Published online May 6, 2017. doi: 10.5527/wjn.v6.i3.111
Peer-review started: November 9, 2016
First decision: November 30, 2016
Revised: January 28, 2017
Accepted: February 18, 2017
Article in press: February 20, 2017
Published online: May 6, 2017
Processing time: 181 Days and 7 Hours
Long-term exposure to bioincompatible peritoneal dialysis (PD) solutions frequently results in peritoneal fibrosis and ultrafiltration failure, which limits the life-long use of and leads to the cessation of PD therapy. Therefore, it is important to elucidate the pathogenesis of peritoneal fibrosis in order to design therapeutic strategies to prevent its occurrence. Peritoneal fibrosis is associated with a chronic inflammatory status as well as an elevated oxidative stress (OS) status. Beyond uremia per se, OS also results from chronic exposure to high glucose load, glucose degradation products, advanced glycation end products, and hypertonic stress. Therapy targeting the cannabinoid (CB) signaling pathway has been reported in several chronic inflammatory diseases with elevated OS. We recently reported that the intra-peritoneal administration of CB receptor ligands, including CB1 receptor antagonists and CB2 receptor agonists, ameliorated dialysis-related peritoneal fibrosis. As targeting the CB signaling pathway has been reported to be beneficial in attenuating the processes of several chronic inflammatory diseases, we reviewed the interaction among the cannabinoid system, inflammation, and OS, through which clinicians ultimately aim to prolong the peritoneal survival of PD patients.
Core tip: Long-term exposure to bioincompatible peritoneal dialysis (PD) solutions frequently results in peritoneal fibrosis and ultrafiltration failure, which limits the life-long use of PD therapy. Beyond uremia per se, oxidative stress (OS) also results from chronic exposure to high glucose load, glucose degradation products, advanced glycation end products, and hypertonic stress in PD patients. Therapy targeting the cannabinoid signaling pathway has been reported in several chronic inflammatory diseases with elevated OS. In this article, we review the interaction among the cannabinoid system, inflammation, and OS, through which the health-care professionals ultimately aim to prolong the peritoneal survival of PD patients.
- Citation: Yang CY, Chau YP, Chen A, Lee OKS, Tarng DC, Yang AH. Targeting cannabinoid signaling for peritoneal dialysis-induced oxidative stress and fibrosis. World J Nephrol 2017; 6(3): 111-118
- URL: https://www.wjgnet.com/2220-6124/full/v6/i3/111.htm
- DOI: https://dx.doi.org/10.5527/wjn.v6.i3.111
As chronic kidney disease progresses to end-stage renal disease, uremia ensues requiring the use of long-term dialysis therapy. Both uremia and dialysis give rise to elevated oxidative stress (OS)[1-4], which is detrimental to the patients’ health. A recent survey indicated that approximately 11% of dialysis patients undergo peritoneal dialysis (PD) therapy worldwide, estimating to be more than 272000 patients with an 8% annual growth rate globally[5]. Beyond uremia per se, PD patients are at an increased risk of inflammation and OS, both systemically and locally, because of the chronic exposure to high glucose load[6-15], advanced glycosylated end products (AGEs)[16-18], glucose degradation products (GDPs)[18-21], and hypertonic stress[9,22-24]. After long-term exposure to various GDPs and AGEs, mesothelial cells of PD patients undergo a de-differentiation process, followed by peritoneal fibrosis and ultrafiltration failure[25-29]. In a large peritoneal biopsy study, peritoneal tissue samples from 212 subjects including healthy controls, hemodialysis and PD patients were examined. They found that peritoneal fibrosis was absent in normal individuals but was present in 28% of samples from hemodialysis patients and up to 56% of biopsies from PD patients[30]. Although whether a uremia-induced chronic inflammation status causes OS or OS leads to a proinflammatory process in uremic patients remains uncertain[1,4,31], therapeutic strategies for peritoneal damage targeting OS have been reported, such as antioxidants[12,18,32,33], scavenging agents for reactive oxygen species (ROS)[34-36], the trace element selenium[37], and gaseous mediators[38,39].
Cannabinoid (CB) signaling has also been reported to be effective in treating a variety of disease entities with elevated OS, including diabetic macrovascular and microvascular complications[40-50], cardiomyopathies[51-57], liver injury and fibrosis[58-64], cholangiopathies[65], colitis[66,67], drug nephrotoxicity[68,69], and autoimmune diseases[70] (Table 1). Since the peritoneum of long-term PD patients is under a chronic pro-inflammatory status, it is possible that the CB receptor (CBR) signaling system may be inappropriately modulated. In this article, we reviewed the sources and influence of OS in PD patients, and the therapeutic rationale and mechanisms of targeting the CB signaling system to reduce OS.
