Published online Jul 6, 2016. doi: 10.5527/wjn.v5.i4.339
Peer-review started: February 14, 2016
First decision: March 21, 2016
Revised: March 31, 2016
Accepted: April 14, 2016
Article in press: April 18, 2016
Published online: July 6, 2016
Processing time: 139 Days and 21.7 Hours
AIM: To determine the temporal expression and pattern of Rel/nuclear factor (NF)-κB proteins in renal tissue in polycystic kidney disease (PKD).
METHODS: The renal expression of Rel/NF-κB proteins was determined by immunohistochemistry, immunofluorescence and immunoblot analysis in Lewis polycystic kidney rats (LPK, a genetic ortholog of human nephronopthsis-9) from postnatal weeks 3 to 20. At each timepoint, renal disease progression and the mRNA expression of NF-κB-dependent genes (TNFα and CCL2) were determined. NF-κB was also histologically assessed in human PKD tissue.
RESULTS: Progressive kidney enlargement in LPK rats was accompanied by increased renal cell proliferation and interstitial monocyte accumulation (peaking at weeks 3 and 10 respectively), and progressive interstitial fibrosis (with α smooth muscle actin and Sirius Red deposition significantly increased compared to Lewis kidneys from weeks 3 to 6 onwards). Rel/NF-κB proteins (phosphorylated-p105, p65, p50, c-Rel and RelB) were expressed in cystic epithelial cells (CECs) of LPK kidneys as early as postnatal week 3 and sustained until late-stage disease at week 20. From weeks 10 to 20, nuclear p65, p50, RelB and cytoplasmic IκBα protein levels, and TNFα and CCL2 expression, were upregulated in LPK compared to Lewis kidneys. NF-κB proteins were consistently expressed in CECs of human PKD. The DNA damage marker γ-H2AX was also identified in the CECs of LPK and human polycystic kidneys.
CONCLUSION: Several NF-κB proteins are consistently expressed in CECs in human and experimental PKD. These data suggest that the upregulation of both the canonical and non-canonical pathways of NF-κB signaling may be a constitutive and early pathological feature of cystic renal diseases.
Core tip: Until now, there has been limited information regarding the specific nuclear factor (NF)-κB proteins involved in polycystic kidney disease (PKD) and their expression throughout disease progression. Our study demonstrated that a diverse array of NF-κB proteins is expressed in the renal cyst-lining cells of a chronic rodent model of PKD, and that NF-κB expression is constitutive over time. NF-κB was also identified in human PKD, suggesting that NF-κB upregulation is common to renal cystic disease models. Our data suggest that components of both the canonical and non-canonical NF-κB pathway are upregulated in PKD. Future studies should be directed at verifying whether specific NF-κB inhibition can attenuate interstitial inflammation and cyst growth, and slow the decline in renal function in in vivo models of PKD.
- Citation: Ta MHT, Schwensen KG, Liuwantara D, Huso DL, Watnick T, Rangan GK. Constitutive renal Rel/nuclear factor-κB expression in Lewis polycystic kidney disease rats. World J Nephrol 2016; 5(4): 339-357
- URL: https://www.wjgnet.com/2220-6124/full/v5/i4/339.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i4.339
Polycystic kidney diseases (PKD) are a group of genetic disorders characterized by the formation of multiple renal cysts and an increased life-time risk for kidney failure[1]. The two most common forms, autosomal dominant and recessive PKD (ADPKD and ARPKD), are due to mutations in PKD1/2 and PKHD1 respectively[2-4]. These genes encode proteins that are localized to the cilia of most cells within the body, including renal tubule epithelia[5,6]. Interstitial inflammation is a universal histological feature associated with renal cyst growth and formation[7-9], and is possibly mediated by the release of pro-inflammatory cytokines from cyst-lining epithelial cells (CECs)[8]. Abnormalities in apoptosis and increased proliferation of CECs[7,10], interstitial fibrosis[11] and hypertension[12] are also typical features of PKD.
The nuclear factor (NF)-κB system is a key regulator of pro-inflammatory and pro-apoptotic gene transcription[13,14]. The Rel proteins, namely p65 (RelA), RelB and c-rel, contain transcription-binding domains (TADs) that allow them to bind DNA[13,14]. In contrast, p50 (NF-κB1) and p52 (NF-κB2), which are derived from the breakdown of p105 and p100 respectively, do not possess TADs and therefore must bind to Rel proteins in order to modulate transcription[13,15]. In the inactive state, NF-κB proteins normally exist as dimers, and are bound to inhibitor of κB (IκB) proteins in the cytoplasm[13]. Upon activation by certain stimuli, IκB kinase (IKK) proteins are activated, phosphorylating the IκB proteins and leading to their degradation, thus freeing the NF-κB proteins to translocate to the nucleus and regulate transcription[13,14]. NF-κB signaling can be broadly classified as canonical or non-canonical[13,16]. The canonical pathway is activated by a wide range of stimuli including tumor necrosis factor (TNF)α and lipopolysaccharide[13,17], and typically involves the p65:p50 dimer and IκBα[13,16,18]. The non-canonical pathway is known to be stimulated by fewer stimuli (e.g., CD40 ligand and lymphotoxin-β2[16]) and usually implicates the RelB:p52 dimer in mediating lymphoid organ development[13,16].
There is an overlap between NF-κB-regulated genes and the pathophysiological features of PKD, such as inflammation (e.g., TNFα, CCL2), cell growth (e.g., cyclin D1), apoptosis (e.g., Bcl-xL) and hypertension (e.g., ANGII)[8,9,12,17,19]. These commonalities provide a theoretical basis for the involvement of NF-κB in PKD. Recent studies have provided preliminary evidence for NF-κB involvement in the pathogenesis of cystic renal disease. For example, the use of small interfering RNA (siRNA) to over-express or delete ciliary proteins in vitro, leads to upregulation of NF-κB signaling[20,21]. Park et al[22] demonstrated that PKD2 transgenic mice have higher levels of renal NF-κB proteins and phosphorylated-IKKα/β compared to wild type controls. Moreover, upregulated NF-κB expression has been identified in the CEC nuclei in Pkd1-/- and PKD2 transgenic mice[22], and in human ADPKD[22].
Despite the complexity of the NF-κB system, the previous studies of NF-κB in PKD mainly or solely focused on p65, and in some cases did not specify the particular NF-κB subunits that were investigated[22,23]. Therefore, the first aim of the current study was to examine the expression and localization of a spectrum of NF-κB proteins, in a chronic disease model of PKD and in human cystic renal disease. We investigated the expression of p65, p50, RelB and c-rel, as well as IκBα and phosphorylated p105 (P-p105, which is synthesized prior to p50 production and accordingly is a marker of NF-κB upregulation[24]). In addition, cyst development in ADPKD is progressive, beginning in utero and continuing through to the later decades of life[9], but no studies have examined whether the NF-κB system changes throughout disease progression in PKD. Thus, the second aim of this study was to characterize NF-κB expression over the time-course of disease in the Lewis polycystic kidney (LPK) rat (a chronic model of ARPKD[25]). Based on data showing that the expression of a NF-κB-dependent gene, TNFα, is upregulated in cpk mice and increases further over time[26], and previous correlations between TNFα and NF-κB signaling in in vitro models of PKD[27], we hypothesized that NF-κB protein expression is elevated in LPK kidneys compared to control kidneys in the early stages of PKD, and increases incrementally throughout the course of the disease.
The LPK rat is a genetic ortholog of NPHP9 (human nephronophthisis) due to a point mutation in Nek8[28]. It is characterized by post-natal distal nephron and collecting duct ectasia which progress over approximately 20 wk, and is associated with hypertension and renal failure[25]. In this study, LPK rats and Lewis/SSN rats were obtained from the breeding colony at Westmead Hospital. This colony was established in 2008 from a single founder homozygous breeding pair from the Animal Resources Centre (Perth, Western Australia). The colony was maintained by mating homozygous male and female breeder pairs. Animals were housed under standard conditions and allowed food and water ad libitum at the Animal Care Department at Westmead Hospital. All protocols and procedures were approved by the Western Sydney Local Health District Animal Ethics Committee, (Protocol number 4100). Since disease is more severe in male than in female LPK rats[25], for this study, male LPK rats were sacrificed at postnatal weeks 1, 2, 3, 4, 6, 10, 16 and 20 (n = 6-9 per timepoint) and were compared to male Lewis animals at the same timepoints (n = 3-6 per timepoint).
Kidney enlargement was determined using the kidney to body weight ratio (KW:BW) at the time of tissue collection. Renal function was assessed in blood at the time of sacrifice, and was analyzed by the Institute of Clinical Pathology and Medical Research. To assess proteinuria and creatinine clearance (CrCl), rats were placed in metabolic cages for 16 h. Creatinine clearance was calculated using CrCl = [Urine creatinine (μmol/L) × Urine volume (mL/min)]/Serum creatinine (μmol/L), and was corrected for body weight. Rats were euthanized as previously described[29].
