Published online Jul 6, 2016. doi: 10.5527/wjn.v5.i4.358
Peer-review started: February 22, 2016
First decision: March 25, 2016
Revised: April 1, 2016
Accepted: May 10, 2016
Article in press: May 11, 2016
Published online: July 6, 2016
Processing time: 131 Days and 9.5 Hours
AIM: To compare anemia prevalence between matched chronic kidney disease (CKD) patients with and without diabetes mellitus (DM) and to assess factors associated with anemia development.
METHODS: This is a nested case-control study of 184 type-2 diabetic and 184 non-diabetic CKD patients from a prospectively assembled database of a Nephrology outpatient clinic, matched for gender, age and estimated glomerular filtration rate (eGFR). Prevalence of anemia (hemoglobin: Men: < 13 g/dL, women: < 12 g/dL and/or use of recombinant erythropoietin) was examined in comparison, in the total population and by CKD Stage. Univariate and multivariate logistic regression analyses were conducted to identify factors associated with anemia.
RESULTS: The total prevalence of anemia was higher in diabetics (47.8% vs 33.2%, P = 0.004). Accordingly, prevalence was higher in diabetics in CKD Stage 3 (53.5% vs 33.1%, P < 0.001) and particularly in Stage 3a (60.4% vs 26.4%, P < 0.001), whereas it was non-significantly higher in Stage 4 (61.3% vs 48.4%; P = 0.307). Serum ferritin was higher in diabetics in total and in CKD stages, while serum iron was similar between groups. In multivariate analyses, DM (OR = 2.206, 95%CI: 1.196-4.069), CKD Stages 3a, 3b, 4 (Stage 4: OR = 12.169, 95%CI: 3.783-39.147) and serum iron (OR = 0.976, 95%CI: 0.968-0.985 per mg/dL increase) were independently associated with anemia.
CONCLUSION: Prevalence of anemia progressively increases with advancing stages of CKD and is higher in diabetic than matched non-diabetic CKD patients and diabetes is independently associated with anemia occurrence. Detection and treatment of anemia in diabetic CKD patients should be performed earlier than non-diabetic counterparts.
Core tip: Anemia is an established complication of chronic kidney disease (CKD) and diabetes mellitus is proposed to further increase anemia occurrence through various mechanisms. However, a direct comparison between diabetic and non-diabetic CKD patients with regards to anemia is currently missing. This study evaluates in comparison the prevalence of anemia in carefully matched CKD patients with and without diabetes mellitus.
- Citation: Loutradis C, Skodra A, Georgianos P, Tolika P, Alexandrou D, Avdelidou A, Sarafidis PA. Diabetes mellitus increases the prevalence of anemia in patients with chronic kidney disease: A nested case-control study. World J Nephrol 2016; 5(4): 358-366
- URL: https://www.wjgnet.com/2220-6124/full/v5/i4/358.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i4.358
Anemia is a major complication of chronic kidney disease (CKD) contributing to the clinical significance and the complex therapeutic approach of the uremic syndrome[1]. The prevalence of anemia (defined as serum hemoglobin levels < 130 g/L for men and < 120 g/L for women) in the general population is estimated at 7.6%, but among patients with CKD anemia is reported at least twice as prevalent, reaching 15%[2]. Anemia is generally associated with the severity of renal insufficiency, as serum hemoglobin levels and estimated glomerular filtration rate (eGFR) present an almost linear correlation[3]. Anemia commonly occurs after CKD Stage 3, with prevalence increasing from 5% in CKD Stage 1, to 75%-80% in pre-dialysis CKD Stage 5[4,5]. The main pathogenetic mechanism for the development of anemia in CKD is the impaired production of erythropoietin from kidney[6]. Iron deficiency or decreased availability, caused mainly by increased levels of hepcidin, due to inflammation accompanying chronic uremia, may constitute another important mechanism[7]. Additionally, folate and vitamin B12 deficiency, due to malnutrition and chronic inflammation result in increased red blood cells and immature erythroblasts apoptosis[6]. Results from observational studies in pre-dialysis CKD patients suggest that anemia is associated with poor quality of life, increased hospital admissions, progression of kidney disease, and elevated mortality[8].
Diabetes mellitus (DM) is the leading cause of CKD and ESRD[9] and is proposed to elevate the risk of anemia development even in the absence of renal impairment. Anemia has been found in about 10% of patients with DM and normal kidney function[10]. In a cohort of > 9000 patients without renal disease, DM was an independent determinant of hemoglobin levels[11]. Many factors have been suggested to contribute in the pathogenesis of anemia in these patients, such as erythropoietin deficiency due to efferent sympathetic denervation of the kidney in the context of diabetic neuropathy, chronic inflammatory reaction leading to functional iron deficiency, non-selective urinary protein excretion leading to transferrin and erythropoietin loss and the use of renin-angiotensin-aldosterone system (RAAS) blockers which are central in the treatment of proteinuric diabetic nephropathy[12].
Preliminary data suggest that anemia may be more common and occurs at earlier CKD stages in diabetic patients[13]. An observational study in 1 million CKD patients of all stages indicated that prevalence of anemia in patients with DM was around 30%[14]. In another study, including patients with type 2 DM and CKD, the prevalence of anemia increased from 15% in Stage 1 to 90% in Stage 5[15]. However, epidemiologic data from a direct comparison between diabetic and non-diabetic CKD patients with regards to anemia are currently missing. On this context, the aim of this study was to examine in comparison the prevalence of anemia in matched CKD patients with and without DM and to evaluate additional factors that may contribute in anemia development.