| Disease | Species | Disease model | Ref. |
| Diabetes mellitus | |||
| Microvascular complications | |||
| Nephropathy | Mouse | In vivo | [49,50] |
| Retinopathy | Mouse, rat | In vivo | [43,44] |
| Neuropathy | Mouse, rat | In vivo | [45-47] |
| Macrovascular complications | Mouse, rat | In vivo | [40,41] |
| Cardiomyopathy | |||
| Diabetic cardiomyopathy | Mouse | In vivo | [57] |
| Human cardiomyocyte | In vitro | [57] | |
| Myocardial ischemia/reperfusion | Mouse | In vivo | [51,53,54] |
| Ageing-related cardiomyopathy | Mouse | In vivo | [55] |
| Doxorubicin-induced cardiomyopathy | Mouse | In vivo | [52,56] |
| Human cardiomyocyte | In vitro | [56] | |
| Rat cardiomyocyte | In vitro | [52] | |
| Cisplatin nephropathy | Mouse | In vivo | [68,69] |
| Liver injury/fibrosis | |||
| Ischemia/reperfusion hepatocyte injury | Mouse | In vivo | [59] |
| CCl4-induced hepatocyte injury | Mouse | In vivo | [59] |
| Alcoholic liver fibrosis | Mouse | In vivo | [62] |
| Fibrotic activation of hepatic stellate cells | Human, rat, mouse | In vitro | [58,60,63,64] |
| Biliary diseases | |||
| Cholangiopathies | Mouse | In vivo | [65] |
| Mouse cholangiocyte | In vitro | [65] | |
| Enteric diseases | |||
| Inflammatory bowel disease | Mouse | In vivo | [66] |
| Colitis | Mouse | In vivo | [67] |
| Autoimmune systemic sclerosis | Mouse | In vivo | [70] |
Uremia per se and dialysis therapy both lead to a pro-oxidant status, which can lead to increased OS in patients receiving hemodialysis or PD therapy[1-4]. In particular, PD patients are exposed to hypertonic glucose solution on a long-term basis, which is not only toxic to mesothelial cells[6,71] but also promotes immune cell apoptosis[72]. Moreover, the high-temperature sterilization process produces GDPs such as methylglyoxal (MGO), acetaldehyde, formaldehyde, and 3-deoxyglucosone in PD dialysate[73-76]. GDPs possess strong oxidative properties and toxicity, and can induce AGEs[77]. In addition, it has been demonstrated that MGO, a key GDP, in PD dialysates inhibits the insulin signaling pathway, resulting in increased endogenous ROS production and subsequent cell injury[78]. Furthermore, 2-33 μmol/L of MGO has been reported to be present in commercial glucose-based PD fluids[79,80].
After long-term exposure to various GDPs and AGEs, mesothelial cells undergo a de-differentiation process and peritoneal fibrosis ensues[25-29]. Furthermore, these sites of chronic inflammatory have been associated with progressive peritoneal angiogenesis[29,81-83], and finally a reduction in the efficacy of PD. However, therapeutic strategies for these pathogenic processes have not been fully developed[81], and so some PD patients still develop peritoneal fibrosis or even encapsulating peritoneal sclerosis, a disastrous and highly fatal condition.
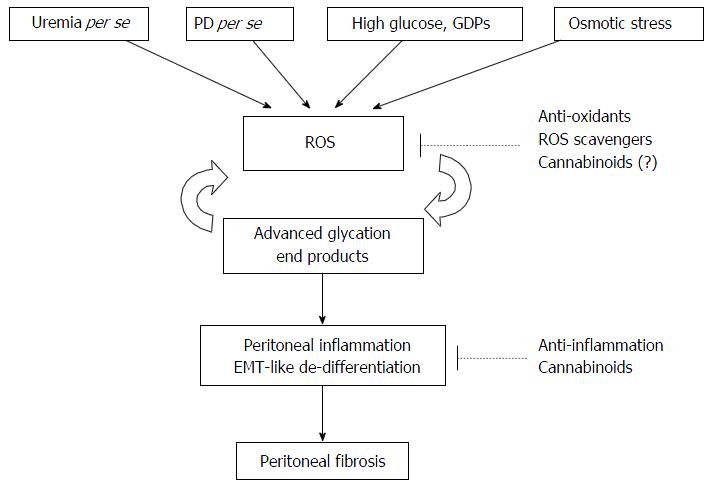
Low GDP PD dialysates can prevent peritoneal injury by PD-induced OS. However, the relatively high cost limits their full implementation. Moreover, even though the concentration of GDPs in the new generation of PD dialysates is low, it still exists[84-86]. Meanwhile, as long as the PD dialysate is glucose-based, glucose load per se results in ROS production[6-15]. Fortunately, therapies reducing peritoneal OS are under investigation, and include antioxidants[12,18,32,33], ROS scavengers[34-36], selenium[37], and gaseous mediators[38,39] (Figure 1).
In addition to low GDP PD dialysates, non-glucose-based PD dialysates such as icodextrin are free of GDPs and have been shown to be beneficial in fluid control and small solute clearance[87]. It has also been reported that peritoneal OS is reduced when using icodextrin compared with conventional PD dialysates[88].
However, other studies have reported conflicting results in that the osmotic stress, a type of stress resulted from hypertonic PD dialysate exposure, leads to oxidative DNA damage of peritoneal mesothelial cells through lipid peroxidation. Such peritoneal oxidative injury may then lead to mesothelial cell death either through apoptosis or necrosis[9,22-24]. Therefore, persistent efforts are warranted to develop an optimal solution.
Our recent study suggested that using CBR ligands as an additive in PD dialysate may be a promising solution to treat dialysis-induced peritoneal inflammation[89]. There are two subtypes of CBRs, type 1 CB receptor (CB1R) and type 2 CB receptor (CB2R). The former mainly exists in the brain and regulates inhibitory neurotransmitters on neurons through the psychoactive drug cannabis or endocannabinoids such as anandamide. Nevertheless, it has recently been found that CB1R also exists in tissues other than that of the central nervous system, and that its function varies in different organs[73]. CB1R antagonists and CB2R agonists have been shown to decrease inflammation and OS[48], and previous studies have also shown that CBR plays an important role in liver fibrogenesis[90-94]. Moreover, hepatic fibrosis can be rescued by knockout of the CB1R gene or by administration of the CB1R antagonist[93,95,96]. In contrast, CB2R is located on immune cells and modulates cytokine release[97,98]. Recent studies have shown that the activation of CB2R ameliorates liver fibrogenesis through inhibiting myofibroblast cell proliferation[92,99]. Furthermore, CBR ligands such as cannabidiol have been proven to be well-tolerated without adverse effects when administered to humans on a long-term basis[48].
Only a few studies have been published on pharmacological modulation targeting peritoneal inflammation and fibrogenesis using CBR ligands[100]. Our recent study indicated that the pharmacological effects of CBR ligands against dialysate-induced peritoneal fibrosis may involve a diverse signaling system including the TGF-β1-PI3K pathway[89], and that this offers a promising therapeutic strategy for the prevention of peritoneal fibrosis in patients receiving long-term PD. Therefore, we suggest that CBR signaling might play an important role in the pathogenesis of dialysis-induced peritoneal inflammation and ROS production, which required further studies.
In vitro data showed that, upon GDP and AGE exposure during PD, cellular OS of human peritoneal mesothelial cells were induced through activation of protein kinase C, nicotinamide adenine dinucleotide phosphate oxidase, and mitochondrial metabolism. In turn, the generated ROS upregulate fibronectin expression by mesothelial cells[7]. An in vivo study demonstrated 8-hydroxy-2’-deoxyguanosine (8-OHdG)-positive cells, indicating cells with increased OS, were observed throughout the fibrotic peritoneal tissue. Further immunofluorescent analysis revealed that 8-OHdG-positive cells also co-stained with mesothelin (mesothelial cells), CD68 (macrophages), CD31 (vascular endothelial cells), and α-smooth muscle actin (fibroblasts)[34], suggesting that OS was also increased in cells other than mesothelial cells. However, whether these fibroblasts with increased cellular OS were derived from an epithelial-mesenchymal transition (EMT)-like process of mesothelial cells is unknown.
As aforementioned, it has been reported that CB2R is located on immune cells and modulates cytokine release[97,98]. The CB2R expression of human lymphocytes was downregulated by TGF-β stimulation[101], which was not seen in human mesothelial cells[89]. These findings suggest that TGF-β1 might have different physiological function in different cell types. Meanwhile, CB2R activation might exert its anti-fibrotic effects not directly to cells undergoing fibrotic change but indirectly through modulating the immune cells. Such interaction among different types of cells underlines the pathophysiological role of CBR signaling pathway in uremic and/or dialysis injuries, which is partly supported by a recent study showing that systemic administration of interleukin-10, an anti-inflammatory cytokines secreted by M2 macrophages, significantly reduced fibrous peritoneal thickening[102]. Therefore, current evidence indicates that beyond mesothelial cells, macrophages and vascular endothelial cells also contribute to ROS production during PD-induced peritoneal fibrosis.
It has been reported that modulating the CB signaling system is beneficial in treating various diseases resulting from increased OS including diabetic macrovascular and microvascular complications[41-43,48,57], cardiomyopathies[51,55-57], liver injury and fibrosis[58,60-64,103], cholangiopathies[65], colitis[66,67], drug nephrotoxicity[68,69], and autoimmune diseases[70]. At present, evidences of the therapeutic benefit of CBR ligands on peritoneal OS are lacking and deserve investigations.
Furthermore, recent studies have also shown significant anti-fibrogenic effects of CBR ligands in the liver[91-93,95,96,99]. However, the effects of CBR ligands on peritoneal tissue have rarely been studied, with only one recent study reporting that the CB2R agonist reduced the number of peritoneal macrophages in a murine peritonitis model induced by thioglycollate, an AGE derivative[104]. Furthermore, we recently demonstrated that both the selective CB1R antagonist (AM281) and the selective CB2R agonist (AM1241) were able to ameliorate MGO-induced peritoneal fibrosis in vivo, indicating that pharmacological modulation of CBR may be a feasible approach to optimize the biocompatibility of peritoneal dialysis fluid. However, ACEA, a CB1R agonist, has been shown to have an opposite effect to AM281 with regards type I collagen expression in cultured mesothelial cells, indicating specific anti-fibrogenic activity of the CB1R antagonist[89].
During peritoneal fibrosis, mesothelial cells undergo a process of myofibroblastic conversion. This is a complex process which has been reported to be associated with increased levels of TGF-β1, leptin, metalloproteinase-2, vascular endothelial growth factor, Snail, and the receptor for advanced glycosylated end products[79,105,106]. TGF-β1 has long been known to play crucial roles in the fibrogenic process of the peritoneum[107,108]. A previous study demonstrated that a high glucose load stimulates the production of TGF-β1 in peritoneal mesothelial cells[109]. Moreover, AGEs have been shown to increase the expression of TGF-β1, contribute to the development of sub-mesothelial fibrosis[110], and significantly contribute to increases in peritoneal OS. Meanwhile, our recent study showed that such EMT-like processes can be attenuated by the selective CB1R antagonist, AM281[89]. It is quite possible that OS is involved in the CBR-related pharmacological effects against peritoneal fibrosis. However, the exact pathogenic mechanisms between the CBR signaling pathway and uremic and/or dialysis injuries remain largely unknown.
Compared with hemodialysis patients, the chronic use of PD dialysate exposes PD patients to additional OS. The influence of such OS on the patients’ health can be both systemic and local, leading to cardiovascular diseases and peritoneal fibrosis, respectively. It has been shown that OS plays a critical role in the pathogenesis of chronic inflammatory diseases, and therefore targeting the CB signaling system may offer a potential therapeutic strategy to reduce dialysis-induced peritoneal fibrosis and eventually to prolong the peritoneal survival of PD patients.
Manuscript source: Unsolicited manuscript
Specialty type: Urology and nephrology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Demonacos C, Navarro-Gonzalez JF, Riutta AA, Swierczynski JT S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Marques de Mattos A, Marino LV, Ovidio PP, Jordão AA, Almeida CC, Chiarello PG. Protein oxidative stress and dyslipidemia in dialysis patients. Ther Apher Dial. 2012;16:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Diepeveen SH, Verhoeven GH, van der Palen J, Dikkeschei BL, van Tits BL, Kolsters G, Offerman JJ, Bilo HJ, Stalenhoef AF. The effect of the initiation of renal replacement therapy on lipid profile and oxidative stress during the first 6 months of treatment. Clin Chim Acta. 2005;361:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1379] [Cited by in RCA: 1461] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 4. | Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 860] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 5. | Li PK, Chow KM, Van de Luijtgaarden MW, Johnson DW, Jager KJ, Mehrotra R, Naicker S, Pecoits-Filho R, Yu XQ, Lameire N. Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol. 2017;13:90-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 382] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 6. | Gotloib L, Shostak A, Wajsbrot V, Kushnier R. High glucose induces a hypertrophic, senescent mesothelial cell phenotype after long in vivo exposure. Nephron. 1999;82:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Lee HB, Yu MR, Song JS, Ha H. Reactive oxygen species amplify protein kinase C signaling in high glucose-induced fibronectin expression by human peritoneal mesothelial cells. Kidney Int. 2004;65:1170-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Hung KY, Liu SY, Yang TC, Liao TL, Kao SH. High-dialysate-glucose-induced oxidative stress and mitochondrial-mediated apoptosis in human peritoneal mesothelial cells. Oxid Med Cell Longev. 2014;2014:642793. [PubMed] |
| 9. | Gotloib L. Mechanisms of cell death during peritoneal dialysis. A role for osmotic and oxidative stress. Contrib Nephrol. 2009;163:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Doñate T, Herreros A, Martinez E, Martinez J, Andrés E, Cabezas A, Ortiz A, de Prado A, Pou JM, Pamplona R. Protein oxidative stress in dialysis patients. Adv Perit Dial. 2002;18:15-17. [PubMed] |
| 11. | Zhu X, Wen F, Yang D, Liu J, Yuan S, Li J, Liu H, Xu X, Sun L, Liu F. [Effect of high glucose peritoneal dialysis solution on PGC-1α expression and mitochondria related oxidative injury in human peritoneal mesothelial cells]. ZhongNan DaXue XueBao YiXue Ban. 2013;38:1085-1091. [PubMed] |
| 12. | Gotloib L, Wajsbrot V, Cuperman Y, Shostak A. Acute oxidative stress induces peritoneal hyperpermeability, mesothelial loss, and fibrosis. J Lab Clin Med. 2004;143:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Duan S, Yu J, Liu Q, Wang Y, Pan P, Xiao L, Ling G, Liu F. Epithelial-to-mesenchymal transdifferentiation of peritoneal mesothelial cells mediated by oxidative stress in peritoneal fibrosis rats. ZhongNan DaXue XueBao YiXue Ban. 2011;36:34-43. [PubMed] |
| 14. | Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3116] [Cited by in RCA: 3103] [Article Influence: 124.1] [Reference Citation Analysis (0)] |
| 15. | Ishibashi Y, Sugimoto T, Ichikawa Y, Akatsuka A, Miyata T, Nangaku M, Tagawa H, Kurokawa K. Glucose dialysate induces mitochondrial DNA damage in peritoneal mesothelial cells. Perit Dial Int. 2002;22:11-21. [PubMed] |
| 16. | Yim MB, Yim HS, Lee C, Kang SO, Chock PB. Protein glycation: creation of catalytic sites for free radical generation. Ann N Y Acad Sci. 2001;928:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685-E694. [PubMed] |
| 18. | Noh H, Kim JS, Han KH, Lee GT, Song JS, Chung SH, Jeon JS, Ha H, Lee HB. Oxidative stress during peritoneal dialysis: implications in functional and structural changes in the membrane. Kidney Int. 2006;69:2022-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Müller-Krebs S, Kihm LP, Zeier B, Gross ML, Wieslander A, Haug U, Zeier M, Schwenger V. Glucose degradation products result in cardiovascular toxicity in a rat model of renal failure. Perit Dial Int. 2010;30:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Diaz-Buxo JA, Gotloib L. Peritoneal dialysis solutions--at a crossroad. Minerva Urol Nefrol. 2006;58:145-160. [PubMed] |
| 21. | Zeier M, Schwenger V, Deppisch R, Haug U, Weigel K, Bahner U, Wanner C, Schneider H, Henle T, Ritz E. Glucose degradation products in PD fluids: do they disappear from the peritoneal cavity and enter the systemic circulation? Kidney Int. 2003;63:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Gotloib L, Wajsbrot V, Shostak A. Mesothelial dysplastic changes and lipid peroxidation induced by 7.5% icodextrin. Nephron. 2002;92:142-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Gastaldello K, Husson C, Dondeyne JP, Vanherweghem JL, Tielemans C. Cytotoxicity of mononuclear cells as induced by peritoneal dialysis fluids: insight into mechanisms that regulate osmotic stress-related apoptosis. Perit Dial Int. 2008;28:655-666. [PubMed] |