Coronal kidney slices were fixed in either 37 g/L formaldehyde or methyl Carnoy’s solution for 24 h, then paraffin embedded. For immunohistochemistry, 4 μm sections were deparaffinized, blocked with 0.03 g/mL hydrogen peroxide, and antigen retrieval was performed by microwave oven heating (for formalin slides only, 100% power, 10 min in 1 × Antigen Decloaker, Biocare Medical, CA). Sections were blocked with 100 mL/L goat serum, and incubated with primary antibodies for 1 h overnight at 4 °C. The primary antibodies used were: (1) anti-Ki67 to assess proliferation (1:100, ab16667, Abcam, Cambridge, United Kingdom); (2) anti-ED-1 for CD68+ monocytes to assess inflammation (1:400, MCA341R, Serotec, United Kingdom); (3) anti-α smooth muscle actin (α-SMA) to assess myofibroblast accumulation (and also a marker of vascular smooth muscle cells, 1:4000, A2547, Sigma-Aldrich, St. Louis, MO); and (4) anti-phosphorylated-p105 (P-p105) to assess NF-κB expression (1:50, #4808 Cell Signaling Technology, Danvers, MA). Secondary biotinylated antibodies were applied for 30 min at room temperature (anti-mouse, 1:200, 65-6440; anti-rabbit, 1:200, 65-6140, Life Technologies, Carlsbad, CA). Vectastain ABC reagent (Vector Laboratories, Burlingame, CA) was applied for 20 min, followed by diaminobenzidine. Sections were counterstained with methyl green (Sigma-Aldrich) then dehydrated. To assess interstitial fibrosis, Sirius Red staining was performed on methyl Carnoy’s fixed sections with 1 g/L Direct Red 80 and 1 g/L Fast Green FCF (Sigma-Aldrich) for 24 h. To quantify immunohistology, whole slide digital images (20 × magnification) were acquired using a scanner (ScanScope CS2, Aperio, CA). Percentage cyst volume was assessed in Periodic Acid Schiff (PAS) stained sections by whole-slide digital analysis in Aperio ImageScope (v11.2.0.780).
For immunofluorescence, formalin-fixed slides were deparaffinized and antigen retrieval was performed by microwave oven heating as described above. Sections were soaked in Tris Buffered Saline (TBS), 4 mL/L TritonX, for 30 min then blocked with 30 g/L BSA in TBS, 2 g/L Tween20 for 1 h. Slides were incubated with primary antibodies for 1h overnight at 4 °C: (1) p65 (1:100, #8242, Cell Signaling); (2) p50/p105 (1:100, ab7971, Abcam); (3) RelB (1:100, bs-3562R, Bioss Antibodies, Woburn, MA); (4) c-rel (1:100, orb5913, Biorbyt, Cambridge, United Kingdom); and (5) γ-H2AX (1:100, ab2893, Abcam). Secondary antibody (1:200, Alexa Fluor 546 goat anti-rabbit IgG, A-11010, Life Technologies) was applied for 30 min at room temperature, following by DAPI for 5 min. Slides were mounted using Fluorescence Mounting Medium (Dako, Glostrup, Denmark). Immunofluorescence was assessed using an Olympus BX53/DP80 microscope (Olympus Corporation, Shinjuku, Japan) and images were taken at 20 × and 40 × magnification using the software (cellSens, v1.6, Olympus).
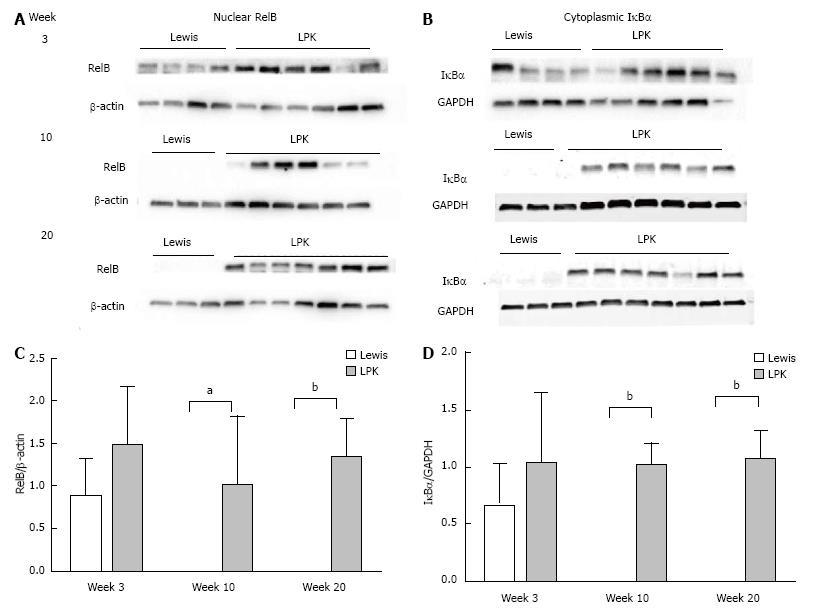
Nuclear and cytosolic extracts were obtained from 100 mg of kidney cortex from Lewis and LPK animals at weeks 3, 10 and 20, using a previously described method[30] and stored at -80 °C. Protein concentration of the extracts was assessed using the DC Protein Assay (Bio-Rad). Western blot was performed as previously described[31]. Primary antibodies used were: p50/105 (1:1000, ab7971, Abcam), p65 (1:1000, #8242, Cell Signaling Technology, Danvers, MA), RelB (1:1000, #4922, Cell Signaling), IκBα (1:1000, #4814, Cell Signaling), β-actin (1:1000, #4970, Cell Signaling), and GAPDH (1:1000, #5174, Cell Signaling). Densitometry was quantified using ImageJ (v1.47, National Institutes of Health, United States) and normalized using β-actin (for nuclear extracts) or GAPDH (for cytoplasmic extracts).
Quantitative real-time polymerase chain reaction (qPCR) was performed to assess the mRNA expression of two pro-inflammatory genes, TNFα and chemokine (C-C motif) ligand 2 (CCL2). Renal tissue was snap-frozen in liquid nitrogen and stored at -80 °C. RNA was extracted using the RNeasy Mini Kit (Qiagen, Venlo, Limburg, Netherlands). RNA was reverse-transcribed into cDNA (SuperScript III First-Strand Synthesis System, Thermo Fisher Scientific, Waltham, MA) using oligo(dT) and dNTP, and using the cDNA Synthesis Mastermix according to manufacturer’s instructions. Real-time quantitative PCR was performed using Platinum SYBR Green qPCR SuperMix-UDG (Thermo Fisher) on a Bio-Rad CFX96 machine (Bio-Rad Laboratories, Hercules, CA). The PCR primers were: TNFα (forward: 5’ GTC GTA GCA AAC CAC CAA GC 3’, reverse: 5’ TGT GGG TGA GGA GCA CAT AG 3’)[32], CCL2 (forward: 5’ AGC CCA GAA ACC AGC CAA CTC 3’, reverse: 5’ GCC GAC TCA TTG GGA TCA TCT T 3’)[33], and GAPDH (forward: 5’ GAA CAT CAT CCC TGC ATC CA 3’, reverse: 5’ CCA GTG AGC TTC CCG TTC A 3’)[34]. PCR parameters were 95 °C for 2 min and 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s for 40 cycles; melting temperature was measured between 65 °C and 95 °C. Data were analyzed using CFX Manager software (v3.1.1517.0823, 2012 release, Bio-Rad). Gene expression was quantified using the ΔΔCT method. The mRNA quantities of TNFα and CCL2 in each sample were normalized using GAPDH mRNA quantity.
Assessment of NF-κB expression in human PKD
Renal tissue was obtained from two ADPKD patients (ID no. P17 and P18) and one ARPKD patient (ID no. ARPKD1). Kidney tissue was also obtained from two non-PKD patients (ID no. C1 and C2; both kidneys were removed due to renal cancer, and the non-cancerous portions used as controls). All patients provided written informed consent, and the study was approved by the Human Research Ethics Committee at Westmead Hospital (HREC/09/WMEAD/305; SSA/12/WMEAD/327). Samples were paraffin-embedded, and PAS staining, immunohistochemistry and immunofluorescence were performed as described for LPK kidneys.
Results were presented as mean ± SD. The data were analyzed with the JMP statistical software package (v4.04, SAS institute, Carey, NC, United States) and graphed in GraphPad Prism (v6.04 for Windows, GraphPad Software, San Diego, CA). Comparisons between the experimental groups were performed by ANOVA, followed by a post-hoc analysis with the Tukey-Kramer HSD test. A P-value of < 0.05 was interpreted as statistically significant.
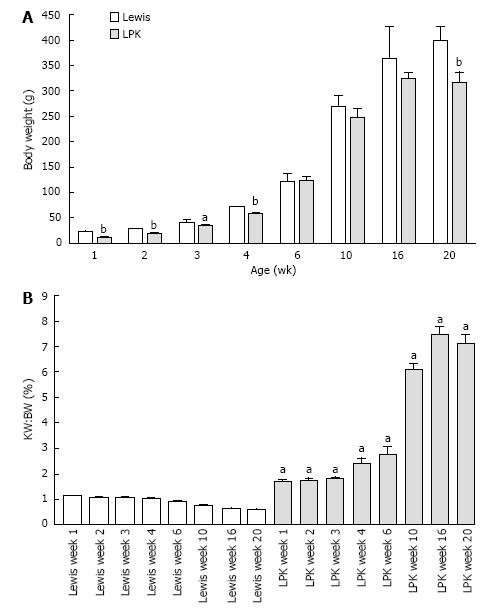
Body weight and kidney enlargement: Body weight increased steadily in both experimental groups from weeks 1 to 20, but was lower in LPK animals (Figure 1A). In LPK rats, the KW:BW was increased compared to Lewis rats as early as postnatal week 1 and continued to rise until week 16 (Figure 1B). The rate of increase in KW:BW was greatest between weeks 6 and 10 (239% increase) whereas between weeks 10 and 20 only a further 16% increase was observed (Figure 1B).
