Published online Sep 6, 2016. doi: 10.5527/wjn.v5.i5.418
Peer-review started: April 29, 2016
First decision: May 17, 2016
Revised: May 31, 2016
Accepted: June 27, 2016
Article in press: June 29, 2016
Published online: September 6, 2016
Processing time: 126 Days and 11.6 Hours
Renal injury or failure may occur in the context of pregnancy requiring special considerations with regard to fetal and maternal health. The condition of pregnancy itself may be a major factor in such injuries. In addition, for many young women previously known to be healthy, pregnancy may be the first presentation for routine urine and blood testing which may yield previously subclinical renal disease. As such, pregnancy may add complexity to considerations in the management of renal disease presenting coincidentally requiring knowledge of the physiologic changes and potential renal disorders that may be encountered during pregnancy.
Core tip: Kidney disease and particularly complications of hypertensive disorders is one of the dire threats to successful pregnancy. This review highlights advances in our understanding of the pathophysiological processes that drive the development of hypertensive disorders’ complications during pregnancy, potential use of biomarkers in predicting these complications, and novel therapeutic approaches under consideration for their great promise in achieving successful pregnancy.
- Citation: Berry C, Atta MG. Hypertensive disorders in pregnancy. World J Nephrol 2016; 5(5): 418-428
- URL: https://www.wjgnet.com/2220-6124/full/v5/i5/418.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i5.418
Pregnancy is a unique arena in the practice of nephrology in which special considerations must be made for a host of factors such as hemodynamics, immunology, metabolism, pharmacology, and embryology. For many young women previously known to be healthy, pregnancy may be the first presentation for routine urine and blood testing which may yield previously subclinical renal disease. Furthermore, renal injury or failure may occur in the context of pregnancy requiring special considerations with regard to fetal and maternal health. The condition of pregnancy itself may be a major factor in such injuries (e.g., as in prerenal injury secondary to hyperemesis gravidarum, or acute cortical necrosis secondary to septic abortion or peripartum hemorrhage) or may simply add complexity to considerations in the management of renal disease presenting coincidentally. The nephrologist may be consulted when patients develop acute kidney injury (AKI) with glomerulonephritic features including refractory hypertension, edema, reduced estimated glomerular filtration rate (eGFR), proteinuria, and occasionally microangiopathy[1], for which preeclampsia is often on the differential diagnosis. This review aims to explore the anticipated physiologic changes in pregnancy before addressing the hypertensive disorders of gestation, of which pre-eclampsia is the most common; affecting approximately 5% of pregnancies worldwide. Our aim is to present a comprehensive overview of the current knowledge on physiologic changes in pregnancy, with special attention paid to the epidemiology, genetics, pathophysiology, diagnosis, and management of preeclampsia.
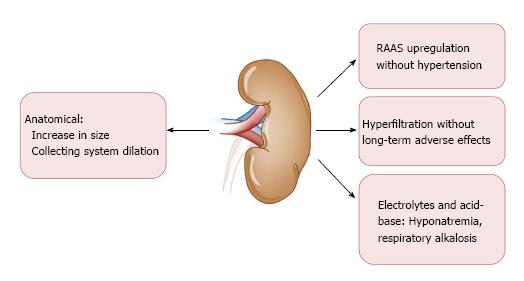
Several unique physiologic changes occur in the context of a normal healthy pregnancy (Figure 1). There is an overall up-regulation of the renin-angiotensin-aldosterone system (RAAS) that begins at the time of the luteal phase of the menstrual cycle and is co-incident with rising estrogen and progesterone levels[2,3]. After fertilization, elaboration of each of these hormones continues in order to support gestation; renin up to eight times, angiotensin up to four times, and aldosterone up to ten to twenty times normal levels[2,3].
Healthy women do not become hypertensive in this context, however, owing either to an estrogen-mediated decrease in vascular responsiveness to these RAAS components, or possibly owing to the counteracting vasodilatory effect of prostacyclins and the ovarian-secreted gestational hormone relaxin[4]. This systemic vasodilation tends to decrease systolic blood pressure by about 10-15 mmHg[5]. This global vasodilation also dilates the renal vasculature resulting in an early and robust increase in glomerular filtration-initially by about 25% and progressing up to 50% by mid-pregnancy. There is an even larger increase in renal plasma flow, around 60%. The result is a state of hyperfiltration that does not actually engender any pathologic injury as in other states of hyperfiltration such as in diabetic kidney disease[6]. Pregnancy is associated with reduction in the single nephron filtration fraction compared to the increase encountered in other hyperfiltration conditions. RAAS up-regulation contributes to sodium and fluid retention, which facilitates plasma volume expansion within the dilated vasculature. Intravascular volume expansion results in a mild dilutional anemia and hyponatremia; serum sodium concentrations may be reduced by approximately 4-5 meq/L[7].
Increased renal volume and length is frequently appreciated on ultrasonography, along with mild non-obstructive hydronephrosis due to uterine compression of the ureters. Pelviectasis is typically more pronounced on the right side, possibly owing to dextrorotation of the uterus and exaggerated by the relative protection of the left ureter provided by the sigmoid colon[8]. This low-grade obstruction may be symptomatic in approximately 30% of pregnant women and can predispose to urinary tract infections.