| 24. | Gotloib L, Wajsbrot V, Shostak A. Icodextrin-induced lipid peroxidation disrupts the mesothelial cell cycle engine. Free Radic Biol Med. 2003;34:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | De Vriese AS, Tilton RG, Mortier S, Lameire NH. Myofibroblast transdifferentiation of mesothelial cells is mediated by RAGE and contributes to peritoneal fibrosis in uraemia. Nephrol Dial Transplant. 2006;21:2549-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Jiménez-Heffernan JA, Aguilera A, Aroeira LS, Lara-Pezzi E, Bajo MA, del Peso G, Ramírez M, Gamallo C, Sánchez-Tomero JA, Alvarez V. Immunohistochemical characterization of fibroblast subpopulations in normal peritoneal tissue and in peritoneal dialysis-induced fibrosis. Virchows Arch. 2004;444:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118:4325-4326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 322] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 28. | Yáñez-Mó M, Lara-Pezzi E, Selgas R, Ramírez-Huesca M, Domínguez-Jiménez C, Jiménez-Heffernan JA, Aguilera A, Sánchez-Tomero JA, Bajo MA, Alvarez V. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med. 2003;348:403-413. [PubMed] |
| 29. | Selgas R, Bajo A, Jiménez-Heffernan JA, Sánchez-Tomero JA, Del Peso G, Aguilera A, López-Cabrera M. Epithelial-to-mesenchymal transition of the mesothelial cell--its role in the response of the peritoneum to dialysis. Nephrol Dial Transplant. 2006;21 Suppl 2:ii2-ii7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, Mackenzie RK, Williams GT. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol. 2002;13:470-479. [PubMed] |
| 31. | Carrero JJ, Axelsson J, Avesani CM, Heimbürger O, Lindholm B, Stenvinkel P. Being an inflamed peritoneal dialysis patient - a Dante’s journey. Contrib Nephrol. 2006;150:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Kihm LP, Müller-Krebs S, Klein J, Ehrlich G, Mertes L, Gross ML, Adaikalakoteswari A, Thornalley PJ, Hammes HP, Nawroth PP. Benfotiamine protects against peritoneal and kidney damage in peritoneal dialysis. J Am Soc Nephrol. 2011;22:914-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Nakayama M, Izumi G, Nemoto Y, Shibata K, Hasegawa T, Numata M, Wang K, Kawaguchi Y, Hosoya T. Suppression of N(epsilon)-(carboxymethyl)lysine generation by the antioxidant N-acetylcysteine. Perit Dial Int. 1999;19:207-210. [PubMed] |
| 34. | Wakabayashi K, Hamada C, Kanda R, Nakano T, Io H, Horikoshi S, Tomino Y. Oral Astaxanthin Supplementation Prevents Peritoneal Fibrosis in Rats. Perit Dial Int. 2014;35:506-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Zareie M, Tangelder GJ, ter Wee PM, Hekking LH, van Lambalgen AA, Keuning ED, Schadee-Eestermans IL, Schalkwijk CG, Beelen RH, van den Born J. Beneficial effects of aminoguanidine on peritoneal microcirculation and tissue remodelling in a rat model of PD. Nephrol Dial Transplant. 2005;20:2783-2792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Inagi R, Miyata T, Ueda Y, Yoshino A, Nangaku M, van Ypersele de Strihou C, Kurokawa K. Efficient in vitro lowering of carbonyl stress by the glyoxalase system in conventional glucose peritoneal dialysis fluid. Kidney Int. 2002;62:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Liu J, Zeng L, Zhao Y, Zhu B, Ren W, Wu C. Selenium suppresses lipopolysaccharide-induced fibrosis in peritoneal mesothelial cells through inhibition of epithelial-to-mesenchymal transition. Biol Trace Elem Res. 2014;161:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Lu Y, Shen H, Shi X, Feng S, Wang Z, Shi Y. Hydrogen sulfide ameliorates high-glucose toxicity in rat peritoneal mesothelial cells by attenuating oxidative stress. Nephron Exp Nephrol. 2014;126:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Terawaki H, Hayashi Y, Zhu WJ, Matsuyama Y, Terada T, Kabayama S, Watanabe T, Era S, Sato B, Nakayama M. Transperitoneal administration of dissolved hydrogen for peritoneal dialysis patients: a novel approach to suppress oxidative stress in the peritoneal cavity. Med Gas Res. 2013;3:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Steffens S, Veillard NR, Arnaud C, Pelli G, Burger F, Staub C, Karsak M, Zimmer A, Frossard JL, Mach F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature. 2005;434:782-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 329] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 41. | Wheal AJ, Cipriano M, Fowler CJ, Randall MD, O’Sullivan SE. Cannabidiol improves vasorelaxation in Zucker diabetic fatty rats through cyclooxygenase activation. J Pharmacol Exp Ther. 2014;351:457-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Wang D, Couture R, Hong Y. Activated microglia in the spinal cord underlies diabetic neuropathic pain. Eur J Pharmacol. 2014;728:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 43. | El-Remessy AB, Rajesh M, Mukhopadhyay P, Horváth B, Patel V, Al-Gayyar MM, Pillai BA, Pacher P. Cannabinoid 1 receptor activation contributes to vascular inflammation and cell death in a mouse model of diabetic retinopathy and a human retinal cell line. Diabetologia. 2011;54:1567-1578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | El-Remessy AB, Al-Shabrawey M, Khalifa Y, Tsai NT, Caldwell RB, Liou GI. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am J Pathol. 2006;168:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 45. | Ulugol A, Karadag HC, Ipci Y, Tamer M, Dokmeci I. The effect of WIN 55,212-2, a cannabinoid agonist, on tactile allodynia in diabetic rats. Neurosci Lett. 2004;371:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Toth CC, Jedrzejewski NM, Ellis CL, Frey WH. Cannabinoid-mediated modulation of neuropathic pain and microglial accumulation in a model of murine type I diabetic peripheral neuropathic pain. Mol Pain. 2010;6:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 47. | Ellington HC, Cotter MA, Cameron NE, Ross RA. The effect of cannabinoids on capsaicin-evoked calcitonin gene-related peptide (CGRP) release from the isolated paw skin of diabetic and non-diabetic rats. Neuropharmacology. 2002;42:966-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Horváth B, Mukhopadhyay P, Haskó G, Pacher P. The endocannabinoid system and plant-derived cannabinoids in diabetes and diabetic complications. Am J Pathol. 2012;180:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 49. | Barutta F, Corbelli A, Mastrocola R, Gambino R, Di Marzo V, Pinach S, Rastaldi MP, Perin PC, Gruden G. Cannabinoid receptor 1 blockade ameliorates albuminuria in experimental diabetic nephropathy. Diabetes. 2010;59:1046-1054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 50. | Barutta F, Piscitelli F, Pinach S, Bruno G, Gambino R, Rastaldi MP, Salvidio G, Di Marzo V, Cavallo Perin P, Gruden G. Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy. Diabetes. 2011;60:2386-2396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 51. | Defer N, Wan J, Souktani R, Escoubet B, Perier M, Caramelle P, Manin S, Deveaux V, Bourin MC, Zimmer A. The cannabinoid receptor type 2 promotes cardiac myocyte and fibroblast survival and protects against ischemia/reperfusion-induced cardiomyopathy. FASEB J. 2009;23:2120-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 52. | Mukhopadhyay P, Bátkai S, Rajesh M, Czifra N, Harvey-White J, Haskó G, Zsengeller Z, Gerard NP, Liaudet L, Kunos G. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol. 2007;50:528-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 174] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 53. | Lim SY, Davidson SM, Yellon DM, Smith CC. The cannabinoid CB1 receptor antagonist, rimonabant, protects against acute myocardial infarction. Basic Res Cardiol. 2009;104:781-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Montecucco F, Lenglet S, Braunersreuther V, Burger F, Pelli G, Bertolotto M, Mach F, Steffens S. CB(2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J Mol Cell Cardiol. 2009;46:612-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 55. | Bátkai S, Rajesh M, Mukhopadhyay P, Haskó G, Liaudet L, Cravatt BF, Csiszár A, Ungvári Z, Pacher P. Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase. Am J Physiol Heart Circ Physiol. 2007;293:H909-H918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 56. | Mukhopadhyay P, Rajesh M, Bátkai S, Patel V, Kashiwaya Y, Liaudet L, Evgenov OV, Mackie K, Haskó G, Pacher P. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res. 2010;85:773-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 57. | Rajesh M, Mukhopadhyay P, Bátkai S, Patel V, Saito K, Matsumoto S, Kashiwaya Y, Horváth B, Mukhopadhyay B, Becker L. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. 2010;56:2115-2125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 411] [Cited by in RCA: 390] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 58. | Siegmund SV, Qian T, de Minicis S, Harvey-White J, Kunos G, Vinod KY, Hungund B, Schwabe RF. The endocannabinoid 2-arachidonoyl glycerol induces death of hepatic stellate cells via mitochondrial reactive oxygen species. FASEB J. 2007;21:2798-2806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 59. | Cao Z, Mulvihill MM, Mukhopadhyay P, Xu H, Erdélyi K, Hao E, Holovac E, Haskó G, Cravatt BF, Nomura DK. Monoacylglycerol lipase controls endocannabinoid and eicosanoid signaling and hepatic injury in mice. Gastroenterology. 2013;144:808-817.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 60. | Wang M, Abais JM, Meng N, Zhang Y, Ritter JK, Li PL, Tang WX. Upregulation of cannabinoid receptor-1 and fibrotic activation of mouse hepatic stellate cells during Schistosoma J. infection: role of NADPH oxidase. Free Radic Biol Med. 2014;71:109-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Wei Y, Kang XL, Wang X. The peripheral cannabinoid receptor 1 antagonist VD60 efficiently inhibits carbon tetrachloride-intoxicated hepatic fibrosis progression. Exp Biol Med (Maywood). 2014;239:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 62. | Patsenker E, Stoll M, Millonig G, Agaimy A, Wissniowski T, Schneider V, Mueller S, Brenneisen R, Seitz HK, Ocker M. Cannabinoid receptor type I modulates alcohol-induced liver fibrosis. Mol Med. 2011;17:1285-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 63. | Siegmund SV, Wojtalla A, Schlosser M, Zimmer A, Singer MV. Fatty acid amide hydrolase but not monoacyl glycerol lipase controls cell death induced by the endocannabinoid 2-arachidonoyl glycerol in hepatic cell populations. Biochem Biophys Res Commun. 2013;437:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Lee PJ, Woo SJ, Jee JG, Sung SH, Kim HP. Bisdemethoxycurcumin Induces apoptosis in activated hepatic stellate cells via cannabinoid receptor 2. Molecules. 2015;20:1277-1292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | DeMorrow S, Francis H, Gaudio E, Ueno Y, Venter J, Onori P, Franchitto A, Vaculin B, Vaculin S, Alpini G. Anandamide inhibits cholangiocyte hyperplastic proliferation via activation of thioredoxin 1/redox factor 1 and AP-1 activation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G506-G519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Borrelli F, Fasolino I, Romano B, Capasso R, Maiello F, Coppola D, Orlando P, Battista G, Pagano E, Di Marzo V. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem Pharmacol. 2013;85:1306-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 67. | Borrelli F, Aviello G, Romano B, Orlando P, Capasso R, Maiello F, Guadagno F, Petrosino S, Capasso F, Di Marzo V. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med (Berl). 2009;87:1111-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 68. | Mukhopadhyay P, Rajesh M, Pan H, Patel V, Mukhopadhyay B, Bátkai S, Gao B, Haskó G, Pacher P. Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radic Biol Med. 2010;48:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 69. | Pan H, Mukhopadhyay P, Rajesh M, Patel V, Mukhopadhyay B, Gao B, Haskó G, Pacher P. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J Pharmacol Exp Ther. 2009;328:708-714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 198] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 70. | Servettaz A, Kavian N, Nicco C, Deveaux V, Chéreau C, Wang A, Zimmer A, Lotersztajn S, Weill B, Batteux F. Targeting the cannabinoid pathway limits the development of fibrosis and autoimmunity in a mouse model of systemic sclerosis. Am J Pathol. 2010;177:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 71. | Yang AH, Chen JY, Lin YP, Huang TP, Wu CW. Peritoneal dialysis solution induces apoptosis of mesothelial cells. Kidney Int. 1997;51:1280-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 72. | Jin HM, Di YD, Xu QJ. Effects of commercial glucose-based peritoneal dialysates on peripheral blood phagocytes apoptosis. Perit Dial Int. 1999;19 Suppl 2:S388-S393. [PubMed] |
| 73. | Kjellstrand P, Martinson E, Wieslander A, Holmquist B. Development of toxic degradation products during heat sterilization of glucose-containing fluids for peritoneal dialysis: influence of time and temperature. Perit Dial Int. 1995;15:26-32. [PubMed] |
| 74. | Nilsson-Thorell CB, Muscalu N, Andrén AH, Kjellstrand PT, Wieslander AP. Heat sterilization of fluids for peritoneal dialysis gives rise to aldehydes. Perit Dial Int. 1993;13:208-213. [PubMed] |
| 75. | Jörres A. Glucose degradation products in peritoneal dialysis: from bench to bedside. Kidney Blood Press Res. 2003;26:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 76. | Linden T, Forsbäck G, Deppisch R, Henle T, Wieslander A. 3-Deoxyglucosone, a promoter of advanced glycation end products in fluids for peritoneal dialysis. Perit Dial Int. 1998;18:290-293. [PubMed] |
| 77. | Nakayama M, Sakai A, Numata M, Hosoya T. Hyper-vascular change and formation of advanced glycation endproducts in the peritoneum caused by methylglyoxal and the effect of an anti-oxidant, sodium sulfite. Am J Nephrol. 2003;23:390-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Riboulet-Chavey A, Pierron A, Durand I, Murdaca J, Giudicelli J, Van Obberghen E. Methylglyoxal impairs the insulin signaling pathways independently of the formation of intracellular reactive oxygen species. Diabetes. 2006;55:1289-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 79. | Hirahara I, Ishibashi Y, Kaname S, Kusano E, Fujita T. Methylglyoxal induces peritoneal thickening by mesenchymal-like mesothelial cells in rats. Nephrol Dial Transplant. 2009;24:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | Schalkwijk CG, ter Wee PM, Teerlink T. Reduced 1,2-dicarbonyl compounds in bicarbonate/lactate-buffered peritoneal dialysis (PD) fluids and PD fluids based on glucose polymers or amino acids. Perit Dial Int. 2000;20:796-798. [PubMed] |
| 81. | Aroeira LS, Aguilera A, Sánchez-Tomero JA, Bajo MA, del Peso G, Jiménez-Heffernan JA, Selgas R, López-Cabrera M. Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol. 2007;18:2004-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 266] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 82. | Stavenuiter AW, Schilte MN, Ter Wee PM, Beelen RH. Angiogenesis in peritoneal dialysis. Kidney Blood Press Res. 2011;34:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | Schilte MN, Celie JW, Wee PM, Beelen RH, van den Born J. Factors contributing to peritoneal tissue remodeling in peritoneal dialysis. Perit Dial Int. 2009;29:605-617. [PubMed] |
| 84. | Jörres A, Bender TO, Witowski J. Glucose degradation products and the peritoneal mesothelium. Perit Dial Int. 2000;20 Suppl 5:S19-S22. [PubMed] |
| 85. | Mortier S, Faict D, Schalkwijk CG, Lameire NH, De Vriese AS. Long-term exposure to new peritoneal dialysis solutions: Effects on the peritoneal membrane. Kidney Int. 2004;66:1257-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 86. | Mittelmaier S, Niwa T, Pischetsrieder M. Chemical and physiological relevance of glucose degradation products in peritoneal dialysis. J Ren Nutr. 2012;22:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 87. | Qi H, Xu C, Yan H, Ma J. Comparison of icodextrin and glucose solutions for long dwell exchange in peritoneal dialysis: a meta-analysis of randomized controlled trials. Perit Dial Int. 2011;31:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 88. | Canepa A, Verrina E, Perfumo F. Use of new peritoneal dialysis solutions in children. Kidney Int Suppl. 2008;108:S137-S144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 89. | Yang CY, Chau YP, Lee HT, Kuo HY, Lee OK, Yang AH. Cannabinoid receptors as therapeutic targets for dialysis-induced peritoneal fibrosis. Am J Nephrol. 