This is a nested case-control study in a prospectively assembled cohort of CKD patients first visiting the Nephrology Outpatient clinic of the General Hospital of Grevena, Greece between 1/01/2007 and 1/05/2015. Inclusion criteria were diagnosis of CKD and a complete dataset for the present analysis. Exclusion criteria were type 1 DM, Stage 5 CKD (eGFR < 15 mL/min per 1.73 m2) or kidney transplant. In total, 184 patients with type 2 DM were included and represented the cases. After this group was formed an equal number of non-diabetic patients were selected from the same cohort by an investigator blinded to patient data apart from matching parameters to form the control group. Matching was performed for gender, age (± 5 years) and eGFR (± 5 mL/min per 1.73 m2) with particular care so that both cases and controls belonged to the same CKD stage. All study procedures belonged to the routine clinical practice of the Nephrology Outpatient clinic and all patients provided informed written consent prior to study enrollment. The study protocol was approved by the Institutional Ethics Committee and all investigations were performed according to the Declaration of Helsinki (2013 amendment).
Study data collection
For the purpose of this study, demographic and anthropometric parameters as well as cardiovascular risk factors and co-morbidities were recorded for each patient on their first outpatient visit within the aforementioned period. These included age, gender, height and weight, from which body mass index (BMI) was calculated according to the formula weight divided by height squared, as well as history of hypertension, dyslipidemia, DM, coronary heart disease, stroke, peripheral vascular disease, and cardiac arrhythmias. Moreover, data with regards to drug therapy were collected, such as medications for the treatment of DM (insulin and/or other non-insulin hypoglycaemic agents), use of medications that may affect erythropoiesis, such as oral iron supplements, recombinant erythropoietin, ACEIs or ARBs, cyclosporine, tacrolimus, etc., and use of drugs interfering in the coagulation process (aspirin, clopidogrel, acenocoumarol, ticlopidine, heparin). During this visit blood samples were also acquired for the evaluation of routine hematological and biochemical parameters, including among others, serum urea, creatinine, sodium, potassium, uric acid, glucose, lipid profile and liver function tests. Patients were also instructed to perform a 24-h urine collection immediately before their next visit so that urine protein excretion would be evaluated.
Anemia was defined as serum hemoglobin levels < 130 g/L for men and < 120 g/L for women, according to the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines for anemia in CKD[1] and/or use of recombinant erythropoietin for known anemia. The diagnosis of DM was based on American Diabetes Association criteria[16], or on the basis of history of type 2 DM under dietary intervention or use of hypoglycaemic agents. Calculation of eGFR was performed from serum creatinine levels using the Modification of Diet in Renal Disease (MDRD) equation[17]. Definition and staging of CKD was performed according to the KDIGO 2012 guidelines[18], i.e., Stage 1 CKD as eGFR ≥ 90 mL/min per 1.73 m2 plus evidence of kidney injury for more than 3 mo; Stage 2 kidney CKD as eGFR ≥ 60 and < 90 mL/min per 1.73 m2 and evidence of kidney injury; Stage 3a CKD as eGFR ≥ 45 and < 60 mL/min per 1.73 m2, Stage 3b CKD as eGFR ≥ 30 and < 45 mL/min per 1.73 m2 and Stage CKD 5 as eGFR < 15 mL/min per 1.73 m2.
Statistical analysis was performed with Statistical Package for Social Sciences 21 (SPSS Inc, Chicago, IL). The Shapiro-Wilk test or Kolmogorov-Smirnov tests were used to examine the normality of distribution for quantitative variables. Continuous variables are presented as mean ± 1 SD or median range (presented in brackets) and categorical variables are described as absolute and relevant frequencies (n, %). χ2 test or Fisher’s exact test for qualitative variables, and Student’s t-test, Mann-Whitney test or analysis of variance (ANOVA) for quantitative variables were used for between-group comparisons. In addition, multiple logistic regression analysis was performed to evaluate the association of various studied parameters (demographic, clinical and laboratory) with anemia. Variables were tested for interactions and included in the multivariate model if P < 0.2 in univariate analysis. Adjusted odd ratios (OR) with 95%CI are reported. Values of P < 0.05 (two-tailed) were considered statistically significant.
A total of 368 patients with CKD (Stages 2-4) were included in this study, forming two groups: The first group consisted of 184 patients with DM and CKD and the second group of 184 matched CKD patients without DM. Baseline demographic, clinical and biochemical characteristics are presented in Table 1. In each group 96 patients (52%) were male and 88 (47.8%) were female. The mean age was 75.91 ± 8.38 and 76.00 ± 9.54 years for patients with and without DM accordingly (P = 0.908). Patients were stratified in CKD Stages as follows: Stage 2, 14.1%; Stage 3a, 28.8%; Stage 3b, 40.2%; and Stage 4, 16.8%. With regards to the existing risk factors and comorbidities smoking habit (39.7% vs 16.8%; P < 0.001) and history of stroke (8.7% vs 0.5%; P < 0.001) were more common in diabetics. As expected, results from routine biochemical tests indicated significant differences in serum glucose levels (diabetics 8.61 ± 2.74 mmol/L, non-diabetics 5.46 ± 0.61 mmol/L; P < 0.001) and 24-h urine protein [diabetics 527 (59-9, 300)] mg, non-diabetics 320 (65-3, 100) mg; P < 0.001].