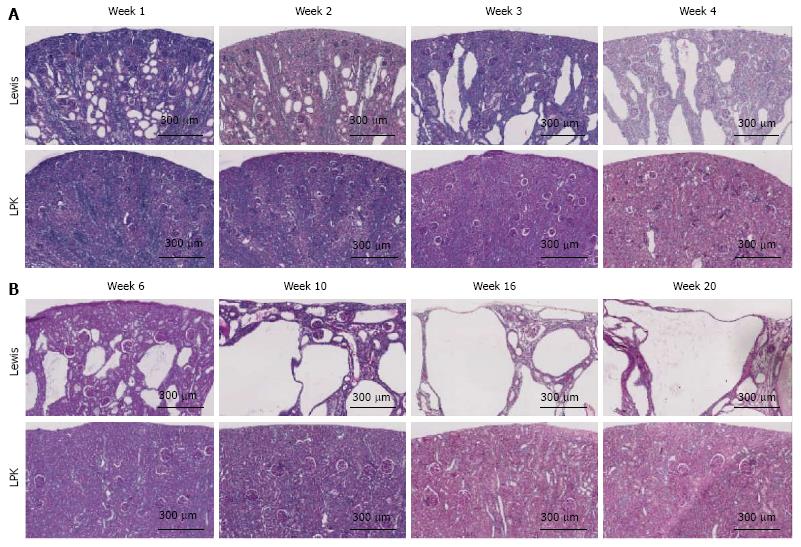
Histological pattern of renal disease: Qualitative histological analysis showed that there was progressive dilatation of collecting ducts and distal tubules over time in LPK kidneys (Figure 2). At week 1, cystic renal disease was mild, characterized by focal areas of rounded collecting duct dilatation in the corticomedullary region. From week 2 onwards, the rounded dilatation developed into rectangular elongation. As shown in Table 1, the mean dimensions (width and length) of the cyst tubular dilatation increased progressively from week 1 to 20. These histological changes were accompanied by time-dependent increases in renal interstitial inflammation (ED-1 immunohistochemistry) and fibrosis (α-SMA immunohistochemistry and Sirius Red staining) in LPK rats (Table 1). As previously shown[35], cell proliferation (Ki-67 positive cells) peaked at week 3 in LPK rats. Interstitial ED-1 and α-SMA were highest at week 10 (Table 1). Collagen deposition (as measured by Sirius Red) was elevated in LPK kidneys from week 6 onwards (Table 1).
| Parameter | Week 1 | Week 3 | Week 6 | Week 10 | Week 16 | Week 20 | |
| Cyst length (μm) | Lewis | ND | ND | ND | ND | ND | ND |
| LPK | 106 ± 4 | 347 ± 88 | 938 ± 280 | 1548 ± 237 | 2252 ± 436 | 3718 ± 922 | |
| Cyst diameter (μm) | Lewis | ND | ND | ND | ND | ND | ND |
| LPK | 69 ± 18 | 101 ± 19 | 279 ± 71 | 442 ± 81 | 681 ± 121 | 1119 ± 256 | |
| Ki-67 (%) | Lewis | 24.3 + 8.3 | 1.9 + 0.6 | 2.4 + 1.5 | 0.2 + 0.1 | 0.3 + 0.1 | 0.2 ± 0.02 |
| LPK | 7.0 ± 3.8a | 9.9 + 2.6a | 2.2 ± 1.5 | 1.1 ± 0.4a | 1.9 ± 0.8a | 0.8 ± 0.3a | |
| ED-1 (%) | Lewis | 1.87 ± 0.61 | 1.26 ± 0.20 | 1.32 ± 0.95 | 0.56 ± 0.08 | 0.22 ± 0.11 | 0.16 ± 0.02 |
| LPK | 1.58 ± 0.15 | 3.81 ± 1.02a | 4.87 ± 1.21a | 12.37 ± 4.10a | 0.81 ± 0.38a | 0.84 ± 0.24a | |
| Sirius Red (%) | Lewis | 0.86 ± 0.38 | 4.32 ± 1.58 | 1.67 ± 0.52 | 2.91 ± 0.77 | 0.51 ± 0.43 | 0.64 ± 0.40 |
| LPK | 1.96 ± 1.15 | 2.88 ± 1.51a | 5.92 ± 4.20b | 16.63 ± 8.12b | 10.34 ± 5.82b | 18.24 ± 6.00b | |
| α-SMA (%) | Lewis | 17.2 ± 2.12 | 1.68 ± 0.42 | 1.87 ± 0.32 | 0.98 ± 0.86 | 1.04 ± 0.06 | 1.09 ± 0.13 |
| LPK | 22.4 ± 2.80 | 8.70 ± 3.70a | 8.98 ± 3.41a | 20.18 ± 3.54a | 13.75 ± 0.99a | 13.28 ± 1.68a |
Renal function: LPK rats developed an increase in 24 h urine volume compared to Lewis rats from week 10 (Table 2). A decline in renal function was indicated by the elevated levels of serum creatinine in LPK compared to Lewis rats from week 10 onwards (Table 2). Creatinine clearance was decreased in LPK rats compared to Lewis at weeks 10 and 16. Serum urea was elevated in LPK rats at all timepoints (Table 2).
| Parameter | Week 3 | Week 6 | Week 10 | Week 16 | Week 20 | |
| 24 h urine volume (mL) | Lewis | ND | 6 ± 1 | 9 ± 2 | 9 ± 2 | ND |
| LPK | ND | 7 ± 1 | 25 ± 2b | 23 ± 9b | ND | |
| Serum creatinine (μmol/L) | Lewis | 24 ± 3 | 22 ± 5 | 29 ± 10 | 24 ± 1 | 27 ± 5 |
| LPK | 20 ± 2a | 28 ± 14 | 33 ± 3b | 63 ± 9 | 191 ± 38b | |
| Endogenous CrCl (mL/min) | Lewis | ND | 0.5 ± 0.2 | 1.8 ± 0.3 | 2.5 ± 0.6 | ND |
| LPK | ND | 0.5 ± 0.2 | 0.8 ± 0.1b | 0.6 ± 0.2b | ND | |
| CrCl/BW (fold-change over Lewis) | Lewis | ND | 1 ± 0.4 | 1.6 ± 0.1 | 1.7 ± 0.3 | ND |
| LPK | ND | 0.9 ± 0.3 | 0.8 ± 0.1b | 0.4 ± 0.1b | ND | |
| Serum urea (mmol/L) | Lewis | 5 ± 1 | 6 ± 1 | 6 ± 1 | 6 ± 1 | 8 ± 1 |
| LPK | 7 ± 0b | 8 ± 1b | 14 ± 1b | 22 ± 3b | 44 ± 4b |
Time-course of renal Rel/NF-κB protein localization in LPK rats
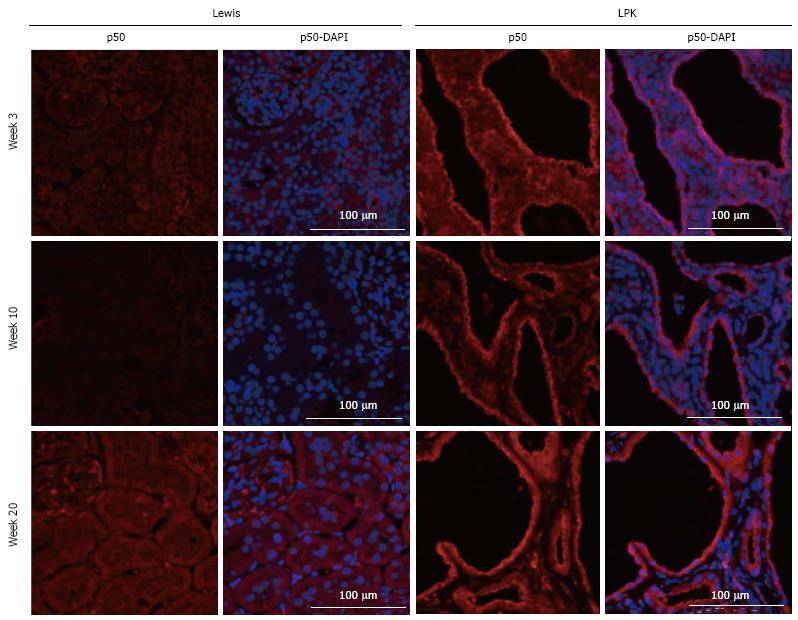
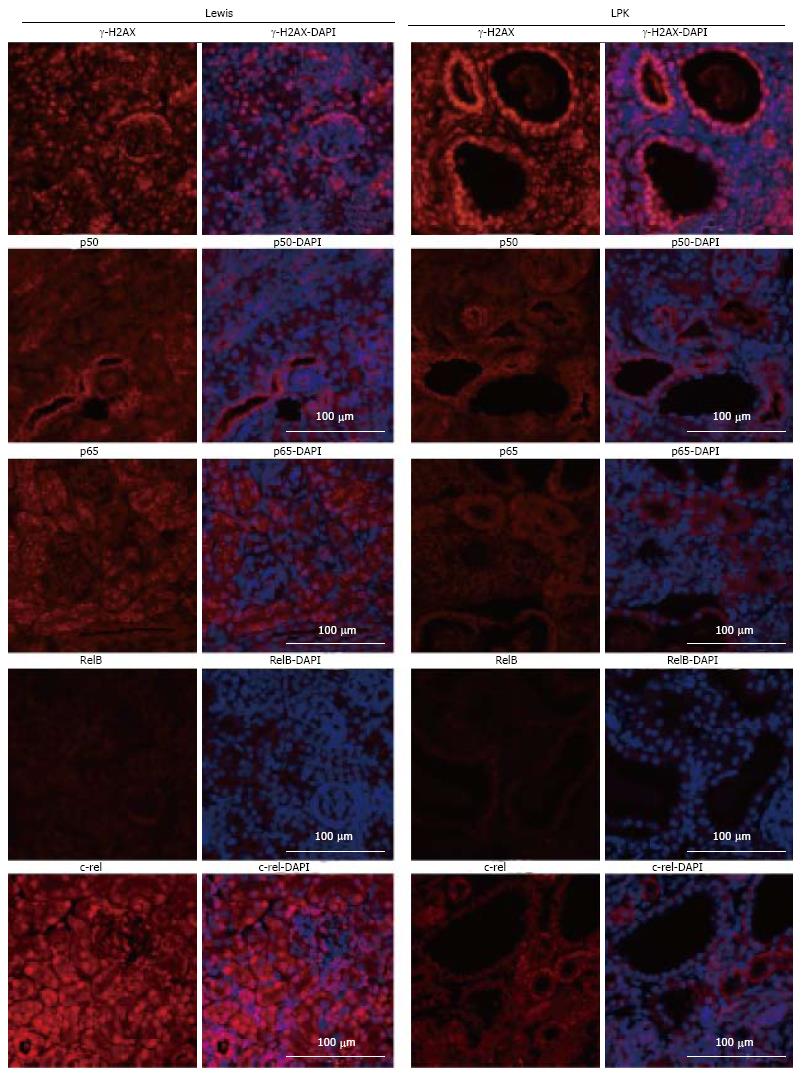
p50: In kidneys from Lewis rats, p50 expression was weak and diffuse and localized predominantly to the cytoplasm of tubular cells (Figure 3). In LPK kidneys, this pattern of expression was maintained, but in addition there was strong staining of epithelial cells lining the dilated tubular cystic segments (Figure 3). The staining, which occurred in the majority of cysts, was detected as early as week 3 and remained persistent at weeks 10 and 20. Populations of intensely staining interstitial cells were also noted in LPK kidneys in the outer medulla at weeks 6 and 20 (data not shown).
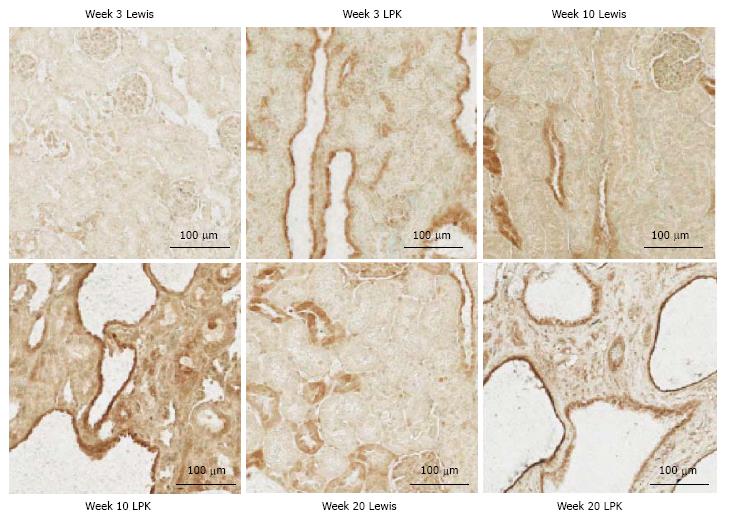
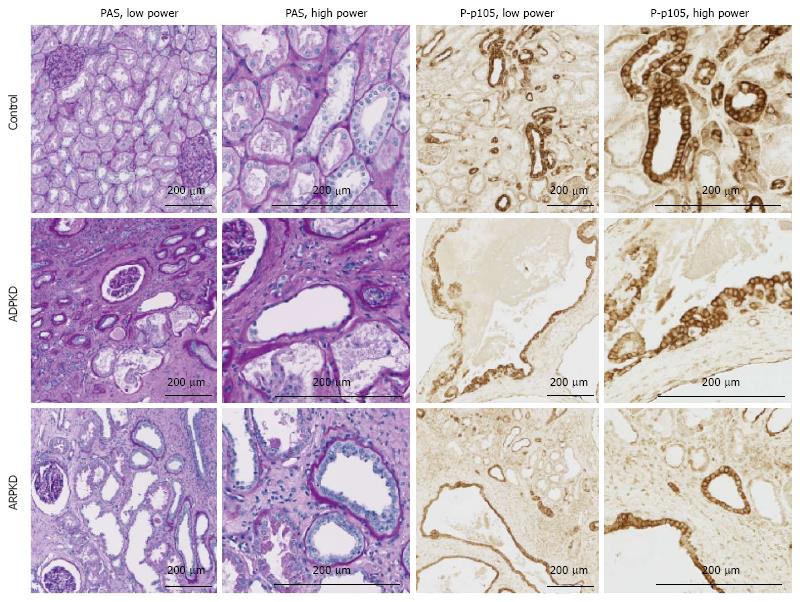
P-p105: In Lewis rats, at week 3, P-p105 staining was observed in focal areas of tubules and in selected cortical glomerular cells (Figure 4). This pattern of staining was stronger at weeks 10 and 20. In LPK rats, P-p105 was strongly expressed in cystic epithelial cells at week 3, 10 and 20 in the majority of cysts (Figure 4).
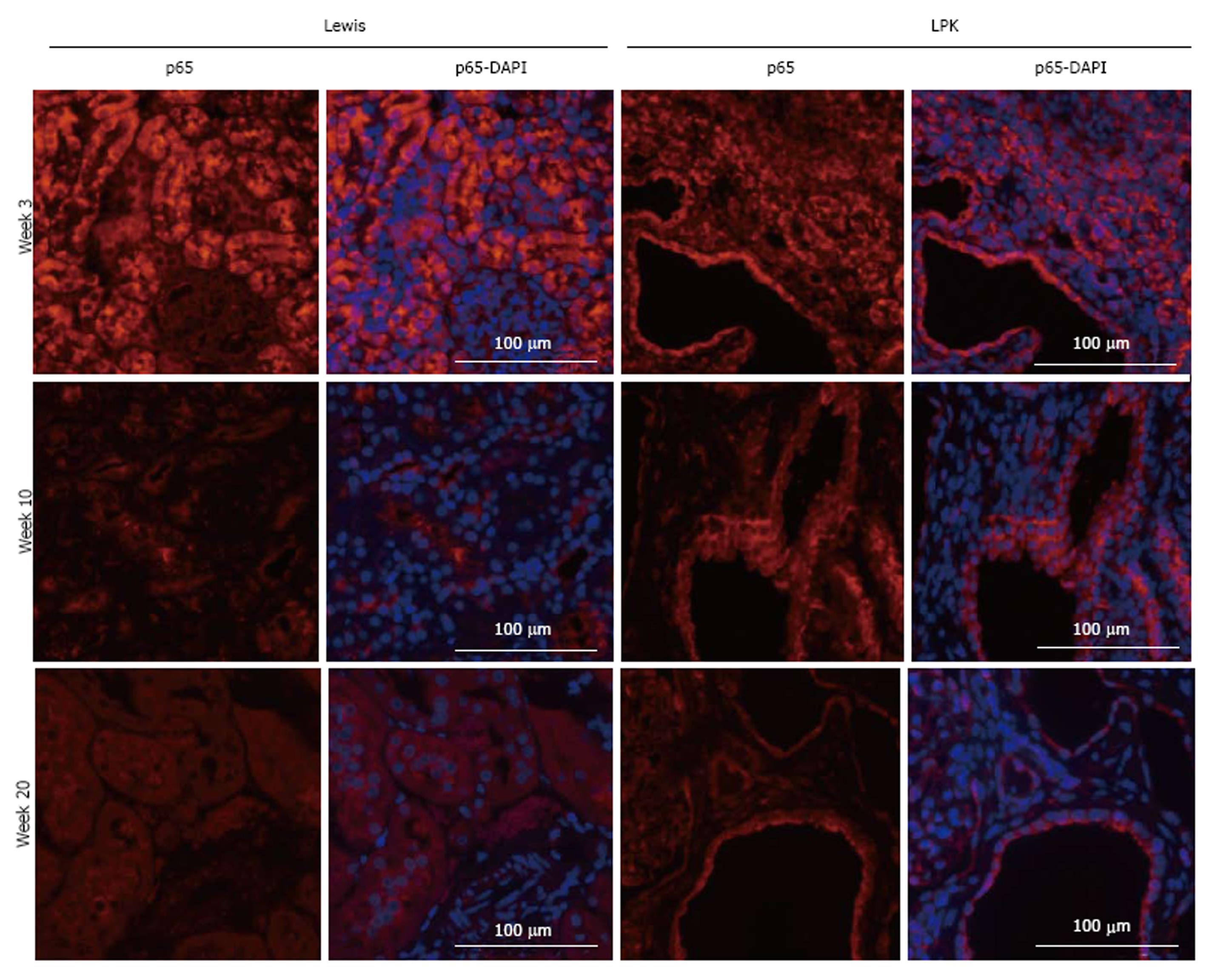
p65: In Lewis rats, p65 was localised to the cytoplasm of tubules. Expression was strongly upregulated at week 3, but declined at weeks 10 and 20 (Figure 5). In LPK kidneys, p65 was strongly expressed in the cytoplasm of cortical CECs in the majority of cysts at all timepoints, starting from week 3 (Figure 5).
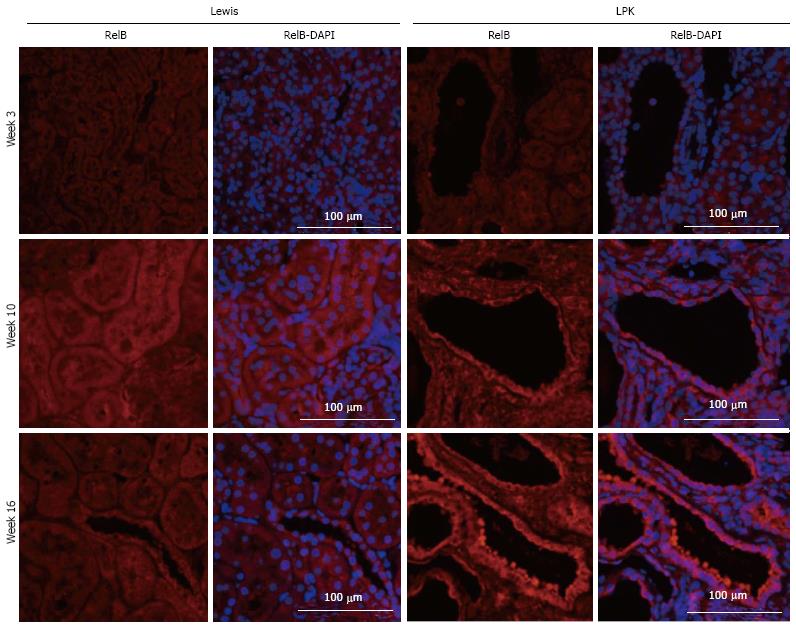
RelB: In Lewis rats, RelB expression in the kidney was weak at all timepoints (Figure 6). In contrast, in LPK there was strong staining of cysts at mid to late disease, particularly at weeks 10 and 16 (Figure 6).
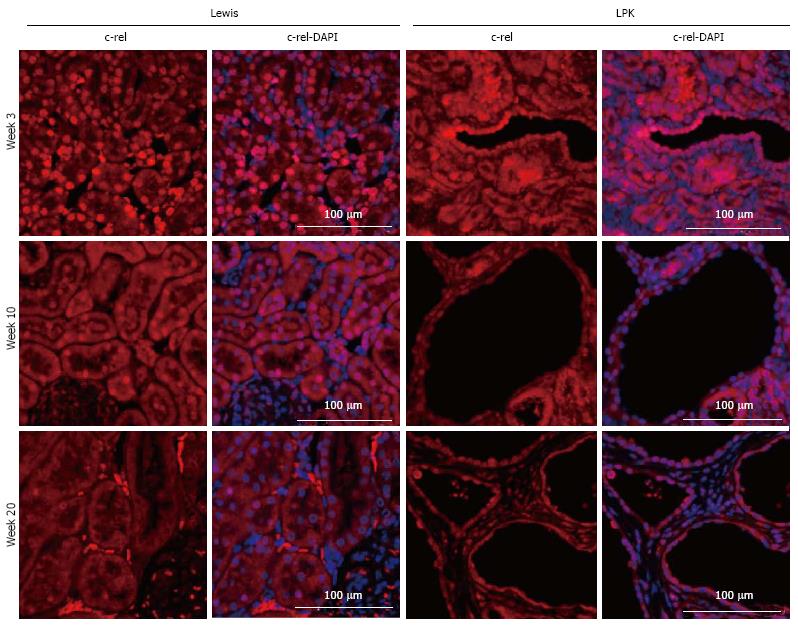
c-rel: In Lewis rats, the renal expression of c-rel was localized to the nuclei and cytoplasm of tubules and interstitial cells (Figure 7). In control animals, nuclear c-rel expression was higher at week 3, declining at weeks 10 and 20, but cytoplasmic c-rel expression remained consistent over time. In LPK rats, cystic epithelial cell staining was evident at weeks 3 to 20, in the nuclei and cytoplasm (Figure 7).
Time-course of nuclear Rel/NF-κB and cytoplasmic IκBαexpression in the kidney
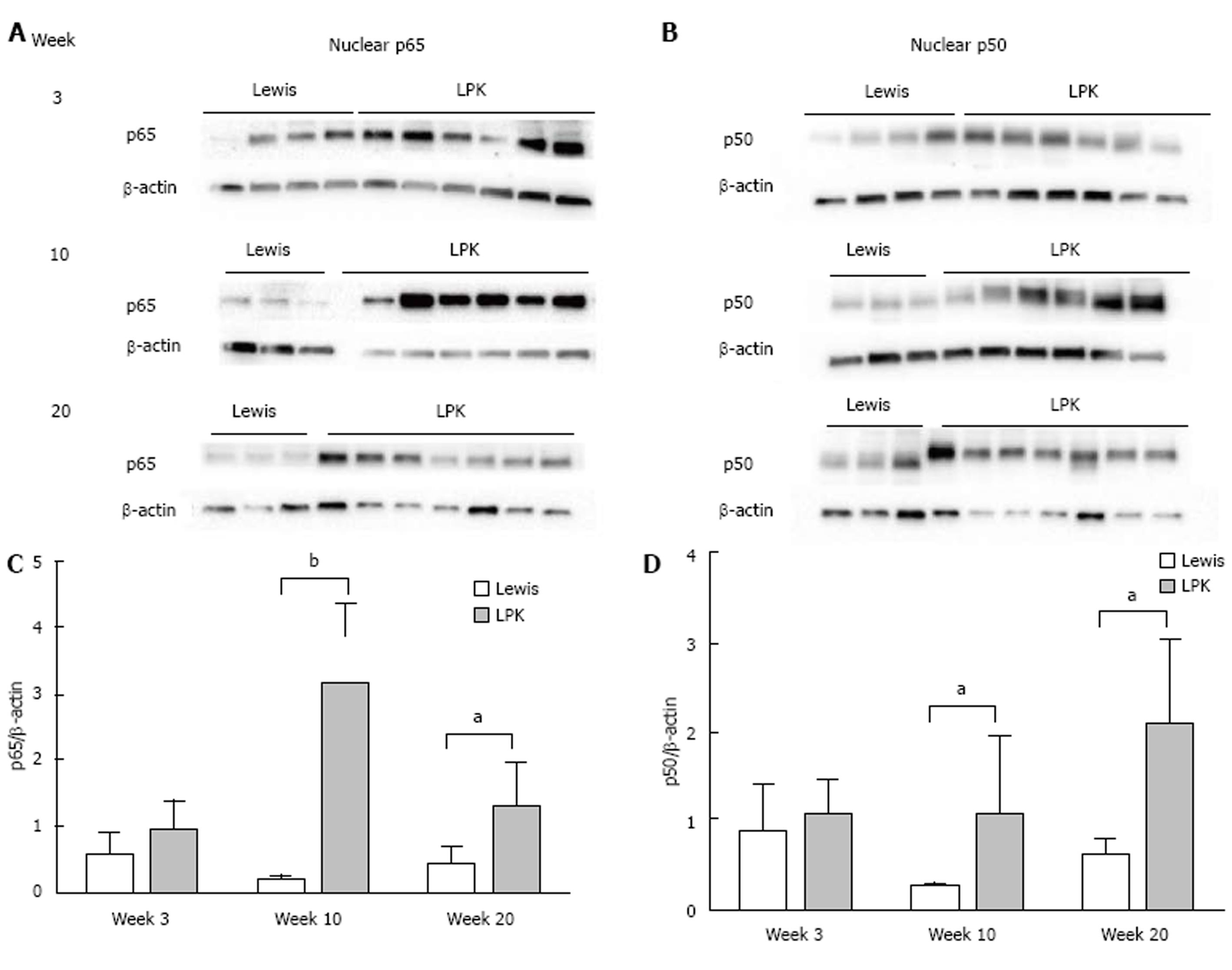
Western blotting of nuclear kidney extracts found that p65 expression was increased in LPK rats, peaking at week 10, when it was 18-fold higher than in Lewis animals (Figure 8). Similarly, p50 was elevated in LPK rats compared to the Lewis group, peaking at week 20 (Figure 8). For RelB, at week 3, nuclear expression was similar between Lewis and LPK rats (Figure 9). However, at weeks 10 and 20, there was a marked upregulation in nuclear RelB expression in LPK rats. Cytoplasmic IκBα displayed the same temporal pattern of expression as nuclear RelB in LPK kidneys (Figure 9).
Time-course of renal NF-κB-dependent gene expression in LPK rats
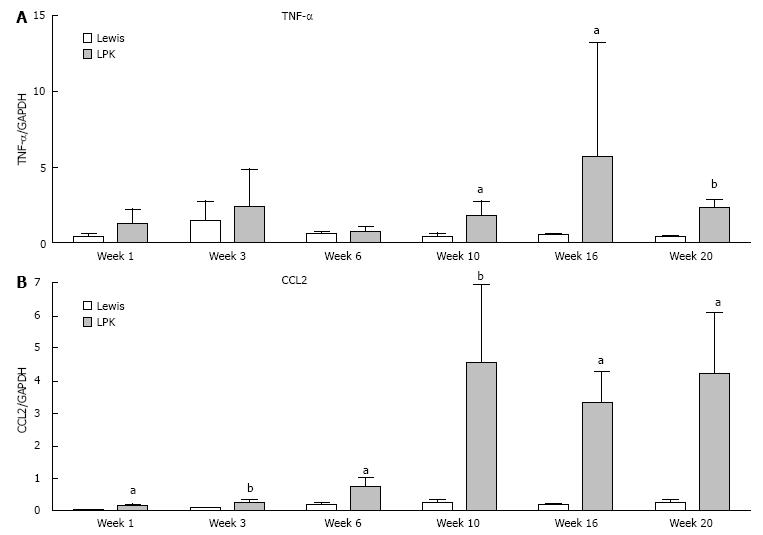
The upregulation of Rel/NF-κB proteins in LPK rats was accompanied by time-dependent increases in the mRNA expression of NF-κB-dependent genes (CCL2 and TNFα mRNA), which were elevated in LPK rats compared to the Lewis group, particularly at the late stages of disease (Figure 10).
Renal expression of NF-κB in human ADPKD and ARPKD
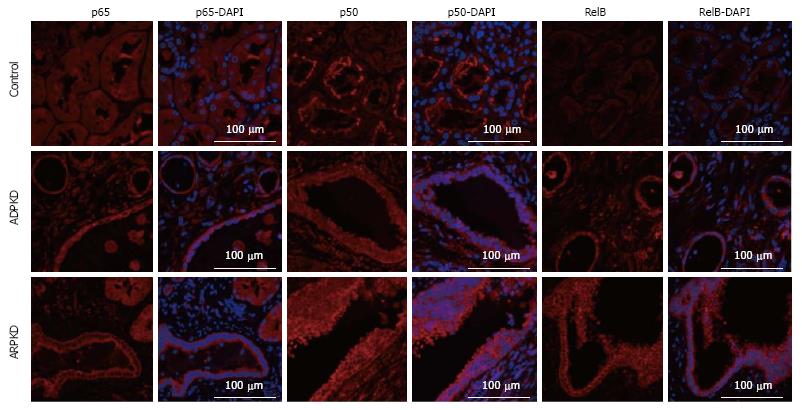
Human ADPKD and ARPKD kidneys displayed cystic dilatations of varying sizes, lined by either flattened single-layer, or hyperplastic epithelial cells (Figure 11). Abnormal glomeruli, interstitial fibrosis and large numbers of infiltrating cells were also observed. By immunofluorescence, in control kidneys, p50 staining was intensely expressed in proximal tubule brush borders, but weak in the glomeruli and in renal tubule cytoplasm (Figure 12). In ADPKD tissue, p50 staining was strong in the cytoplasm and moderate in the nuclei of CECs. Strong expression was also detected in intraluminal and interstitial cells. A similar pattern of staining was observed in ARPKD, with strong expression in CEC cytoplasm and moderate expression in nuclei (Figure 12). Among the CECs, p50 expression was notably more intense in areas of hyperplasia than in areas where the epithelial layer was single-cell thick. Staining was also strong and diffuse in interstitial cells (Figure 12). A similar pattern of staining was observed for P-p105, p65 and RelB (Figures 11 and 12).
γ-H2AX expression in experimental and human PKD kidneys
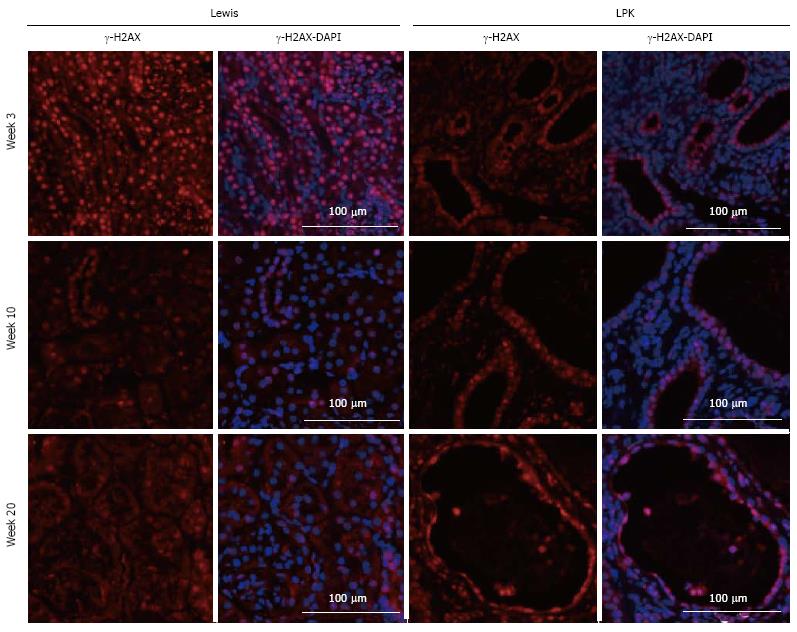
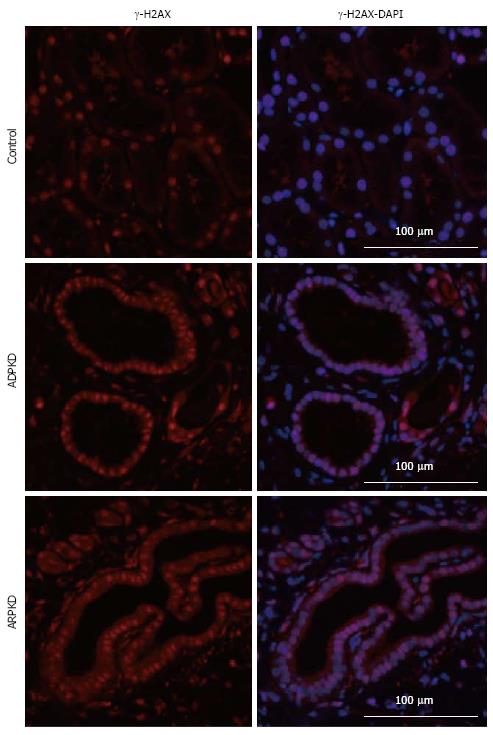
Previous studies in Nek8 mice revealed that DNA damage is evident early in diseased animals with this mutation[36]. Therefore, to elucidate whether there is a relationship between NF-κB activation and DNA damage, we assessed the expression of a phosphorylated histone H2A variant, γ-H2AX, which is produced during double-strand DNA breakage[37]. A time-course study of LPK kidneys showed that γ-H2AX was strongly expressed in tubular nuclei of Lewis kidneys at week 3, and weakly expressed in later life (Figure 13). In contrast, γ-H2AX expression in the CECs of LPK kidneys was consistent throughout disease progression (Figure 13). We also found that γ-H2AX was strongly expressed in the cyst-lining cells at postnatal week 1 when NF-κB/Rel proteins were minimally expressed (Figure 14). In ADPKD and ARPKD, γ-H2AX was expressed in virtually all CEC nuclei (Figure 15).
In the past decade, in vivo studies have demonstrated an upregulation of NF-κB proteins in PKD, but there has been limited information regarding the specific NF-κB proteins involved and their expression throughout disease progression[22,23]. The first main finding of this study is that a diverse array of NF-κB proteins, including p50, P-p105, p65, RelB and c-rel, is present in LPK kidneys. The localization of these NF-κB proteins to the CECs in LPK kidneys concurs with previous in vivo studies of Pkd1-/- and PKD2 mice[22,23]. Notably, p65, p50 and RelB were predominantly localized to the cytoplasm rather than to the nuclei of CECs. Since NF-κB transcription factor activity is critically dependent on the translocation of NF-κB proteins to the nucleus[13], western blotting for NF-κB proteins was also performed in LPK renal nuclear extracts. This confirmed that p65, p50 and RelB proteins were present in LPK nuclei.
The second major finding was that in the LPK rat, renal NF-κB protein expression occurs early in the disease and is constitutive over time. Expression of p65 was high in both Lewis and LPK kidneys in early life, suggesting that a basal level of NF-κB activation may be required for development in the normal kidney. Indeed, NF-κB inhibition in ex vivo embryonic kidneys has been shown to impair ureteric bud branching, which is critical for collecting duct development[38,39]. Whereas in Lewis rats p65 expression was low from week 10 onwards, in LPK rats the high expression of p65 was sustained throughout life. Similarly, P-p105, p50, RelB and c-rel were also identified in CECs of LPK rats at all stages of disease. Western blotting revealed that nuclear p65, p50 and RelB levels were elevated in LPK compared to Lewis kidneys at week 10 and week 20.
We hypothesized that NF-κB signaling is upregulated in LPK compared to Lewis kidneys, and therefore predicted that cytoplasmic IκBα would be absent in LPK kidneys, since degradation of IκBα is necessary for NF-κB activation[13]. However, we found the converse; cytoplasmic IκBα was consistently identified in all assessed timepoints in LPK kidneys but only present in Lewis kidneys at week 3. Since cytokines can stabilize newly resynthesized IκBα, decreasing its susceptibility to further degradation[40,41], it is possible that there is a chronic upregulation of NF-κB in LPK kidneys that results in the stabilization of IκBα.
Notably, the histological data showed that NF-κB proteins are strongly expressed in the cytoplasm of LPK kidneys in early life (week 3), while immunoblotting suggested that nuclear NF-κB levels are comparable between LPK and Lewis kidneys at this timepoint and do not significantly increase in LPK compared to Lewis until weeks 10 and 20. Overall, this suggests that NF-κB proteins are present in the CEC cytoplasm at early stages of disease, but that a significant increase in NF-κB activation does not occur until later in life. However, the persistent nature of NF-κB protein expression in LPK kidneys indicates that it is a chronic, rather than transient feature of renal cystic disease in this model.
Since NF-κB regulates the transcription of TNFα and CCL2[17], and as the cytokine products of these genes are commonly found in models of PKD[8], we sought to determine the expression of these genes throughout the time-course of disease in LPK rats. TNFα expression was higher in LPK rats compared to Lewis rats, concurring with work by Nakamura et al[42] which found elevated TNFα mRNA expression in cpk mice compared to wild-type controls. Nakamura et al[42] demonstrated a steady increase in TNFα from early to late-stage disease. In contrast, LPK kidneys displayed a biphasic pattern of TNFα expression, wherein TNFα was upregulated at the early (week 1) and then at the late stages of disease (weeks 10 and 20). We also demonstrated an elevation in CCL2 expression in LPK compared to Lewis at almost all timepoints, which concurs with previous findings of increased CCL2 expression in homozygous Han:SPRD rats compared to wild-type controls[43].
To confirm the results observed in LPK rats, we also examined NF-κB expression in human cystic renal disease. Similar to LPK rats, NF-κB was localized to CECs in human ADPKD and ARPKD kidneys, and this paralleled with the findings of Park et al[22] which demonstrated strong staining for phosphorylated NF-κB in the CECs of ADPKD tissue. NF-κB expression was particularly intense in regions of CEC hyperplasia, suggesting that the NF-κB system regulates transcription in highly proliferating areas. In a related disorder, acquired cystic disease-associated renal cell carcinoma, phosphorylated NF-κB was identified in hyperplastic epithelial cyst-lining cells[44]. While our in vitro previous work found no association between the rate of proliferation and degree of NF-κB activation in human ADPKD cells[31], siRNA-induced expression of a polycystin-1 cytoplasmic terminal tail in human embryonic kidney cells led to increased NF-κB activation and proliferation[20]. Further study is therefore required to verify the relationship between NF-κB and cell proliferation in PKD. We also performed preliminary western blotting for NF-κB in normal, ADPKD and ARPKD renal tissue, and immunohistostaining for NF-κB in Pkd2 knockout mouse kidneys, but the results were inconclusive due to small sample sizes (data not shown). Overall, the current data in LPK rats and human PKD provide a basis for future studies which may confirm the increase in NF-κB transcription activity by examining the DNA:protein binding activity of p65, p50 and RelB in human PKD tissue.
It is unclear whether NF-κB activation directly contributes to the pathogenesis of PKD, or whether it is secondary to the disease, stimulated in response to cytokines and inflammatory cells. Although our study did not directly address this question, it provided some information on the relationship between NF-κB expression and markers of PKD progression. Firstly, NF-κB proteins were observed in LPK CECs as early as week 1, and were strongly expressed at week 3, coinciding with cystic expansion and the increases in cell proliferation, inflammation and fibrosis, and continued throughout the disease time-course. The chronological order of these events may suggest that NF-κB activation contributes to cyst expansion via the upregulation of cell proliferation and interstitial inflammation. Qin et al[23] found that NF-κB inhibition decreases cyst growth in kidney explants, supporting the theory that cyst growth is NF-κB-dependent. Secondly, western blotting indicated that NF-κB protein levels were elevated in mid- to late-stage disease, coinciding with the elevations in TNFα and CCL2 at weeks 10 and 20. Previous work has suggested that in Pkd1 null mouse embryonic kidney cells, TNFα regulates its own transcription via the NF-κB pathway[27], and that receptor activator of NF-κB ligand (RANKL, a cytokine of the TNF family) activates the transcription of TNFα[45]. Taken together, these results support the notion that TNFα may contribute to NF-κB upregulation in polycystic kidneys, and furthermore suggest there may be a positive-feedback loop in which NF-κB regulates TNFα and vice versa. Further study is required to prove or disprove a causal relationship between NF-κB and cystic renal disease.
Our study provides preliminary data for a role of non-canonical NF-κB signaling in PKD. At weeks 10 and 20, RelB (a protein typically associated with the non-canonical pathway[13]) was absent in Lewis but present in LPK kidney extracts, suggesting that while RelB may be involved in NF-κB signaling in early normal renal development, cystic renal disease involves chronic RelB activity. Thus far, the non-canonical pathway has been associated with fewer physiological functions compared to the canonical pathway, and is mainly known for its role in B- and T-cell organogenesis[14,15]. However, studies have suggested that non-canonical NF-κB signaling may also be involved in acute kidney injury[46] and IgA nephropathy[47]. Interestingly, RANKL is a stimulus of the non-canonical NF-κB pathway and has been implicated in NF-κB signaling in Pkd1-/- murine cells[45,46]. However since LPK kidneys also displayed elevations in p65 and TNFα, which are typically associated with canonical NF-κB signaling[13,16], it is likely that PKD involves a combination of canonical and non-canonical NF-κB upregulation. Future investigation of other NF-κB family members normally associated with the non-canonical pathway (e.g., p52 and IKKα[16]) and NEMO (which is exclusive to the canonical pathway[14]) would be useful to further characterize NF-κB signaling in PKD.
Since DNA damage has been proposed to play a role in renal ciliopathies[48-50], we investigated the DNA damage marker, γ-H2AX, in polycystic kidneys. Our study demonstrated that γ-H2AX was strongly expressed in ADPKD and ARPKD CEC nuclei, suggesting that DNA damage may be a component of human renal cystic disease. Furthermore, γ-H2AX was apparent in CECs of LPK kidneys throughout the time-course of disease. Given that the LPK rat possesses a mutation in Nek8[28], and abnormalities in this gene lead to DNA double-strand breaks (DSBs) and abnormal structuring of epithelial cells in 3D spheroid cultures[36], our data add credence to the theory that Nek8-linked DNA damage is involved in the pathogenesis of renal ciliopathies and renal cystic diseases[48]. Interestingly, γ-H2AX was strongly expressed at week 1 in LPK kidneys, when the expression of NF-κB proteins was low (Figure 14). Since Tilstra et al[51] have demonstrated that NF-κB inhibition can slow the progression of DNA damage in an animal model of senescence, there is potential for NF-κB inhibition to be investigated as a strategy to ameliorate DNA injury as well as inflammation in PKD.
One limitation of this study was the small sample size and lack of genotype data for the human ADPKD and ARPKD specimens. Since in ADPKD, the PKD2 mutation is associated with a slower progression to renal failure compared to the PKD1 mutation[52], future studies may determine whether NF-κB activation differs according to the genotypic form and/or type of mutation (e.g., missense vs nonsense) in ADPKD patients[53]. Also, although we demonstrated that NF-κB proteins are constitutively present in CECs of LPK rats throughout the time-course of disease, it remains unknown whether this holds true in human PKD progression. Further characterization of renal NF-κB signaling in Pkd1 and/or Pkd2 knockout mice (which are orthologous to human ADPKD[54]) may aid to bridge our understanding of NF-κB in human PKD. Future studies may also employ southwestern histochemistry or electrophoretic mobility shift assays (EMSA) to confirm that DNA:protein binding activity is upregulated in LPK kidneys and human PKD tissue.
In conclusion, this study found that several NF-κB proteins are expressed in the CECs of kidney tissue of LPK rats and human ADPKD and ARPKD patients. Taking into account previous studies, NF-κB upregulation has now been identified in human PKD or animal models that possess mutations in PKD1, PKD2, PKHD1, and NEK8, suggesting that it is a shared feature of cystic renal diseases and independent of genotypic variation[22,23]. Our study demonstrated that in the LPK rat, NF-κB proteins are expressed in early disease and are constitutively present throughout life. This may suggest that NF-κB inhibiting drugs need to be commenced in the early stages of PKD, in order to reduce or delay the increases in cell proliferation, interstitial inflammation and cyst volume. Our study also highlighted that RelB and non-canonical NF-κB signaling may be involved in the late stages of PKD. Although these data provide a basis for the role of NF-κB throughout disease progression in PKD, a direct causal relationship between NF-κB and PKD has not yet been proved. Future studies should address this question through cross-breeding studies of IKK knockout and Pkd1-/- mice, or by testing selective NF-κB inhibitors (e.g., IKK inhibitors) in in vivo models of PKD.
The nuclear factor (NF)-κB transcription factor system is a key regulator of genes controlling inflammation and growth. The aim of this study was to determine the temporal expression of Rel/NF-κB proteins in renal tissue in polycystic kidney disease (PKD).
To date, there has been limited information regarding the particular NF-κB subunits involved in PKD and their expression throughout the time-course of disease progression. Further studies are required to elucidate whether NF-κB signaling directly contributes to cyst growth in PKD.
This study found that NF-κB protein expression is upregulated in a rodent model of PKD, the LPK rat, and that this expression occurred early, was constitutive, and trended toward an increase over time. NF-κB upregulation was also identified in the cyst-lining cells of human autosomal dominant and recessive PKD, suggesting that this transcription factor system may regulate inflammation in cystic renal disease.
Although this study provides promising data regarding NF-κB as a possible target for PKD, it should be noted that functional data are required to demonstrate that selective NF-κB inhibition is effective in reducing renal cystic disease in animal models. There are no selective NF-κB inhibitors approved for human use. Currently approved drugs that possess NF-κB-inhibiting properties, such as disulfiram, may be potential therapies, but require further investigation in experimental models of PKD.
Cystic epithelial cells: Epithelial cells that line the cyst, separating the cyst lumen from the renal interstitium. These cells rapidly proliferate, contributing to cyst expansion, and are thought respond to stimulation by intraluminal cytokines. Non-canonical NF-κB signaling: A pathway of NF-κB signaling that typically regulates the development of immune organs. Southwestern histochemistry: A technique which allows the expression of DNA-binding transcription factors to be assessed in situ. Selective NF-κB inhibition: A strategy of NF-κB inhibition by targeting specific components of NF-κB.
The paper is interesting and well written. The methods are sound and the conclusions are consistent with the results.
P- Reviewer: Mocellin S, Wong KL S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Harris PC, Torres VE. Polycystic kidney disease. Annu Rev Med. 2009;60:321-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 681] [Cited by in RCA: 607] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 2. | Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millán JL, Gamble V, Harris PC. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet. 1995;10:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 641] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 3. | Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1040] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 4. | Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 2002;30:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 520] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 5. | Patel V, Chowdhury R, Igarashi P. Advances in the pathogenesis and treatment of polycystic kidney disease. Curr Opin Nephrol Hypertens. 2009;18:99-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Yoder BK. Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2007;18:1381-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Ibrahim S. Increased apoptosis and proliferative capacity are early events in cyst formation in autosomal-dominant, polycystic kidney disease. ScientificWorldJournal. 2007;7:1757-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Ta MH, Harris DC, Rangan GK. Role of interstitial inflammation in the pathogenesis of polycystic kidney disease. Nephrology (Carlton). 2013;18:317-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Grantham JJ, Mulamalla S, Swenson-Fields KI. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2011;7:556-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 10. | Lanoix J, D’Agati V, Szabolcs M, Trudel M. Dysregulation of cellular proliferation and apoptosis mediates human autosomal dominant polycystic kidney disease (ADPKD). Oncogene. 1996;13:1153-1160. [PubMed] |
| 11. | Norman J. Fibrosis and progression of autosomal dominant polycystic kidney disease (ADPKD). Biochim Biophys Acta. 2011;1812:1327-1336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Wilson PD. Polycystic kidney disease. N Engl J Med. 2004;350:151-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 536] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 13. | Hayden MS, Ghosh S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1359] [Article Influence: 104.5] [Reference Citation Analysis (0)] |
| 14. | Tergaonkar V. NFkappaB pathway: a good signaling paradigm and therapeutic target. Int J Biochem Cell Biol. 2006;38:1647-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680-6684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1589] [Cited by in RCA: 1699] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 16. | Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12:695-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1500] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 17. | Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853-6866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Sanz AB, Sanchez-Niño MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A. NF-kappaB in renal inflammation. J Am Soc Nephrol. 2010;21:1254-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 456] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 19. | Goilav B. Apoptosis in polycystic kidney disease. Biochim Biophys Acta. 2011;1812:1272-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Banzi M, Aguiari G, Trimi V, Mangolini A, Pinton P, Witzgall R, Rizzuto R, del Senno L. Polycystin-1 promotes PKCalpha-mediated NF-kappaB activation in kidney cells. Biochem Biophys Res Commun. 2006;350:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Mangolini A, Bogo M, Durante C, Borgatti M, Gambari R, Harris PC, Rizzuto R, Pinton P, Aguiari G, del Senno L. NF-kappaB activation is required for apoptosis in fibrocystin/polyductin-depleted kidney epithelial cells. Apoptosis. 2010;15:94-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Park EY, Seo MJ, Park JH. Effects of specific genes activating RAGE on polycystic kidney disease. Am J Nephrol. 2010;32:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Qin S, Taglienti M, Cai L, Zhou J, Kreidberg JA. c-Met and NF-κB-dependent overexpression of Wnt7a and -7b and Pax2 promotes cystogenesis in polycystic kidney disease. J Am Soc Nephrol. 2012;23:1309-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Salmerón A, Janzen J, Soneji Y, Bump N, Kamens J, Allen H, Ley SC. Direct phosphorylation of NF-kappaB1 p105 by the IkappaB kinase complex on serine 927 is essential for signal-induced p105 proteolysis. J Biol Chem. 2001;276:22215-22222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Phillips JK, Hopwood D, Loxley RA, Ghatora K, Coombes JD, Tan YS, Harrison JL, McKitrick DJ, Holobotvskyy V, Arnolda LF. Temporal relationship between renal cyst development, hypertension and cardiac hypertrophy in a new rat model of autosomal recessive polycystic kidney disease. Kidney Blood Press Res. 2007;30:129-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Nakamura T, Ebihara I, Fukui M, Osada S, Tomino Y, Masaki T, Goto K, Furuichi Y, Koide H. Increased endothelin and endothelin receptor mRNA expression in polycystic kidneys of cpk mice. J Am Soc Nephrol. 1993;4:1064-1072. [PubMed] |
| 27. | Fan LX, Zhou X, Sweeney WE, Wallace DP, Avner ED, Grantham JJ, Li X. Smac-mimetic-induced epithelial cell death reduces the growth of renal cysts. J Am Soc Nephrol. 2013;24:2010-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | McCooke JK, Appels R, Barrero RA, Ding A, Ozimek-Kulik JE, Bellgard MI, Morahan G, Phillips JK. A novel mutation causing nephronophthisis in the Lewis polycystic kidney rat localises to a conserved RCC1 domain in Nek8. BMC Genomics. 2012;13:393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Ta MH, Rao P, Korgaonkar M, Foster SF, Peduto A, Harris DC, Rangan GK. Pyrrolidine dithiocarbamate reduces the progression of total kidney volume and cyst enlargement in experimental polycystic kidney disease. Physiol Rep. 2014;2:pii: e12196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Rangan GK, Wang Y, Tay YC, Harris DC. Inhibition of nuclear factor-kappaB activation reduces cortical tubulointerstitial injury in proteinuric rats. Kidney Int. 1999;56:118-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 134] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Ta MH, Liuwantara D, Rangan GK. Effects of pyrrolidine dithiocarbamate on proliferation and nuclear factor-κB activity in autosomal dominant polycystic kidney disease cells. BMC Nephrol. 2015;16:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Elks CM, Mariappan N, Haque M, Guggilam A, Majid DS, Francis J. Chronic NF-{kappa}B blockade reduces cytosolic and mitochondrial oxidative stress and attenuates renal injury and hypertension in SHR. Am J Physiol Renal Physiol. 2009;296:F298-F305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Mu W, Ouyang X, Agarwal A, Zhang L, Long DA, Cruz PE, Roncal CA, Glushakova OY, Chiodo VA, Atkinson MA. IL-10 suppresses chemokines, inflammation, and fibrosis in a model of chronic renal disease. J Am Soc Nephrol. 2005;16:3651-3660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 34. | de Borst MH, van Timmeren MM, Vaidya VS, de Boer RA, van Dalen MB, Kramer AB, Schuurs TA, Bonventre JV, Navis G, van Goor H. Induction of kidney injury molecule-1 in homozygous Ren2 rats is attenuated by blockade of the renin-angiotensin system or p38 MAP kinase. Am J Physiol Renal Physiol. 2007;292:F313-F320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Schwensen KG, Burgess JS, Graf NS, Alexander SI, Harris DC, Phillips JK, Rangan GK. Early cyst growth is associated with the increased nuclear expression of cyclin D1/Rb protein in an autosomal-recessive polycystic kidney disease rat model. Nephron Exp Nephrol. 2011;117:e93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Choi HJ, Lin JR, Vannier JB, Slaats GG, Kile AC, Paulsen RD, Manning DK, Beier DR, Giles RH, Boulton SJ. NEK8 links the ATR-regulated replication stress response and S phase CDK activity to renal ciliopathies. Mol Cell. 2013;51:423-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 37. | Sharma A, Singh K, Almasan A. Histone H2AX phosphorylation: a marker for DNA damage. Methods Mol Biol. 2012;920:613-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 534] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 38. | Takayama M, Miyatake K, Nishida E. Identification and characterization of retinoic acid-responsive genes in mouse kidney development. Genes Cells. 2014;19:637-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Nagalakshmi VK, Yu J. The ureteric bud epithelium: morphogenesis and roles in metanephric kidney patterning. Mol Reprod Dev. 2015;82:151-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Place RF, Haspeslagh D, Hubbard AK, Giardina C. Cytokine-induced stabilization of newly synthesized I(kappa)B-alpha. Biochem Biophys Res Commun. 2001;283:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Place RF, Haspeslagh D, Giardina C. Induced stabilization of IkappaBalpha can facilitate its re-synthesis and prevent sequential degradation. J Cell Physiol. 2003;195:470-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Nakamura T, Ebihara I, Nagaoka I, Tomino Y, Nagao S, Takahashi H, Koide H. Growth factor gene expression in kidney of murine polycystic kidney disease. J Am Soc Nephrol. 1993;3:1378-1386. [PubMed] |
| 43. | Cowley BD, Ricardo SD, Nagao S, Diamond JR. Increased renal expression of monocyte chemoattractant protein-1 and osteopontin in ADPKD in rats. Kidney Int. 2001;60:2087-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Konda R, Sugimura J, Sohma F, Katagiri T, Nakamura Y, Fujioka T. Over expression of hypoxia-inducible protein 2, hypoxia-inducible factor-1alpha and nuclear factor kappaB is putatively involved in acquired renal cyst formation and subsequent tumor transformation in patients with end stage renal failure. J Urol. 2008;180:481-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Zhou JX, Fan LX, Li X, Calvet JP, Li X. TNFα signaling regulates cystic epithelial cell proliferation through Akt/mTOR and ERK/MAPK/Cdk2 mediated Id2 signaling. PLoS One. 2015;10:e0131043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Sanz AB, Sanchez-Niño MD, Izquierdo MC, Jakubowski A, Justo P, Blanco-Colio LM, Ruiz-Ortega M, Selgas R, Egido J, Ortiz A. TWEAK activates the non-canonical NFkappaB pathway in murine renal tubular cells: modulation of CCL21. PLoS One. 2010;5:e8955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 47. | Jin J, Xiao Y, Chang JH, Yu J, Hu H, Starr R, Brittain GC, Chang M, Cheng X, Sun SC. The kinase TBK1 controls IgA class switching by negatively regulating noncanonical NF-κB signaling. Nat Immunol. 2012;13:1101-1109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 48. | Jackson PK. Nek8 couples renal ciliopathies to DNA damage and checkpoint control. Mol Cell. 2013;51:407-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Chaki M, Airik R, Ghosh AK, Giles RH, Chen R, Slaats GG, Wang H, Hurd TW, Zhou W, Cluckey A. Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell. 2012;150:533-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 308] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 50. | Polci R, Peng A, Chen PL, Riley DJ, Chen Y. NIMA-related protein kinase 1 is involved early in the ionizing radiation-induced DNA damage response. Cancer Res. 2004;64:8800-8803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Tilstra JS, Robinson AR, Wang J, Gregg SQ, Clauson CL, Reay DP, Nasto LA, St Croix CM, Usas A, Vo N. NF-κB inhibition delays DNA damage-induced senescence and aging in mice. J Clin Invest. 2012;122:2601-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 366] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 52. | Hou X, Mrug M, Yoder BK, Lefkowitz EJ, Kremmidiotis G, D’Eustachio P, Beier DR, Guay-Woodford LM. Cystin, a novel cilia-associated protein, is disrupted in the cpk mouse model of polycystic kidney disease. J Clin Invest. 2002;109:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 53. | Tan YC, Blumenfeld J, Rennert H. Autosomal dominant polycystic kidney disease: genetics, mutations and microRNAs. Biochim Biophys Acta. 2011;1812:1202-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | Happé H, Peters DJ. Translational research in ADPKD: lessons from animal models. Nat Rev Nephrol. 2014;10:587-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |