Published online Jul 6, 2016. doi: 10.5527/wjn.v5.i4.308
Peer-review started: March 9, 2016
First decision: March 25, 2016
Revised: March 31, 2016
Accepted: May 17, 2016
Article in press: May 27, 2016
Published online: July 6, 2016
Processing time: 114 Days and 8.6 Hours
This review revises the reclassification of the membranoproliferative glomerulonephritis (MPGN) after the consensus conference that by 2015 reclassified all the glomerulonephritis basing on etiology and pathogenesis, instead of the histomorphological aspects. After reclassification, two types of MPGN are to date recognized: The immunocomplexes mediated MPGN and the complement mediated MPGN. The latter type is more extensively described in the review either because several of these entities are completely new or because the improved knowledge of the complement cascade allowed for new diagnostic and therapeutic approaches. Overall the complement mediated MPGN are related to acquired or genetic cause. The presence of circulating auto antibodies is the principal acquired cause. Genetic wide association studies and family studies allowed to recognize genetic mutations of different types as causes of the complement dysregulation. The complement cascade is a complex phenomenon and activating factors and regulating factors should be distinguished. Genetic mutations causing abnormalities either in activating or in regulating factors have been described. The diagnosis of the complement mediated MPGN requires a complete study of all these different complement factors. As a consequence, new therapeutic approaches are becoming available. Indeed, in addition to a nonspecific treatment and to the immunosuppression that has the aim to block the auto antibodies production, the specific inhibition of complement activation is relatively new and may act either blocking the C5 convertase or the C3 convertase. The drugs acting on C3 convertase are still in different phases of clinical development and might represent drugs for the future. Overall the authors consider that one of the principal problems in finding new types of drugs are both the rarity of the disease and the consequent poor interest in the marketing and the lack of large international cooperative studies.
Core tip: The complement pathway dysregulation has been recognized as the main cause of some membranoproliferative glomerulonephritis (MPGNs). This fact is at the basis of the new classification of the disease and of the findings of new entities as the complement factor H related protein nephropathy. Genetic studies as well as improvement in proteomics allowed recognizing the complement dysregulation as the cause of some renal diseases as the MPGN and the atypical hemolytic uremic syndrome that may be considered as strictly related diseases. The anti-complement drugs represent a new approach in the treatment of these diseases and their use in larger evidence based randomized trials is required.
- Citation: Salvadori M, Rosso G. Reclassification of membranoproliferative glomerulonephritis: Identification of a new GN: C3GN. World J Nephrol 2016; 5(4): 308-320
- URL: https://www.wjgnet.com/2220-6124/full/v5/i4/308.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i4.308
By 2015, nephrologists and renal pathologists held a consensus meeting to formulate a new etiology/pathogenesis-based system to classify glomerulonephritis (GN)[1]. According to the consensus report, GNs have been classified into five etiology/pathogenesis-based categories (Table 1).
| Pathogenetic type | Specific disease entity | Pattern of injury: Focal or diffuse | Score or class |
| Immune-complex GN | IgA nephropathy, IgA vasculitis, lupus nephritis, | Mesangial, endocapillary, exudative, membranoproliferative, necrotizing, crescentic, sclerosing or multiple | Oxford/MEST scores for IgA nephropathy |
| infection-related GN, fibrillary GN with polyclonal Ig deposits | ISN/RPS class for lupus nephritis | ||
| Pauci-immune GN | MPO-ANCA GN, | Necrotizing, crescentic, sclerosing, or multiple | Focal, crescentic, mixed, or sclerosing class (Berden/EUVAS class) |
| proteinase 3-ANCA GN, | |||
| ANCA-negative GN | |||
| Anti-GBM GN | Anti-GBM GN | Necrotizing, crescentic, sclerosing, or mixed | |
| Monoclonal Ig GN | Monoclonal Ig deposition disease, proliferative GN with monoclonal Ig deposits, | Mesangial, endocapillary, exudative, membranoproliferative, necrotizing, crescentic, sclerosing or multiple | |
| immunotactoid glomerulopathy, fibrillary GN with monoclonal Ig deposits | |||
| C3 glomerulopathy | C3 GN, dense deposit disease | Mesangial, endocapillary, exudative, membranoproliferative, necrotizing, crescentic, sclerosing or multiple |
According to the new classification, membranoproliferative GNs (MPGN) have been reclassified and divided into different chapters on the basis of pathophysiology. In addition, new entities have been found. This review will discuss the new classification of MPGNs and will principally describe the complement-dysregulation dependent C3 glomerulopathies (C3G).
Until recently, the MPGNs have been distinguished according the histological and ultra structural findings and were classified as MPGN type I, type II and type III. The glomerular lesions include mesangial hypercellularity, endocapillary proliferation and duplication of glomerular basement membrane (GBM) lesions[2]. Sub-endothelial and mesangial deposits are predominant in MPGN type I[3]. Highly osmiophylic electron-dense intramembranous deposits characterize type II GN[4], which is also known as dense deposits disease (DDD). In type III MPGN deposits may be found in the sub-endothelial and sub-epithelial spaces[5].
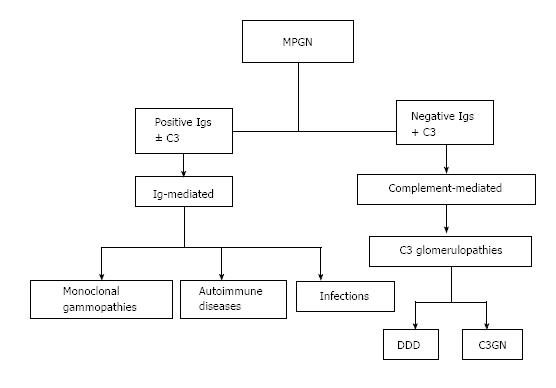
With the discovery of the complement role in generating glomerular diseases[6], a new classification of MPGN was developed, based on pathophysiology and considering whether immunoglobulins accompany the complement using immunofluorescence on biopsy specimens[7,8] (Figure 1).
This new classification resulted in three principal consequences: (1) to identify new entities, which until now were unknown or misdiagnosed; (2) to highlight new diagnostic approaches. Indeed in the case of Ig-mediated MPGN, a work-up for infections, autoimmune diseases and monoclonal gammopathies should be adopted. In the case of complement-mediated GN, a complete study of the complement alternative pathway (AP) should be performed; and (3) to differentiate the therapeutic approach according to the type of MPGN. In summary, the three different forms of MPGN are now recognized as follows: (1) Immunocomplexes-associated MPGN with complement over activation (old MPGN type I); (2) MPGN with intramembranous dense deposits (old MPGN type II); and (3) C3GN, a new entity complement-mediated GN. DDD and C3GN are both related to complement dysregulation and are “de facto” included in the same chapter.
Immune-complexes mediated MPGN is caused by the deposition of immunocomplexes in the glomeruli. The immunocomplexes activate the classical pathway (CP) of complement and cause the deposition of complement factors or of the membrane attack complex (MAC) in the mesangium and capillary loops[9].
The MPGN is an uncommon cause of nephropathy (approximately 5 per million persons per year) and is more often secondary to infections, autoimmune disease and monoclonal gammopathy[10].
MPGN and infections: Hepatitis C and B, which are often accompanied by circulating cryoglobulins, are a frequent cause of MPGN[11-14]. In addition, chronic bacterial infections, fungal and parasitic infections may also cause MPGN[15-17].
Immunocomplexes depositions are the first step. Consequently, CP is activated and in addition to the direct damage cause by MAC, C3a and C5a are generated that favor leukocyte accumulation, cytokine release and a further glomerular damage.
MPGN and autoimmune diseases: Mixed cryoglobulinemia is frequently associated with hepatitis C infection, systemic lupus erythematosus, sclerodermia, Sjögren syndrome and rheumatoid arthritis. These are the autoimmune diseases that more frequently cause MPGN due to the persistence of circulating immunocomplexes[18-21]. Under these conditions, circulating immunocomplexes may also activate the complement CP with the abovementioned subsequent events described for MPGN due to infections.