| Parameter | Diabetic CKD patients | Non-diabetic CKD patients | P |
| n | 184 | 184 | - |
| Age (yr) | 75.91 ± 8.38 | 76.00 ± 9.54 | 0.908 |
| Gender n (%) | |||
| Female | 88 (47.8) | 88 (47.8) | 1 |
| Male | 96 (52.2) | 96 (52.2) | |
| Weight (kg) | 79.78 ± 14.51 | 78.51 ± 12.58 | 0.373 |
| Height (m) | 1.67 ± 0.09 | 1.66 ± 0.08 | 0.121 |
| BMI (kg/m2) | 28.34 ± 4.16 | 28.33 ± 3.26 | 0.979 |
| Urea Nitrogen (mmol/L) | 10.95 ± 5.06 | 10.90 ± 4.82 | 0.927 |
| Creatinine (μmol/L) | 136.14 ± 45.97 | 134.37 ± 46.85 | 0.826 |
| eGFR (mL/min per 1.73 m2) | 43.3 ± 14.8 | 43.7 ± 14.9 | 0.778 |
| Glucose (mmol/L) | 8.61 ± 2.74 | 5.46 ± 0.61 | < 0.001 |
| 24 h urine protein excretion (mg) | 527 (59-9, 300) | 320 (65-3, 100) | < 0.001 |
| CKD Stages n (%) | |||
| Stage 2 | 26 (14.1) | 26 (14.1) | 1 |
| Stage 3a | 53 (28.8) | 53 (28.8) | |
| Stage 3b | 74 (40.2) | 74 (40.2) | |
| Stage 4 | 31 (16.8) | 31 (16.8) | |
| Hypertension n (%) | 171 (92.9) | 175 (95.1) | 0.379 |
| Dyslipidemia n (%) | 103 (56) | 86 (46.7) | 0.076 |
| Coronary heart disease n (%) | 65 (35.3) | 60 (32.6) | 0.582 |
| Heart failure n (%) | 30 (16.3) | 34 (18.5) | 0.583 |
| Arrhythmia n (%) | 20 (10.9) | 25 (13.6) | 0.426 |
| Stroke history n (%) | 16 (8.7) | 1 (0.5) | < 0.001 |
| Peripheral vascular disease n (%) | 17 (9.2) | 13 (7.1) | 0.446 |
| Smoking n (%) | 73 (39.7) | 31 (16.8) | < 0.001 |
Prevalence of anemia in total and in two study groups
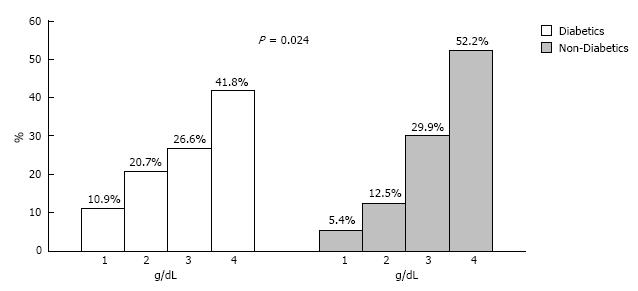
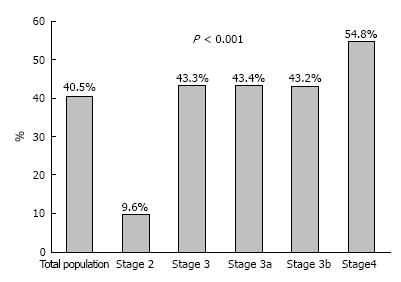
As Table 2 depicts the mean hematocrit and hemoglobin levels were 39.02% ± 4.3% vs 40.07% ± 4.0% (P = 0.015) and 128.7 ± 15.6 g/L vs 131.9 ± 14.0 g/L (P = 0.036) for diabetic and the non-diabetic CKD patients respectively. Figure 1 presents the distribution of patients from the two study groups over the continuum of hemoglobin levels; the distribution was in general towards lower values in patients with DM (P = 0.024). Anemia was present in 149 patients accounting for 40.5% of the total population studied (Figure 2). A trend of increasing anemia prevalence was found with the progression of CKD from Stage 2 towards Stage 4, i.e., Stage 2, 9.6%; Stage 3, 43.3%; Stage 4, 54.8% (P < 0.001).
| Parameter | Total studypopulation | P | Stage 2 | P | Stage 3 | P | Stage 3a | P | Stage 3b | P | Stage 4 | P |
| Hematocrit (%) | ||||||||||||
| Diabetics | 39.02 ± 4.30 | 0.015 | 42.27 ± 4.79 | 0.324 | 38.69 ± 3.97 | 0.001 | 38.92 ± 4.08 | < 0.001 | 38.53 ± 3.91 | 0.278 | 37.63 ± 4.02 | 0.687 |
| Non-diabetics | 40.07 ± 4 | 41.18 ± 2.83 | 40.34 ± 4.09 | 41.93 ± 4.10 | 39.21 ± 3.71 | 38.04 ± 3.9 | ||||||
| Hemoglobin (g/L) | ||||||||||||
| Diabetics | 128.7 ± 15.6 | 0.036 | 141.8 ± 17.4 | 0.21 | 127.4 ± 14.1 | 0.003 | 128.2 ± 14.8 | 0.001 | 126.9 ± 13.6 | 0.383 | 122.7 ± 14.1 | 0.58 |
| Non-diabetics | 131.9 ± 14.0 | 136.7 ± 10.9 | 132.7 ± 14.1 | 138.2 ± 14.0 | 128.8 ± 13.0 | 124.7 ± 13.5 | ||||||
| MCV (fL) | ||||||||||||
| Diabetics | 87.62 ± 6.99 | 0.739 | 87.76 ± 74.2 | 0.536 | 87.70 ± 7.49 | 0.633 | 87.78 ± 5.88 | 0.81 | 87.65 ± 8.5 | 0.457 | 87.11 ± 6.86 | 0.527 |
| Non-diabetics | 87.9 ± 6.99 | 86 ± 13.7 | 88.20 ± 8.83 | 87.45 ± 8.34 | 88.73 ± 9.17 | 88.29 ± 7.68 | ||||||
| MCH (pg/cell) | ||||||||||||
| Diabetics | 29.91 ± 5.08 | 0.748 | 29.53 ± 2.21 | 0.684 | 30.28 ± 5.89 | 0.494 | 29.52 ± 2.24 | 0.792 | 30.82 ± 7.45 | 0.526 | 28.76 ± 2.42 | 0.415 |
| Non-diabetics | 29.78 ± 2.64 | 29.83 ± 3 | 29.89 ± 2.55 | 29.4 ± 2.75 | 30.25 ± 2.35 | 29.3 ± 2.73 | ||||||
| MCHC (g/L) | ||||||||||||
| Diabetics | 323.8 ± 17.6 | 0.523 | 333.6 ± 12.0 | 0.03 | 321.6 ± 19.0 | 0.362 | 323.0 ± 21.7 | 0.982 | 320.6 ± 16.9 | 0.174 | 324.8 ± 11.6 | 0.282 |
| Non-diabetics | 322.6 ± 20.1 | 319.5 ± 29.5 | 323.7 ± 18.4 | 322.9 ± 20.9 | 324.3 ± 16.4 | 320.3 ± 19.7 | ||||||
| Serum iron (μmol/L) | ||||||||||||
| Diabetics | 12.35 | 0.783 | 2.75 | 0.027 | 12.17 | 0.351 | 12.71 | 0.86 | 11.01 | 0.194 | 10.92 | 0.559 |
| (1.61-35.73) | (1.09-4.23) | (1.61-35.73) | (1.61-28.28) | (2.15-35.73) | (3.83-20.23) | |||||||
| Non-diabetics | 12.53 | 2.21 | 12.53 | 12.35 | 12.71 | 11.99 | ||||||
| (2.69-27.03) | (0.38-4.13) | (2.69-27.03) | (4.47-23.27) | (2.69-27.03) | (3.94-23.81) | |||||||
| Ferritin (ng/mL) | ||||||||||||
| Diabetics | 200 | < 0.001 | 230.3 | 0.01 | 175.3 | 0.003 | 175.3 | 0.013 | 175.3 | 0.061 | 220.2 | 0.011 |
| (17.3-1048.7) | (62.9-570.7) | (17.3-1048.7) | (23.4-1048.7) | (7.7-1015.6) | (25.6-867.3) | |||||||
| Non-diabetics | 148.3 | 155.1 | 148.3 | 155.1 | 143.8 | 143.8 | ||||||
| (7.2-993.2) | (78.6-435.9) | (22.5-993.2) | (22.5-294.4) | (26.9-993.2) | (7.2-441.4) | |||||||
| 24 h urine protein Excretion (mg) | ||||||||||||
| Diabetics | 527 | < 0.001 | 283 | 0.126 | 530 | < 0.001 | 545 | < 0.001 | 525 | < 0.001 | 670 | 0.647 |
| (59-9300) | (68-5100) | (59-9300) | (129-1700) | (59-9300) | (95-3800) | |||||||
| Non-diabetics | 320 | 245 | 300 | 250 | 375 | 560 | ||||||
| (65-3100) | (110-780) | (65-3100) | (65-1500) | (104-3100) | (117-3100) | |||||||
| Smoking (n, %) | ||||||||||||
| Diabetics | 73 (39.7) | < 0.001 | 10 (38.5) | 0.375 | 57 (44.9) | < 0.001 | 32 (60.4) | 0.011 | 25 (33.8) | < 0.001 | 6 (19.4) | 0.255 |
| Non-diabetics | 31 (16.8) | 7 (26.9) | 22 (17.3) | 19 (35.8) | 3 (4.1) | 2 (6.5) | ||||||
| Use of erythropoietin (n, %) | ||||||||||||
| Diabetics | 15 (8.2) | 0.851 | 0 (0) | n/a | 9 (7.1) | 0.271 | 4 (7.5) | 0.118 | 5 (6.8) | 1 | 6 (80.6) | 0.155 |
| Non-diabetics | 16 (8.7) | 0 (0) | 5 (3.9) | 0 (0) | 5 (6.8) | 11 (35.5) | ||||||
| Iron supplements therapy (n, %) | ||||||||||||
| Diabetics | 27 (14.7) | 0.152 | 1 (3.8) | 1 | 19 (15) | 0.076 | 8 (15.1) | 0.111 | 11 (14.9) | 0.314 | 7 (22.6) | 1 |
| Non-diabetics | 18 (9.8) | 1 (3.8) | 10 (7.9) | 3 (5.7) | 7 (9.5) | 7 (22.6) | ||||||
| ACEIs/ARBs (n, %) | ||||||||||||
| Diabetics | 121 (65.8) | 0.372 | 22 (84.6) | 1 | 88 (69.3) | 0.784 | 38 (71.7) | 0.831 | 50 (67.6) | 0.592 | 11 (35.5) | 0.075 |
| Non-diabetics | 129 (70.1) | 21 (80.8) | 90 (70.9) | 37 (69.8) | 53 (71.6) | 18 (58.1) | ||||||
| Antiplatelet/anticoagulant drugs (n, %) | ||||||||||||
| Diabetics | 86 (46.7) | 0.034 | 9 (34.6) | 0.199 | 62 (48.8) | 0.165 | 27 (50.9) | 0.171 | 35 (47.3) | 0.508 | 15 (48.4) | 0.303 |
| Non-diabetics | 66 (35.9) | 4 (15.4) | 51 (40.2) | 20 (37.7) | 31 (41.9) | 11 (35.5) | ||||||
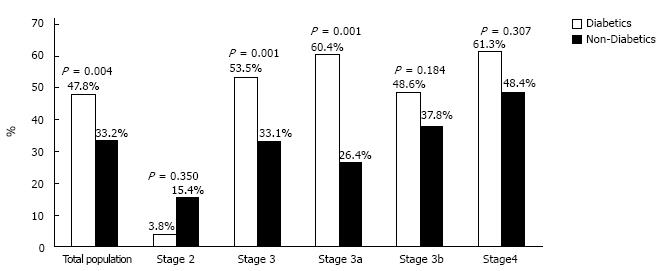
With regards to between-group differences, anemia was significantly more prevalent in the diabetic patient group in total (diabetics 47.8%, non-diabetics 33.2%; P = 0.004). As shown in Figure 3, prevalence of anemia was higher in non-diabetics but statistically not different between the two groups in CKD Stage 2 (3.8% vs 15.4%, P = 0.350) and thereafter higher in diabetic patients: Stage 3, 53.5% vs 33.1% (P = 0.001); Stage 3a, 60.4% vs 26.4% (P = 0.001); Stage 3b, 48.6% vs 37.8% (P = 0.184); Stage 4, 61.3% vs 48.4% (P = 0.307) for patients with and without DM accordingly.
Results for all other anemia-related parameters are presented in Figure 2. In both groups no significant differences were noted with regards to red blood cell indices, such as mean corpuscular volume, mean corpuscular hemoglobin and mean corpuscular hemoglobin concentration. However, serum ferritin levels were significantly higher in patients with DM both in total and in all CKD stages, while serum iron levels were equal between groups, with the exception of CKD Stage 2, in which patients with DM had 15.39 (6.09-23.63) μmol/L and patients without DM 12.35 (2.15-23.09) μmol/L (P = 0.027).
Use of recombinant erythropoietin was similar between the two study groups in total (diabetics 8.2%, non-diabetics 8.7%; P = 0.851) and in CKD stages separately and the use of oral iron supplementary therapy was similar (diabetics 14.7%, non-diabetics 9.8%, P = 0.152). Regarding other medications that may interfere with development of anemia, use of RAAS-blockers did not differ significantly between diabetics (65.8%) and non-diabetics (70.1%, P = 0.372) in total and in CKD stages respectively. Finally, the use of drugs interfering in the coagulation process was higher for patients with DM in total (diabetics 46.7%, non-diabetics 35.9%; P = 0.034), but differences were not significant between the two groups in CKD stages.
Univariate and multivariate regression analyses in the total population studied is presented in Table 3. Anemia was the dependent variable, while several demographic, clinical and laboratory factors that can interfere with development of anemia were the independent variables. Diabetes was an independent factor for anemia occurrence in the total population (OR = 2.206, 95%CI: 1.196-4.069). Advancing stage of CKD was associated with progressively increasing risk for anemia development both in univariate and multivariate analysis; i.e., Stage 3a (OR = 6.068, 95%CI: 2.112-17.430), Stage 3b (OR = 7.499, 95%CI: 2.604-21.597), Stage 4 (OR = 12.169, 95%CI: 3.783-39.147). Serum iron levels were also associated with occurrence of anemia (OR = 0.976, 95%CI: 0.968-0.985 per mg/dL increase). Interestingly, female gender was associated with decreased risk for anemia occurrence (OR = 0.389, 95%CI: 0.224-0.675), but this may be related to the lower threshold of hemoglobin for females in the definition used. With regards to other existing comorbidities no significant correlations were observed. Similarly, use of RAAS-blockers and antiplatelet or anticoagulant drugs, as well as the degree of 24-h urine protein excretion were not found to be associated with the development of anemia.
| Parameter | Univariate analysis | Multivariate analysis | ||
| Unadjusted odds ratio (95%CI) | P | Adjusted odds ratio (95%CI) | P | |
| ΒΜΙ Groups | ||||
| Normal (18.5-25) | Reference group | |||
| Underweight (< 18.5) | 0.545 (0.046-6.443) | 0.63 | ||
| Overweight (25-30) | 0.714 (0.377-1.350) | 0.3 | ||
| Obese (> 30) | 0.708 (0.348-1.442) | 0.342 | ||
| Age | ||||
| < 75 yr | Reference group | Reference group | ||
| ≥ 75 yr | 1.623 (1.028-2.564) | 0.038 | 1.198 (0.694-2.069) | 0.517 |
| Gender | ||||
| Male | Reference group | Reference group | ||
| Female | 0.546 (0.357-0.833) | 0.005 | 0.389 (0.224-0.675) | 0.001 |
| CKD Stages | ||||
| Stage 2 | Reference group | Reference group | ||
| Stage 3a | 7.207 (2.656-19.566) | < 0.001 | 6.068 (2.112-17.430) | 0.001 |
| Stage 3b | 7.162 (2.694-19.038) | < 0.001 | 7.499 (2.604-21.597) | < 0.001 |
| Stage 4 | 11.414 (3.999-32.582) | < 0.001 | 12.169 (3.783-39.147) | < 0.001 |
| Diabetes | 1.848 (1.212-2.818) | 0.004 | 2.206 (1.196-4.069) | 0.011 |
| Hypertension | 0.663 (0.280-1.573) | 0.351 | ||
| Dyslipidemia | 0.745 (0.491-1.130) | 0.166 | 0.659 (0.404-1.074) | 0.094 |
| Coronary heart disease | 1.446 (0.934-2.239) | 0.098 | 1.048 (0.506-1.960) | 0.883 |
| Heart failure | 1.725 (1.003-2.967) | 0.049 | 1.228 (0.628-2.398) | 0.548 |
| Arrhythmia | 0.788 (0.412-1.509) | 0.472 | ||
| Smoking | 1.051 (0.662-1.667) | 0.834 | ||
| Serum glucose levels (per mg/dL increase) | 1.006 (1.002 to 1.011) | 0.009 | 0.999 (0.992-1.005) | 0.736 |
| Serum iron (per mg/dL increase) | 0.978 (0.970-0.986) | < 0.001 | 0.976 (0.968-0.985) | < 0.001 |
| Ferritin (per ng/mL increase) | 0.998 (0.995-1.001) | 0.209 | ||
| 24 h urine protein excretion (per mg increase) | 1.000 (1.000-1.003) | 0.146 | 1.000 (1.000-1.001) | 0.772 |
| ACEIs/ARBs | 0.690 (0.443-1.075) | 0.101 | 0.963 (0.565-1.641) | 0.888 |
| Antiplatelet/anticoagulant drugs | 1.413 (0.927-2.156) | 0.108 | 1.161 (0.669-2.015) | 0.595 |
This study examined in comparison the prevalence of anemia in matched CKD patients with and without DM and further aimed to evaluate the possible association of demographic, clinical and laboratory factors with the development of anemia. The overall prevalence of anemia in the population studied was high (40.5%), while the prevalence in patients with DM was about 15% higher than that in non-diabetic counterparts (47.8% vs 33.2%). With the exception of Stage 2, where the overall prevalence was low (9.5%), anemia was more prevalent in the diabetic patients group in the rest CKD stages, with the difference between groups being particularly large at CKD Stage 3a, where diabetic patients had more than two times higher anemia occurrence (60.4% vs 26.4%). Serum ferritin levels, but not iron, was higher in diabetic than in non-diabetic patients in all stages; as the former also had higher rates of anemia, increased ferritin may mirror its role as an acute phase reactant, signifying higher subclinical inflammation in diabetic patients. In multivariate analyses, among a wide set of demographic, co-morbid, laboratory and medication parameters studied presence of DM, CKD Stages 3a, 3b and 4 and serum iron levels were independently associated with anemia occurrence.
Anemia is an established complication of CKD and is per se associated with the severity of renal insufficiency, mostly due to impaired production of endogenous erythropoietin and true deficiency or decreased availability of serum iron[7,8]. This study further supports this principle, as our results indicated progressing increase in prevalence of anemia with the progression of CKD from Stage 2 (9.6%) to Stage 3 (43.3%) and Stage 4 (54.8%). Moreover, advancing stage of CKD was independently associated with progressively higher OR levels for the development of anemia from CKD Stage 3a (O R= 6.068), CKD Stage 3b (OR = 7.499) and CKD Stage 4 (OR = 12.169). These results are in accordance to the National Health and Nutrition Examination Survey (NHANES) in which prevalence of anemia was 5% in patients with CKD Stage 1 and reached progressively 80% in pre-dialysis Stages 4-5 patients[4]. Similarly, in another cross-sectional study of 5000 individuals with CKD, prevalence of anemia in overall was 48% and was associated with eGFR deterioration as it increased from 27% to 75% with the progression from CKD Stage 2 to CKD Stage 5[5].
Previous indirect data suggested that diabetic patients with CKD may exhibit higher rates of anemia in relation to patients without DM. Patients with type 2 DM may experience anemia even in the absence of nephropathy, as indicated by a previous observational study, in which 16% of the individuals who had type 2 DM but no CKD developed anemia in a 7-year follow up[10]. In a cross-sectional study of > 1 million patients with CKD of Stages 1-5, in which 5% were diabetics, prevalence of anemia was twice as high in diabetics (30% vs 15%) in total, but prevalence in each CKD stage with regards to diabetes presence was not evaluated[14]. In a cohort study of type-2 diabetic CKD patients, prevalence of anemia was 15% in Stage 1, 25% in Stage 2, 50% in Stage 3 and 90% in Stages 4-5[15]. Two other studies have associated DM with increased occurrence of anemia in CKD. The first, including almost 5400 individuals with CKD, of whom 27% had DM, indicated an overall prevalence of anemia 11.6% among diabetics, with its frequency increasing about 45% from CKD Stage 1 to Stage 5[19]. The second studied 468 unmatched CKD patients of whom 44% were type 1 or type 2 diabetics and prevalence of anemia in patients with DM was 17% in CKD Stages 1-2, 51% in CKD Stage 3 and 59% in CKD Stages 4-5, while DM was associated with a significant fourfold increase in risk of anemia in the regression analysis[20]. In contrast, results from the Pre-dialysis Survey of Anemia Management Study indicated no significant differences between patients with and without DM regarding the correlation of serum hemoglobin levels and creatinine clearance rate[21]. Our study further clarifies this issue, showing higher prevalence of anemia in diabetic than carefully matched non-diabetic CKD patients, particularly in Stage 3a, where the majority of individuals with CKD belongs.
As discussed above, several mechanisms promoting anemia in diabetic individuals have been previously described. Erythropoietin deficiency due to efferent sympathetic denervation of the kidney as a result of diabetic neuropathy, subclinical inflammation leading to functional iron deficiency through increased hepcidin levels, increased non-selective proteinuria excretion resulting in transferrin and erythropoietin loss, increased red blood cell destruction because of disorders in the cellular structure caused by DM and advanced glycation end products (AGEs) possibly decreasing erythrocyte lifespan are among them[11-13,22,23]. Further, increased use of RAAS-blockers in diabetic patients, may promote anemia occurrence through inhibition of the physiologic erythropoietic action of angiotensin II[24]. In our study, proteinuria was significantly higher in diabetic patients, but it did not display significant associations with anemia in multivariate analysis. Further, the use of RAAS-blockers was practically equal between the two groups, thus it could not significantly affect the results; use of ACEIs or ARBs was also not associated with anemia in multivariate analysis.
A role of chronic inflammation affecting anemia in DM is also proposed. Recent findings suggest that diabetic patients have higher ferritin and hepcidin levels than matched non-diabetic individuals[25]. Levels of ferritin as a marker of inflammation and hepcidin were shown to correlate strongly in various populations including patients with DM[26] and CKD of various types[27]. Increased hepcidin following subclinical inflammation has also been observed in obese individuals[28]. Hepcidin is the key factor causing functional iron deficiency reducing the efflux of recycled iron from both splenic and hepatic macrophages and also inhibits iron absorption from the gut; the overall reduction of iron available for erythropoiesis leads to anemia[28]. Our findings support this mechanism of chronic inflammation as ferritin levels were significantly higher in diabetics in overall (200.0 pmol/L vs 148.3 pmol/L; P < 0.001) and in almost every CKD stage. In addition, although an increase in serum iron was associated with less anemia occurrence in multivariate analysis, ferritin levels displayed no significant associations, a finding suggesting that ferritin could not be considered as a marker of iron stores. Further examination of this pathway including measurement of hepcidin levels could be useful.
This study has methodologic strengths. Although prevalence of anemia in CKD and DM has been examined previously, a direct comparison in patients with and without DM in CKD, to the best of our knowledge, was absent. Apart from the careful matching of individuals to form the two study groups, the capture of several factors that may theoretically affect the development of anemia in DM and a careful multiple logistic regression analysis further strengthen our results. However, there are also some limitations. This is an observational study, thus definite cause and effect associations cannot be established. The use of a unique hemoglobin measurement to determine the diagnosis of anemia may have misclassified some individuals. Finally, observed frequencies and significance levels in some comparisons may have been affected to an extent by the relatively small sample sizes in some of the subgroup analyses.
In conclusion, this study has confirmed that anemia is common in CKD outpatients and increases steadily with advancing Stages of CKD. Furthermore, the prevalence of anemia is higher in diabetic patients with CKD compared to matched non-diabetic counterparts. The difference between diabetic and non-diabetic patients with CKD was more prominent in CKD Stage 3a, where the majority of individuals with CKD belongs. Subclinical inflammation in diabetic patients with moderate CKD may be the most important underlying factor for this association, as indicated by increased ferritin levels in diabetics in our study. As anemia is associated with significant morbidity and mortality, both detection and treatment of anemia in diabetic CKD patients should be performed earlier than in non-diabetic counterparts.
Anemia is a major complication of chronic kidney disease (CKD) and diabetes mellitus (DM) is proposed to elevate the risk of anemia development. However, epidemiologic data from a direct comparison between diabetic and non-diabetic CKD patients with regards to anemia are currently missing.
Prevalence of anemia has been extensively studied in patients with CKD. However, current evidence about the role of DM in anemia development in CKD derive only from observational studies in CKD population in which diabetics constitute only a small proportion. DM has been found to further elevate the prevalence of anemia in CKD, but not in all studies. On this context, a study examining in comparison the prevalence of anemia in matched CKD diabetic and non-diabetic patients would further clarify the role of DM in anemia development.
This study is the first to evaluate prevalence of anemia with a case control design in carefully matched diabetic and non-diabetic CKD patients.
Both detection and treatment of anemia in diabetic CKD patients should be performed earlier than in non-diabetics, in order to prevent anemia-associated complications.
The study deals with a common issue in clinical practice; (i.e., diabetic patients with moderate CKD often appear with low Hb levels for their eGFR levels and have already been investigated for anemia from internists or hematologists for years with no results). Although there are some previous data pointing to the fact that anemia (among many factors studied) is more common in diabetics with CKD, this study adds to current knowledge.
P- Reviewer: Elisaf MS, Friedman EA, Olowu WA, Papagianni A, Trimarchi H S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | 1 Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. Guideline for Anemia in Chronic Kidney Disease. Kidney Int. 2012;2:279-335. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9:e84943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 401] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 3. | Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2002;13:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Centers for Disease Control and Prevention. Prevalence of chronic kidney disease and associated risk factors--United States, 1999-2004. MMWR Morb Mortal Wkly Rep. 2007;56:161-165. [PubMed] |
| 5. | McClellan W, Aronoff SL, Bolton WK, Hood S, Lorber DL, Tang KL, Tse TF, Wasserman B, Leiserowitz M. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin. 2004;20:1501-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 284] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 6. | National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am J Kidney Dis. 2006;47:S11-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 370] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 7. | Zaritsky J, Young B, Wang HJ, Westerman M, Olbina G, Nemeth E, Ganz T, Rivera S, Nissenson AR, Salusky IB. Hepcidin--a potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1051-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 229] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 8. | McClellan WM, Jurkovitz C, Abramson J. The epidemiology and control of anaemia among pre-ESRD patients with chronic kidney disease. Eur J Clin Invest. 2005;35 Suppl 3:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, Bragg-Gresham J, Balkrishnan R, Chen JL, Cope E. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2016;67:A7-A8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 386] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 10. | Craig KJ, Williams JD, Riley SG, Smith H, Owens DR, Worthing D, Cavill I, Phillips AO. Anemia and diabetes in the absence of nephropathy. Diabetes Care. 2005;28:1118-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Grossman C, Dovrish Z, Koren-Morag N, Bornstein G, Leibowitz A. Diabetes mellitus with normal renal function is associated with anaemia. Diabetes Metab Res Rev. 2014;30:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Deray G, Heurtier A, Grimaldi A, Launay Vacher V, Isnard Bagnis C. Anemia and diabetes. Am J Nephrol. 2004;24:522-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Mehdi U, Toto RD. Anemia, diabetes, and chronic kidney disease. Diabetes Care. 2009;32:1320-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 14. | Dmitrieva O, de Lusignan S, Macdougall IC, Gallagher H, Tomson C, Harris K, Desombre T, Goldsmith D. Association of anaemia in primary care patients with chronic kidney disease: cross sectional study of quality improvement in chronic kidney disease (QICKD) trial data. BMC Nephrol. 2013;14:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Chen CX, Li YC, Chan SL, Chan KH. Anaemia and type 2 diabetes: implications from a retrospectively studied primary care case series. Hong Kong Med J. 2013;19:214-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33 Suppl 1:S62-S69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3654] [Cited by in RCA: 4280] [Article Influence: 285.3] [Reference Citation Analysis (0)] |
| 17. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11183] [Cited by in RCA: 11814] [Article Influence: 454.4] [Reference Citation Analysis (0)] |
| 18. | Summary of Recommendation Statements. Kidney Int Suppl (2011). 2013;3:5-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 308] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 19. | El-Achkar TM, Ohmit SE, McCullough PA, Crook ED, Brown WW, Grimm R, Bakris GL, Keane WF, Flack JM. Higher prevalence of anemia with diabetes mellitus in moderate kidney insufficiency: The Kidney Early Evaluation Program. Kidney Int. 2005;67:1483-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 20. | Al-Khoury S, Afzali B, Shah N, Covic A, Thomas S, Goldsmith DJ. Anaemia in diabetic patients with chronic kidney disease--prevalence and predictors. Diabetologia. 2006;49:1183-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Valderrábano F, Hörl WH, Macdougall IC, Rossert J, Rutkowski B, Wauters JP. PRE-dialysis survey on anaemia management. Nephrol Dial Transplant. 2003;18:89-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Thomas MC, Tsalamandris C, MacIsaac R, Medley T, Kingwell B, Cooper ME, Jerums G. Low-molecular-weight AGEs are associated with GFR and anemia in patients with type 2 diabetes. Kidney Int. 2004;66:1167-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Iwata H, Ukeda H, Maruyama T, Fujino T, Sawamura M. Effect of carbonyl compounds on red blood cells deformability. Biochem Biophys Res Commun. 2004;321:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Vlahakos DV, Marathias KP, Madias NE. The role of the renin-angiotensin system in the regulation of erythropoiesis. Am J Kidney Dis. 2010;56:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Jiang F, Sun ZZ, Tang YT, Xu C, Jiao XY. Hepcidin expression and iron parameters change in Type 2 diabetic patients. Diabetes Res Clin Pract. 2011;93:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Andrews M, Soto N, Arredondo-Olguín M. Association between ferritin and hepcidin levels and inflammatory status in patients with type 2 diabetes mellitus and obesity. Nutrition. 2015;31:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Rumjon A, Sarafidis P, Brincat S, Musto R, Malyszko J, Bansal SS, Macdougall IC. Serum hemojuvelin and hepcidin levels in chronic kidney disease. Am J Nephrol. 2012;35:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Sarafidis PA, Rumjon A, MacLaughlin HL, Macdougall IC. Obesity and iron deficiency in chronic kidney disease: the putative role of hepcidin. Nephrol Dial Transplant. 2012;27:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |