Published online Sep 6, 2016. doi: 10.5527/wjn.v5.i5.429
Peer-review started: May 3, 2016
First decision: July 6, 2016
Revised: August 2, 2016
Accepted: August 17, 2016
Article in press: August 19, 2016
Published online: September 6, 2016
Processing time: 123 Days and 10.9 Hours
The large prevalence of respiratory acid-base disorders overlapping metabolic acidosis in hemodialysis population should prompt nephrologists to deal with the partial pressure of carbon dioxide (pCO2) complying with the reduced bicarbonate concentration. What the most suitable formula to compute pCO2 is reviewed. Then, the neglected issue of CO2 content in the dialysis fluid is under the spotlight. In fact, a considerable amount of CO2 comes to patients’ bloodstream every hemodialysis treatment and “acidosis by dialysate” may occur if lungs do not properly clear away this burden of CO2. Moreover, vascular access recirculation may be easy diagnosed by detecting CO2 in the arterial line of extracorporeal circuit if CO2-enriched blood from the filter reenters arterial needle.
Core tip: Partial pressure of carbon dioxide (pCO2) should be always taken into account for comprehensive assessment of acid-base imbalances of hemodialysis patients, also because respiratory disorders are very common in this population. To infer a respiratory disorder superimposing to metabolic acidosis, nephrologists should compute the expected pCO2 complying with the reduced bicarbonate concentration. Moreover, they have to take in account CO2 load from dialysis solution, because this burden may be harmful if ventilatory compensation does not properly occur. Finally, checking an increase of pCO2 in arterial line of extracorporeal circuit is an easy and reliable method to discover vascular access recirculation.
- Citation: Marano M, D’Amato A, Cantone A. Carbon dioxide: Global warning for nephrologists. World J Nephrol 2016; 5(5): 429-436
- URL: https://www.wjgnet.com/2220-6124/full/v5/i5/429.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i5.429
There is widespread awareness about carbon dioxide’s (CO2) effects on global warming of the Earth. A similar recognition would be desirable about the key role of CO2 in nephrology, but this topic is actually under-recognized. This review aims to issue a global warning about CO2 in the field of renal replacement therapies.
To date, nephrologists have always focused on serum bicarbonate (HCO3) concentration and the latter, as marker of metabolic acidosis, has been associated with mortality risk in hemodialysis patients. The finding of a low HCO3 value has been always regarded as a sign of metabolic acidosis, but respiratory alkalosis also is featured by decreased HCO3 concentration. Hence, diagnosing metabolic acidosis based on the latter parameter clearly neglects serum HCO3 modifications that are secondary to respiratory disorders. However, as this “respiratory bias” on serum HCO3 has been recently highlighted, from now on, a comprehensive assessment of acid-base parameters should be taken into account; it is mandatory, therefore, to include partial pressure of CO2 (pCO2) as an important parameter to characterize acid-base imbalances and estimate mortality risk in hemodialysis population. In these patients, respiratory acid-base disorders have been recently found in a large percentage and this should further prompt nephrologists to deal with the pCO2 complying with the reduced HCO3 concentration. Mixed disorders occur if measured pCO2 is not consistent with the expected value.
Next point that will be discussed in this review is the forgotten issue of CO2 load from dialysis solution. Dialysis solution needs to be acidic to avoid salt precipitations; at the same time, it has to increase patient’s blood pH. CO2 allows to meet both goals, if patients’ lungs function is not impaired. In fact the considerable amount of CO2 in the final diluted dialysis fluid keeps the pH low, preventing salt precipitation. Then, this volatile acid easily and quickly reaches patient’s bloodstream and it is cleared by lung ventilation as well. As a result of CO2 clearance and of HCO3 addition from dialysis solution, patient’s blood pH increases. When this clearance does not happen properly, “acidosis by dialysate” may occur. This syndrome is characterized by early hypercapnia followed by typical, i.e., hypoxic, respiratory failure.
Finally, we will point out that the large amount of CO2 moving from dialysis solution to the extracorporeal circuit may allow to detect vascular access recirculation if blood returning from the filter reenters arterial needle. Basics of “RecirCO2lation test” based upon detecting CO2 in the arterial line of extracorporeal circuit will be outlined.
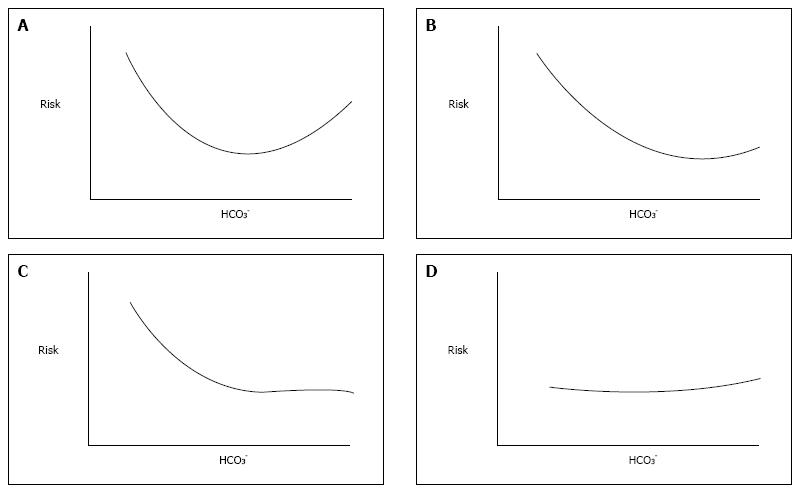
Since a slightly decreased pre-dialysis HCO3 concentration has been proven to lead to lower risk of death in hemodialysis patients[1], many efforts have been made to better characterize such risk. Results from Dialysis Outcomes and Practice Patterns (DOPPS) study[2] depicted such relationship as a U-shape curve (Figure 1A): Either very low and very high serum HCO3 concentrations were associated with higher risk of death. The authors of this landmark study concluded that moderate predialysis acidosis seems to be associated with lower relative mortality risk than what observed in patients with normal ranges of midweek predialysis serum HCO3 concentration or severe acidosis[2].
In fact the acid-base status of hemodialysis patients was inferred by serum HCO3, alone; neither pH or pCO2 were taken into account, because they were unavailable. Furthermore, true serum HCO3 concentration had not even been measured as the authors dealt with total CO2 content, however the latter amount is only slightly changed by large fluctuations of partial pressure of CO2 so that this parameter may be properly used as tantamount to serum HCO3 concentration. Conversely, it should be noted that serum HCO3 concentration changes are not exclusively due to metabolic disorders and that this assumption may be misleading because completely neglects the effects of respiratory acid-base disorders on HCO3 value. These disorders have never been taken into account, but likely exist because DOPPS population was characterized by a burden of comorbidity conditions, including heart and lung diseases known to be associated both with respiratory acidosis and alkalosis.
Another large population study[3] is based on the same assumption. This study confirmed the high risk of death associated with low HCO3 concentration, however if it was higher than the reference range risk did not increase (Figure 1B). Again acid-base status was inferred by the HCO3 value alone, but to answer the question whether it is better for an hemodialysis patient to be acidotic or alkalotic - that authors asked - a complete assessment of acid-base parameters is mandatory. Similar findings (Figure 1C) were later reported by Tentori et al[4] also in DOPPS cohort, again lacking complete acid-base assessment.
More recently, Yamamoto et al[5] failed to find any relationship between serum HCO3 concentration and mortality risk in a Japanese hemodialysis population (Figure 1D), but remarkably they found a strong association between pre-dialysis pH and mortality risk. Moreover, and above all, they provided all acid-base parameters and, in turn, allowed us to have for the first time the picture of acid-base disorders in a large hemodialysis population. As largely expected, the mean pH value was close to the lower limit of normal reference range, mean HCO3 concentration was 20.5 mEq/L and pCO2 was slightly under its normal value[5]. At a first glance, it would seem to be nothing else but mild metabolic acidosis with normal ventilatory response, but looking deeper into their data an unexpected presence of respiratory disorders may be predicted. In fact, patients in the lowest quartile of pH have the lowest mean value of HCO3 concentration but higher pCO2 value than patients in the highest pH quartile. This conflicting pattern of pCO2 with respect to that of HCO3 can be exclusively due to a superimposing respiratory acidosis in the lowest pH quartile group. Moreover, in the highest pH quartile group (i.e., pH ≥ 7.40) HCO3 concentration was lower than, not higher than, the normal range and also pCO2 was decreased as for coexisting respiratory alkalosis. Unfortunately, more detailed data are lacking, hence the existence of respiratory acid-base disorders in hemodialysis patients may be only conjectured. This notwithstanding, it should be acknowledged that Yamamoto et al[5] moved the spotlight away from serum HCO3 concentration.
Finally, in a much smaller cohort of patients we have reported the prevalence of all kinds of simple and mixed acid-base disorders[6]. As expected, metabolic acidosis was the most common acid-base disorder. It was observed that metabolic acidosis as simple disorder was found in 38.7% of measurements and was coupled with respiratory acid-base disturbances in further 23.2%. The latter, as simple or complex disorders, were found in 41% of analyzed blood samples. This finding might be surprising, but the large and growing prevalence in hemodialysis patients of heart[7] and lung diseases[8] - known to be possibly associated with respiratory acidosis and alkalosis - accounts for such results. It should be needless to say that to characterize acid-base pattern of hemodialysis patients, as well of all other patients, pCO2 should always be measured; however, here we want to emphasize that looking at HCO3 concentration alone is not enough. This is not an academic issue, because a superimposing lung or heart disease can move up or down HCO3 concentration toward the lower risk zone, but mortality risk of hemodialysis patient likely increases rather than decreases. Even though these results need further confirmation, the era of blood gas measurements in hemodialysis patients begins, and it perhaps occurs with some delay.
In conclusion, CO2 as respiratory component of acid-base pattern is at least as important as the metabolic component in acid-base assessment also in hemodialysis patients.
Metabolic acidosis is the commonest acid-base disorder occurring in hemodialysis patients[9-11]. Often it results in acidemia and consequently in increased ventilation to keep pH close to normal. As a result, pCO2 decreases, and the magnitude of this reduction is closely dependent on how much serum HCO3 concentration is decreased. Clearly, concurrent respiratory disorders may affect ventilatory compensation to metabolic acidosis, but this issue never received attention in this population. However mixed acid-base disorders - i.e., respiratory acid-base disturbances superimposing to metabolic disorder - likely occur and, according to recent reports[6], they are not a rare occurrence. This finding is all but unexpected, as these patients carry a burden of heart and lung comorbid conditions[2]. Accordingly, mixed disorders deserve full and prompt recognition, also in hemodialysis patients. To infer and diagnose mixed acid-base disorders clinicians must first evaluate the physiologic respiratory response to metabolic acidosis, namely they must estimate the value of partial pressure of pCO2 complying with the reduced HCO3 concentration. Ventilatory response to chronic metabolic acidosis is very predictable indeed; if the measured pCO2 value is greater or lower than the computed “expected” one, then the presence of a mixed disorder can be inferred. Ventilatory response to chronic metabolic acidosis is independent of the disease causing acid-base derangement[12], hence rules for the general population and all other patient also apply to hemodialysis population. However, in textbooks[13-15] and in current literature[10,16] more than one formula and rule are available, but recommendations on what should be used are lacking. As formulas are different each other, results are often inconsistent; this notwithstanding, selecting the proper formula, i.e., computing the proper value - is mandatory, to avoid wrong diagnosis and inappropriate treatment.
According to the long-lasting and widely used Winters’ formula[17,18] pCO2 can be predicted as serum HCO3× 1.5 + 8. This formula was derived by Albert, Dell and Winters in the 60’ in patients with severe acidosis and nowadays is still recommended, even though it lacks at all of any validation in patients with minor reductions of HCO3 concentration. Intuitively, a slight reduction of HCO3 is consistent with minor activation of the compensatory mechanisms whereas sizable decrease of serum HCO3 elicits large increase of ventilation, hence a linear relationship - as Winters’ formula is - might be not reliable throughout the acidosis spectrum.
Taking into account that serum HCO3 in modern hemodialysis patients ranges around 20 mmol/L[2,3,6,11] which is exactly twice the mean value in Albert’s population[17] - applying Winters’ formula in this scenario is at least questionable. Even though it is recommended across-the-board to apply Winters’ formula to hemodialysis population, that was associated with a larger error in prediction than other formulas.
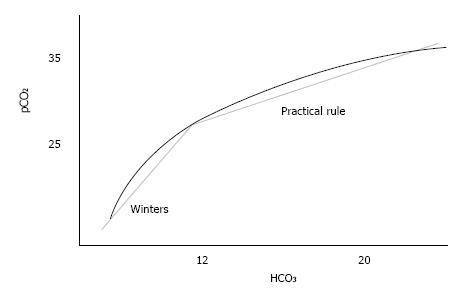
A reliable alternative may be the common practical rule that reads “the reduction of pCO2 with respect to its normal value equals 1.2 multiplied by the reduction of bicarbonate with respect to its normal value”[11,12,15,16]. This rule reliably predicts pCO2 in mild-to-moderate acidosis; as a matter of fact, it has always been adopted in hemodialysis population[11,10]. If 40 mmHg and 24 mmol/L are the normal values of pCO2 and of HCO3, respectively, the rule can be read as pCO2 = 40 - (24-HCO3) × 1.2 and equivalently rewritten as pCO2 = 1.2 × HCO3 + 11.2. Besides, it requires quite a few computations - and therefore the label practical is not very fitting - also this rule is a linear relationship between pCO2 and HCO3, hence it cannot be conveniently applied to all degrees of severity of metabolic acidosis.
In this case the slope of linear equation is reduced to 1.2. The use of different multipliers for acidosis of different degree fulfills the concept that activation of compensatory mechanisms is gradual and progressive, hence non-linear. In other words ventilatory compensation to chronic metabolic acidosis varies with severity of acidosis and a quadratic or cubic equation, i.e., a curve, better depicts the whole relationship between pCO2 and HCO3[12].
Unfortunately, this is an unfeasible option for physicians. However, as Bushinsky et al[12] highlighted, by restricting the analysis to HCO3 values below 10 mmol/L ventilatory response can be predicted with good approximation by the linear equation with a slope equal to 1.5 - just the multiplier of Winters’ formula - whereas if HCO3 values range between 10.1 and 24 mmol/L the linear equation with a slope close to 1.2 - the multiplier of practical rule - allows to properly calculate the expected pCO2 value. Accordingly, as we already suggested elsewhere[19,20], a reliable method to correctly predict pCO2 may be the use of two different linear formulas depending on severity of metabolic acidosis (Figure 2).
Beyond the well-known and widely used above-mentioned formulas, several textbooks provide some tips to easy calculate the expected pCO2. One of these rules - quite surprisingly - allows a very easy and valid prediction of pCO2 value in hemodialysis population[19]. It simply suggests to add “15” to HCO3 concentration to obtain the expected pCO2 value, the so called “Bicarbonate plus 15” rule. With this very simple formula only 1 mmHg difference arises compared to practical rule when HCO3 ranges between 14 and 24 mmol/L, as commonly occurs in almost all hemodialysis patients. In this population the very simple formula was associated with same (low) mean error exhibited by the practical rule (Table 1)[19] and therefore in this scenario it could be suggested as a valid and reliable alternative formula as it has the undeniable advantage of making CO2 prediction easier and also attractive to physicians reluctant to approach the acid-base troubles.
| Blood samples featuring HCO3 < 24 mmol/L | Blood samples claimed for metabolic acidosis | |
| “Winters’ formula” pCO2 = 1.5 HCO3 + 8 | 4.85 | 2.06 |
| “Practical rule” pCO2 = 1.2 HCO3 + 11.2 | 3.14 | 1.50 |
| “Very simple formula” pCO2 = HCO3 + 15 | 3.09 | 1.56 |
The acid-base pattern of dialysate and of blood coming back from dialyzer to patient during bicarbonate hemodialysis has been recently recalled and has been labeled “dialysis-related acidemia”[21].
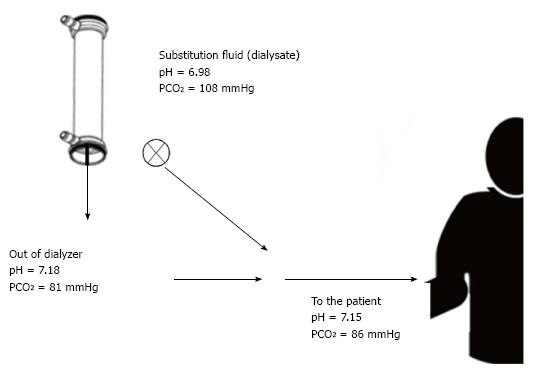
It has been above mentioned the compensatory response to metabolic acidosis that ultimately leads to hypocapnia - a common feature of hemodialysis patient - here we want to recall that pCO2 in the final diluted dialysate is two-to-three folds the quantity found in the uremic blood entering the extracorporeal circuit. This large dialysate-blood difference accounts for very high CO2 dialysance and in turn for the sizeable transfer of CO2 from dialysate into the blood coming back to patient[22]. Even though high HCO3 concentration, blood reaching patient’s bloodstream is featured by low pH due to very high pCO2[22,23]. This pattern looks like respiratory acidosis but it has nothing to do with the lung. Moreover in hypercapnic acidosis partial pressure of oxygen (pO2) is always decreased, whereas in dialysis-related acidemia does not, because a gain of oxygen across the filtering membrane also occurs. Dialysis-related acidemia vanishes as soon as CO2 is breathed away by lung (hyper)ventilation, thus HCO3 coming from dialyzer counteracts uremic acidosis. The source of CO2 is dialysate itself, indeed mixing acid concentrate with HCO3-containing solution the acid - commonly acetic acid - reacts with buffer leading to acetate anion and CO2. The more the acid in acid concentrate, the more the CO2 in the final diluted dialysate. As a typical example 3 mmol/L of acetic acid (or a mixture of citric and acetic acid) are in the concentrate and as a result 3 mmol/L of CO2 are in dialysate. This leads to pCO2 ranging between 80 and 100 mmHg and in turn to dialysate pH lower than 7.30. This allows calcium and magnesium bicarbonate salts to remain in their soluble form. The presence of CO2 is actually mandatory and in the same way “an adequate ventilatory capacity is imperative to excrete the excess CO2 generated during high efficiency bicarbonate hemodialysis”[23].
If patients are unable to increase their ventilatory rate and in turn to breath away CO2 overload from dialysate, then systemic pCO2 increases leading to reduction of peripheral vascular resistance[24,25], harmful hypotension and severe dyspnea poorly relieved by oxygen administration for the time being. Dialysis treatment should be slowed down or even stopped to avoid more severe effects. As hypercapnia superimposes to metabolic acidosis, a mixed (metabolic plus respiratory) acidosis occurs with abrupt fall of blood pH. Hypoxia is only a later event. A few of such cases are reported in the literature[26-28], likely due to poor awareness of the syndrome, recently labeled “acidosis by dialysate”[21].
The issue of CO2 load during renal replacement therapy has been for long time neglected and has not in depth investigated. However theoretical considerations and some findings from literature allow to briefly comment on.
Acetate-free hemodiafiltration is an alternative dialysis technique claimed for allowing better hemodynamic stability and paucity of dialysis-related symptoms. It is featured by lack of any buffer in dialysate, indeed any acid is needed. Accordingly, the final diluted dialysate is “CO2-free” other than “acetate-free” and this represents an important difference between acetate-free biofiltration and all other dialysis techniques. Even though some amount of CO2 comes back to patients from sodium bicarbonate infusion, acetate-free biofiltration should be claimed for providing a lighter CO2 load compared to conventional bicarbonate hemodialysis[29]. Outstandingly, pCO2 in blood from dialyzer is very close to physiological amount, meaning that AFB might be suggested as the more advisable technique for patients unable to handle CO2 overload as those with chronic obstructive lung disease, an increasingly prevalent comorbid condition[8].
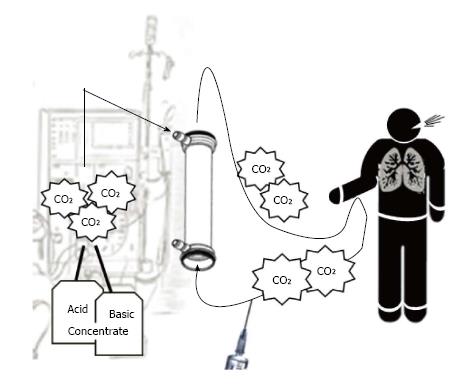
On the other side online hemodiafiltration - regarded as the new gold standard of renal replacement therapy - implies an heavier CO2 load than bicarbonate hemodialysis does[30]. An additional CO2 load is delivered by infusing dialysate, with its burden of CO2, directly in patient’s bloodstream (Figure 3). As the largest infusion volume possible has been recommended[31], the issue of CO2 overload during online hemodiafiltration should be taken in account. Whether different CO2 loads should be taken in account to withhold or in the opposite to recommend a certain replacement therapy to a certain hemodialysis patient is a question never asked.
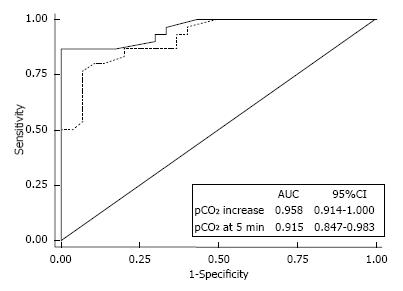
If CO2-enriched blood coming from the dialyzer reenters extracorporeal circuit, then vascular access recirculation may be detected by means of gas analysis of blood withdrawn from arterial line[32] (Figure 4). The typical acid-base picture of blood out the dialyzer - “dialysis-related acidemia” - is actually found in arterial line. As hypercapnic acidosis is coupled with normal or high pO2, this acid-base pattern is unique and it is not suggestive of any human illness. Accordingly, vascular access recirculation may be easy and profitably discovered by means of easy blood sampling from arterial line of dialysis circuit. A pCO2-increase > 4.5 mmHg (with respect to pre-dialysis value: “two samples technique”) discovers vascular access recirculation with absolute specificity (100%) and high sensitivity (86.7%). A reliable alternative chance (“one sample technique”) consists of a single blood sampling (5 min from dialysis start) to check whether pCO2 is over or below a certain threshold. For both approaches, receiver operating characteristic analysis showed remarkable areas under curves (Figure 5). As a special feature of this novel test - labeled “RecirCO2lation test” - the use of CO2 as indicator offers the undeniable chance of overcoming the issue of cardiopulmonary recirculation, because the excess of CO2 coming from the dialyzer is time by time cleared away by lungs and therefore if recirculation does not occur, it can never reaches arterial line.
CO2 as respiratory component of acid-base pattern is at least as important as the metabolic component in acid-base assessment also in hemodialysis patients. To infer and diagnose mixed acid-base disorders, physiologic respiratory response to metabolic acidosis should be considered and the expected pCO2 value should be computed. To do it, a very simple formula - “bicarbonate plus 15” - is a reliable alternative to the common practical rule, not so practical.
The acid-base pattern of blood coming back from dialyzer to patient during bicarbonate hemodialysis is featured by low pH due to very high pCO2. Increasing ventilation rate is mandatory to excrete CO2 overload, otherwise harmful “acidosis by dialysate” may occur. Among renal replacement therapies, acetate-free biofiltration is featured by a more physiological load of CO2, whereas online hemodiafiltration implies an additional CO2 load.
Finally, vascular access recirculation may be detected by means of gas analysis performed on blood withdrawn from arterial line of extracorporeal circuit. This novel method has been labeled “RecirCO2lation test”.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P- Reviewer: Marino IR, Muench MO, Marickar YMF S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1422] [Cited by in RCA: 1306] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 2. | Bommer J, Locatelli F, Satayathum S, Keen ML, Goodkin DA, Saito A, Akiba T, Port FK, Young EW. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004;44:661-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Wu DY, Shinaberger CS, Regidor DL, McAllister CJ, Kopple JD, Kalantar-Zadeh K. Association between serum bicarbonate and death in hemodialysis patients: is it better to be acidotic or alkalotic? Clin J Am Soc Nephrol. 2006;1:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Tentori F, Karaboyas A, Robinson BM, Morgenstern H, Zhang J, Sen A, Ikizler TA, Rayner H, Fissell RB, Vanholder R. Association of dialysate bicarbonate concentration with mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2013;62:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 5. | Yamamoto T, Shoji S, Yamakawa T, Wada A, Suzuki K, Iseki K, Tsubakihara Y. Predialysis and Postdialysis pH and Bicarbonate and Risk of All-Cause and Cardiovascular Mortality in Long-term Hemodialysis Patients. Am J Kidney Dis. 2015;66:469-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Marano M, Marano S, Gennari FJ. Beyond bicarbonate: complete acid-base assessment in patients receiving intermittent hemodialysis. Nephrol Dial Transplant. 2016; Mar 21; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States: Cardiovascular Disease in Patients With. Am J Kidney Dis. 2016;67 Suppl 3:S49-S56. |
| 8. | Kent BD, Eltayeb EE, Woodman A, Mutwali A, Nguyen HT, Stack AG. The impact of chronic obstructive pulmonary disease and smoking on mortality and kidney transplantation in end-stage kidney disease. Am J Nephrol. 2012;36:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Vashistha T, Kalantar-Zadeh K, Molnar MZ, Torlén K, Mehrotra R. Dialysis modality and correction of uremic metabolic acidosis: relationship with all-cause and cause-specific mortality. Clin J Am Soc Nephrol. 2013;8:254-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Oh MS, Uribarri J, Weinstein J, Schreiber M, Kamel KS, Kraut JA, Madias NE, Laski ME. What unique acid-base considerations exist in dialysis patients? Semin Dial. 2014;17:351-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Gennari FJ. Acid-base homeostasis in dialysis. Handbook of dialysis therapy. Philadelphia: Saunders Elsevier 2008; 673-684. [DOI] [Full Text] |
| 12. | Bushinsky DA, Coe FL, Katzenberg C, Szidon JP, Parks JH. Arterial PCO2 in chronic metabolic acidosis. Kidney Int. 1982;22:311-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Du Bose Jr TD. Acidbase disorders. Brenner & Rector’s the kidney. Philadelphia: Saunders 2008; 505-546. |
| 14. | Du Bose Jr TD. Acidosis and alkalosis. Harrison’s principles of internal medicine. New York: McGrawHill 2011; 363-373. |
| 15. | Palmer BF, Alpern RJ. Metabolic acidosis. Comprehensive clinical nephrology. Saunders: St. Louis Missouri 2010; 155-166. [DOI] [Full Text] |
| 16. | Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol. 2010;21:920-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Albert MS, Dell RB, Winters RW. Quantitative displacement of acid-base equilibrium in metabolic acidosis. Ann Intern Med. 1967;66:312-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 102] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Berend K, de Vries AP, Gans RO. Physiological approach to assessment of acid-base disturbances. N Engl J Med. 2014;371:1434-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 19. | Marano M, D’Amato A, Marano S. A very simple formula to compute pCO2 in hemodialysis patients. Int Urol Nephrol. 2015;47:691-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Marano M. [On the use of Winters’ formula in chronic metabolic acidosis]. Rev Psiquiatr Salud Ment. 2015;8:45-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Marano M, Borrelli S, Zamboli P. Dialysis-Related Acidemia and Acidosis by Dialysate: The Forgotten Issue of CO2 Overload from Dialysate. Blood Purif. 2016;41:313-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Sombolos KI, Bamichas GI, Christidou FN, Gionanlis LD, Karagianni AC, Anagnostopoulos TC, Natse TA. pO2 and pCO2 increment in post-dialyzer blood: the role of dialysate. Artif Organs. 2005;29:892-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Symreng T, Flanigan MJ, Lim VS. Ventilatory and metabolic changes during high efficiency hemodialysis. Kidney Int. 1992;41:1064-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Cullen DJ, Eger EI. Cardiovascular effects of carbon dioxide in man. Anesthesiology. 1974;41:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 129] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Curley G, Laffey JG, Kavanagh BP. Bench-to-bedside review: carbon dioxide. Crit Care. 2010;14:220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Latchford K, Cowperthwaite J. Shortness of breath during dialysis--a role of bicarbonate in dialysis fluid? J Ren Care. 2008;34:2-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Hamm LL, Lawrence G, DuBose TD. Sorbent regenerative hemodialysis as a potential cause of acute hypercapnia. Kidney Int. 1982;21:416-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Marano M. [Gas analysis in bicarbonate dialysis]. G Ital Nefrol. 2013;30:pii: gin/30.5.8. [PubMed] |
| 29. | Marano M, D’Amato A, Patriarca A, Di Nuzzi LM, Giordano G, Iulianiello G. Carbon Dioxide and Acetate-Free Biofiltration: A Relationship to be Investigated. Artif Organs. 2015;39:960-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Marano M, Capasso M, Cicchella T. On-Line Hemodiafiltration: All That Glitters Is Not Gold. Blood Purif. 2016;41:315-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Maduell F. Is There an ‘Optimal Dose’ of Hemodiafiltration? Blood Purif. 2015;40 Suppl 1:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Marano M, Borrelli S, Zamboli P. pCO2 Reveals Arteriovenous Fistula Recirculation in Bicarbonate Hemodialysis (RecirCO2lation Test). Blood Purif. 2016;41:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |