Published online Jul 6, 2016. doi: 10.5527/wjn.v5.i4.378
Peer-review started: February 19, 2016
First decision: March 25, 2016
Revised: April 7, 2016
Accepted: June 14, 2016
Article in press: June 16, 2016
Published online: July 6, 2016
Processing time: 132 Days and 16.8 Hours
AIM: To evaluate methods measuring the intestinal per-meability in chronic kidney disease (CKD) and clarify whether there is an increased intestinal permeability in CKD.
METHODS: We reviewed the literature in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) protocol and performed a systematic literature search through MEDline and EMBASE. All controlled trials and cohort studies using non-invasive methods to assess intestinal permeability in CKD patients were included. Excluded were: Conference abstracts and studies including patients younger than 18 years or animals. From the included studies we summarized the used methods and their advantages and disadvantages. For the comparison of their results we divided the included studies in two categories based on their included patient population, either assessing the intestinal permeability in mild to moderate CKD patients or in end stage renal disease (ESRD) patients. Results were graphically displayed in two plots, one comparing the intestinal permeability in mild to moderate CKD patients to healthy controls and one comparing the intestinal permeability in ESRD patients to healthy controls.
RESULTS: From the 480 identified reports, 15 met our inclusion criteria. Methods that were used to assess the intestinal permeability varied from markers measured in plasma to methods based on calculating the urinary excretion of an orally administered test substance. None of the applied methods has been validated in CKD patients and the influence of decreased renal function on the different methods remains unclear to a certain extent. Methods that seem the least likely to be influenced by decreased renal function are the quantitative PCR (qPCR) for bacterial DNA in blood and D-lactate. Considering the results published by the included studies; the studies including patients with mild to moderate CKD conducted conflicting results. Some studies did report an increase in intestinal permeability whilst other did not find a significant increased permeability. However, despite the variety in used methods among the different studies, all studies measuring the intestinal permeability in ESRD point out a significant increased intestinal permeability. Results should nevertheless be interpreted with caution due to the possible influence of a decreased glomerular filtration rate on test results.
CONCLUSION: The intestinal permeability in CKD: (1) could be measured by qPCR for bacterial DNA in blood and D-lactate; and (2) seems to be increased in ESRD.
Core tip: Several methods are currently being used to measure the intestinal permeability, there is however no gold standard. In addition to this, most methods are influenced by renal function. We suggest that preferred methods to assess the intestinal permeability in chronic kidney disease patients could be quantitative PCR for bacterial DNA in blood and D-lactate. Independent of the used method, all studies measuring the intestinal permeability in patients with end stage renal disease (ESRD) reported a significantly increased intestinal permeability. Even though these results should be interpret with caution due to the disadvantages of the applied methods, it seems likely that there is a connection between ESRD and intestinal barrier dysfunction.
- Citation: Terpstra ML, Singh R, Geerlings SE, Bemelman FJ. Measurement of the intestinal permeability in chronic kidney disease. World J Nephrol 2016; 5(4): 378-388
- URL: https://www.wjgnet.com/2220-6124/full/v5/i4/378.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i4.378
Within the last three decades an increasing number of studies highlight the role of chronic systemic inflammation in the progression of chronic kidney disease (CKD) to end stage renal disease (ESRD) and its associated complications, such as cardiovascular disease[1,2]. Even though the inflammatory status has been pointed out as an important prognostic factor in CKD, the pathophysiology has not been elucidated. Factors that appear to be involved are retained uremic toxins, hypervolemia, hypertension, underlying disease (diabetes, autoimmune disease, etc.) and infection[3,4]. In addition to this, more recent studies have been opposing alterations in the gut as possible source of inflammation[5-7].
An important aspect of the alterations in the gut is a decreased barrier function causing an increased intestinal permeability, which possibly leads to diffusion of endotoxins and bacterial DNA through the epithelial barrier into the circulation. An interesting finding was reported in uremic rats; gut bacteria and their DNA fragments were found in the intestinal wall and the mesenteric lymph nodes, whilst their non-uremic controls showed no signs of these fragments in the obtained biopsies[8]. The entry of uremic retention solutes, bacterial DNA, endotoxins and other possibly noxious compounds from the intestinal lumen into the circulation is likely to contribute to the inflammatory status of CKD patients and thus their prognosis.
The suggestion of an increased intestinal permeability as a prognostic factor in CKD has led to an increased interest in non-invasive methods measuring the intestinal permeability in CKD patients. There are numerous approved ways to assess the intestinal permeability, which was outlined in 2010 by Grootjans et al[9]. There is however no gold standard and each method comes with its own advantages and disadvantages. An important aspect is how renal function interferes with the test results; most studies assessing the intestinal permeability exclude CKD patients to prevent possible bias obtained by a decreased estimated glomerular filtration rate (eGFR).
Presently there is no overview available on the results of studies assessing the intestinal permeability in CKD.
This systematic review provides an overview of the studies assessing the intestinal permeability of the small and large intestine in CKD patients. We will answer two research questions: (1) what is the best available method to determine the intestinal permeability in CKD patients; and (2) what is currently known on intestinal permeability in CKD.
We discuss the methods used to assess the intestinal permeability in CKD patients and their advantages and disadvantages, specifically focusing on the influence of renal function. In addition to this we extracted the data derived from these studies in order to summarize the results of the currently available evidence of what is known about the intestinal permeability in CKD.