2013;37:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 90. | Bátkai S, Járai Z, Wagner JA, Goparaju SK, Varga K, Liu J, Wang L, Mirshahi F, Khanolkar AD, Makriyannis A. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med. 2001;7:827-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 255] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 91. | Julien B, Grenard P, Teixeira-Clerc F, Van Nhieu JT, Li L, Karsak M, Zimmer A, Mallat A, Lotersztajn S. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology. 2005;128:742-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 359] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 92. | Lotersztajn S, Teixeira-Clerc F, Julien B, Deveaux V, Ichigotani Y, Manin S, Tran-Van-Nhieu J, Karsak M, Zimmer A, Mallat A. CB2 receptors as new therapeutic targets for liver diseases. Br J Pharmacol. 2008;153:286-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 93. | Teixeira-Clerc F, Julien B, Grenard P, Tran Van Nhieu J, Deveaux V, Li L, Serriere-Lanneau V, Ledent C, Mallat A, Lotersztajn S. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med. 2006;12:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 414] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 94. | Caraceni P, Pertosa AM, Giannone F, Domenicali M, Grattagliano I, Principe A, Mastroleo C, Perrelli MG, Cutrin J, Trevisani F. Antagonism of the cannabinoid CB-1 receptor protects rat liver against ischaemia-reperfusion injury complicated by endotoxaemia. Gut. 2009;58:1135-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 95. | Giannone FA, Baldassarre M, Domenicali M, Zaccherini G, Trevisani F, Bernardi M, Caraceni P. Reversal of liver fibrosis by the antagonism of endocannabinoid CB1 receptor in a rat model of CCl(4)-induced advanced cirrhosis. Lab Invest. 2012;92:384-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 96. | Yang YY, Lin HC, Huang YT, Lee TY, Hou MC, Wang YW, Lee FY, Lee SD. Effect of chronic CB1 cannabinoid receptor antagonism on livers of rats with biliary cirrhosis. Clin Sci (Lond). 2007;112:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 97. | Demuth DG, Molleman A. Cannabinoid signalling. Life Sci. 2006;78:549-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 336] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 98. | Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006;147 Suppl 1:S163-S171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 476] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 99. | Montecucco F, Burger F, Mach F, Steffens S. CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am J Physiol Heart Circ Physiol. 2008;294:H1145-H1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 100. | González-Mateo GT, Aroeira LS, López-Cabrera M, Ruiz-Ortega M, Ortiz A, Selgas R. Pharmacological modulation of peritoneal injury induced by dialysis fluids: is it an option? Nephrol Dial Transplant. 2012;27:478-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 101. | Gardner B, Zu LX, Sharma S, Liu Q, Makriyannis A, Tashkin DP, Dubinett SM. Autocrine and paracrine regulation of lymphocyte CB2 receptor expression by TGF-beta. Biochem Biophys Res Commun. 2002;290:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Onishi A, Akimoto T, Urabe M, Hirahara I, Muto S, Ozawa K, Nagata D, Kusano E. Attenuation of methylglyoxal-induced peritoneal fibrosis: immunomodulation by interleukin-10. Lab Invest. 2015;95:1353-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 103. | Siegmund SV, Uchinami H, Osawa Y, Brenner DA, Schwabe RF. Anandamide induces necrosis in primary hepatic stellate cells. Hepatology. 2005;41:1085-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 104. | Willecke F, Zeschky K, Ortiz Rodriguez A, Colberg C, Auwärter V, Kneisel S, Hutter M, Lozhkin A, Hoppe N, Wolf D. Cannabinoid receptor 2 signaling does not modulate atherogenesis in mice. PLoS One. 2011;6:e19405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 105. | Hirahara I, Kusano E, Yanagiba S, Miyata Y, Ando Y, Muto S, Asano Y. Peritoneal injury by methylglyoxal in peritoneal dialysis. Perit Dial Int. 2006;26:380-392. [PubMed] |
| 106. | Yang AH, Huang SW, Chen JY, Lin JK, Chen CY. Leptin augments myofibroblastic conversion and fibrogenic activity of human peritoneal mesothelial cells: a functional implication for peritoneal fibrosis. Nephrol Dial Transplant. 2007;22:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 107. | van Westrhenen R, Aten J, Hajji N, de Boer OJ, Kunne C, de Waart DR, Krediet RT. Cyclosporin A induces peritoneal fibrosis and angiogenesis during chronic peritoneal exposure to a glucose-based, lactate-buffered dialysis solution in the rat. Blood Purif. 2007;25:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 108. | Yang AH, Chen JY, Lin JK. Myofibroblastic conversion of mesothelial cells. Kidney Int. 2003;63:1530-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 109. | Kang DH, Hong YS, Lim HJ, Choi JH, Han DS, Yoon KI. High glucose solution and spent dialysate stimulate the synthesis of transforming growth factor-beta1 of human peritoneal mesothelial cells: effect of cytokine costimulation. Perit Dial Int. 1999;19:221-230. [PubMed] |
| 110. | De Vriese AS, Flyvbjerg A, Mortier S, Tilton RG, Lameire NH. Inhibition of the interaction of AGE-RAGE prevents hyperglycemia-induced fibrosis of the peritoneal membrane. J Am Soc Nephrol. 2003;14:2109-2118. [PubMed] |