MPGN and monoclonal gammopathy: The renal deposition of monoclonal immunoglobulins (MIg) may determine a wide spectrum of renal lesions as recently described[22]. Monoclonal gammopathy as well as light chain and heavy chain diseases may result in MPGN[23]. These lesions may hide a variety of severe hematological diseases ranging from low-grade B cell lymphoma, chronic lymphocyte leukemia to multiple myeloma[9]. In a recent monocenter study of MIg-associated MPGN after excluding infections and autoimmune disease, 26 out of 28 patients were serum electrophoresis-positive and 27 out of 28 patients were urine electrophoresis-positive[24]. In this monocentric study, out of 126 patients affected by MPGN, 41% were urine- or serum-positive for monoclonal gammopathy.
Monoclonal gammopathies are associated with complement activation; indeed, the abnormal immunoglobulin might activate the AP[10].
Recently, cases of C3 glomerulopathies, including C3GN and DDD (see below) associated with MIgs have been described[25]. In these patients the monoclonal immunoglobulin causes a complement dysregulation by interfering with the function of complement-regulating proteins, such as factor H or acting as an autoantibody against factor H or factor B[26-28].
Immunocomplex-mediated MPGNs principally affect children and young adults. Its clinical presentation may range from nephrotic syndrome and acute nephritic syndrome, to asymptomatic proteinuria and hematuria. Renal dysfunction frequently occurs, and 40% of the patients progress to end stage renal disease (ESRD) in approximately 10 years.
The efficacy of the different therapeutic approach is difficult to evaluate due to the small number of patients and because several trials include the three different types of MPGN[29,30]. The therapy most widely used is based on anti-cell proliferation agents[31]. Over-activation of complement is often present, but whether anticomplement drugs might be useful in this context remains to be elucidated.
According to different studies, renal transplantation is a viable option in patients with ESRD, even if the disease recurs after transplantation with a frequency ranging from 27% to 65%[32-34]. In a recent study, after the exclusion of patients with DDD, a recurrence rate of 41% has been reported[34]. Such a high recurrence rate has been confirmed by a study published in 2016, which evaluated the recurrence rate using the new classification[35]. In another study the recurrence of Ig-mediated MPGN was lower (23.5%) and after a follow-up of 15 years, the graft survival rate of MPGN patients was similar to those of controls affected by different diseases[36].
MPGN patients that have on renal biopsy clear glomerular C3 staining with few or no immunoglobulin deposition are referred to as complement-mediated MPGN and are defined as C3G. C3Gs are less common than immune-complex-mediated MPGNs and are further divided into two groups according to the presence or absence of highly electron-dense deposits into the GBM.
The disease with intramembranous deposits corresponds to the DDD (previously called MPGN type II). The disease without dense deposits, with C3 prevalence and no Igs on the glomeruli and with MPGN aspect on normal histology, is referred to a recently recognized entity: the C3GN. The distinction between the two diseases often requires the use of electron microscopy. C3GN was initially described by Servais et al[37] who described a series of 19 patients and proposed the term C3GN to highlight a disease that is characterized by C3 prevalence on the glomeruli, without intramembranous deposits. In addition, Servais et al[37] observed that this new entity often shares common genetic risk factors with atypical hemolytic uremic syndrome (aHUS).
Overall the term C3G was introduced to define all MPGNs that are characterized by the prevalence of C3 in the glomeruli[38], including DDD.
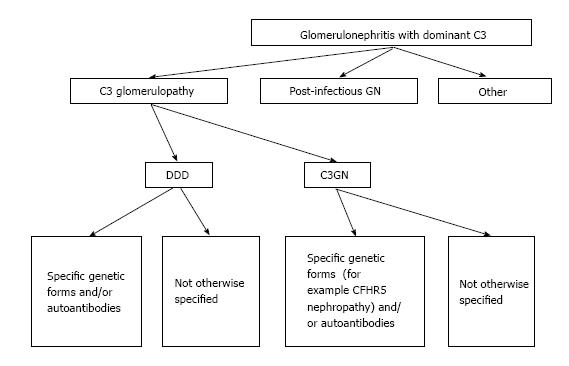
The term C3G has also been introduced because C3 isolated accumulation was recognized to include several heterogeneous entities and due to our improvement in the understanding of complement-mediated kidney injuries. Consequently, several complement factor abnormalities resulting in glomerular lesions have been identified. In 2013, a first consensus meeting on C3G was held to better clarify the pathogenic aspects and terminology[39]. The consensus conference resulted in an improved classification (Figure 2) that also documented that need of future work.
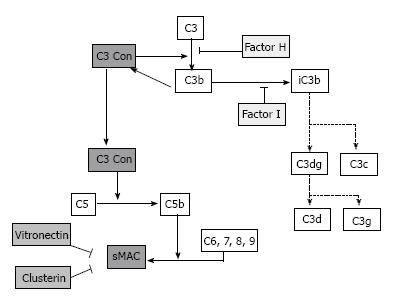
C3G are all caused by dysregulation of the complement AP and of the terminal complement complex (TCC)[40] (Figure 3).
DDD has an estimated prevalence of 2 to 3 per million populations[41] and prevails in childhood and in young adults[42]. C3GN prevalence is difficult to be evaluated, as this disease is new and as time progresses, more patients are identified with family studies and with an improvement in the Genetic wide association studies.
Overall, patients affected by DDD are younger with respect to patients affected by C3GN[43]. Both diseases affect males and females with the same frequency[44-46]. Renal manifestations are similar in DDD and C3GN[43] and include hypertension, hematuria and proteinuria more often in the nephrotic range. Non renal manifestations of DDD include ocular lipoproteinaceous deposition and acquired lipodystrophy[47,48].
MIg in the serum may also be associated with both DDD and C3GN[25,49-51]. These patients often have a poor renal prognosis.
Progression to ESRD is common in both DDD and C3GN. Renal transplantation is feasible but with a high rate of disease recurrence[52].
Complement factor H related protein (CFHR5) nephropathy is a subtype that is well identified in C3GN caused by the presence of an abnormal CFHR5 protein. The disease is inherited and was first identified in Cypriot families[53]. The disease may often occur with clinical manifestations that are similar to IgA nephropathy with microscopic hematuria or macroscopic hematuria after an acute upper respiratory tract disease[54]. Progression to ESRD is common. Interestingly, ten patients affected by CFHR5 nephropathy received a successful renal transplantation[54].
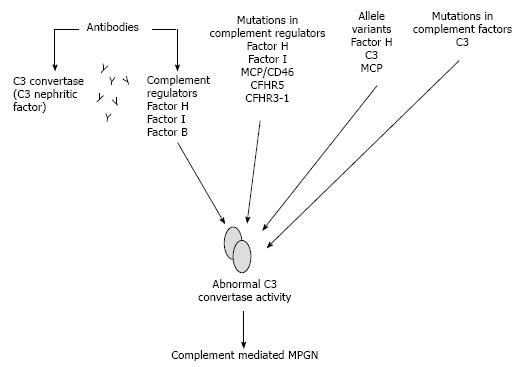
Dyregulation of the complement AP may occur principally due to acquired or genetic abnormalities[9] (Figure 4).
The auto-antibodies are the most frequently acquired abnormality. Auto-antibodies may be directed against the complement-regulating factors, such as factor H, factor I, factor B as well as against C3 convertase itself[55,56].
The first described autoantibody was the C3 nephritic factor (C3 NeF), which binds and stabilizes C3 convertase[57]. A second type of C3 NeF properdin dependent has also been described[58]. C3 NeFs are principally present in patients affected by DDD but are less frequently found in C3GN and absent in CFHR5 nephropathy[43].
In DDD, auto-antibodies that bind factor B and target C3B have been described in patients affected by MPGN type II[56,59]. Anti CFH auto-antibodies have also been found in patients affected by DDD and C3GN[60,61].
Anti CFH auto antibodies are also frequently present in aHUS. A recent study[62] highlights that anti-factor H antibodies are equally present in C3G and aHUS, but that the auto-antibody structure is different in the two diseases. Indeed, in C3G, the auto-antibody principally binds to the amino terminal domains, while in aHUS, it binds to the carboxyterminal domain[10]. As previously mentioned, the two diseases are strictly related, but several differences are present.