We reviewed the literature in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol[10]. The study protocol was registered in the PROSPERO international registry; registration number CRD42015025101. PROSPERO is an international database of prospectively registered reviews in health and social care in which key features from the review protocol are recorded and maintained as a permanent record. PROSPERO aims to provide a comprehensive listing of systematic reviews registered at inception to help avoid unplanned duplication and enable comparison of reported review methods with what was planned in the protocol[11].
Two co-authors (Terpstra ML and Singh R) performed a systematic literature search through MEDLINE and EMBASE, combined with a search through personal databases of all co-authors. Search terms for each database are described in supplementary tables.
The references obtained through the search were stored within Endnote X7 file. Titles and abstracts of the obtained articles were screened by two co-authors independently, Terpstra ML and Singh R. In case of discussion about inclusion, a third investigator was consulted (Bemelman FJ). All trials and cohort studies using non-invasive methods to assess the permeability of the small and large intestine in CKD patients were included. Only methods directly reflecting the intestinal permeability of a patient at a specific time were included, studies demonstrating the effect of different compounds on the intestinal barrier were excluded. Furthermore studies only assessing the permeability of the stomach were excluded since there are no uremic and other noxious compounds produced here and the environment is almost sterile.
Other exclusion criteria were: Conference abstracts, patients younger than 18 years and animal studies. Studies reporting data that had been previously published were also excluded.
Whilst analyzing the publications possibly meeting the inclusion criteria; articles cited in the included studies were also assessed on their relevance and included when meeting the eligibility criteria.
Each reference was categorized in Endnote according to the inclusion/exclusion criteria.
For each included study the methodological quality assessment was provided by using the Newcastle - Ottowa quality assessment scale for cohort studies[12]. For this scorings system not all items were applicable to the type of included studies; points were only given for those sections that were relevant. Hence, the maximum amount of stars that could be obtained was 6.
From the included studies the following data were retrieved: Data on CKD etiology and renal function, sample size of the subgroups, description of control group, method(s) used to assess the permeability, part of the intestine that is evaluated by this method, mean or median levels of the used marker per subgroup (if provided) and P value of the statistical test that was used to compare the groups. If applicable, the interaction between the measurement outcome and renal function was evaluated; whether or not the measurement outcome was corrected for the renal function.
All data were summarized in two tables: Table 1 summarizes the mechanism of action and (dis)advantages of each method and Table 2 summarizes the results obtained by each study.
| Marker for intestinal permeability | Mechanism of action | Advantages | Disadvantages | Influence renal function | Part of the intestine evaluated | Ref. |
| D-lactate (plasma) | Produced by bacteria in the colon. Present in human blood at very low concentrations as a product of methylglyoxal metabolism. In case of increased intestinal permeability levels will rise due to increased translocation across the intestinal mucosa | Non-invasive Low levels in healthy subjects, high specificity Mainly large intestine; thus focusing on part of the bowel with the highest bacterial load | Possibly increased fermentation of undigested carbohydrates to D-lactate in case of bacterial overgrowth | Influenced by renal function to some extent | Mainly large intestine | [18,19] |
| Sugar absorption test (urine) | Method based on calculating the urinary excretion of orally administered test substance that reflects the non-mediated diffusion of that probe across the intestinal barrier. Most commonly used combination of sugars is a oligosaccharide or disaccharide (lactulose, cellobiose) combined with a monosaccharide (mannitol). By adding sucralose to the test, which is not degraded by the bacteria of the colon, the colonic permeability can be assessed | Non-invasive Different sugar combinations can assess different parts of the gastrointestinal tract | Relative impractical in use Results could be influenced by decreased bowel motility 32 Used according to different protocols and different combinations of sugars which makes the comparison of studies difficult Relative large inter- and intra-individual variety | Influenced by renal function. Corrected by using the ratio of administered sugars. It is however not clarified whether this correction is sufficient due to possible different renal clearance of the administered sugars | Small intestine, large intestine (only if sucralose, is added) | [14-17] |