An increasing subset of women enter into pregnancy with pre-existing hypertension in the setting of the usual risk factors for essential hypertension such as obesity, race, and advanced maternal age. An estimated 25% of these patients may develop a superimposed preeclampsia syndrome. Hypertensive disorders of pregnancy increase maternal risk of developing AKI in addition to other etiologies as stratified in Table 1. In this relatively young demographic, essential hypertension is less likely to have been present long enough to manifest any clinically apparent end-organ damage so the development of any proteinuria or other renal dysfunction would potentially point to the onset of an overlapping preeclampsia syndrome. In women entering pregnancy with pre-existing renal disease, which may potentially be masked by the effects of hyperfiltration on conventional markers of renal function (e.g., serum creatinine) risks to the mother and fetus can be stratified by the severity of renal insufficiency and modes of renal replacement therapy, if applicable (Table 2)[9].
| Renal disease by trimester | ||
| 1st trimester | Hyperemesis gravidarum | Worsening of preexisting renal disease |
| Week 1-12 | Cortical necrosis due to septic abortion | ↓ |
| Preeclampsia (> after 20 wk) | ↓ | |
| AFLP | ↓ | |
| 2nd trimester | Preeclampsia | ↓ |
| Week 13-28 | HELLP syndrome | ↓ |
| TTP | ↓ | |
| 3rd trimester | Preeclampsia | ↓ |
| Week 29-40 | Polyhydramnios | ↓ |
| Extraureteral obstructive hydronephrosis | ↓ | |
| Post-partum | Post-partum hemolytic uremic syndrome | ↓ |
| Preeclampsia | ↓ | |
| Maternal and fetal risk by degree of renal impairment | ||
| Stage | Pregnancy/fetal outcomes | Renal/maternal outcomes |
| Early CKD I-II sCr < 1.4 mg/dL eGFR < 70 mL/min Normal BP Minimal proteinuria | Higher risk than general population for preeclampsia, SGA, preterm delivery Counseling: May need specialized care Generally good outcomes | Lower risks for accelerated progression |
| Moderate CKD II-III sCr 1.4-2.4 mg/dL eGFR 40-70 mL/min | With more advanced CKD and higher proteinuria: Higher risks of caesarian section, preterm delivery, SGA, and need for NICU | Increased risk of progression during pregnancy and within 6 wk postpartum Counseling: Pregnancy termination doesn’t reliably reduce risks for progression |
| Severe CKD III-IV sCr > 2.4 mg/dL eGFR < 40 mL/min | With more advanced CKD and higher proteinuria: Higher risks of caesarian section, preterm delivery, SGA, and need for NICU care | Increased risk of progression during pregnancy and within 6 wk postpartum |
| ESRD | Decreased fertility and high fetal mortality except with more intensive hemodialysis Higher risks of preeclampsia, SGA, cervical incompetence, and need for NICU care persist | Increased need for transfusion, worsening hypertension |
| Post-transplant | ± increased risk of fetal loss Increased risk of low birth weight and preterm delivery Significantly increased risk of preeclampsia if hypertensive | Blunted renal physiologic adaptations No anticipated decrease in graft survival but may be associated with decreased maternal life span Increased risk of diabetes, urinary tract infection (due to anatomy, insulin resistance, and immunosuppression) |
Preeclampsia is a heterogeneous, multi-system disorder characterized by widespread dysfunction, including glomerular endothelium; it is the most common glomerular disorder in pregnancy. The criteria for diagnosis includes two blood pressure readings at least 4-6 h apart that are greater than 140/90 occurring after 20 wk’ gestation in a woman not known to be previously hypertensive[10]. The syndrome may also develop in the 4-6 wk postpartum period[10]. Consequently, preeclampsia is best categorized into early onset/placental (< 34 wk of gestation) vs late onset/maternal (> 34 wk of gestation) reflecting that there are potentially different triggering events in pathogenesis as well as the worse maternal-fetal prognoses of early vs late preeclampsia. Edema and elevated uric acid levels are also frequently among the constellation of findings but are not strictly part of the definition[10] (Table 3). Either new onset or worsening of pre-existing proteinuria greater than 300 mg in 24 h may be present, but proteinuria itself has been removed from the definition since it is a relatively late marker of glomerular injury. Higher levels of proteinuria above 5 g/g were once considered to be a marker of severity as well but this has fallen out of favor for the same reason. In the absence of proteinuria, preeclampsia is confirmed when de novo hypertension after 20 wk of gestation is associated with maternal or fetal end organ damage which may include thrombocytopenia, elevated serum aminotransferase levels, AKI, pulmonary edema, new onset of cerebral or visual disturbances, or uteroplacental dysfunction. AKI or renal failure can occur, however identifying AKI may be fraught with its own challenges given the lack of a consensus definition of AKI in the pregnant population[10]. Approximately 10%-20% of cases of preeclampsia are severe enough to manifest hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome, a thrombotic microangiopathic process (TMA) named for its most notable features of red cell lysis and thrombocytopenia[10]. As such, HELLP is likely a form of atypical HUS that is triggered by pregnancy although transaminitis can occur alone or as part of this syndrome. Adverse cardiovascular and cerebrovascular outcomes may develop if blood pressure is not adequately controlled.
| Updated definition | Supportive clinical signs |
| 2 blood pressure readings | Edema |
| ≥ 140/90 | ± |
| Taken ≥ 4-6 h apart | Uric acid level ≥ 7.8 mg/dL |
| After 20 wk gestation | ± |
| + | Proteinuria (severe ≥ 5 g/g) |
| Patient not previously known to be | |
| hypertensive | Thrombocytopenia |
| Elevated serum aminotransferase levels | |
| Acute kidney injury | |
| Pulmonary edema | |
| Cerebral/visual disturbances (new onset) |
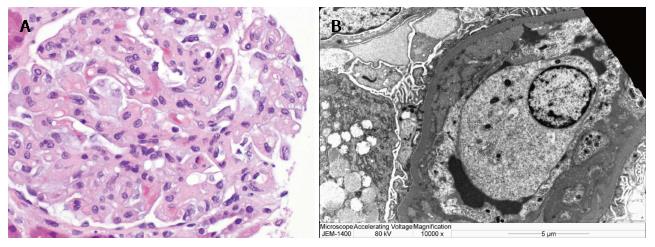
Morphologically, endothelial swelling (Figure 2A) is the cardinal feature on light microscopy which typically resolves approximately eight weeks after delivery, along with the proteinuria and hypertension. However, persistent damage can follow preeclampsia in the form of focal segmental glomerulosclerosis (FSGS) with collapsing features. Loss of glomerular endothelial fenestrae with relative preservation of the podocyte foot processes is expected on electron microscopy along with possible electron-dense deposits in subendothelial and mesangial tissue (Figure 2B). In severe cases or in healing stages one may note increased glomerular cellularity and mesangial interposition. FSGS has been one of the dominant histopathologic lesions in renal biopsies sampled from women with persistent proteinuria following a preeclamptic pregnancy.