The discovery of familial cases of C3G highlights that in several cases, a familial genetic basis of the disease occurs.
In 2010, Martínez-Barricarte et al[63] described a family in which some members were affected by a mutant form of C3 resistant to cleavage by C3 convertase. Consequently, this caused an AP dysregulation restricted to the fluid phase and these patients continuously produced and consumed C3 produced by the normal C3 allele. These patients were affected by the classic DDD. Complement factor H-related (CFHR) genes are often involved. There are five CFH-related proteins (CFHR1-5 and genetic abnormalities of these proteins have been recognized and may cause disease. Recently, Chen et al[64] described two patients from the same family affected by DDD and with an abnormal deletion in the complement factor H-related (CFHR) gene cluster. This resulted in a hybrid CFHR protein that inhibited the complement decay-factor H-mediated.
Another genetic cause of C3G has been reported by Gale[53]. Gale et al[53] described two families of Cypriot origin whose members were affected by a mutation in CFHR protein 5. These patients were affected by a C3G that was defined as CFHR5 nephropathy. Indeed, genome-wide linked analysis (GWLA) allowed localization of a genetic abnormality in chromosome 1q31-32. In these patients, a larger CFHR5 protein is generated that is less effective in associating with surface-bound C3b. The resulting disease was known as CFHR5 nephropathy.
Recently, Malik et al[65] described an autosomal dominant complement-mediated C3G associated with abnormal copies in the CFHR3 and CFHR1 loci.
Finally, Habbig et al[66] described two siblings affected by renal disease. Both children had a homozygous deletion of 224 lysine of CFH. This deletion led to a defective complement control[67]. The renal disease was compatible with C3G. The authors proposed the name of C3 deposition glomerulopathy (C3DG) due to the absence of DDD.
Overall, these families highlight the genetic origin of several C3Gs related to a dysregulation of the AP and TCC.
Summarizing, the disease mechanisms in C3G caused by genetic defects identified in family studies may be classified into three categories: (1) homozygous deficiency dysfunction of CFH resulting in excessive C3 activation; (2) hyperfunctional C3 producing excessive C3 activation despite normal CFH activity; and (3) abnormal CFHR protein that enhances CFH dysregulation and consequent excessive C3 activation.
The diagnosis of C3G and differential diagnosis between DDD and C3GN should include a comprehensive pathological analysis and a complete work-up on the genetic and biochemical aspects of complement pathways, with particular regard to the AP.
Using light microscopy, in the case of C3 prevailing without Ig on glomeruli, only a suspicious diagnosis of C3G may be formulated. The definitive diagnosis might only rely on ultra-structural basis.
Overall DDD, is characterized by dense osmiophilic band-like deposits within the GBM. C3GN may be characterized by sub endothelial and mesangial deposits, though intramembranous and sub epithelial deposits may also be present[68]. Several patients may present an overlap in the ultra-structural findings and are difficult to be classified. Proteomic studies may be useful for their identification[50,69].
The evaluation of the complement AP is essential for an improved diagnosis. The evaluation may be performed in several ways: (1) evaluating the total hemolytic complement assay[70]; (2) evaluating the complement alternative pathway assay[71]; and (3) evaluating the complement factor H functional assay[72].
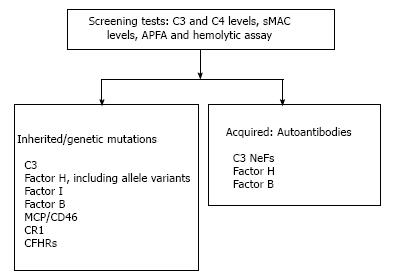
In addition, the C3, C4 and serum MAC (sMAC) levels should be determined. In the case of positivity of these tests, genetic and enzyme-linked immunosorbent assays for complement abnormalities should be performed[8] (Figure 5).
Mutations in the CFH, CFI and CD46 genes have been reported in some patients affected by DDD[39,43]. Changes in factor B and C3 genes may also be present[56,63]. In CFHR5 nephropathy, an internal duplication in the CFHR5 gene is present[53]. Other rearrangements of the CFHR2-CFHR5 hybrid gene and other abnormalities in CFHR1 and CFHR5 have been reported[73-75].
An interpretation of identified variants may be difficult to be understood for several reasons[76]. The pathogenic variants accounts for only 25% of patients affected by DDD and C3GN[43,46]. In addition, mutations in other genes, such as thrombomodulin (THBD), diacylglycerol kinase-epsilon (DGKE), and the CFHR gene family have been recently found to be implicated to contribute to these diseases[77,78].
Moreover, further studies did not confirm a pathogenic role for several missense variants that were originally thought to be at the basis of the disease. Consequently, several amino acid changes in the gene structure are not “de facto” related to the disease[79].
Finally, most variants have a low penetrance and combined variants have been reported in 3% to 12% of patients[80].
The non-genetic causes of C3G are principally auto-antibodies: (1) C3 NeF: It binds directly to C3 convertase prolonging its survival. C3NeFs are found in 80% of patients affected by DDD and in 50% of patients affected by C3GN[43,59]. A C3NeF can stabilize C5 convertase in addition to C3 convertase, which has been previously described[81]. The detection of C3 NeF may be performed in several ways[82]. Further studies are needed to better correlate the presence of C3 NeF with the cause of the diseases and with treatment efficacy; (2) C4 NeF: The role of C4 NeF is still unclear, even if this auto-antibody has been found in some patients affected by MPGN[83]; (3)Anti-factor H auto-antibodies: Have been described in patients affected by DDD[41] and C3GN[46]. They may be detected using an enzyme-linked immunosorbent assay. If an anti-factor H is found, then monoclonal gammopathy should be excluded principally in older people[28,50]; and (4) Anti-factor B auto-antibodies are not frequently found and their research by enzyme-like immunosorbent assay is not easy and is often not available[56]. Overall, the suggested complement investigations in C3G are indicated in Table 2 as suggested by the previously cited consensus report[39].
| Test | Interpretation | Limitations |
| C3 and C4 levels | C3 frequently depressed and support diagnosis; Normal C4 suggests an alternative pathway process | Non-specific |
| Soluble C5b-9 | May be indicator of active disease; May identify patients who will benefit from C5 blockade | Test not widely available |
| C3 nephritic factor | Associated with C3 glomerulopathy; May identify patients who will benefit from B cell targeted therapies | Levels do not correlate with disease activity; also seen in MPGN type I |
| Factor H protein levels | May identify underlying mechanism of alternative pathway activity; May identify patients who will benefit from plasma infusion/exchange | |
| Autoantibodies to factor H and factor B | May identify underlying mechanism of alternative pathway activity; May identify patients who will benefit from B cell targeted therapies | Test not widely available |
| Genetic mutation screening Factor H CFHR1, 2, and 5 Factor I C3 Factor B | May identify underlying mechanism of alternative pathway activity | Not widely available; Clinical implications unknown |
Several treatments for C3Gs may be attempted. According to evidence-based medicine, to date, most of the treatments have not yet been proven to be effective in C3G (Table 3).
| Nonspecific treatment |
| Replace deficient gene products |
| Plasma infusion |
| Liver Transplantation |
| Eliminate autoantibodies and/or mutant proteins |
| Plasma exchange |
| Immunosuppression |
| Treatment of plasma cell dyscrasia |
| Inhibition of complement activation |
| Eculizumab (anti C5) |
| Inhibition of the C3 Convertase |
| Renal transplantation |
| New trials ongoing |
Non-specific or supportive measures: By extrapolating from the treatment of other chronic renal diseases, blood pressure control, reduction of proteinuria and the lowering serum lipid levels should have a beneficial effect in patients affected by C3G, and principally in those affected by a low disease progression[46].
In the previously mentioned French study[43], the renin-angiotensin-aldosterone system (RAAS) blockade was associated with prolonged renal survival, but these findings have not been confirmed by a United States study[49]. In the latter study, the RAAS blockade had beneficial effects only when associated with steroids. In another study, Maisch et al[84] documented the efficacy of a lipid-lowering strategy by statins.
Replacement of deficient gene products: Due to the unavailability of purified complement regulating factors, often a functioning factor may be administered by plasma infusion. The limitation is the need of lifelong substitution therapy.
Plasma infusion is not beneficial in patients affected by a mutation in the membrane cofactor protein because the factor is membrane-bound and not circulating[85]. Plasma infusion is similarly ineffective or even contraindicated in patients affected by gain-of-function mutations or in patients affected by a C3 convertase resistant to factor H[63]. Because CFH, CFI, CFB and C3 are produced by the liver, a simultaneous liver-kidney transplantation may be effective and therapeutically useful[86].
In consideration of frequent short-term complications, of the mortality rate of 15% and of the growing experience with eculizumab, an anti-complement drug, a combined liver-kidney transplantation will lose indication[87,88].
Elimination of the auto-antibodies and/or mutant protein: The use of plasma exchange has a strong rationale[89], but to date, its efficacy has only been confirmed by single case reports. Three patients with DDD had a beneficial effect from plasma exchange, but they were also treated with immunosuppression[90-92]. However, McCaughan et al[93] reported the lack of efficacy of plasma exchange, despite the complete removal of C3NeF. Moreover, in the eculizumab era, the plasma exchange will continue to be used after evaluation of individual patients. Efficacy of immunosuppression is not yet established.
Treatment with steroids led to a clinical improvement in children affected by C3G treated on the basis of a renal biopsy, revealing signs of acute glomerular inflammation with crescents, but a similar improvement was similarly observed in non-treated patients[94]. The combination of steroids with other immunosuppressants has been reported to have a higher beneficial effect[95-97]. These effects have been principally documented in the aHUS. Treatment with an anti-CD20 monoclonal antibody has been effective in one patient affected by DDD with documented anti-CFB auto-antibodies[59].
Very recently, the beneficial effect of mycophenolate mofetil (MMF) in C3G has been reported in a randomized Spanish study[98]. However, due to the lack of controlled trials, treatment with immunosuppressants should be restricted to patients with proteinuria, progressive loss of glomerular filtration rate (GFR) and those with signs of severe inflammation on renal biopsy[89].
An immunosuppressant-based strategy should also be attempted in patients with C3G associated with monoclonal gammopathy, even if the result of such a treatment differed according to different authors[50,51].
Inhibition of complement activation: The most adequate approach to the treatment should be the complement cascade blockade. Eculizumab is a recombinant, fully humanized monoclonal antibody that binds to the C5 complement protein and blocks C5 cleavage[89]. In recent years, eculizumab was highly effective in several kidney diseases, including aHUS and antibody-mediated rejection (ABMR) after renal transplantation[99]. The efficacy of eculizumab in C3Gs to date is only based on the report of single patients, on an open label proof of concept study in 6 patients, and on one ongoing randomized clinical trial (RCT) whose results are unknown to date[100]. Overall, 14 patients affected either by DDD or C3GN treated with eculizumab have been reported. Eight of these patients were described in single case reports and the treatment was successful in seven patients[93,101-107]. In addition to the clinical response, an improvement in renal histology has been observed in patients who underwent a repeated renal biopsy. However, such good results were not confirmed by the proof-of-concept study[108,109]. In this study, a clinical response to eculizumab has been observed in only three patients.
Furthermore, in a recent study, three more patients affected by rapidly progressive C3G have been reported[110]. All these patients responded to eculizumab with an improvement in renal function, a regression of proteinuria and an improvement of glomerular lesions. The phenotypic expression of C3G (DDD vs C3GN) does not predict the response to treatment, even if in biomarkers studies, a higher terminal pathway activity in C3GN has been found[111].
Overall, these results revealed disparate results to the treatment and highlight the possibility that complement dysregulation is not always the same in these patients and that in some of the patients, a resistance to C5 cleavage blockade might exist. The unresponsiveness to eculizumab may have different explanations.
Recently, Nishimura et al[112] documented that some patients affected by paroxysmal nocturnal hemoglobinuria (PNH) had a missense mutation at arginine 885 at the level of the C5 gene. This mutation caused a resistance to C5 cleavage by eculizumab.
In addition, patients affected by C3G, after eculizumab administration, may have a persistent fluid phase C3 convertase activity in the absence of terminal complement activity, which has been documented in a patient with C3G caused by a hybrid CFHR2/CHFR5 protein[64]. In this patient, after eculizumab administration, a block of C5 cleavage and sMAC generation has been obtained, but the hyperfunctioning C3 convertase remained active. Consequently, patients with a C3 convertase dysregulation greater than C5 dysregulation should not be treated with C5 blockade[113]. Moreover, has been documented that this block might aggravate the C3 convertase activity via a feed-back mechanism. Consequently, patients affected by C3G with a prevailing C3 convertase activity should be treated with drugs inhibiting C3 convertase. Blocking the complement AP at the C3 level might be essential in several patients affected by C3G, but the usefulness of such a blockade should be weighed against potential drawbacks as the block of C3b with its critical role in innate immunity.
To date, there are essentially 3 drugs aimed to exert a blockade at the C3 level. The compstatin analog Cp40 was documented to be effective in inhibiting complement dysregulation in vitro in C3G[114]. Compstatin binds to C3 and C3b, preventing the complement dysregulation caused by genetic mutations or by auto-antibodies. To date, compstatin is used in trials for macular degeneration and PNH. Similarly, a monoclonal antibody, which inhibits C3 convertase induced by C3NeF by binding to C3b is currently in the preclinical phase[115]. The most advanced drug among the C3 inhibitors is CDX1135, which is also known as TP10 and the soluble complement receptor 1 (sCR1). CR1 is a cell surface glycoprotein expressed on several cells, including immune cells. sCR1 is a protein that can regulate C3 convertase. Under normal conditions, only small quantities of sCR1 are in circulation. Administration of a high quantity of sCR1 in patients undergoing cardiac surgery revealed that this protein is able to exert a complement inhibition effective and safe[116,117]. Recently, at Iowa University, the efficacy of sCR1 has been documented in vitro and in mice affected by C3G[118].
Renal transplantation: C3G has a frequent evolution towards ESRD. Renal transplantation has been proposed for ESRD patients affected by C3G. Renal transplantation in such patients has two principal challenges: (1) whether to perform a dual liver-kidney transplantation; and (2) The high recurrence rates of the disease and its treatment.
The question of liver-kidney transplantation has been previously mentioned above[86-88] and has been documented that in the eculizumab era, the liver-kidney transplantation will lose its relevance.
In the case of the kidney transplant alone the principal challenge is the high recurrence rate. In the case of DDD, the risk of recurrence is over 70%[119], with a high risk of graft loss[120,121]. These data confirmed a retrospective United States study including 75 children affected by DDD[122] and a more recent Irish cohort, including 33 patients affected by DDD[123]. Fewer data have been reported on the recurrence risk of C3GN. The most relevant study has been published by Zand et al[124] from the Mayo clinic. They report 21 renal transplant patients affected by C3GN. The recurrence rate was as high as 70% and the graft failure occurred in 50% of the patients. Importantly, all these reports with high recurrence rates also include patients transplanted in the pre anti-complement era. Transplants in patients affected by CFHR nephropathy has been reported in 11 subjects. All transplants were successful, despite the histological recurrence in three patients[125].
The treatment of recurrent disease has not yet been the object of clinical trials. Close monitoring is mandatory following renal transplantation to promptly detect the clinical signs of recurrence. Patients with circulating auto-antibodies might be treated by agents targeting T and B cells, but we should remember that the transplanted patients are already on immunosuppressant drugs.
Anti-complement drugs are promising. McCaughan et al[93] described the first report of a transplanted patient affected by recurrent DDD and who was successfully treated by eculizumab. However, the long-term dependence on eculizumab and the long-term safety of the drug remain open questions and the object of future RCTs. Another interesting approach is the use of sCR1, but its use to date has been limited to the native disease and not to its recurrence after transplantation.
Clinical trials ongoing: C3G is a rare disease and it is not surprising that ongoing RCTs are scarce. From one perspective the market interest is poor due to the few numbers of patients. However, a wide comprehensive multinational network among centers should be developed to include a significant number of patients for a RCT.
To date, four clinical trials are ongoing on C3G. Two trials aimed to evaluate eculizumab therapy in DDD and C3GN[100,126]. Two other RCTs are evaluating the effect of two different formulations of sCR1 on C3G[127,128].
Other drugs, such as compstatin and monoclonal antibody against C3 convertase, are still in the pre-clinical phase.
The Mayo Clinic/Renal Pathology Society Consensus Conference held in 2015 allowed the elaboration of a new etiology-pathology based classification of the GN, which substitutes for the old morphologic-based classification (1). In addition, before the Consensus Conference, the MPGNs had been the object of new classifications for several years. To date, it is clear that the MPGNs should be distinguished into two principal categories: The immune-complex-mediated MPGN and the complement-dysregulation-mediated MPGN. This finding is principally relevant, not only from a taxonomic perspective, but also from a diagnostic and therapeutic approach. New findings in the complement related pathways and in genetics allowed for an improved understanding and definition of complement related MPGN, in addition to the discovery of new entities, such as C3GN and CFHR5 GN. To date, the MPGNs have a new diagnostic approach with a new network that applies to the immune-complexes related MPGN and complement-related MPGN.
Currently, fewer new drugs are available for the treatment of immune-complexes MPGN.
With the discovery of complement-inhibitor drugs, there has been more progress for the complement related MPGN. However, due to the rarity of the disease, well conducted RCTs are scarce. This finding supports the need to perform more multinational cooperative studies to identify an evidence-based medicine therapeutic approach.
P- Reviewer: Fujigaki Y, Pedersen E, Trimarchi H, Watanabe T S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Sethi S, Haas M, Markowitz GS, D’Agati VD, Rennke HG, Jennette JC, Bajema IM, Alpers CE, Chang A, Cornell LD. Mayo Clinic/Renal Pathology Society Consensus Report on Pathologic Classification, Diagnosis, and Reporting of GN. J Am Soc Nephrol. 2016;27:1278-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 197] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 2. | Cook HT, Pickering MC. Histopathology of MPGN and C3 glomerulopathies. Nat Rev Nephrol. 2015;11:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Levy M, Gubler MC, Sich M, Beziau A, Habib R. Immunopathology of membranoproliferative glomerulonephritis with subendothelial deposits (Type I MPGN). Clin Immunol Immunopathol. 1978;10:477-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Habib R, Gubler MC, Loirat C, Mäiz HB, Levy M. Dense deposit disease: a variant of membranoproliferative glomerulonephritis. Kidney Int. 1975;7:204-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 130] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Strife CF, McEnery PT, McAdams AJ, West CD. Membranoproliferative glomerulonephritis with disruption of the glomerular basement membrane. Clin Nephrol. 1977;7:65-72. [PubMed] |
| 6. | Salvadori M, Rosso G, Bertoni E. Complement involvement in kidney diseases: From physiopathology to therapeutical targeting. World J Nephrol. 2015;4:169-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Bomback AS, Appel GB. Pathogenesis of the C3 glomerulopathies and reclassification of MPGN. Nat Rev Nephrol. 2012;8:634-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis--a new look at an old entity. N Engl J Med. 2012;366:1119-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 329] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 10. | Noris M, Remuzzi G. Glomerular Diseases Dependent on Complement Activation, Including Atypical Hemolytic Uremic Syndrome, Membranoproliferative Glomerulonephritis, and C3 Glomerulopathy: Core Curriculum 2015. Am J Kidney Dis. 2015;66:359-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | Smith KD, Alpers CE. Pathogenic mechanisms in membranoproliferative glomerulonephritis. Curr Opin Nephrol Hypertens. 2005;14:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Rennke HG. Secondary membranoproliferative glomerulonephritis. Kidney Int. 1995;47:643-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 69] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Alpers CE, Smith KD. Cryoglobulinemia and renal disease. Curr Opin Nephrol Hypertens. 2008;17:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Roccatello D, Fornasieri A, Giachino O, Rossi D, Beltrame A, Banfi G, Confalonieri R, Tarantino A, Pasquali S, Amoroso A. Multicenter study on hepatitis C virus-related cryoglobulinemic glomerulonephritis. Am J Kidney Dis. 2007;49:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Rodrigues VL, Otoni A, Voieta I, Antunes CM, Lambertucci JR. Glomerulonephritis in schistosomiasis mansoni: a time to reappraise. Rev Soc Bras Med Trop. 2010;43:638-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Ohara S, Kawasaki Y, Takano K, Isome M, Nozawa R, Suzuki H, Hosoya M. Glomerulonephritis associated with chronic infection from long-term central venous catheterization. Pediatr Nephrol. 2006;21:427-429. [PubMed] [DOI] [Full Text] |
| 17. | Vella J, Carmody M, Campbell E, Browne O, Doyle G, Donohoe J. Glomerulonephritis after ventriculo-atrial shunt. QJM. 1995;88:911-918. [PubMed] |
| 18. | Adam FU, Torun D, Bolat F, Zumrutdal A, Sezer S, Ozdemir FN. Acute renal failure due to mesangial proliferative glomerulonephritis in a pregnant woman with primary Sjögren’s syndrome. Clin Rheumatol. 2006;25:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Khan MA, Akhtar M, Taher SM. Membranoproliferative glomerulonephritis in a patient with primary Sjögren’s syndrome. Report of a case with review of the literature. Am J Nephrol. 1988;8:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Korpela M, Mustonen J, Teppo AM, Helin H, Pasternack A. Mesangial glomerulonephritis as an extra-articular manifestation of rheumatoid arthritis. Br J Rheumatol. 1997;36:1189-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1335] [Cited by in RCA: 1343] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 22. | Sethi S, Fervenza FC, Rajkumar SV. Spectrum of manifestations of monoclonal gammopathy-associated renal lesions. Curr Opin Nephrol Hypertens. 2016;25:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Mutluay R, Aki SZ, Erten Y, Konca C, Yagci M, Barit G, Sindel S. Membranoproliferative glomerulonephritis and light-chain nephropathy in association with chronic lymphocytic leukemia. Clin Nephrol. 2008;70:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Sethi S, Zand L, Leung N, Smith RJ, Jevremonic D, Herrmann SS, Fervenza FC. Membranoproliferative glomerulonephritis secondary to monoclonal gammopathy. Clin J Am Soc Nephrol. 2010;5:770-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Sethi S, Sukov WR, Zhang Y, Fervenza FC, Lager DJ, Miller DV, Cornell LD, Krishnan SG, Smith RJ. Dense deposit disease associated with monoclonal gammopathy of undetermined significance. Am J Kidney Dis. 2010;56:977-982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Sethi S, Rajkumar SV. Monoclonal gammopathy-associated proliferative glomerulonephritis. Mayo Clin Proc. 2013;88:1284-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Jokiranta TS, Solomon A, Pangburn MK, Zipfel PF, Meri S. Nephritogenic lambda light chain dimer: a unique human miniautoantibody against complement factor H. J Immunol. 1999;163:4590-4596. [PubMed] |
| 28. | Meri S, Koistinen V, Miettinen A, Törnroth T, Seppälä IJ. Activation of the alternative pathway of complement by monoclonal lambda light chains in membranoproliferative glomerulonephritis. J Exp Med. 1992;175:939-950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 154] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Alchi B, Jayne D. Membranoproliferative glomerulonephritis. Pediatr Nephrol. 2010;25:1409-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (2)] |
| 30. | Levin A. Management of membranoproliferative glomerulonephritis: evidence-based recommendations. Kidney Int Suppl. 1999;70:S41-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Hewins P, Smith RJH, Savage COS Idiopathic membranoproliferative glomerulonephritis. In: Berl; Himmelfarb; Mitch; Murphy; Pioli; Wilcox; Salant; Yu, editors. Therapy in Nephrology and Hypertension. UK: Elsevier Inc 2007; 249-256. |
| 32. | Andresdottir MB, Assmann KJ, Hoitsma AJ, Koene RA, Wetzels JF. Recurrence of type I membranoproliferative glomerulonephritis after renal transplantation: analysis of the incidence, risk factors, and impact on graft survival. Transplantation. 1997;63:1628-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Karakayali FY, Ozdemir H, Kivrakdal S, Colak T, Emiroğlu R, Haberal M. Recurrent glomerular diseases after renal transplantation. Transplant Proc. 2006;38:470-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Lorenz EC, Sethi S, Leung N, Dispenzieri A, Fervenza FC, Cosio FG. Recurrent membranoproliferative glomerulonephritis after kidney transplantation. Kidney Int. 2010;77:721-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Alasfar S, Carter-Monroe N, Rosenberg AZ, Montgomery RA, Alachkar N. Membranoproliferative glomerulonephritis recurrence after kidney transplantation: using the new classification. BMC Nephrol. 2016;17:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Moroni G, Casati C, Quaglini S, Gallelli B, Banfi G, Montagnino G, Messa P. Membranoproliferative glomerulonephritis type I in renal transplantation patients: a single-center study of a cohort of 68 renal transplants followed up for 11 years. Transplantation. 2011;91:1233-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Servais A, Frémeaux-Bacchi V, Lequintrec M, Salomon R, Blouin J, Knebelmann B, Grünfeld JP, Lesavre P, Noël LH, Fakhouri F. Primary glomerulonephritis with isolated C3 deposits: a new entity which shares common genetic risk factors with haemolytic uraemic syndrome. J Med Genet. 2007;44:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 217] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 38. | Fakhouri F, Frémeaux-Bacchi V, Noël LH, Cook HT, Pickering MC. C3 glomerulopathy: a new classification. Nat Rev Nephrol. 2010;6:494-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 251] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 39. | Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, Alpers CE, Bajema IM, Bedrosian C, Braun M. C3 glomerulopathy: consensus report. Kidney Int. 2013;84:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 449] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 40. | Sethi S, Nester CM, Smith RJ. Membranoproliferative glomerulonephritis and C3 glomerulopathy: resolving the confusion. Kidney Int. 2012;81:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 41. | Smith RJ, Harris CL, Pickering MC. Dense deposit disease. Mol Immunol. 2011;48:1604-1610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Galle P, Mahieu P. Electron dense alteration of kidney basement membranes. A renal lesion specific of a systemic disease. Am J Med. 1975;58:749-764. [PubMed] |
| 43. | Servais A, Noël LH, Roumenina LT, Le Quintrec M, Ngo S, Dragon-Durey MA, Macher MA, Zuber J, Karras A, Provot F. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82:454-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 401] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 44. | Barbour TD, Pickering MC, Terence Cook H. Dense deposit disease and C3 glomerulopathy. Semin Nephrol. 2013;33:493-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Lu DF, Moon M, Lanning LD, McCarthy AM, Smith RJ. Clinical features and outcomes of 98 children and adults with dense deposit disease. Pediatr Nephrol. 2012;27:773-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 46. | Sethi S, Fervenza FC, Zhang Y, Zand L, Vrana JA, Nasr SH, Theis JD, Dogan A, Smith RJ. C3 glomerulonephritis: clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int. 2012;82:465-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 47. | Duvall-Young J, MacDonald MK, McKechnie NM. Fundus changes in (type II) mesangiocapillary glomerulonephritis simulating drusen: a histopathological report. Br J Ophthalmol. 1989;73:297-302. [PubMed] |
| 48. | Barbour TD, Pickering MC, Cook HT. Recent insights into C3 glomerulopathy. Nephrol Dial Transplant. 2013;28:1685-1693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Nasr SH, Valeri AM, Appel GB, Sherwinter J, Stokes MB, Said SM, Markowitz GS, D’Agati VD. Dense deposit disease: clinicopathologic study of 32 pediatric and adult patients. Clin J Am Soc Nephrol. 2009;4:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 50. | Bridoux F, Desport E, Frémeaux-Bacchi V, Chong CF, Gombert JM, Lacombe C, Quellard N, Touchard G. Glomerulonephritis with isolated C3 deposits and monoclonal gammopathy: a fortuitous association? Clin J Am Soc Nephrol. 2011;6:2165-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 51. | Zand L, Kattah A, Fervenza FC, Smith RJ, Nasr SH, Zhang Y, Vrana JA, Leung N, Cornell LD, Sethi S. C3 glomerulonephritis associated with monoclonal gammopathy: a case series. Am J Kidney Dis. 2013;62:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 52. | Angelo JR, Bell CS, Braun MC. Allograft failure in kidney transplant recipients with membranoproliferative glomerulonephritis. Am J Kidney Dis. 2011;57:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Gale DP, de Jorge EG, Cook HT, Martinez-Barricarte R, Hadjisavvas A, McLean AG, Pusey CD, Pierides A, Kyriacou K, Athanasiou Y. Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet. 2010;376:794-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 255] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 54. | Athanasiou Y, Voskarides K, Gale DP, Damianou L, Patsias C, Zavros M, Maxwell PH, Cook HT, Demosthenous P, Hadjisavvas A. Familial C3 glomerulopathy associated with CFHR5 mutations: clinical characteristics of 91 patients in 16 pedigrees. Clin J Am Soc Nephrol. 2011;6:1436-1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 55. | Sethi S, Fervenza FC, Zhang Y, Nasr SH, Leung N, Vrana J, Cramer C, Nester CM, Smith RJ. Proliferative glomerulonephritis secondary to dysfunction of the alternative pathway of complement. Clin J Am Soc Nephrol. 2011;6:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 56. | Strobel S, Zimmering M, Papp K, Prechl J, Józsi M. Anti-factor B autoantibody in dense deposit disease. Mol Immunol. 2010;47:1476-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 57. | Daha MR, Fearon DT, Austen KF. C3 nephritic factor (C3NeF): stabilization of fluid phase and cell-bound alternative pathway convertase. J Immunol. 1976;116:1-7. [PubMed] |
| 58. | Tanuma Y, Ohi H, Hatano M. Two types of C3 nephritic factor: properdin-dependent C3NeF and properdin-independent C3NeF. Clin Immunol Immunopathol. 1990;56:226-238. [PubMed] |
| 59. | Chen Q, Müller D, Rudolph B, Hartmann A, Kuwertz-Bröking E, Wu K, Kirschfink M, Skerka C, Zipfel PF. Combined C3b and factor B autoantibodies and MPGN type II. N Engl J Med. 2011;365:2340-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Zhang Y, Meyer NC, Wang K, Nishimura C, Frees K, Jones M, Katz LM, Sethi S, Smith RJ. Causes of alternative pathway dysregulation in dense deposit disease. Clin J Am Soc Nephrol. 2012;7:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 61. | Goodship TH, Pappworth IY, Toth T, Denton M, Houlberg K, McCormick F, Warland D, Moore I, Hunze EM, Staniforth SJ. Factor H autoantibodies in membranoproliferative glomerulonephritis. Mol Immunol. 2012;52:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 62. | Blanc C, Togarsimalemath SK, Chauvet S, Le Quintrec M, Moulin B, Buchler M, Jokiranta TS, Roumenina LT, Fremeaux-Bacchi V, Dragon-Durey MA. Anti-factor H autoantibodies in C3 glomerulopathies and in atypical hemolytic uremic syndrome: one target, two diseases. J Immunol. 2015;194:5129-5138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 63. | Martínez-Barricarte R, Heurich M, Valdes-Cañedo F, Vazquez-Martul E, Torreira E, Montes T, Tortajada A, Pinto S, Lopez-Trascasa M, Morgan BP. Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J Clin Invest. 2010;120:3702-3712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 64. | Chen Q, Wiesener M, Eberhardt HU, Hartmann A, Uzonyi B, Kirschfink M, Amann K, Buettner M, Goodship T, Hugo C. Complement factor H-related hybrid protein deregulates complement in dense deposit disease. J Clin Invest. 2014;124:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 65. | Malik TH, Lavin PJ, Goicoechea de Jorge E, Vernon KA, Rose KL, Patel MP, de Leeuw M, Neary JJ, Conlon PJ, Winn MP. A hybrid CFHR3-1 gene causes familial C3 glomerulopathy. J Am Soc Nephrol. 2012;23:1155-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 66. | Habbig S, Mihatsch MJ, Heinen S, Beck B, Emmel M, Skerka C, Kirschfink M, Hoppe B, Zipfel PF, Licht C. C3 deposition glomerulopathy due to a functional factor H defect. Kidney Int. 2009;75:1230-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 67. | Licht C, Heinen S, Józsi M, Löschmann I, Saunders RE, Perkins SJ, Waldherr R, Skerka C, Kirschfink M, Hoppe B. Deletion of Lys224 in regulatory domain 4 of Factor H reveals a novel pathomechanism for dense deposit disease (MPGN II). Kidney Int. 2006;70:42-50. [PubMed] |
| 68. | Sethi S, Fervenza FC, Smith RJ, Haas M. Overlap of ultrastructural findings in C3 glomerulonephritis and dense deposit disease. Kidney Int. 2015;88:1449-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 69. | Sethi S, Gamez JD, Vrana JA, Theis JD, Bergen HR, Zipfel PF, Dogan A, Smith RJ. Glomeruli of Dense Deposit Disease contain components of the alternative and terminal complement pathway. Kidney Int. 2009;75:952-960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 70. | Yamamoto S, Kubotsu K, Kida M, Kondo K, Matsuura S, Uchiyama S, Yonekawa O, Kanno T. Automated homogeneous liposome-based assay system for total complement activity. Clin Chem. 1995;41:586-590. [PubMed] |
| 71. | Sethi S, Smith RJ, Dillon JJ, Fervenza FC. C3 glomerulonephritis associated with complement factor B mutation. Am J Kidney Dis. 2015;65:520-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 72. | Sánchez-Corral P, González-Rubio C, Rodríguez de Córdoba S, López-Trascasa M. Functional analysis in serum from atypical Hemolytic Uremic Syndrome patients reveals impaired protection of host cells associated with mutations in factor H. Mol Immunol. 2004;41:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 73. | Chen Q, Wiesener M, Eberhrdt H, Hartmann A, Hugo C, Skerka C, Zipfel PF. A novel hybrid CFHR2/CFHR5 gene develops MPGN II and provides insights into disease mechanism and therapeutic implications. Immunobiology. 2012;217:1131-1132. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 74. | Tortajada A, Yébenes H, Abarrategui-Garrido C, Anter J, García-Fernández JM, Martínez-Barricarte R, Alba-Domínguez M, Malik TH, Bedoya R, Cabrera Pérez R. C3 glomerulopathy-associated CFHR1 mutation alters FHR oligomerization and complement regulation. J Clin Invest. 2013;123:2434-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 75. | Medjeral-Thomas N, Malik TH, Patel MP, Toth T, Cook HT, Tomson C, Pickering MC. A novel CFHR5 fusion protein causes C3 glomerulopathy in a family without Cypriot ancestry. Kidney Int. 2014;85:933-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 76. | Angioi A, Fervenza FC, Sethi S, Zhang Y, Smith RJ, Murray D, Van Praet J, Pani A, De Vriese AS. Diagnosis of complement alternative pathway disorders. Kidney Int. 2016;89:278-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 77. | Noris M, Mele C, Remuzzi G. Podocyte dysfunction in atypical haemolytic uraemic syndrome. Nat Rev Nephrol. 2015;11:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 78. | Barbour TD, Ruseva MM, Pickering MC. Update on C3 glomerulopathy. Nephrol Dial Transplant. 2014; Oct 17; Epub ahead of print. [PubMed] |
| 79. | Tortajada A, Pinto S, Martínez-Ara J, López-Trascasa M, Sánchez-Corral P, de Córdoba SR. Complement factor H variants I890 and L1007 while commonly associated with atypical hemolytic uremic syndrome are polymorphisms with no functional significance. Kidney Int. 2012;81:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 80. | Bresin E, Rurali E, Caprioli J, Sanchez-Corral P, Fremeaux-Bacchi V, Rodriguez de Cordoba S, Pinto S, Goodship TH, Alberti M, Ribes D. Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype. J Am Soc Nephrol. 2013;24:475-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 280] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 81. | Skattum L, Mårtensson U, Sjöholm AG. Hypocomplementaemia caused by C3 nephritic factors (C3 NeF): clinical findings and the coincidence of C3 NeF type II with anti-C1q autoantibodies. J Intern Med. 1997;242:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Paixão-Cavalcante D, López-Trascasa M, Skattum L, Giclas PC, Goodship TH, de Córdoba SR, Truedsson L, Morgan BP, Harris CL. Sensitive and specific assays for C3 nephritic factors clarify mechanisms underlying complement dysregulation. Kidney Int. 2012;82:1084-1092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 83. | Ohi H, Yasugi T. Occurrence of C3 nephritic factor and C4 nephritic factor in membranoproliferative glomerulonephritis (MPGN). Clin Exp Immunol. 1994;95:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | Maisch NM, Pezzillo KK. HMG-CoA reductase inhibitors for the prevention of nephropathy. Ann Pharmacother. 2004;38:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | Caprioli J, Noris M, Brioschi S, Pianetti G, Castelletti F, Bettinaglio P, Mele C, Bresin E, Cassis L, Gamba S. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006;108:1267-1279. [PubMed] |
| 86. | Tran H, Chaudhuri A, Concepcion W, Grimm PC. Use of eculizumab and plasma exchange in successful combined liver-kidney transplantation in a case of atypical HUS associated with complement factor H mutation. Pediatr Nephrol. 2014;29:477-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 87. | Saland J. Liver-kidney transplantation to cure atypical HUS: still an option post-eculizumab? Pediatr Nephrol. 2014;29:329-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | Coppo R, Bonaudo R, Peruzzi RL, Amore A, Brunati A, Romagnoli R, Salizzoni M, Galbusera M, Gotti E, Daina E. Liver transplantation for aHUS: still needed in the eculizumab era? Pediatr Nephrol. 2016;31:759-768. [PubMed] |
| 89. | De Vriese AS, Sethi S, Van Praet J, Nath KA, Fervenza FC. Kidney Disease Caused by Dysregulation of the Complement Alternative Pathway: An Etiologic Approach. J Am Soc Nephrol. 2015;26:2917-2929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 90. | Banks RA, May S, Wallington T. Acute renal failure in dense deposit disease: recovery after plasmapheresis. Br Med J (Clin Res Ed). 1982;284:1874-1875. [PubMed] |
| 91. | Krmar RT, Holtbäck U, Linné T, Berg UB, Celsi G, Söderberg MP, Wernerson A, Szakos A, Larsson S, Skattum L. Acute renal failure in dense deposit disease: complete recovery after combination therapy with immunosuppressant and plasma exchange. Clin Nephrol. 2011;75 Suppl 1:4-10. [PubMed] |
| 92. | Kurtz KA, Schlueter AJ. Management of membranoproliferative glomerulonephritis type II with plasmapheresis. J Clin Apher. 2002;17:135-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 93. | McCaughan JA, O’Rourke DM, Courtney AE. Recurrent dense deposit disease after renal transplantation: an emerging role for complementary therapies. Am J Transplant. 2012;12:1046-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 94. | West CD, McAdams AJ, Witte DP. Acute non-proliferative glomerulitis: a cause of renal failure unique to children. Pediatr Nephrol. 2000;14:786-793. [PubMed] |
| 95. | Dragon-Durey MA, Sethi SK, Bagga A, Blanc C, Blouin J, Ranchin B, André JL, Takagi N, Cheong HI, Hari P. Clinical features of anti-factor H autoantibody-associated hemolytic uremic syndrome. J Am Soc Nephrol. 2010;21:2180-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 96. | Sinha A, Gulati A, Saini S, Blanc C, Gupta A, Gurjar BS, Saini H, Kotresh ST, Ali U, Bhatia D. Prompt plasma exchanges and immunosuppressive treatment improves the outcomes of anti-factor H autoantibody-associated hemolytic uremic syndrome in children. Kidney Int. 2014;85:1151-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 97. | Boyer O, Balzamo E, Charbit M, Biebuyck-Gougé N, Salomon R, Dragon-Durey MA, Frémeaux-Bacchi V, Niaudet P. Pulse cyclophosphamide therapy and clinical remission in atypical hemolytic uremic syndrome with anti-complement factor H autoantibodies. Am J Kidney Dis. 2010;55:923-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 98. | Rabasco C, Cavero T, Román E, Rojas-Rivera J, Olea T, Espinosa M, Cabello V, Fernández-Juarez G, González F, Ávila A. Effectiveness of mycophenolate mofetil in C3 glomerulonephritis. Kidney Int. 2015;88:1153-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 99. | Frémeaux-Bacchi V, Legendre CM. The emerging role of complement inhibitors in transplantation. Kidney Int. 2015;88:967-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 100. | Columbia University. Eculizumab Therapy for Dense Deposit Disease and C3 Nephropathy. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https: //www.clinicaltrials.gov/ct2/show/NCT01221181. |
| 101. | Radhakrishnan S, Lunn A, Kirschfink M, Thorner P, Hebert D, Langlois V, Pluthero F, Licht C. Eculizumab and refractory membranoproliferative glomerulonephritis. N Engl J Med. 2012;366:1165-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 102. | Daina E, Noris M, Remuzzi G. Eculizumab in a patient with dense-deposit disease. N Engl J Med. 2012;366:1161-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 103. | Vivarelli M, Pasini A, Emma F. Eculizumab for the treatment of dense-deposit disease. N Engl J Med. 2012;366:1163-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 104. | Rousset-Rouvière C, Cailliez M, Garaix F, Bruno D, Laurent D, Tsimaratos M. Rituximab fails where eculizumab restores renal function in C3nef-related DDD. Pediatr Nephrol. 2014;29:1107-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 105. | Ozkaya O, Nalcacioglu H, Tekcan D, Genc G, Meydan BC, Ozdemir BH, Baysal MK, Keceligil HT. Eculizumab therapy in a patient with dense-deposit disease associated with partial lipodystropy. Pediatr Nephrol. 2014;29:1283-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 106. | Sánchez-Moreno A, De la Cerda F, Cabrera R, Fijo J, López-Trascasa M, Bedoya R, Rodríguez de Córdoba S, Ybot-González P. Eculizumab in dense-deposit disease after renal transplantation. Pediatr Nephrol. 2014;29:2055-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 107. | Gurkan S, Fyfe B, Weiss L, Xiao X, Zhang Y, Smith RJ. Eculizumab and recurrent C3 glomerulonephritis. Pediatr Nephrol. 2013;28:1975-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 108. | Bomback AS, Smith RJ, Barile GR, Zhang Y, Heher EC, Herlitz L, Stokes MB, Markowitz GS, D’Agati VD, Canetta PA. Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin J Am Soc Nephrol. 2012;7:748-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 263] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 109. | Herlitz LC, Bomback AS, Markowitz GS, Stokes MB, Smith RN, Colvin RB, Appel GB, D’Agati VD. Pathology after eculizumab in dense deposit disease and C3 GN. J Am Soc Nephrol. 2012;23:1229-1237. [PubMed] |
| 110. | Le Quintrec M, Lionet A, Kandel C, Bourdon F, Gnemmi V, Colombat M, Goujon JM, Frémeaux-Bacchi V, Fakhouri F. Eculizumab for treatment of rapidly progressive C3 glomerulopathy. Am J Kidney Dis. 2015;65:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 111. | Zhang Y, Nester CM, Martin B, Skjoedt MO, Meyer NC, Shao D, Borsa N, Palarasah Y, Smith RJ. Defining the complement biomarker profile of C3 glomerulopathy. Clin J Am Soc Nephrol. 2014;9:1876-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 112. | Nishimura J, Yamamoto M, Hayashi S, Ohyashiki K, Ando K, Brodsky AL, Noji H, Kitamura K, Eto T, Takahashi T. Genetic variants in C5 and poor response to eculizumab. N Engl J Med. 2014;370:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 293] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 113. | Nester CM, Smith RJ. Treatment options for C3 glomerulopathy. Curr Opin Nephrol Hypertens. 2013;22:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 114. | Zhang Y, Shao D, Ricklin D, Hilkin BM, Nester CM, Lambris JD, Smith RJ. Compstatin analog Cp40 inhibits complement dysregulation in vitro in C3 glomerulopathy. Immunobiology. 2015;220:993-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 115. | Paixão-Cavalcante D, Torreira E, Lindorfer MA, Rodriguez de Cordoba S, Morgan BP, Taylor RP, Llorca O, Harris CL. A humanized antibody that regulates the alternative pathway convertase: potential for therapy of renal disease associated with nephritic factors. J Immunol. 2014;192:4844-4851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | Lazar HL, Keilani T, Fitzgerald CA, Shapira OM, Hunter CT, Shemin RJ, Marsh HC, Ryan US. Beneficial effects of complement inhibition with soluble complement receptor 1 (TP10) during cardiac surgery: is there a gender difference? Circulation. 2007;116:I83-I88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 117. | Li JS, Sanders SP, Perry AE, Stinnett SS, Jaggers J, Bokesch P, Reynolds L, Nassar R, Anderson PA. Pharmacokinetics and safety of TP10, soluble complement receptor 1, in infants undergoing cardiopulmonary bypass. Am Heart J. 2004;147:173-180. [PubMed] |
| 118. | Zhang Y, Nester CM, Holanda DG, Marsh HC, Hammond RA, Thomas LJ, Meyer NC, Hunsicker LG, Sethi S, Smith RJ. Soluble CR1 therapy improves complement regulation in C3 glomerulopathy. J Am Soc Nephrol. 2013;24:1820-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 119. | Barbour S, Gill JS. Advances in the understanding of complement mediated glomerular disease: implications for recurrence in the transplant setting. Am J Transplant. 2015;15:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 120. | Ponticelli C, Glassock RJ. Posttransplant recurrence of primary glomerulonephritis. Clin J Am Soc Nephrol. 2010;5:2363-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 121. | Sprangers B, Kuypers DR. Recurrence of glomerulonephritis after renal transplantation. Transplant Rev (Orlando). 2013;27:126-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 122. | Braun MC, Stablein DM, Hamiwka LA, Bell L, Bartosh SM, Strife CF. Recurrence of membranoproliferative glomerulonephritis type II in renal allografts: The North American Pediatric Renal Transplant Cooperative Study experience. J Am Soc Nephrol. 2005;16:2225-2233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 123. | Little MA, Dupont P, Campbell E, Dorman A, Walshe JJ. Severity of primary MPGN, rather than MPGN type, determines renal survival and post-transplantation recurrence risk. Kidney Int. 2006;69:504-511. [PubMed] |
| 124. | Zand L, Lorenz EC, Cosio FG, Fervenza FC, Nasr SH, Gandhi MJ, Smith RJ, Sethi S. Clinical findings, pathology, and outcomes of C3GN after kidney transplantation. J Am Soc Nephrol. 2014;25:1110-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 125. | Vernon KA, Gale DP, de Jorge EG, McLean AG, Galliford J, Pierides A, Maxwell PH, Taube D, Pickering MC, Cook HT. Recurrence of complement factor H-related protein 5 nephropathy in a renal transplant. Am J Transplant. 2011;11:152-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 126. | MarioNegri Institute for Pharmacological Research. Eculizumab in Primary MPGN (EAGLE). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://www.clinicaltrials.gov/ct2/show/NCT02093533 NLM Identifier: NCT02093533. |
| 127. | University of Iowa. TP10 use in Patients with C3 Glomerulopathy (C3G). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://www.clinicaltrials.gov/ct2/show/NCT02302755 NLM Identifier: NCT 02302755. |
| 128. | University of Iowa. Clinical Trial of CDX-1135 in Pediatric and Adult Patients with Dense Deposit Disease. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://www.clinicaltrials.gov/ct2/show/NCTNCT01791686 NLM Identifier: NCT01791686. |