| 5¹Cr-EDTA (urine) | Method based on calculating the urinary excretion of orally administered test substance that reflects the non-mediated diffusion of that probe across the intestinal barrier | Not degraded by bacteria in the colon, useful marker for both the small and large intestinal permeability | Radioactivity Not commonly used nowadays due to radioactivity | Influenced by renal function. Corrected in included studies: 24-h Cr-EDTA excretion = 100% of the total oral dose excreted in the urine in 24 h/creatinine | Both small and large intestine | [20-23] |
| Endotoxin level (blood), LPS (plasma) | Indirect measurement of translocation of bacterial products | High specificity | Not eligible to use among patients with inflammation in the GI tract | Unlikely to be influenced by renal function | Both small and large intestine | [18,25,26] |
| Bacterial derived DNA (16S rRNA PCR) (blood) | Direct measurement of bacterial products in blood | Optimal tool for detection and identification of bacterial isolates | Not eligible to use among patients with inflammation in the GI tract | Unlikely to be influenced by renal function | Both small and large intestine | [18,19,24] |
| Polyethylene glycols (PEG) (urine) | Method based on calculating the urinary excretion of orally administered test substance that reflects the non-mediated diffusion of that probe across the intestinal barrier. It is hypothesized that, as saccharides in sugar absorption test, molecular PEG will only cross the intestinal mucosa to the circulation in case of barrier integrity loss. Increased urinary levels of large PEGs therefore reflect an increased intestinal permeability | Biologically inert and not degraded by bacteria, thus providing information of the whole intestinal permeability | High inter- and intra-individual variations have been reported, even in healthy controls[34] | Influenced by renal function | Both small and large intestine | [28] |
| Ref. | Population | Study size | Marker used to assess intestinal permeability (values provided as mean ± SD) | Results | Part of the intestine evaluated |
| Shi et al[18] | ESRD (both HD and non-HD) vs healthy controls ESRD group further divided patients with bacterial DNA and without bacterial DNA in their blood samples | ESRD n = 52 (HD n = 22, ND n = 30) Controls n = 10 | D-lactate (plasma) Endotoxins (blood) Bacterial DNA (blood) | D-lactate plasma levels higher: ESRD HD vs controls P = 0.039 ESRD non-HD vs controls P = 0.044 HD vs non-HD P > 0.05 ESRD with bacterial DNA vs ESRD without bacterial DNA P < 0.05 ESRD HD with bacterial DNA vs ESRD non-HD with bacterial DNA P > 0.05 Endotoxin significantly higher: ESRD HD vs controls P < 0.05 ESRD non-HD vs controls P < 0.05 ESRD HD 0.95 ± 0.12 EU/mL ESRD non-HD 0.70 ± 0.15 EU/mL Controls 0.17 ± 0.10 EU/mL Presence of bacterial 16S rDNA: ESRD HD 6/22 patients ESRD non-HD 6/30 patients Controls: 0/10 patients | Large intestine Mostly large intestine Mostly large intestine |
| Wang et al[19] | ESRD patients (non-HD) vs healthy controls ESRD group further divided patients with bacterial DNA and without bacterial DNA in their blood samples | ESRD n = 30 Controls n = 10 | D-lactate (plasma) Bacterial 16s rDNA (blood) | Plasma D-lactate higher: ESRD with bacterial DNA vs ESRD without bacterial DNA P = 0.0233 ESRD with bacterial DNA vs controls P =0.067 ESRD with bacterial DNA: 13.53 ± 1.47 μg/mL ESRD without bacterial DNA: 5.71 ± 2.28 μg/mL Controls: 4.82 ± 0.93 μg/mL D-lactate plasma levels both ESRD groups combined: 7.274 ± 2.16 μg/mL1 ESRD: 6/30 bacterial DNA in blood Controls: no bacterial DNA in blood | Large intestine |
| Bossola et al[24] | HD patients (AVF en CVC) vs healthy controls | HD n = 58 (AVF n = 44, CVC n = 14) Controls n = 30 | Bacterial 16S rDNA (blood) | HD patients: 12/58 bacterial DNA in blood (= 20.7%) Healthy controls: No bacterial DNA in blood AVF patients 5/44 (= 15.9%) CVC patients 5/14 (35.7) P = 0.22 | Both small and large intestine |
| McIntyre et al[25] | HD patients, PD patients, CKD patients (stage 3-5) vs healthy controls | HD n = 120 PD n =25 CKD stage 3-5 n = 90 Controls n = 14 | Endotoxin level (blood) | Significant higher endotoxin levels in HD vs PD P < 0.008 Dialysis patients (HD + PD) vs CKD P < 0.001 CKD vs controls P > 0.05 HD patients: 0.64 EU/mL PD patients: 0.56 EU/mL HD + PD patients: 0.62 ± 0.37 EU/mL CKD patients: 0.11 ± 0.68 EU/mL Controls: Not provided | Both small and large intestine |
| Feroze et al[26] | HD patients, follow up for 42 mo | HD n = 303 | Endotoxin level (blood) | No significant association between elevated circulating endotoxin levels and mortality Mean endotoxin levels: 2.31 ± 3.10 EU/mL | Both small and large intestine |
| Zuckerman et al[20] | No control group CAPD patients vs healthy controls | CAPD patients n = 11 (5 with significant urine output) Controls n = 32 | Cr-EDTA recovery (24 h urine + dialysate) | Significant less recovery of Cr-EDTA: CAPD vs controls P < 0.0005 | Both small and large intestine |
| Szeto et al[27] | New PD patients vs IgAN patients (mild to moderate CKD) and healthy controls Mean creatinine level IgAN group: 151.2 ± 116.68 μmol/L | PD n = 30 IgAN n = 10 Controls n = 6 | LPS (plasma) | CAPD patients: Mean 0.57% (0%-1.24%) Healthy controls: Mean 1.99% (0.59-3.48) Significantly higher LPS levels PD vs IgAN P < 0.0001 PD vs controls P < 0.0001 IgAN vs controls: Not provided PD: 0.44 ± 0.18 EU/mL IgAN: 0.0035 ± 0.009 EU/mL Controls: 0.013 ± 0.007 EU/mL | Both small and large intestine |
| Cobden et al[17] | CKD patients vs healthy controls CKD group: Serum creatinine levels ranging from 140 to 1050 μmol/L | CKD n = 6 Controls n = 55 | Cellobiose and mannitol recovery (urine) | No significant difference recovery cellobiose and mannitol CKD vs controls P > 0.05 Cellobiose: CKD: Recovery range 0.09%-0.44% Controls: Not provided Mannitol: CKD: Recovery range 12.8%-52.3% Controls: Not provided | Small intestine |
| Magnusson et al[28] | Asymptomatic uremic CKD vs healthy volunteers Mean serum creatinine level IgAN group: 503 μmol/L, range 274-796 μmol/L | CKD n = 9 Controls n = 6 | PEGs (urine) Computer model was used to predict the PEG recovery adjusted for eGFR | Significant lower urinary recovery of PEG’s CKD vs controls P < 0.05 More heavy PEG’s were harvest in urine CKD patients: indicating that intestinal permeability in CKD patients is more increased for larger molecules | Both small and large intestine |
| Kovacs et al[21] and Kovacs et al[23] | IgAN patients (both uremic and non-uremic) vs healthy controls | 1989: IgAN patients n = 29: (uremic n = 24 non-uremic n = 5) Controls n = 20 | Cr-EDTA recovery (urine) | Significantly higher Cr-EDTA recovery in IgAN patients vs controls P < 0.005, both in 1989 and in follow up after 5 yr | Both small and large intestine |
| These two studies published results measured in the same patient group. Provided data by the two articles are summarized | Both in 1989 and after a four year follow up in 1994 No mean serum creatinine levels of total IgAN group provided | 1996: IgAN patients n = 21 No controls Follow up patients further divided an analyzed in two groups; increased intestinal permeability group vs non-increased intestinal permeability | IgAN (1989): 3.86% ± 0.29% IgAN (1994): 4.57% ± 0.63% Controls: 2.72% ± 0.23% Only in the increased permeability group significant decrease in eGFR (Baseline eGFR 84.4 ± 6.1 mL/min vs 65.4 ± 8.6 mL/min after four years, P < 0.01) | ||
| Rostoker et al[22] | Patients with Primary IgA glomerulonefritis and permanent proteinuria (IgA GN), INS IC-GN: Membranous + membranoproliferative) vs healthy controls and alcohol abusers (positive controls) | IgA GN n = 30 INS n = 25 IC-GN n = 20 Controls n = 20 Alcohol abusers n = 5 | Cr-EDTA recovery (urine) | Significantly higher Cr-EDTA recovery in IgA GN vs controls P < 0.005 INS vs controls P < 0.005 IC-GN vs controls P < 0.005 Alcohol abusers vs controls P < 0.005 IgA GN: Median 3.25% (0.7-17.8) INS: Median 3.71% (0.82-10) IC-GN: 3.40% (0.30-16) Alcohol abusers: 4.9% (7-30) Controls: 2% (0.4-3.9) | Both small and large intestine |
| Layward et al[15] | Histologically proven IgAN with proteinuria and microscopic hematuria vs healthy No mean serum creatinine levels provided controls | IgAN patients n = 18 Controls n = 17 | Cellobiose/mannitol ratio (urine) | No significant difference cellobiose/mannitol ratio IgA NP patients vs controls P = 0.42 IgA NP: 0.015 ± 0.008 Controls: 0.022 ± 0.015 | Small intestine |
| De Maar et al[14] | Renal transplant patients assessed before transplantation and in the follow up during active CMV infection and CMV negative controls | Permeability assessed before transplantation n = 104 Permeability assessed during active infection n = 12 (primary infections: 5, secondary infections: 7) Controls (CMV-): n = 9 | Lactulose/mannitol ratio (urine) | L/M ratio increased during active CMV infection in 9/12 patients P < 0.01 L/M ratio active CMV infection compared to patients without CMV P < 0.01 | Small intestine Small intestine |
| Ponda et al[16] | CKD stadium III patients vs healthy controls CKD patients: mean eGFR: 51 mL/min per 1.73² All patients and controls had a vitamin D deficiency | CKD n = 5 Controls n = 4 | Endotoxin activity; expressed as fraction of the maximum response to endotoxin (plasma) Lactulose/mannitol ratio (urine) | No significant difference endotoxin activity CKD vs controls P > 0.05 CKD: 0.23 ± 0.15 Healthy controls: 0.20 ± 0.13 L/M ratio increased with D3 therapy P = 0.02 (reflecting an increase in permeability) L/M ratio not assessed in control group |
In attempt to compare results studies were divided in two categories: Studies comparing the intestinal permeability in mild to moderate CKD patients (eGFR 15-90) to healthy controls and studies comparing intestinal permeability in ESRD [eGFR < 15; both hemodialysis (HD) and non-hemodialysis (non-HD]] patients to healthy controls. For each study providing the mean and standard deviation the standardized mean difference was calculated through Review Manager 5.3. Biostatistics analysis was performed after consultation of a biomedical epidemiologist. In case of missing data the authors of the studies were contacted in order to obtain the required data. If studies only provided mean and standard deviation values of subgroups, we calculated the mean and standard deviation for the entire group with the following formula: sqrt[(6-1)*1.47^2+(24-1)*2.28^2]/(6-1+24-1). Sp = √(n1 – 1) x S1² + (N2 - 1) x S2²/ (n1 - 1 + n2 - 1)[13].
Results were graphically displayed in two plots, one comparing the intestinal permeability in mild to moderate CKD patients to healthy controls and one comparing the intestinal permeability in ESRD patients to healthy controls. Since different methods were used among the different included studies, results were not pooled and no meta-analysis was performed.
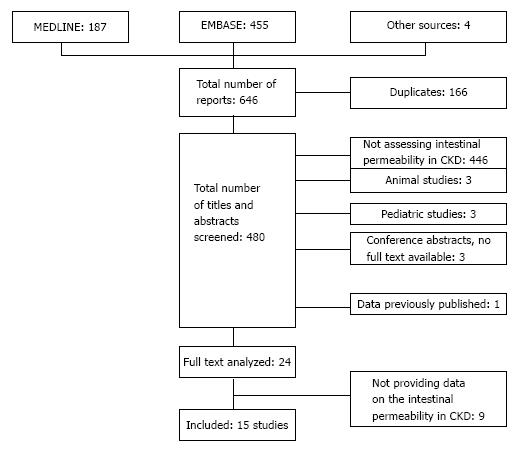
Our search through MEDLINE and EMBASE yielded 646 articles. The personal databases retrieved one more article and the search through the references lists of the relevant studies yielded three more studies. After removing duplicates, 480 articles remained and were screened for meeting the inclusion criteria. In 24 articles the full text was assessed. Reasons for exclusion are summarized in Figure 1. A total number of 15 studies were included in our study.
For each included study the methodological study was assessed through the Newscastle - Ottowa quality assessment scale[12]. The amount of stars scored by each study is summarized in supplementary tables. The mean amount of stars obtained by each study was 4.7 with a range from 4 to 6 stars. Methods that were used to assess the intestinal permeability varied from markers measured in plasma to methods based on calculating the urinary excretion of an orally administered test substance. The used methods, their mechanisms of action and (dis)advantages are summarized in Table 1. Most commonly used were the sugar absorption test[14-17], D-lactate (plasma)[18,19] and chromium-51 labeled ethylenediamine tetra acetic acid (51Cr-EDTA) (plasma)[20-23]. More recent studies focused on bacterial DNA[18,19,24] and endotoxins or LPS[16,18,25-27] in blood as a projection of intestinal permeability. Few studies used other methods such as polyethylene glycols (PEGs) in urine[28].
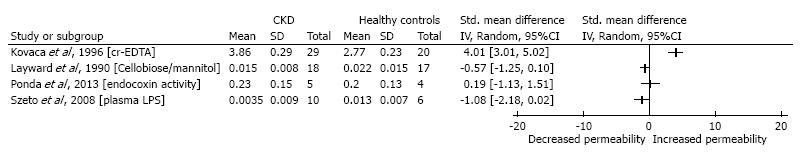
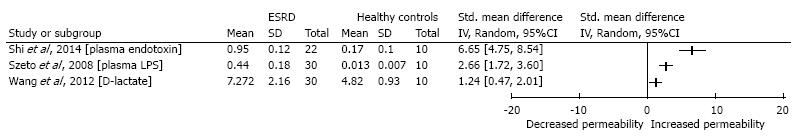
Results provided by each included study are summarized in Table 2. From the 15 included studies, 7 studies provided sufficient data to calculate the mean differences: 4 studies comparing the mild to moderate CKD patients to healthy controls and 3 studies comparing the ESRD patients to the healthy control population (Figures 2 and 3).
Despite the variety in used methods among the different studies, results considering the ESRD patient population are uniform. As displayed in Figure 3, all studies comparing ESRD patients to healthy controls point out a significantly increased intestinal permeability, independent of the used method.
Figure 2 shows the results considering the mild to moderate CKD patients; results are less convincing, whilst some do point out a significant increased intestinal permeability other studies did not report a statistical difference.
There was one study also comparing the peritoneal dialysis (PD) and HD groups[25]. They reported increased endotoxin levels in the HD compared to the PD group (P < 0.008), reflecting a higher permeability in the HD group.
In this review we focused on the intestinal permeability, how it can be assessed and whether there is an increased intestinal permeability in CKD. The currently used methods for intestinal permeability assessment are primarily used and validated in gastroenterological research; patient with renal failure are often excluded due to the possible bias caused by the reduced eGFR. Discriminating between an altered renal clearance of the used marker and an actual increased permeability is challenging, since for most methods the influence of renal function loss has not been evaluated.
Table 1 summarizes the possible influence of renal function on each test. For most used markers and substances the influence of a decreased renal function remains unclear to a certain extent since there are no data evaluating the renal clearance of the substance.
The tests measuring bacterial products such as endotoxins and bacterial derived DNA are the least likely to be influenced by renal function as they are not actively excreted by the kidney. However these methods are not validated in renal failure. Furthermore, whether these products determined in the circulation actually represent an increased intestinal permeability is open for discussion, as the source of those bacterial products is not precisely known and could for example be the dialysate in dialysis patients. However, the hypothesis that these bacterial products in plasma are derived from the gut and are trans located into the bloodstream due to an increased intestinal permeability is supported by several findings. Shi et al[18] compared the endotoxin levels of plasma to the levels in the dialysate of HD patients. Endotoxin levels were markedly lower in the dialysate than in the plasma samples, suggesting another bacterial source than the dialysate. Bacterial phyla in the blood samples appeared to be similar to the samples obtained from the gut, which supports the hypothesis that these bacterial compounds are derived from the intestinal tract. Furthermore Bossola et al[24] reported that only five out of twelve plasma samples from HD patients contained the same bacteria as those in the dialysate, also suggestive for another source of the blood bacteria than the dialysate. They proposed the biofilm on the surface of the central venous catheter (CVC) as a possible source, as the percentages of patients with circulating bacterial DNA fragment tended to be higher in patients with CVCs (4 out of 15) than in patients with an arteriovenous fistula (AVF) (7 out of 44). This difference was however not statistically significant, which can be the result of the small number of patients. However, the species found in the patients with a CVC were Escherichia coli (2 patients), Proteus mirabilis (1 patient), Enterococcus faecalis (1 patient) and Streptococcus Haemolyticus (1 patients). These strains indicate rather an intestinal source. Interestingly and in accordance with the results published by Shi et al[18]: In none of the blood samples obtained from healthy controls bacterial DNA was identified.
It is likely that direct demonstration of bacterial DNA in blood through qPCR is more accurate for determining the intestinal permeability compared to endotoxin level measurement, since endotoxins are bacterial surface products while presence of bacterial DNA in blood definitively indicates bacterial presence.
Another marker that is possibly valuable in the CKD population is D-lactate. D-lactate is usually present in human blood at very low concentrations as a product of methylglyoxal metabolism, which is produced in small amounts from fat, protein and carbohydrate metabolism. However, it is also produced by bacteria in the gastrointestinal tract and absorbed in the small intestine and colon. Only 10% of D-lactate is excreted in urine[29], marking a relatively low influence of renal clearance on plasma levels. In case of bacterial overgrowth a possible increased fermentation of undigested carbohydrates to D-lactate is nevertheless an important factor that might cause bias.
Considering the sugar absorption test, various combinations of oligosaccharides (lactulose, cellobiose) and monosaccharides (mannitol, L-rhamnose) are being used. The percentage of the substance excreted in urine is defined as the urinary recovery and is often expressed as the ratio of the recovery of the administered sugars. Even though renal clearance of these sugars is assumed to be of little or no influence on the ratio since both sugars are equally affected by a reduced eGFR[17], van Nieuwenhuizen et al[30] observed different results in their study evaluating the influence of pre- and postabsorptive factors on the lactulose/rhamnose ratio. The urinary excretion of lactulose and rhamnose was measured in 10 healthy males after intravenous administration of different quantities of each sugars. Equal renal clearance of both sugars would assure an unchanged ratio after administering a higher dose of both sugars. The investigators found a significant (P = 0.021) increase in lactulose/rhamnose ratio after administration of the high dose compared to the regular dose; a higher quantity of lactulose administration resulted in a lower recovery. These findings suggest that the process of renal clearance is different for the two sugars and thus that renal function might influence test results. Furthermore, in a study in endotoxaemic rats[31], fluid loading increased the urinary recovery of lactulose, but not of L-rhamnose. This also suggests that renal clearance of both sugars might not be equal. In conclusion, literature results on the recovery of both sugars are conflicting[30,32]. Differences in administration methods and dosage might be an explanation. The exact renal excretion of the different sugars is not clarified and thus might be affected differently when the eGFR is altered.
Furthermore, a decreased bowel motility has been reported to influence test results[33]. This test could however be valuable as a follow up method with patients being their own controls.
The studies that used 51Cr-EDTA as a marker for intestinal permeability corrected for renal function by dividing the 24 h 51Cr-EDTA excretion by the plasma creatinine level[20-23,34]. The radioactivity is nevertheless a major disadvantage that has caused this method to be considered out of date.
For the urinary recovery of different sized polyethylene glycols (PEGs), large inter- and intra-individual variations have been reported, even in healthy controls[35]. Combined with the influence of renal function on this test we consider it to be less suitable for the CKD patient population than other available methods.
Considering the results provided by the included studies, we divided the studies in categories based on the included patient population before results were compared. In our forest plots both the mild to moderate CKD patients (eGFR 15-90) and the ESRD patients (eGFR < 15) were compared to healthy controls. Seven studies have been published comparing the intestinal permeability specifically in patients with end stage renal disease, with or without dialysis, to healthy controls[18-20,24,27,36]. One of these studies included both HD and PD patients and also compared these groups.
From the studies comparing ESRD to healthy controls, three were providing sufficient data to calculate the standardized mean difference. Markers that were used in these studies were D-lactate, bacterial DNA, and endotoxins levels. Independent of the method that was used, all studies showed a significantly increased permeability in the ESRD group. These consistent results, despite the variety in the methods used, supports the hypothesis that renal failure is associated with increased intestinal permeability. The significant results published by studies measuring bacterial DNA and endotoxins are unlikely to be influenced by renal function.
The study also comparing the PD and HD groups[25] reported a significant increased permeability in the HD group compared to the PD group, P < 0.008. This was however the only study evaluating the difference between these two groups. All included studies including HD or PD patients reported a significant difference compared to the healthy controls. Further research is required to evaluate difference between the influence of HD vs PD on the intestinal permeability.
Studies assessing the intestinal permeability in mild to moderate CKD, mostly IgA nephropathy patients[15,23,27,34], yielded conflicting results. Even though some studies[21,34] reported a significantly increased permeability compared to the healthy controls, other studies could not confirm this finding[15,16,27]. Not all studies provided data on the exact renal function, but in general the eGFR was mildly decreased. This is an important difference compared to the studies assessing the intestinal permeability in end stage renal disease. Szeto et al[27] compared new peritoneal dialysis (PD) patients to both patients with mild to moderate CKD due to IgA nephropathy and healthy controls. Average serum creatinine levels of the IgA nephropathy group were 151.3 ± 116.2 µmol/L. He found significant higher endotoxin levels when comparing the PD patients (who suffer from a later stage of CKD) to the IgA group and the healthy controls. There was no significant difference between de IgA group and the healthy control group. This suggests that the intestinal permeability might only increase in later stages of CKD.
This systematic review outlines the lack of a gold standard to determine the intestinal permeability in the CKD patient population. Even though we aim to oppose the most reliable method, the lack of a gold standard is a limitation of this systematic review. In addition to this, unfortunately none of the included studies used more than one method to measure the intestinal permeability in CKD patients in order to be able to actually compare different methods.
In conclusion, assessing the intestinal permeability in CKD patients remains challenging as the influence of decreased renal function on the test results remains unclear. Quantitative PCR for bacterial DNA in blood and D-lactate levels in plasma seem the least likely to be influenced by a decreased eGFR. It should be noted though that also these methods have not been validated in the CKD patient population and results should still be interpret with caution[37].
However each included study measuring the intestinal permeability in patients with ESRD pointed out a significant increased permeability. Thus, it seems likely that there is a connection between renal failure and an increased intestinal permeability. How the permeability evolves in time, the possible link with (recurrent) infection(s), cardiovascular complications and prognosis of these patients has not yet been made and requires further exploration.
In the recent years numerous studies have been published evaluating the intestinal permeability in chronic kidney disease (CKD). Different methods are being used whilst the influence of a decreased renal clearance on these tests is unclear, complicating the interpretation of test results published by these studies. Her aim of this review is: (1) to determine what the best available method to measure the intestinal permeability in CKD; and (2) whether there is an increased intestinal permeability in CKD.
Noninvasive methods to measure the intestinal permeability have been used for many decades, with the first studies published in the 1950s. Only since the 90s there has however been an increasing interest in the intestinal alterations in renal failure and the possible clinical relevance of this aspect. Even though methods have been improved over the years, still none of the currently available methods has been validated in patients with renal failure. Furthermore it is still unclear whether there actually is an increased intestinal permeability in CKD and what the clinical relevance of this decreased barrier function is. Even though an increased intestinal permeability is proposed as an important prognostic factor, studies evaluating the influence of the intestinal permeability on the long-term prognosis of CKD patients have not yet been published.
Since 2009, three studies have been published using the quantitative amount of bacterial DNA in blood as a marker for intestinal permeability in CKD patients. This method to evaluate the intestinal permeability is unlikely to be influenced by a decreased renal clearance and also points out the exact consequence of an increased intestinal permeability; bacterial translocation into the bloodstream. This is likely to trigger an inflammatory response and could thus be an important prognostic factor for patients with renal failure.
This review opposes the most reliable methods to determine the intestinal permeability in CKD and points out that there possibly is a link between an increased intestinal permeability and renal failure. The overview of the advantages and disadvantages of the currently available methods could help fellow researcher to determine what the most reliable method to measure the intestinal permeability in their study population. Future research is necessary specifically considering the role of the intestinal permeability as a prognostic factor in CKD. In this prospect restoration of the intestinal barrier function could also become a possible therapeutic target.
Even though the exact pathophysiology is not yet clarified, CKD is accompanied by a chronic inflammatory response, meaning that the immune system appears to be constantly triggered. A chronic inflammatory status is associated with many complications such as cardiovascular disease, which are in turn frequently observed in CKD.
In this systematic review the authors have presented a critical analyse of the currently available methods to determine the intestinal permeability in CKD in order to guide fellow researcher in their choice of methods applicable to measure the intestinal permeability in CKD in future research projects. Furthermore the need for studies evaluating the intestinal permeability with reliable methods is emphasised, especially considering the lack of knowledge on the prognostic consequence of an increased intestinal permeability.
P- Reviewer: Akyuz F, Bellomo G, Chang CC, Duan SB, Friedman EA S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 860] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 2. | Cachofeiro V, Goicochea M, de Vinuesa SG, Oubiña P, Lahera V, Luño J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008;S4-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 462] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 3. | Vaziri ND. CKD impairs barrier function and alters microbial flora of the intestine: a major link to inflammation and uremic toxicity. Curr Opin Nephrol Hypertens. 2012;21:587-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 185] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 4. | Himmelfarb J, Hakim RM. Oxidative stress in uremia. Curr Opin Nephrol Hypertens. 2003;12:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 822] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 6. | Vaziri ND, Zhao YY, Pahl MV. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol Dial Transplant. 2016;31:737-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 272] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 7. | Wong J, Piceno YM, Desantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol. 2014;39:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 481] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 8. | de Almeida Duarte JB, de Aguilar-Nascimento JE, Nascimento M, Nochi RJ. Bacterial translocation in experimental uremia. Urol Res. 2004;32:266-270. [PubMed] |
| 9. | Grootjans J, Thuijls G, Verdam F, Derikx JP, Lenaerts K, Buurman WA. Non-invasive assessment of barrier integrity and function of the human gut. World J Gastrointest Surg. 2010;2:61-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 137] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 10. | Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7463] [Cited by in RCA: 8228] [Article Influence: 822.8] [Reference Citation Analysis (0)] |
| 11. | University of York Centre for Reviews and Disseminations. PROSPERO: International prospective register of systematic reviews. Available from: http//www.york.ac.uk/crd/. |
| 12. | Wells G, Shea B, O’Connell D, Petersen J, Welch V; The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute 2012; . |
| 13. | Twisk J; Inleiding in de toegepaste biostatistiek. 1th ed. Nederland: Elsevier Gezondheidszorg Maarssen 2007; 63. |
| 14. | de Maar EF, Kleibeuker JH, Boersma-van Ek W, The TH, van Son WJ. Increased intestinal permeability during cytomegalovirus infection in renal transplant recipients. Transpl Int. 1996;9:576-580. [PubMed] |
| 15. | Layward L, Hattersley JM, Patel HR, Tanner MS, Feehally J. Gut permeability in IgA nephropathy. Nephrol Dial Transplant. 1990;5:569-571. [PubMed] |
| 16. | Ponda MP, Breslow JL. Vitamin D3 repletion in chronic kidney disease stage 3: effects on blood endotoxin activity, inflammatory cytokines, and intestinal permeability. Ren Fail. 2013;35:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Cobden I, Hamilton I, Rothwell J, Axon AT. Cellobiose/mannitol test: physiological properties of probe molecules and influence of extraneous factors. Clin Chim Acta. 1985;148:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Shi K, Wang F, Jiang H, Liu H, Wei M, Wang Z, Xie L. Gut bacterial translocation may aggravate microinflammation in hemodialysis patients. Dig Dis Sci. 2014;59:2109-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 19. | Wang F, Jiang H, Shi K, Ren Y, Zhang P, Cheng S. Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology (Carlton). 2012;17:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 20. | Zuckerman MJ, Buchwald D, Watts MT. Intestinal permeability to 51Cr-EDTA in patients on chronic ambulatory peritoneal dialysis. Nephron. 1994;68:408-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Kovács T, Kun L, Schmelczer M, Wagner L, Davin JC, Nagy J. Do intestinal hyperpermeability and the related food antigens play a role in the progression of IgA nephropathy? I. Study of intestinal permeability. Am J Nephrol. 1996;16:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Rostoker G, Wirquin V, Terzidis H, Chaumette MT, Lang P, Weil B. Study of intestinal permeability in IgA mesangial nephritis and primary glomerulonephritis. Adv Exp Med Biol. 1995;371B:839-841. [PubMed] |
| 23. | Kovács T, Mette H, Per B, Kun L, Schmelczer M, Barta J, Jean-Claude D, Nagy J. [Relationship between intestinal permeability and antibodies against food antigens in IgA nephropathy]. Orv Hetil. 1996;137:65-69. [PubMed] |
| 24. | Bossola M, Sanguinetti M, Scribano D, Zuppi C, Giungi S, Luciani G, Torelli R, Posteraro B, Fadda G, Tazza L. Circulating bacterial-derived DNA fragments and markers of inflammation in chronic hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | McIntyre CW, Harrison LE, Eldehni MT, Jefferies HJ, Szeto CC, John SG, Sigrist MK, Burton JO, Hothi D, Korsheed S. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 349] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 26. | Feroze U, Kalantar-Zadeh K, Sterling KA, Molnar MZ, Noori N, Benner D, Shah V, Dwivedi R, Becker K, Kovesdy CP. Examining associations of circulating endotoxin with nutritional status, inflammation, and mortality in hemodialysis patients. J Ren Nutr. 2012;22:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Szeto CC, Kwan BC, Chow KM, Lai KB, Chung KY, Leung CB, Li PK. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2008;3:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Magnusson M, Magnusson KE, Sundqvist T, Denneberg T. Impaired intestinal barrier function measured by differently sized polyethylene glycols in patients with chronic renal failure. Gut. 1991;32:754-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Herrera DJ, Morris K, Johnston C, Griffiths P. Automated assay for plasma D-lactate by enzymatic spectrophotometric analysis with sample blank correction. Ann Clin Biochem. 2008;45:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | van Nieuwenhoven MA, de Swart EA, van Eijk HM, Deutz NE, Brouns F, Brummer RJ. Effects of pre- and post-absorptive factors on the lactulose/rhamnose gut permeability test. Clin Sci (Lond). 2000;98:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Hallemeesch MM, Lamers WH, Soeters PB, Deutz NE. Increased lactulose/rhamnose ratio during fluid load is caused by increased urinary lactulose excretion. Am J Physiol Gastrointest Liver Physiol. 2000;278:G83-G88. [PubMed] |
| 32. | Maxton DG, Bjarnason I, Reynolds AP, Catt SD, Peters TJ, Menzies IS. Lactulose, 51Cr-labelled ethylenediaminetetra-acetate, L-rhamnose and polyethyleneglycol 400 [corrected] as probe markers for assessment in vivo of human intestinal permeability. Clin Sci (Lond). 1986;71:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 153] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Oudemans-van Straaten HM, van der Voort PJ, Hoek FJ, Bosman RJ, van der Spoel JI, Zandstra DF. Pitfalls in gastrointestinal permeability measurement in ICU patients with multiple organ failure using differential sugar absorption. Intensive Care Med. 2002;28:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Rostoker G, Wirquin V, Terzidis H, Petit-Phar M, Chaumette MT, Delchier JC, Belghiti D, Lang P, Dubert JM, Meignan M. Mucosal immunity in primary glomerulonephritis. III. Study of intestinal permeability. Nephron. 1993;63:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Philipsen EK, Batsberg W, Christensen AB. Gastrointestinal permeability to polyethylene glycol: an evaluation of urinary recovery of an oral load of polyethylene glycol as a parameter of intestinal permeability in man. Eur J Clin Invest. 1988;18:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Vaziri ND, Goshtasbi N, Yuan J, Jellbauer S, Moradi H, Raffatellu M, Kalantar-Zadeh K. Uremic plasma impairs barrier function and depletes the tight junction protein constituents of intestinal epithelium. Am J Nephrol. 2012;36:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 37. | Turner DA, Brien FS. The measurement of absorption from the intestine and the xylose tolerance test. Can J Med Technol. 1953;15:41-46. [PubMed] |