Risk factors: Risk of preeclampsia is increased in the setting of maternal endothelial dysfunction. For example, diabetes mellitus can double or quadruple the risk of developing preeclampsia[10,11]. There is also an increased risk for preeclampsia in patients with a first degree relative with preeclampsia[12]. Patients with a history of preeclampsia are known to carry an increased risk of cardiovascular and renal morbidity and there is some evidence that affected women continue to have impaired endothelial-dependent vasorelaxation which may account for this increased risk. However, there are many shared risk factors for preeclampsia and cardiovascular disease such as diabetes, hypertension, obesity, and metabolic syndrome. Another area of growing evidence is the exposure to environmental and innate risk factors that may contribute to increased susceptibility to hypertensive disorders in pregnancy. For example, seasonal variation in preeclampsia and hypertensive disorders of pregnancy have been observed for the better part of a century. Combined findings in a systematic review of 20 preeclampsia studies suggested an increased frequency of episodes during the rainy seasons of tropical climates and the cold seasons of non-tropical climates[13]. Plausible biological mechanisms explaining this association include higher blood pressures as well as wider daily blood pressure and body temperature variations during these seasons, reduced physical activity and dietary changes, decreased vitamin D levels, and increased infections. Along the same line and not surprisingly, plant-based diets higher in fiber and potassium, cereals, dark bread, and low-fat dairy may be associated with reduced preeclampsia risk[14]. Similarly, vegetable-laden low protein diets (not more than 0.6-0.7 g/kg per day) seem to confer beneficial effects on clinical variables of renal health during pregnancies in cohorts of women with chronic kidney disease (CKD)[15].
Genetics and paternity: While much has been published about the maternal and placental pathophysiologic roles in preeclampsia, a growing area of research is contributing to knowledge about the paternal role as well, as summarized in a recent review by Katsi et al[16]. Among the early implications for a paternal role are the early observations that a family history of preeclampsia places one at increased risk for the syndrome and that multiparity (if prior pregnancies were uncomplicated) decreases one’s risk of preeclampsia, unless there is a change in paternity. This latter fact has been understood to be due to maternal mucosal immune tolerance mechanisms as mediated by human leukocyte antigens. Supporting this hypothesis are reports of relatively increased risks of preeclampsia in women using barrier contraceptive methods, couples with shorter durations of sexual relationships prior to conception, and in women undergoing fertility assistive therapies who receive oocytes fertilized with surgically obtained sperm rather than their partner’s ejaculated sperm. Other observations suggest that immune tolerance is not likely to be the only key in understanding the paternal role. Interestingly, men who father one preeclamptic pregnancy may be more likely to father a preeclamptic pregnancy with a different woman. Also, in a bimodal pattern, paternal age > 45 or < 25 appears to be another risk factor for preeclamptic pregnancy. Paternal race may factor into risk of preeclampsia, such as when differing from maternal ethnicity; and there appear to be decreased rates of preeclampsia in cases of Asian paternal ethnicity.
Pathophysiology: “Disease of theories”: Numerous mechanisms have been proposed regarding the etiologies of preeclampsia, which has earned it the nickname “the disease of theories[12]”. Mapping these different mechanisms may help one day to elucidate the different phenotypes and their respective mechanisms. Preeclampsia is a very heterogeneous disease, and it may be better thought of as an umbrella term for multiple different subtypes driven by a variety of different processes to varying extents which have yet to be fully elucidated. The predominant paradigm at present is that there is a fundamental imbalance between proangiogenic drivers and antiangiogenesis. Research in recent years has yielded significant advances in identifying early biomarkers, especially within the proposed pro- and anti-angiogenic pathways. The pathogenesis involves a two stage process wherein in the first stage uterine spiral arteries are incompletely remodeled leading to vasospasm and placental ischemia and in the second stage ischemic placental tissue releases systemic mediators or angiogenesis and inflammation in the the maternal circulation with resulting downstream endothelial injury.
Fetal endovascular cytotrophoblastic cells implant in the uterine endometrium and myometrium during the phase of embryologic development known as placentation, occurring between 8 and 18 wk of gestation. Cytotrophoblasts subsequently invade and remodel uterine spiral arteries to augment blood flow to meet the oxygen and nutrient demands of fetal growth. Remodeling reduces smooth muscle cells in the infiltrated vascular segments, facilitating dilatation and a resulting reduction in ureteroplacental pressure and velocity of flow. In the placentas of preeclamptic women-for reasons that are not entirely clear - this trophoblastic invasion fails to transpire adequately and the result is tortuous, thick-walled, incompletely remodeled vessels prone to spasms among retained vasoactive smooth muscle. The consequence is placental hypoxia, oxidative stress, and subsequent intermittent fetal hypoperfusion. The placental response is expression and secretion of anti-angiogenic and pro-inflammatory factors which may induce maternal hypertension and proteinuria[18].
Angiogenic imbalance: For more than a decade the preeclampsia syndrome has been understood to be associated with elevated circulating levels the molecule “sFlt-1”; the placental synthesis and release of which is believed to be triggered by the placental ischemic and reperfusion injury alluded to above. Abbreviated for “soluble fms-like tyrosine kinase”, sFlt-1 is a circulating receptor for vascular endothelial growth factor (VEGF), on which it has an antagonistic effect. In the healthy kidney, VEGF is produced by epithelial podocytes to maintain slit diaphragm integrity and to regulate the health of endothelial cells that express receptors to VEGF. Circulating proangiogenic molecules such as VEGF and placental growth factor (PlGF) are scavenged by sFlt-1 (the soluble receptor to VEGF) and as a result perturbs the balance between pro- and antiangiogenesis, skewing this balance towards the latter. Binding sFlt-1 to proangiogenic regulators prevents these vasodilatory effectors from interacting with their receptors on the endothelial cell surface, impairing nitric oxide-mediated vasodilation. The result is widespread endothelial dysfunction with multiorgan implications including notably the renal manifestations of preeclampsia such as hypertension and proteinuric glomerular dysfunction.
Automated assays for the detection and measurement of circulating of sFlt-1 and PlGF levels have been developed but are not yet in widespread use[19]. In a clinical setting the ratio of these markers may perhaps soon provide indices of overall antiangiogenic activity, offering a potential early prediction tool for the development of preeclampsia well before the clinical onset of disease. Another diagnostic dilemma is the later gestational exacerbations of preexisting hypertension and proteinuria which can be difficult to interpret and presents a diagnostic challenge in the identification of superimposed preeclampsia in this population. Bramham et al[20] recently published their evaluation of the performance of PlGF concentrations as predictors of superimposed preeclampsia (with a clinical endpoint of requiring delivery within 14 d) in patients with CKD and/or chronic hypertension[20]. In this study, PlGF levels less than the fifth centile performed well in the detection of superimposed preeclampsia in pregnant women with CKD and/or chronic hypertension, with specificity and negative predictive values greater than 80%. sFlt-1, B-type natriuretic peptide, neutrophil gelatinase-associated lipocalin (NGAL), and relaxin were also evaluated but were concluded to have less promising diagnostic discriminatory potential[20].
Soluble endoglin (sEng) is another known detectable antiangiogenic molecule that behaves as an inhibitory receptor for the cytokine TGF-β1[21]. Similar to sFlt-1, it can be found to be at notably increased serum levels as early as 2 to 3 mo prior the clinical onset of preeclampsia. It may offer improved predictive accuracy when used in combination with the ratio of sFlt-1 to PlGF. In normal pregnancies, the sFlt-1 to PlGF ratio is an s-shaped curve with a steep rise in the first 5-10 wk followed by a stagnation and a subsequent third trimester rise that progresses until labor and delivery.
In a nested case control study serum levels of total sFlt-1, free PlGF, and free VEGF in 120 women with preeclampsia were tracked throughout pregnancy and matched to normotensive controls by gestational age[22]. In the preeclamptic group mean sFlt-1 levels were noted to rise in late gestation; this group also consistently had lower PlGF levels (demonstrating the high sFlt-1 to PlGF ratio associated with an antiantiogenic milieu). Circulating levels of sFlt-1 were noted to increase on average five weeks before the clinical onset of preeclampsia and the degree of rise correlated with disease severity.
Oncology patients treated with drugs targeting VEGF can develop a “preeclampsia-like syndrome” with severe hypertension, proteinuria, and edema[23]. Anti-VEGF drugs have been an important mainstay in cancer therapy owing to their anti-angiogenic effects, exerted through a variety of pharmacologic mechanisms[23]. Examples include anti-VEGF monoclonal antibodies (e.g., bevacizumab), decoy receptors (i.e., VEGF-Trap drugs), and multi-target tyrosine kinase inhibitors (MTKIs), which interfere with the VEGF signaling pathway (e.g., sorafenib, sunitinib and brivanib)[23]. These drugs have been used successfully in renal and gastrointestinal malignancies[23]. Hypertension is an important side effect, along with GI and skin toxicities. Renal side effects that have been reported include proteinuria and acute renal failure, specifically with bevacizumab and sunitinib[23]. In some cases, there have been associated renal failure and biopsy-proven TMA along with endotheliosis and effacement of foot processes[23]. These side effects seem to occur in a dose-dependent manner and have been observed to resolve with treatment cessation[23].
Podocyte shedding: Another feature noted on kidney biopsy specimens in patients on these anti-VEGF therapies is a down-regulation in the expression of podocyte slit diaphragm proteins such as synaptopodin, nephrin, and podocin[24]. This helps to strengthen the link between the upstream antiangiogenic environment in preeclampsia and the downstream glomerular injury and proteinuria[24]. Although proteinuria is no longer considered essential for the diagnosis of preeclampsia, it remains a hallmark of the disorder, differentiating it from other hypertensive disorders of pregnancy as well as from other proteinuric diseases that may be co-incident with pregnancy. Urine sediment is typically “bland” in preeclampsia-without cells or casts-but there may be detectable podocytes and podocyte specific proteins[25]. There has been a recent diagnostic focus on detecting these sloughed podocytes in the urine of preeclamptic women even before proteinuria develops[25]. The degree of podocyturia correlates positively with that of proteinuria; and podocyte damage and shedding may affect renal function for years following a pregnancy complicated by preeclampsia[25,26]. This may someday serve as another methodology for early detection, however currently available lab techniques need more development[25]. Podocytes can be cultured from urine samples, although not quickly enough to be of clinical utility[25]. Cytospin techniques for detection may be automated, however there is a loss of sensitivity and specificity owing to contamination with other cellular debris[25]. Polymerase chain reaction (PCR) and mass spectrometry are anticipated to provide the most sensitive and specific detection methods but are not yet clinically available[25].
Anti-angiotensin II type 1 receptors: Agonistic antibodies to angiotensin II type 1 receptors (AT1-AA) were first described in 1999[27]. These are immunoglobulins of the IgG3 subclass which, by agonizing AT1 receptors, lead to enhanced sensitivity to angiotensin II thus influencing increased sodium retention and vasoconstriction. It remains unclear whether these antibodies are the cause or effect; however agonistic antibodies to AT1-AA may be an upstream trigger of increased sFlt-1 expression[28].
Vasodilatory gases and heme oxygenase pathway: Other vasodilatory gases (in addition to nitric oxide discussed above) may offer mechanistic insight and therapeutic value[29]. There exists growing evidence that the enzyme heme oxygenase and its byproduct carbon monoxide may play a protective role in preeclampsia[29]. Heme oxygenase converts heme to bilirubin and biliverdin; both of which are potent antioxidants[29]. Carbon monoxide (CO) is released in this process and is thought to be an important mediator in maintaining placental vasodilation and healthy development[29]. Supporting this are studies demonstrating reduced end-tidal CO levels in women with preeclampsia (perhaps demonstrating decreased heme oxygenase activity); further, women who smoke and live in areas with higher ambient CO appear to have less epidemiologic risk of preeclampsia[12,29].
Asymmetric dimethylarginine (ADMA) is an endogenous molecule known to competitively inhibit the activity of nitric oxide synthase. Its metabolism is closely associated with homocysteine (Hcy); which, along with ADMA can be found at elevated concentrations in disease patterns characterized by endothelial injury[30]. Hcy is an upstream effector of oxidative stress associated with ischemic injury and CKD[31] and can be found at increased levels in both obesity and vitamin B deficiencies[30]. López-Alarcón et al[30] recruited 411 women from two obstetric hospitals in Mexico focused on high risk pregnancies (excluding smokers, diabetics, and women with hypertension) to monitor monthly serum levels of these potential biomarkers. Approximately 20% of the follow-up group went on to develop preeclampsia and tended to have higher Hcy and ADMA concentrations at baseline despite having values within the normal ranges reported for healthy pregnant women. Though there were no detectable differences between groups with varying degrees of preeclampsia severity, serum levels gradually increased throughout pregnancy in the preeclampsia group compared to women who did not develop pregnancy complications (even after adjusting for obesity and nutritional status), allowing authors to postulate that the detection of increases in serum concentrations of these molecules may allow for early prediction of preeclampsia risk[30].
Placental protein-13: Placental protein 13 (PP-13), first discovered in 1983 by Dr Hans Bohn, is produced by the syncytiotrophoblastic layer in early placental implantation and remodeling, and is thought to be shed in the setting of ischemic placental stress and inflammation[18]. It has been evaluated in an ever-growing body of literature with regards to its capacity to be used as a clinical marker of placental pathology. Second and third trimester levels of PP-13 have been shown to rise in preeclampsia compared to normal pregnancy in a manner correlated with severity[18]; however, conflicting reports exist regarding whether detectable levels can be associated in a predictable way with preeclampsia and may vary demographically when accounting for age, ethnicity, and maternal ABO blood type[32]. According to Seravalli et al[32], however, first trimester levels of PP-13 are not likely to independently identify increased risk of preeclampsia in a population at low risk for placental dysfunction although in their cohort of 908 women at low risk for preeclampsia, lower levels of first trimester PP-13 were identified in women with higher BMI, perhaps reflective of metabolic syndrome which is thought to be a risk factor for adverse pregnancy outcomes[33]. Confusing this significance somewhat is the finding that cigarette smoking was associated with a profound decrease in first trimester PP-13 levels; cigarette use has been an environmental exposure consistently associated with a reduced risk of preeclampsia despite other negative placental effects such as fetal growth restriction[34,35].
Urine congophilia: Recently reported findings by McCarthy et al[36] appear to confirm the presence of increased levels of amyloid protein in the urine of women with preeclampsia, CKD, and CKD with superimposed preeclampsia compared to healthy pregnant women and women with chronic and gestational hypertension. “Congophilia” is a term used to describe the retention of Congo red dye in a specimen which indicates the presence of amyloid, an aggregate of inappropriately folded proteins thought to be generated by stressed endoplasmic reticulum in the ischemic placenta. Also noted was a significant positive correlation between the magnitude of congophilia and urine protein to Creatinine ratios. Further research is needed to elucidate how this method may be utilized clinically to distinguish between renal impairment, early and late term preeclampsia, and other pathologic processes that activate the unfolded protein response pathway in endoplasmic reticulum[36].
Preeclampsia is primarily a placenta-driven disease process; thus delivery is the only definitive treatment. Indeed, levels of key mediators such as sFlt-1 have been noted to fall within 48 h post-partum. The desirability and safety of delivery may depend on clinical considerations such as fetal gestational age, signs of fetal or maternal distress, or severity such as progression to eclampsia as indicated by the presence of seizures[10].
Though it does little to reverse or correct the placental under-perfusion that is thought to be driving preeclampsia, aggressive blood pressure control is another essential mainstay in preeclampsia management[11]. The primary goals are to prolong gestation in order to allow further fetal growth and development and to prevent maternal cerebro- and cardiovascular catastrophes[11]. Angiotensin-converting enzyme (ACE) inhibitors and angiotension receptor blockade (ARB) therapies are very effective but contraindicated during any trimester of pregnancy[37]. Their use is associated with “fetal renin-angiotensin system blockade syndrome” characterized by impaired tubular development and oligohydramnios, among multiple devastating other effects[37]. Angiotensin II is essential in the regulation of umbilical-placental blood flow and maintenance of GFR in the low-pressure circulation of the fetus[37]. Drugs that inhibit renin directly, such as the drug “aliskiren” are expected to have similar effects to ACE inhibitors and ARBs[37]. There are no case reports of fetal exposure to these drugs, but at present they should be avoided[37]. Labetalol, hydralazine, methyldopa, and nifedipine are the antihypertensives that have the best safety profile and are the typical go-to agents in pregnancy[11,38].
Magnesium: Intravenous magnesium sulfate (MgSO4) is the treatment of choice for prevention and treatment of recurrent seizures. Infusions are often started 48-72 h prior to delivery induction once preeclampsia is suspected or diagnosed. This allows time for fetal lung maturation after dosing corticosteroids, typically given concomitantly. Dosing may be approached more empirically in patients with normal renal function, however close serum (at least every 6 h) and clinical monitoring of magnesium levels is advised in renal insufficiency to avoid toxicity. While neuroprotective at therapeutic levels, MgSO4 levels above 4.8 mg/dL can lead to central and respiratory depression or cardiac arrest. Calcium gluconate is the appropriate antidote[39]. MgSO4 also has synergistic blood pressure lowering activity with nifedipine[40] and may have anti-inflammatory effects as well via AT1-AA which has yet to be further elucidated[17].
Aspirin: Aspirin administration to reduce preeclampsia risk has been an important research question since the 1970s, with more than 50 published trials and several recent meta-analyses[41]. It has been hypothesized that aspirin facilitates trophoblastic invasion of the uterine spiral arteries[41]. Some of its benefit may be due to the inhibition of synthesis of platelet thromboxane, a potent vasoconstrictor produced by endothelial cells. However, data to support this strategy has been conflicting[41]. Since there may be up to 50% risk reduction and there is little harm other than the usual contraindications to aspirin, guidelines currently recommend initiating aspirin in the highest risk patients (such as women with pre-existing diabetes)[11] early on, ideally in the first trimester[41,42].
Statin: Statin use has also been under evaluation-plausibility lies with their known anti-inflammatory properties as well as their demonstrated ability in mouse and in vitro studies to inhibit cytokine-mediated release of sFlt-1[43,44]. Statins are also thought to have a positive influence on endothelial health by increasing the bioavailability of nitric oxide, PlGF, and VEGF[44]. Pravastatin has emerged as the only possible safe agent from this class, due to its inability to cross fetal membranes into the embryonic compartments[43]. Simvastatin, lovastatin, and atorvastatin are all lipophilic and able to equilibrate between maternal and fetal compartments where these agents may interfere with cholesterol-mediated cell signaling and result in fetal central nervous system, renal, and limb defects[43].
Metformin: Metformin is known to be safe in pregnancy and is currently used to treat gestational diabetes mellitus[45]. It is hypothesized to reduce sFlt-1 and sEng secretion by way of its effect on reduction of mitochondrial electron transport chain activity and downstream inhibition of hypoxic inducible factor 1α[45]. Recently, in vitro and ex vivo experimentation demonstrated reduced sFlt-1 secretion from metformin-treated endothelial and placental cells in a dose-dependent manner[45]. Clinical trials assessing the effect of metformin on primary outcomes such as hypertension and preeclampsia have yet to be published[45].
Calcium: As of 2013, the World Health Organization recommends 1.5-2.0 g of elemental calcium daily in three divided doses from 20 wk’ gestation until term in all pregnant women in areas where calcium intake is low, particularly those at higher risk of gestational hypertension[46]. Evidence to support calcium supplementation to prevent preeclampsia has been conflicting and remains controversial. In 1996, Bucher et al[47] evaluated 14 randomized controlled trials involving 2459 women and found benefits in blood pressure and preeclampsia incidence reduction supporting the use. The following year, however, the NIH study Calcium for Preeclampsia Prevention (CPEP) concluded from a randomized controlled clinical trial of twice as many healthy nulliparous women that calcium supplementation did not reduce blood pressure, adverse perinatal outcomes, or the incidence or of preeclampsia, nor did it delay onset[48]. In the decade following, subsequent large-scale meta-analyses have supported the practice, particularly in developing countries where dietary calcium intake may be relatively lacking, as well as in otherwise healthy high-risk populations[49,50]. Calcium supplementation in this context has not been shown to increase risk of adverse effects such as nephrolithiasis[48].
Heparin: Heparin has been explored as a possible way to augment the excretion of sFlt-1. A recent systematic review and meta-analysis evaluated six randomized, controlled trials and concluded that the use of low molecular-weight heparin (LMWH) resulted in risk reductions in women who had any previous history of placenta-mediated pregnancy complications[51]. The mechanism of the potential benefit of LMWH is not yet well understood, but the LMWH molecule is thought to mobilize sFtl-1 into circulation from heparan-bound sites in extracellular matrix[51]. Heparan is a polysaccharide structurally similar to heparin which is known to sequester and regulate the release of VEGF and other cytokines involved in neovascularization[51,52].
Potential therapeutic solutions may lie within restoration of angiogenic balance, for example via the antagonism of sFlt-1 and subsequent blockade of its pathologic effects. One strategy involves infusion of sFlt-1’s natural ligands VEGF and PlGF at doses high enough to provide systemic saturation. Attempts have been made in animal trials to induce adenoviral synthesis of VEGF as well as by direct infusion of VEGF in mice[53,54]. Alternative potential therapeutic strategies may include the administration of anti-sFlt-1 antibodies or small molecules that reduce sFlt-1 production (such as small interfering “siRNA”)[55].
Given the potential adverse effects of novel agents introduced into maternal circulation and unknowns regarding the ability of such molecules to traverse the placenta, early experiments have instead attempted to remove circulating sFlt-1 with an extracorporeal device. A recent open pilot study was conducted to evaluate the safety and potential efficacy of therapeutic apheresis with a plasma-specific dextran sulfate column to remove circulating sFlt-1 in 11 pregnant women with very preterm preeclampsia[55]. At physiologic pH, sFlt-1 circulates in blood with a strongly positive charge. The dextran columns used are negatively charged, are approved for safe use in pregnancy, and have already been used in therapeutic apheresis for familial hyperlipidemia. Circulating sFlt-1 can be removed selectively, leaving placental sFlt-1 in vivo, which may be essential for placental health maintenance. In the treated group, the average sFlt-1 reduction was 18% and the average proteinuria was decreased by an average of 44%[55]. Pregnancy continued for eight days in women treated once and 15 d in women treated multiple times. There were no observed adverse effects or infant deaths. Both groups demonstrated similar short-term neonatal outcomes; neonates in the treatment group required fewer days on supplemental oxygen. Without a controlled trial, it remains unknown whether or not some of the therapeutic benefit derives from the removal of other unmeasured factors by the dextran columns, such as LDL and fibrinogen. Studies using ligand-specific apheresis columns (e.g., configured with anti-sFlt-1 Ab or VEGF) would be informative in determining the relative contribution of sFlt-1 depletion vs depletion of other potential mediators[55].
Pregnancy is marked by several key physiologic RAAS driven changes that should not result in hypertensive pathology in normal gestation, yet hypertensive disorders in pregnancy abound. Preeclampsia is the most common among these; it is an exceptionally heterogeneous disease that contributes to at least three million pre-term births each year. Placental dysfunction is the fundamental etiology of preeclampsia and mediates the features of the syndrome via the systemic release of angiogenic molecules, typically late in pregnancy and signified by the principal clinical findings of hypertension and proteinuria[56]. Angiogenic imbalance seems to be at the root of this disorder, resulting in maternofetal endothelial pathology and renal end-organ damage with an almost glomerulonephritic or nephrotic phenotype[1]. In recent years, knowledge regarding the pathophysiology of preeclampsia has increased markedly. Understanding of this disease process has been significantly advanced by the discovery of the factor sFlt-1 and its placental source, antiangiogenic behavior, and role in diminished glomerular endothelial health and likely holds the key to future advances in prognostication, diagnosis, and treatment of a condition associated with a significant amount of cardiovascular and renal morbidity[56]. To date, preventative measures and screening tools are relatively lacking, treatments are directed at the management of overt clinical manifestations, and delivery remains the only definitive cure; thus, a strong need persists for the expansion of detection and treatment options for this disease which has seen few therapeutic advances in recent decades.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bellomo G, Charoenphandhu N S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | August P. Preeclampsia: a “nephrocentric” view. Adv Chronic Kidney Dis. 2013;20:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | O'Donnell E, Floras JS, Harvey PJ. Estrogen status and the renin angiotensin aldosterone system. Am J Physiol Regul Integr Comp Physiol. 2014;307:R498-R500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Chidambaram M, Duncan JA, Lai VS, Cattran DC, Floras JS, Scholey JW, Miller JA. Variation in the renin angiotensin system throughout the normal menstrual cycle. J Am Soc Nephrol. 2002;13:446-452. [PubMed] |
| 4. | Conrad KP. Emerging role of relaxin in the maternal adaptations to normal pregnancy: implications for preeclampsia. Semin Nephrol. 2011;31:15-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 5. | Scantlebury DC, Schwartz GL, Acquah LA, White WM, Moser M, Garovic VD. The treatment of hypertension during pregnancy: when should blood pressure medications be started? Curr Cardiol Rep. 2013;15:412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Piccoli GB, Attini R, Vigotti FN, Parisi S, Fassio F, Pagano A, Biolcati M, Giuffrida D, Rolfo A, Todros T. Is renal hyperfiltration protective in chronic kidney disease-stage 1 pregnancies? A step forward unravelling the mystery of the effect of stage 1 chronic kidney disease on pregnancy outcomes. Nephrology (Carlton). 2015;20:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Gyamlani G, Geraci SA. Kidney disease in pregnancy: (Women’s Health Series). South Med J. 2013;106:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Fainaru O, Almog B, Gamzu R, Lessing JB, Kupferminc M. The management of symptomatic hydronephrosis in pregnancy. BJOG. 2002;109:1385-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Hladunewich MA, Melamad N, Bramham K. Pregnancy across the spectrum of chronic kidney disease. Kidney Int. 2016;89:995-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Acharya A, Santos J, Linde B, Anis K. Acute kidney injury in pregnancy-current status. Adv Chronic Kidney Dis. 2013;20:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Mathiesen ER, Ringholm L, Feldt-Rasmussen B, Clausen P, Damm P. Obstetric nephrology: pregnancy in women with diabetic nephropathy--the role of antihypertensive treatment. Clin J Am Soc Nephrol. 2012;7:2081-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology (Bethesda). 2009;24:147-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 319] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 13. | TePoel MR, Saftlas AF, Wallis AB. Association of seasonality with hypertension in pregnancy: a systematic review. J Reprod Immunol. 2011;89:140-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Pistollato F, Sumalla Cano S, Elio I, Masias Vergara M, Giampieri F, Battino M. Plant-Based and Plant-Rich Diet Patterns during Gestation: Beneficial Effects and Possible Shortcomings. Adv Nutr. 2015;6:581-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Piccoli GB, Attini R, Vasario E, Gaglioti P, Piccoli E, Consiglio V, Deagostini C, Oberto M, Todros T. Vegetarian supplemented low-protein diets. A safe option for pregnant CKD patients: report of 12 pregnancies in 11 patients. Nephrol Dial Transplant. 2011;26:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Katsi V, Felekos I, Siristatidis C, Kasioni S, Drakontaidis A, Farmakides G, Makris T, Aggeli C, Nihoyannopoulos P, Tousoulis D. Preeclampsia: What Does the Father Have to Do with It? Curr Hypertens Rep. 2015;17:60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Lamarca B, Brewer J, Wallace K. IL-6-induced pathophysiology during pre-eclampsia: potential therapeutic role for magnesium sulfate? Int J Interferon Cytokine Mediat Res. 2011;2011:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Than NG, Balogh A, Romero R, Kárpáti E, Erez O, Szilágyi A, Kovalszky I, Sammar M, Gizurarson S, Matkó J. Placental Protein 13 (PP13) - A Placental Immunoregulatory Galectin Protecting Pregnancy. Front Immunol. 2014;5:348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Schiettecatte J, Russcher H, Anckaert E, Mees M, Leeser B, Tirelli AS, Fiedler GM, Luthe H, Denk B, Smitz J. Multicenter evaluation of the first automated Elecsys sFlt-1 and PlGF assays in normal pregnancies and preeclampsia. Clin Biochem. 2010;43:768-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Bramham K, Seed PT, Lightstone L, Nelson-Piercy C, Gill C, Webster P, Poston L, Chappell LC. Diagnostic and predictive biomarkers for pre-eclampsia in patients with established hypertension and chronic kidney disease. Kidney Int. 2016;89:874-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 21. | Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1379] [Cited by in RCA: 1361] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 22. | Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2659] [Cited by in RCA: 2723] [Article Influence: 129.7] [Reference Citation Analysis (0)] |
| 23. | Vigneau C, Lorcy N, Dolley-Hitze T, Jouan F, Arlot-Bonnemains Y, Laguerre B, Verhoest G, Goujon JM, Belaud-Rotureau MA, Rioux-Leclercq N. All anti-vascular endothelial growth factor drugs can induce ‘pre-eclampsia-like syndrome’: a RARe study. Nephrol Dial Transplant. 2014;29:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Kandasamy Y, Watson D, Rudd D. Biomarker of Early Glomerular Injury in Pre-eclampsia. Hypertens Pregnancy. 2015;34:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Penning ME, Bloemenkamp KW, van der Zon T, Zandbergen M, Schutte JM, Bruijn JA, Bajema IM, Baelde HJ. Association of preeclampsia with podocyte turnover. Clin J Am Soc Nephrol. 2014;9:1377-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Unverdi S, Ceri M, Unverdi H, Yilmaz R, Akcay A, Duranay M. Postpartum persistent proteinuria after preeclampsia: a single-center experience. Wien Klin Wochenschr. 2013;125:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jüpner A, Baur E, Nissen E, Vetter K, Neichel D. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 611] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 28. | Amaral LM, Cunningham MW, Cornelius DC, LaMarca B. Preeclampsia: long-term consequences for vascular health. Vasc Health Risk Manag. 2015;11:403-415. [PubMed] |
| 29. | Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, Devey LR, Wigmore SJ, Abbas A, Hewett PW. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 325] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 30. | López-Alarcón M, Montalvo-Velarde I, Vital-Reyes VS, Hinojosa-Cruz JC, Leaños-Miranda A, Martínez-Basila A. Serial determinations of asymmetric dimethylarginine and homocysteine during pregnancy to predict pre-eclampsia: a longitudinal study. BJOG. 2015;122:1586-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Ostrakhovitch EA, Tabibzadeh S. Homocysteine in Chronic Kidney Disease. Adv Clin Chem. 2015;72:77-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Seravalli V, Grimpel YI, Meiri H, Blitzer M, Baschat AA. Relationship between first-trimester serum placental protein-13 and maternal characteristics, placental Doppler studies and pregnancy outcome. J Perinat Med. 2016;44:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Lei Q, Niu J, Lv L, Duan D, Wen J, Lin X, Mai C, Zhou Y. Metabolic risk factors clustering and adverse pregnancy outcomes: a prospective cohort study. Diabetes Metab Res Rev. 2016; Apr 2; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Karumanchi SA, Levine RJ. How does smoking reduce the risk of preeclampsia? Hypertension. 2010;55:1100-1101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | England L, Zhang J. Smoking and risk of preeclampsia: a systematic review. Front Biosci. 2007;12:2471-2483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | McCarthy FP, Adetoba A, Gill C, Bramham K, Bertolaccini M, Burton GJ, Girardi G, Seed PT, Poston L, Chappell LC. Urinary congophilia in women with hypertensive disorders of pregnancy and preexisting proteinuria or hypertension. Am J Obstet Gynecol. 2016; Apr 29; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Darby M, Martin JN, LaMarca B. A complicated role for the renin-angiotensin system during pregnancy: highlighting the importance of drug discovery. Expert Opin Drug Saf. 2013;12:857-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Podymow T, August P. Update on the use of antihypertensive drugs in pregnancy. Hypertension. 2008;51:960-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 39. | Leeman L, Dresang LT, Fontaine P. Hypertensive Disorders of Pregnancy. Am Fam Physician. 2016;93:121-127. [PubMed] |
| 40. | Hussain A, Karovitch A, Carson MP. Blood pressure goals and treatment in pregnant patients with chronic kidney disease. Adv Chronic Kidney Dis. 2015;22:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 41. | Bujold E, Roberge S, Nicolaides KH. Low-dose aspirin for prevention of adverse outcomes related to abnormal placentation. Prenat Diagn. 2014;34:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 42. | American College of Obstetricians and Gynecologists. Task Force on Hypertension in Pregnancy, American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Washington, DC: American College of Obstetricians and Gynecologists 2013; . |
| 43. | Edison RJ, Muenke M. Mechanistic and epidemiologic considerations in the evaluation of adverse birth outcomes following gestational exposure to statins. Am J Med Genet A. 2004;131:287-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Kumasawa K, Ikawa M, Kidoya H, Hasuwa H, Saito-Fujita T, Morioka Y, Takakura N, Kimura T, Okabe M. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci USA. 2011;108:1451-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 287] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 45. | Brownfoot FC, Hastie R, Hannan NJ, Cannon P, Tuohey L, Parry LJ, Senadheera S, Illanes SE, Kaitu’u-Lino TJ, Tong S. Metformin as a prevention and treatment for preeclampsia: effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am J Obstet Gynecol. 2016;214:356.e1-356.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 46. | World Health Organization. Guideline calcium supplementation in pregnant women. Geneva: World Health Organization 2013; . |
| 47. | Bucher HC, Guyatt GH, Cook RJ, Hatala R, Cook DJ, Lang JD, Hunt D. Effect of calcium supplementation on pregnancy-induced hypertension and preeclampsia: a meta-analysis of randomized controlled trials. JAMA. 1996;275:1113-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 89] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Levine RJ, Esterlitz JR, Raymond EG, DerSimonian R, Hauth JC, Ben Curet L, Sibai BM, Catalano PM, Morris CD, Clemens JD. Trial of Calcium for Preeclampsia Prevention (CPEP): rationale, design, and methods. Control Clin Trials. 1996;17:442-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Hofmeyr GJ, Lawrie TA, Atallah AN, Duley L, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2014;6:CD001059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 50. | DerSimonian R, Levine RJ. Resolving discrepancies between a meta-analysis and a subsequent large controlled trial. JAMA. 1999;282:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Hagmann H, Bossung V, Belaidi AA, Fridman A, Karumanchi SA, Thadhani R, Schermer B, Mallmann P, Schwarz G, Benzing T. Low-molecular weight heparin increases circulating sFlt-1 levels and enhances urinary elimination. PLoS One. 2014;9:e85258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Searle J, Mockel M, Gwosc S, Datwyler SA, Qadri F, Albert GI, Holert F, Isbruch A, Klug L, Muller DN. Heparin strongly induces soluble fms-like tyrosine kinase 1 release in vivo and in vitro--brief report. Arterioscler Thromb Vasc Biol. 2011;31:2972-2974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placental ischemia-induced hypertension. Hypertension. 2010;55:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 54. | Craici IM, Wagner SJ, Weissgerber TL, Grande JP, Garovic VD. Advances in the pathophysiology of pre-eclampsia and related podocyte injury. Kidney Int. 2014;86:275-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 55. | Thadhani R, Hagmann H, Schaarschmidt W, Roth B, Cingoez T, Karumanchi SA, Wenger J, Lucchesi KJ, Tamez H, Lindner T. Removal of Soluble Fms-Like Tyrosine Kinase-1 by Dextran Sulfate Apheresis in Preeclampsia. J Am Soc Nephrol. 2016;27:903-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 56. | Sircar M, Thadhani R, Karumanchi SA. Pathogenesis of preeclampsia. Curr Opin Nephrol Hypertens. 2015;24:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |