174) | 0.8264 |
| Associated comorbidities, % (n) | |||
| Ischemic heart disease2 | 31.3 (435) | 30.4 (414) | 0.6460 |
| Chronic heart failure2 | 10.7 (149) | 10.2 (139) | 0.6787 |
| Cardiac dysrhythmias2 | 11.5 (160) | 13.5 (183) | 0.1193 |
| Atrial fibrillation and flutter2 | 7.5 (104) | 8.8 (120) | 0.1946 |
| Peripheral vascular disease2 | 12.1 (168) | 11.3 (153) | 0.5034 |
| Cerebrovascular disease2 | 12.3 (172) | 11.6 (158) | 0.5509 |
| Dyslipidemia2 | 35.0 (487) | 31.9 (434) | 0.0875 |
| Diabetes2 | 21.1 (294) | 20.2 (275) | 0.5599 |
| Chronic kidney disease at grade 1 to 34 | 12.7 (177) | 12.7 (173) | 0.9968 |
| Acute renal failure2 | 4.3 (60) | 4.2 (57) | 0.8769 |
| Pulmonary disease (COPD/asthma)2 | 31.4 (437) | 30.1 (410) | 0.4787 |
| Dementia2 | 2.9 (40) | 2.4 (33) | 0.4656 |
| Rheumatic disease2 | 5.0 (69) | 7.7 (105) | 0.0029 |
| Gastrointestinal disease2 | 7.5 (105) | 8.3 (113) | 0.4571 |
| Drug use for gout treatment3, % (n) | |||
| NSAIDs3 | 37.8 (526) | 47.9 (652) | < 0.0001 |
| Colchicine3 | 26.9 (374) | 31.8 (432) | 0.0048 |
| Intra-articular corticosteroids3 | 6.4 (89) | 5.4 (74) | 0.2899 |
| Oral corticosteroids3 | 1.9 (27) | 2.8 (38) | 0.1400 |
| Narcotics3 | 12.9 (179) | 12.8 (174) | 0.9593 |
| Baseline | During follow-up | |
| Patients (n) | 2752 | 2570 |
| Allopurinol | ||
| Mean dose used during the period (± SD) | 210.9 (± 97.5) | 243.3 (± 128.2) |
| Proportion of patients taking < 300 mg (%) | 64.7 (1782) | 53.5 (1374) |
| Proportion of patients taking 300 mg (%) | 33.5 (921) | 40.8 (1049) |
| Proportion of patients taking > 300 mg (%) | 1.8 (49) | 5.7 (147) |
| Febuxostat | ||
| Proportion of patients who received at least 1 claim of febuxostat (%) | 0 (0) | 0 (0) |
| Mean dose used during the period (± SD) | NA | NA |
As shown in Table 4, among the 22 cases (matched for age, sex, and time period), CVD and associated risk factors, as well as other comorbidities were higher among controls compared with cases. In contrast, CKD at a grade lower than 4, acute renal failure, and severity of hypertension were higher among cases than controls.
| Cases occurring in the follow-up and their controls | ||
| Cases (n = 22) | Controls (n = 319) | |
| Characteristics, % (n) unless otherwise noted | ||
| Allopurinol adherence ≥ 80% | 31.8 (7) | 57.1 (182) |
| Age (mean, continuous)1 | 74.2 (60-78) | 74.8 (60-78) |
| Older than 75 yr1 | 36.4 (8) | 38.6 (123) |
| Ischemic heart disease2 | 18.2 (4) | 24.8 (79) |
| Heart failure2 | 9.1 (2) | 8.5 (27) |
| Cardiac dysrhythmias2 | 0 (0) | 8.2 (26) |
| Atrial fibrillation2 | 0 (0) | 4.7 (15) |
| Peripheral arterial disease2 | 9.1 (2) | 12.5 (40) |
| Cerebrovascular disease2 | 18.2 (4) | 14.7 (47) |
| Dyslipidemia2 | 13.6 (3) | 29.8 (95) |
| Diabetes2 | 18.2 (4) | 19.1 (258) |
| Chronic kidney disease with grade 1 to 33 | 54.6 (12) | 12.9 (41) |
| Acute renal failure2 | 9.1 (2) | 3.1 (10) |
| Pulmonary disease (COPD/asthma)2 | 22.7 (5) | 37.6 (120) |
| Dementia2 | 4.6 (1) | 1.6 (5) |
| Rheumatic disease2 | 4.6 (1) | 5.6 (18) |
| Gastrointestinal disease2 | 4.6 (1) | 11.3 (36) |
| Severity of hypertension (1-yr prior to ESRD) | ||
| No use of antihypertensive agent | 4.6 (1) | 8.8 (28) |
| Monotherapy | 36.4 (8) | 43.0 (137) |
| 2 antihypertensive agents | 36.4 (8) | 35.1 (112) |
| ≥ 3 antihypertensive agents | 22.7 (5) | 13.2 (42) |
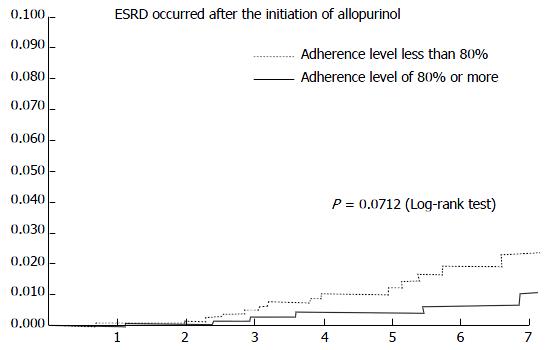
The incidence rate of ESRD for high adherence subjects compared with lower adherence is shown in Figure 2. The mean (SD) adherence level to allopurinol was 65.7% (32.6%). The adjusted RR of ESRD was significantly lower in the high adherence group compared with the lower adherence group (RR = 0.35; 95%CI: 0.13-0.91) (Table 5). We identified 22 cases with ESRD during follow-up. High adherence (≥ 80%) to allopurinol therapy compared with lower adherence (< 80%) was associated with a reduction of ESRD. Major risk factors for ESRD were CKD at stage 1 to 3 (RR = 8.00; 95%CI: 3.16-22.3) and a trend for severity of hypertension (RR = 1.19; 95%CI: 0.38-3.74). Adjusted variables were limited in number, given the low number of cases, and subgroup analyses were unable to be conducted.
| Rate ratio | ||
| Cases occurring at follow-up and their controls | ||
| Crude | Adjusted | |
| Allopurinol adherence level | ||
| < 80% | Reference | Reference |
| ≥ 80% | 0.35 (0.14-0.89) | 0.35 (0.13-0.91) |
| Chronic kidney disease at grade 1 to 31 | 8.06 (3.28-19.77) | 8.00 (3.16-20.25) |
| Severity of hypertension (1 yr prior to ESRD) | ||
| < 3 antihypertensive agents | Reference | Reference |
| ≥ 3 antihypertensive agents | 1.94 (0.68-5.51) | 1.19 (0.38-3.74) |
An adherence rate of > 80% to proton pump inhibitors (RR = 0.90; 95%CI: 0.20-4.01) or benzodiazepines (RR = 1.24; 95%CI: 0.35-4.39) did not impact onset of ESRD. High allopurinol adherence specifically was associated with reduction of ESRD; however, with non-renal protective drugs, this effect was not present.
This study shows that allopurinol treatment adherence of ≥ 80% as a proxy for gout control in this newly-treated population is associated with a significant 65% decrease in the risk of ESRD onset. The association seems to take more than 3 years of exposure, suggesting that allopurinol efficacy in preventing ESRD may need many years before manifesting. This aligns with the medical literature and supports the assertion that the development of ESRD occurs over an extended time period[33].
Our results are in accordance with recent publications, which suggest that lowering sUA levels may slow progression of renal disease. Siu et al[34] reported that the treatment of asymptomatic hyperuricemia in patients with mild renal disease (CKD stage 3) resulted in delayed disease progression. Likewise, Kanbay et al[35] reported that treatment with asymptomatic hyperuricemia improved renal function.
The present study results indicate that CKD at a lower stage and the severity of hypertension disease are associated with increased risk of ESRD onset[36-41]. The most difficult aspect of assessing the impact of allopurinol and disease control in the development or progression of CKD is the number of confounders. Here, we were limited in the number of adjusted variables given the low number of cases, and we were unable to conduct a subgroup analysis. In addition, we were limited to control for severity of hypertension. The concept of an ideal blood pressure target for patients with kidney disease is highly debated[42]. Two critical factors arise: Follow-up time and proteinuria; both factors appear to be important in influencing blood pressure effects in nephroprotection. Our study design does not allow for the verification of the proteinuria hypotheses; however, the importance of time can be agreed upon, as a more significant effect of good allopurinol adherence over more than 3 years was observed. We were also unable to discern what the targeted or achieved blood pressure levels were for our cohort. If lower blood pressure does not convey a lower ESRD risk (a controversial subject), and if our chronic renal failure patients and controls had in fact ended up with lower blood pressures (< 130/80), then this may decrease the strength of the observed association.
The study design took the potential for some methodology-based limitations into consideration. In order to avoid selection bias, only incident allopurinol users were included. Nevertheless, additional limitations potentially remain. First, to control for certain risk factors involved in CKD development, we included any variables that were relevant within the regression model. Since those with higher amounts of comorbidities have a higher likelihood for ESRD, we adjusted for limited risk factors given the number of observed cases. Regardless, one cannot exclude residual confounding due to inaccurate/incomplete measurement of covariates or unmeasured confounders. As an example, patients not adhering to treatment could likely have additional factors contributing to worse outcomes, such as lower socioeconomic status, adverse health behaviors, and depression[43]. Nonetheless, we were able to adjust for these factors.
Our sensitivity analyses regarding the differential class effects of drug adherence on ESRD suggest that adherence-related benefits are associated with medication that lowered sUA levels. The benefit on reduction of ESRD in our study was seen specifically with better adherence to medication that lowered sUA levels, but not with other types of medications such as proton pump inhibitors or benzodiazepines. Still, the possibility remained for “healthy user bias”[44,45]. This term refers to a population of patients that exhibit good adherence to medication as part of an overall healthy lifestyle, and therefore, have less comorbidities and, as a result, exhibit decreased risk of disease development overall. Of note, recent literature discussing differential drug adherence class effects on long-term survival proposed that the associated benefits related to pharmacotherapeutic adherence are mediated more so by the effects of the drugs rather than by healthy adherence behaviors[46]. Another possibility could have been reverse causality. Each patient’s glomerular filtration rate were unknown based on the information available from the administrative databases utilized in this observational study; however, renal function decline was likely to be gradual over the course of a few years, while adherence remained quite constant after the first year and until the index date in both cases and controls. Hence, it is not believed that decreasing renal function resulted in patients becoming more compliant to medication.
Additionally, the evidence surrounding the safety and efficacy of allopurinol among patients with CKD presents an inconsistency of results[47]. We should be aware of the potential risk of allopurinol use among those patients, and we need to titrate according to target sUA level or switch to febuxostat[48]. In our study, we do not suspect that allopurinol non-adherence was related to CKD progression; this is because febuxostat was available to the physician, but the opportunity to switch the patient was not carried out.
Characteristics potentially influencing a doctor’s medication choice, such as unmeasured comorbidities and missing sUA level/blood pressure control data, were not able to be controlled for in our analysis; this potentially could result in residual confounding effects. In addition, adjusting for clinical severity, e.g., precise glomerular filtration rate, CKD etiology, proteinuria, is not allowable with the use of databases. However, these conditions per se are not traditionally associated with better adherence. Also, due to diagnostic coding errors, it is possible that history of renal disease may not have been identified in certain individuals. The probability of this occurring, however, would have been low, as for all individuals, relevant medical/drug information was accessible for all individuals for a several-year period prior to cohort entry. For instance, 12 of the 22 ESRD events had previous CKD (classified as grade 1 to 3); 2 had a history of acute renal failure, and the 8 remaining cases developed CKD during follow-up as the cause of ERSD. Patients with previously diagnosed gout (as opposed to those with newly diagnosed gout) also may have greater motivation to take their medication based on prior gout attack experiences[49]. Patients with previously diagnosed gout may be at higher risk, but this could not be adjusted because of the low number of cases. NSAID use may also be a confounder since its use may cause progression among patients who have pre-existing kidney disease[50]. After adjusting for age, gender, and comorbidities, high cumulative NSAID use was associated with a 26% risk of a rapid decline in estimated glomerular filtration rate relative to those who did not use NSAIDs (OR = 1.26, 95%CI: 1.04–1.53). For instance, in our study, we noticed a difference in NSAID users between adherent (37.8%) and non-adherent (47.9%) participants; however, we could not adjust because of the low number of cases. Nevertheless, we do not expect that this difference could have a major impact, given that there was no difference among patients with CKD at grade 1 to 3 for the low and high allopurinol adherence levels.
Finally, prescription refill patterns were used to assess exposure; therefore, whether the dispensed medication was actually taken by the patient cannot be ascertained. Nevertheless, evidence suggests a good correlation between cumulative drug exposure, pharmacy dispensing records, and gaps in medication supply[51]. Furthermore, no information with respect to potential adverse drug effects was available within the data, which could potentially explain early medication discontinuation. Available evidence has shown that adverse drug effects generally occur in the earlier, rather than later, phases of treatment; therefore, it is unlikely to significantly account for non-adherence of preventive therapy[52].
To summarize, this Canadian population-based study showed an association with significant benefit between good allopurinol adherence, as a proxy for disease control, and ESRD onset, but further research should be conducted to confirm this potential associated risk.
A key factor to determining success with various treatment approaches is improved adherence to pharmacotherapy. As a result, greater awareness should be taken accordingly, as improved patient outcomes can result. Assessing medication adherence should be a routine part of clinical practice. Devising strategies to optimize long-term allopurinol adherence, as well as other measures of adequate disease control, must be encouraged in order to lower or, at a minimum, delay the occurrence of ESRD in patients with gout.
Editorial support was provided by Albert M Balkiewicz, MSc, of PAREXEL, and was funded by AstraZeneca.
Gout is characterized by elevated serum uric acid (sUA) levels and is commonly treated with urate-lowering treatment allopurinol; however, allopurinol adherence is suboptimal and therefore a therapeutic challenge in gout. Some evidence from large data sets has reported sUA to be associated with the risk of end-stage renal disease (ESRD), and hyperuricemia in general is common in chronic kidney disease (CKD).
Several observational studies have been either inconclusive or conflicting as to whether elevated sUA is an independent risk factor for the development and progression of CKD. The pathogenic role of sUA in the onset and progression of CKD, and the relationship of sUA management in this context, remains largely unanswered.
This study examines a large cohort of over 220000 individuals followed for up to 9 years and utilizes long-term real-world evidence to examine the impact of gout control on the onset of ESRD.
This study found a potential association with significant benefit between good adherence to allopurinol and ESRD onset. Optimizing long-term allopurinol adherence and other measures of disease control must be encouraged in order to lower or, as a minimum, delay the occurrence of ESRD in patients with gout.
In their manuscript Perreault et al studied an important topic; the possible role of allopurinol non-adherence in the development of end-stage renal disease in gout patients. The authors used very sophisticated statistics in their case control study on a high patient population.
Manuscript source: Unsolicited manuscript
Specialty type: Urology and nephrology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Nakhoul FM, Navarro-Gonzalez JF, Nemcsik J S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Choi HK, Curhan G. Gout: epidemiology and lifestyle choices. Curr Opin Rheumatol. 2005;17:341-345. [PubMed] |
| 2. | Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136-3141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1247] [Article Influence: 103.9] [Reference Citation Analysis (0)] |
| 3. | Khanna D, Khanna PP, Fitzgerald JD, Singh MK, Bae S, Neogi T, Pillinger MH, Merill J, Lee S, Prakash S. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64:1447-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 507] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 4. | Zhang W, Doherty M, Pascual E, Bardin T, Barskova V, Conaghan P, Gerster J, Jacobs J, Leeb B, Lioté F. EULAR evidence based recommendations for gout. Part I: Diagnosis. Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65:1301-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 402] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 5. | Wu EQ, Patel PA, Mody RR, Yu AP, Cahill KE, Tang J, Krishnan E. Frequency, risk, and cost of gout-related episodes among the elderly: does serum uric acid level matter? J Rheumatol. 2009;36:1032-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Li-Yu J, Clayburne G, Sieck M, Beutler A, Rull M, Eisner E, Schumacher HR. Treatment of chronic gout. Can we determine when urate stores are depleted enough to prevent attacks of gout? J Rheumatol. 2001;28:577-580. [PubMed] |
| 7. | Doherty M, Jansen TL, Nuki G, Pascual E, Perez-Ruiz F, Punzi L, So AK, Bardin T. Gout: why is this curable disease so seldom cured? Ann Rheum Dis. 2012;71:1765-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 8. | Neogi T, Hunter DJ, Chaisson CE, Allensworth-Davies D, Zhang Y. Frequency and predictors of inappropriate management of recurrent gout attacks in a longitudinal study. J Rheumatol. 2006;33:104-109. [PubMed] |
| 9. | Chen JH, Chuang SY, Chen HJ, Yeh WT, Pan WH. Serum uric acid level as an independent risk factor for all-cause, cardiovascular, and ischemic stroke mortality: a Chinese cohort study. Arthritis Rheum. 2009;61:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 252] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 10. | Niskanen LK, Laaksonen DE, Nyyssönen K, Alfthan G, Lakka HM, Lakka TA, Salonen JT. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med. 2004;164:1546-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 492] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 11. | Wertheimer A, Morlock R, Becker MA. A revised estimate of the burden of illness of gout. Curr Ther Res Clin Exp. 2013;75:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | De Vera MA, Marcotte G, Rai S, Galo JS, Bhole V. Medication adherence in gout: a systematic review. Arthritis Care Res (Hoboken). 2014;66:1551-1559. [PubMed] |
| 13. | Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28:437-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 451] [Cited by in RCA: 441] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 14. | Saito T, Mochizuki T, Uchida K, Tsuchiya K, Nitta K. Metabolic syndrome and risk of progression of chronic kidney disease: a single-center cohort study in Japan. Heart Vessels. 2013;28:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Nishino M, Mori N, Yoshimura T, Nakamura D, Lee Y, Taniike M, Makino N, Kato H, Egami Y, Shutta R. Higher serum uric acid and lipoprotein(a) are correlated with coronary spasm. Heart Vessels. 2014;29:186-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Chonchol M, Shlipak MG, Katz R, Sarnak MJ, Newman AB, Siscovick DS, Kestenbaum B, Carney JK, Fried LF. Relationship of uric acid with progression of kidney disease. Am J Kidney Dis. 2007;50:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 310] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 17. | Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811-1821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1874] [Cited by in RCA: 1863] [Article Influence: 109.6] [Reference Citation Analysis (0)] |
| 18. | Kusano E. Mechanism by which chronic kidney disease causes cardiovascular disease and the measures to manage this phenomenon. Clin Exp Nephrol. 2011;15:627-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Berger L, Yü TF. Renal function in gout. IV. An analysis of 524 gouty subjects including long-term follow-up studies. Am J Med. 1975;59:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 84] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Beck LH. Requiem for gouty nephropathy. Kidney Int. 1986;30:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 66] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Fleeman N, Pilkington G, Dundar Y, Dwan K, Boland A, Dickson R, Anijeet H, Kennedy T, Pyatt J. Allopurinol for the treatment of chronic kidney disease: a systematic review. Health Technol Assess. 2014;18:1-77, v-vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44:642-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 292] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 23. | Nacak H, van Diepen M, de Goeij MC, Rotmans JI, Dekker FW. Uric acid: association with rate of renal function decline and time until start of dialysis in incident pre-dialysis patients. BMC Nephrol. 2014;15:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Kabul S, Shepler B. A review investigating the effect of allopurinol on the progression of kidney disease in hyperuricemic patients with chronic kidney disease. Clin Ther. 2012;34:2293-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Goicoechea M, Garcia de Vinuesa S, Verdalles U, Verde E, Macias N, Santos A, Pérez de Jose A, Cedeño S, Linares T, Luño J. Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am J Kidney Dis. 2015;65:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 26. | Bérard A, Lacasse A. Validity of perinatal pharmacoepidemiologic studies using data from the RAMQ administrative database. Can J Clin Pharmacol. 2009;16:e360-e369. [PubMed] |
| 27. | Plante C, Goudreau S, Jacques L, Tessier F. Agreement between survey data and Régie de l’assurance maladie du Québec (RAMQ) data with respect to the diagnosis of asthma and medical services use for asthma in children. Chronic Dis Inj Can. 2014;34:256-262. [PubMed] |
| 28. | Tamblyn R, Lavoie G, Petrella L, Monette J. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Québec. J Clin Epidemiol. 1995;48:999-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 415] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 29. | Tamblyn R, Reid T, Mayo N, McLeod P, Churchill-Smith M. Using medical services claims to assess injuries in the elderly: sensitivity of diagnostic and procedure codes for injury ascertainment. J Clin Epidemiol. 2000;53:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 115] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004;57:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 342] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 31. | Morlock R, Flores NM, Annunziata K, Chapnick J, Ramachandran S. Resource use and health related quality of life of gout exacerbated by common comorbidities. Arthritis Rheum. 2014;66:S45. |
| 32. | Morlock R, Storgard C, Schabert VF, Ogbonnaya A, Chevalier P, Hines D, Ramachandran S. Evaluation of symptom control among treated gout patients in the United States, United Kingdom, and Germany. Arthritis Rheum. 2014;66:S512. |
| 33. | Roy L, White-Guay B, Dorais M, Dragomir A, Lessard M, Perreault S. Adherence to antihypertensive agents improves risk reduction of end-stage renal disease. Kidney Int. 2013;84:570-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 578] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 35. | Kanbay M, Ozkara A, Selcoki Y, Isik B, Turgut F, Bavbek N, Uz E, Akcay A, Yigitoglu R, Covic A. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearance, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39:1227-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 234] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 36. | Avram Z, Krishnan E. Hyperuricaemia--where nephrology meets rheumatology. Rheumatology (Oxford). 2008;47:960-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Cass A, Cunningham J, Wang Z, Hoy W. Social disadvantage and variation in the incidence of end-stage renal disease in Australian capital cities. Aust N Z J Public Health. 2001;25:322-326. [PubMed] |
| 38. | Mangione F, Dal Canton A. The epidemic of chronic kidney disease: looking at ageing and cardiovascular disease through kidney-shaped lenses. J Intern Med. 2010;268:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Naicker S, Fabian J. Risk factors for the development of chronic kidney disease with HIV/AIDS. Clin Nephrol. 2010;74 Suppl 1:S51-S56. [PubMed] |
| 40. | Najafi I, Attari F, Islami F, Shakeri R, Malekzadeh F, Salahi R, Gharavi MY, Hosseini M, Broumand B, Haghighi AN. Renal function and risk factors of moderate to severe chronic kidney disease in Golestan Province, northeast of Iran. PLoS One. 2010;5:e14216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Palmer Alves T, Lewis J. Racial differences in chronic kidney disease (CKD) and end-stage renal disease (ESRD) in the United States: a social and economic dilemma. Clin Nephrol. 2010;74 Suppl 1:S72-S77. [PubMed] |
| 42. | Lewis JB. Blood pressure control in chronic kidney disease: is less really more? J Am Soc Nephrol. 2010;21:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5316] [Cited by in RCA: 5583] [Article Influence: 279.2] [Reference Citation Analysis (0)] |
| 44. | Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 541] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 45. | Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, Johnson JA. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 948] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 46. | Daskalopoulou SS, Delaney JA, Filion KB, Brophy JM, Mayo NE, Suissa S. Discontinuation of statin therapy following an acute myocardial infarction: a population-based study. Eur Heart J. 2008;29:2083-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 47. | Thurston MM, Phillips BB, Bourg CA. Safety and efficacy of allopurinol in chronic kidney disease. Ann Pharmacother. 2013;47:1507-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Tsuruta Y, Mochizuki T, Moriyama T, Itabashi M, Takei T, Tsuchiya K, Nitta K. Switching from allopurinol to febuxostat for the treatment of hyperuricemia and renal function in patients with chronic kidney disease. Clin Rheumatol. 2014;33:1643-1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Sarawate CA, Brewer KK, Yang W, Patel PA, Schumacher HR, Saag KG, Bakst AW. Gout medication treatment patterns and adherence to standards of care from a managed care perspective. Mayo Clin Proc. 2006;81:925-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 50. | Gooch K, Culleton BF, Manns BJ, Zhang J, Alfonso H, Tonelli M, Frank C, Klarenbach S, Hemmelgarn BR. NSAID use and progression of chronic kidney disease. Am J Med. 2007;120:280.e1-280.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 197] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 51. | Choo PW, Rand CS, Inui TS, Lee ML, Cain E, Cordeiro-Breault M, Canning C, Platt R. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37:846-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 430] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 52. | Atthobari J, Brantsma AH, Gansevoort RT, Visser ST, Asselbergs FW, van Gilst WH, de Jong PE, de Jong-van den Berg LT. The effect of statins on urinary albumin excretion and glomerular filtration rate: results from both a randomized clinical trial and an observational cohort study. Nephrol Dial Transplant. 2006;21:3106-3114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |