Published online May 6, 2016. doi: 10.5527/wjn.v5.i3.233
Peer-review started: November 3, 2015
First decision: December 4, 2015
Revised: January 16, 2016
Accepted: March 9, 2016
Article in press: March 14, 2016
Published online: May 6, 2016
Processing time: 180 Days and 22.7 Hours
Chronic kidney disease (CKD) is a significant public health problem as risk factors such as advanced age, obesity, hypertension and diabetes rise in the global population. Currently there are no effective pharmacologic treatments for this disease. The role of diet is important for slowing the progression of CKD and managing symptoms in later stages of renal insufficiency. While low protein diets are generally recommended, maintaining adequate levels of intake is critical for health. There is an increasing appreciation that the source of protein may also be important. Soybean protein has been the most extensively studied plant-based protein in subjects with kidney disease and has demonstrated renal protective properties in a number of clinical studies. Soy protein consumption has been shown to slow the decline in estimated glomerular filtration rate and significantly improve proteinuria in diabetic and non-diabetic patients with nephropathy. Soy’s beneficial effects on renal function may also result from its impact on certain physiological risk factors for CKD such as dyslipidemia, hypertension and hyperglycemia. Soy intake is also associated with improvements in antioxidant status and systemic inflammation in early and late stage CKD patients. Studies conducted in animal models have helped to identify the underlying molecular mechanisms that may play a role in the positive effects of soy protein on renal parameters in polycystic kidney disease, metabolically-induced kidney dysfunction and age-associated progressive nephropathy. Despite the established relationship between soy and renoprotection, further studies are needed for a clear understanding of the role of the cellular and molecular target(s) of soy protein in maintaining renal function.
Core tip: This review summarizes the data, both animal and limited human studies, that support the hypothesis that a soy-enriched diet is protective against chronic kidney disease. While the clinical studies have small subject numbers, the data suggest that soy improves renal function, or attenuates the progression of chronic renal dysfunction. The potential mechanisms of action, from both experimental and clinical studies, is also discussed, including positive effects on lipid and blood glucose profiles, improved vascular function and reduced inflammation. Consideration is also given to the potential active ingredients within soy, including both protein and isoflavones, that may mediate the renoprotective effect of the botanical.
- Citation: McGraw NJ, Krul ES, Grunz-Borgmann E, Parrish AR. Soy-based renoprotection. World J Nephrol 2016; 5(3): 233-257
- URL: https://www.wjgnet.com/2220-6124/full/v5/i3/233.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i3.233
Chronic kidney disease (CKD) is characterized by kidney damage and/or dysfunction that is present for more than 3 mo and that leads to complications in other organ systems[1,2]. The leading causes of CKD are Type 2 diabetes and hypertension[3-7] with less frequent causes being glomerulonephritis, nephrolithiasis, polycystic kidney disease (PKD), systemic lupus and hepatitis[7,8]. Five stages of CKD severity are determined using measures of estimated glomerular filtration rate (eGFR), with measures of urinary albumin excretion rate (AER) conferring additional prognostic classification[1,2]. These stages range from an asymptomatic condition (eGFR > 60 mL/min per 1.73 m2 and urinary albumin < 30 mg/g) to end-stage renal disease (ESRD) (eGFR < 15 mL/min per 1.73 m2 and urinary albumin > 300 mg/g) requiring kidney dialysis or transplantation. The prevalence of CKD varies between 2.5%-11.2% of adults in Europe, Asia, North America and Australia[9]. In the United States the prevalence was reported to be 13.7% between 2007 and 2012[10]. Hoerger et al[11] predicts the prevalence to increase to 14.4% in 2020 and 16.7% in 2030. Currently, incidence of CKD ranges from 200 cases per million to up to 400 cases per million in the United States and Taiwan[12]. Studies show that CKD patients experience poorer quality of life and loss of function vs healthy individuals[13-15].
Hypertension is defined as systolic blood pressure greater than 140 mmHg and diastolic blood pressure higher than 90 mmHg[16] and is the second leading cause of ESRD[10]. Therapeutic goals for blood pressure are influenced by patient age as well as presence of comorbidities such as diabetes and CKD[17]. Data collected from Chronic Renal Insufficiency Cohort study participants indicated that hypertension affects 67%-92% of CKD patients and its prevalence increases with deteriorating renal function[18-20].
Besides CKD resulting from metabolic risk factors, renal dysfunction can result from PKD. PKD is an inherited genetic disorder that is either autosomal dominant (ADPKD) or autosomal recessive (ARPKD) and is characterized by the development of fluid-filled cysts from the epithelial lining of the nephron which causes renal enlargement and obstructive tissue fibrosis that can lead to ESRD[21]. As the fourth leading cause of kidney failure, PKD afflicts more than 500000 individuals in the United States[22].
Mechanistically, CKD is marked by fibrosis, an accumulation of extracellular matrix (ECM)[23]. Given the importance of renal fibrosis in the loss of renal function in CKD, much effort has focused on the mechanisms underlying fibrosis. Fibrosis results from abnormal accumulation of matrix, predominantly collagen, which is associated with loss of organ function as normal tissue is replaced by scar tissue[24]. CKD is a prototypical example of progressive fibrosis leading to organ failure[25,26].
The kidney may be uniquely sensitive to inflammation as it is a source for cytokine and chemokine synthesis within the tubular epithelium[27], and due to the high blood flow, it is continually exposed to circulating pro-inflammatory mediators. There are significant data linking inflammation to the loss of renal function[28]. A candidate signaling pathway that links inflammation and fibrosis in the kidney is nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). This transcription factor has a long-recognized role as a pro-inflammatory mediator[29]. A number of humoral stimuli induce NF-κB activity in the kidney, including tumor necrosis factor (TNF-α)[30] and angiotensin II[31] - both of which are associated with CKD[32,33].
Primary interventions for treating and preventing the progression of CKD are focused on reducing the increased vascular disease risk in the early stages of this disease (Stages 1-3) and involve exercise, dietary changes, smoking cessation, glycemic and blood pressure control and lipid management[7] which are also steps used in treating diabetes which itself is a risk factor for CKD[5]. Dietary changes focus on managing salt/sodium, phosphorus, potassium and protein intakes[2]. Glycemic control with various anti-diabetic drugs has been shown to reduce the risk and slow the progression of renal disease in both Type 1 and Type 2 diabetics[5] with metformin being a first choice of therapy. Blood pressure reduction can dramatically reduce progression of renal disease[20] and there is a good body of data showing that angiotensin-converting enzyme (ACE) inhibitors are effective in reducing proteinuria in nondiabetic[34] and diabetic[3] renal disease patients. Dual blockade of the renin-angiotensin-aldosterone-system is often used to achieve blood pressure targets in hypertensive subjects, however, combined use of ACE inhibitors and angiotensin-receptor-blockers are not recommended as there is insufficient evidence of any further benefit to decrease CKD progression[2], and several studies with combinations in subjects with diabetic kidney disease showed an increased risk of adverse events[3,5].
Lipid management is of particular importance in CKD as many patients present with hyperlipidemia regardless of etiology[8,35] and are at higher risk for cardiovascular events[2,36-38]. Lipid lowering is particularly effective in the early stages of CKD where atherogenic risk factors have a much greater impact on disease progression[7]. Moreover, it is now well recognized that dyslipidemia is an independent contributing factor in the progression of renal injury and dysfunction[39-42]. There may be three pathophysiologic mechanisms of renal injury related to dyslipidemia[41]. First, kidney mesangial cells exposed to oxidized lipoproteins (that are more prevalent in dyslipidemia) can stimulate the secretion of chemokines and adhesion molecules promoting the infiltration of macrophages which results in glomerulosclerosis and tubular fibrosis. This process has been suggested to be similar to the process of atherosclerosis[42]. Second, uptake of oxidized lipoproteins by the infiltrating macrophages increases lipid deposition in the kidney and promotes the release of reactive oxygen species (ROS) and prosclerotic and proliferative cytokines [e.g., transforming growth factor-β (TGF-β) and platelet-derived growth factor-AB]. Lastly, macrophage secreted cytokines promote mesangial expansion through increased ECM protein synthesis[41].
Dyslipidemia associated with CKD is most often characterized by elevated triglycerides, small dense low density lipoprotein (LDL) particles and dysfunctional high density lipoprotein (HDL)[43-45]. Triglyceride-rich lipoproteins are elevated in CKD due to a variety of causes. Diabetic CKD patients tend to have more dysfunction of the vascular endothelium, leading to a relative deficiency of lipoprotein lipase (LPL)[43]. Hepatic lipase is also lower in all CKD patients[43] and may result from elevated parathyroid hormone in these patients, which increases hepatic calcium concentrations and deranges normal hepatocyte cell function[37,46]. Finally, apolipoprotein (apo) CIII and angiopoietin-like 4 protein, both inhibitors of LPL function, are also higher in CKD patients[43,47].
Various clinical practice guidelines for treating dyslipidemia in CKD have been published[38] and most recommend the use of statins in early stages of the disease, as they are considered the safest lipid lowering agent in early stage kidney disease[37,41]. The use of statins in early stage CKD has been shown to reduce the cardiovascular risk associated with the dyslipidemia, however the data do not support any benefit of statin in the long term reduction of renal disease progression[37,48,49]. Statins can also, in certain cases, induce proteinuria[49] so caution is recommended in the use of high dose or potent statin drugs. Clinical practice guidelines also do not recommend initiating statins in patients undergoing dialysis based on the results of three pivotal trials (4D, AURORA and SHARP trials)[50-52] which all failed to show efficacy of statins in reducing cardiovascular endpoints in dialysis patients as a whole. Nonetheless, statins have been shown to be helpful in reducing overall morbidity in renal transplant patients who are often on immunosuppressive medications which cause elevations in lipids[37].
The lack of statin benefit to reduce overall cardiovascular mortality in dialysis patients has led some investigators to propose that other means of lipid lowering may be beneficial in this population[53]. Since dialysis patients experience a high absorption of sterols, Silbernagel et al[53] suggest that other lipid lowering agents other than statins (e.g., bile acid binding compounds) should be considered in this population. To this end, it has been demonstrated that pharmacologic doses of omega-3 polyunsaturated fatty acids can be prescribed at all stages of CKD even in combination with a statin to successfully reduce hypertriglyceridemia[36].
Nutritional interventions can be viewed as consisting of two phases in renal dysfunction - diet plans that address the underlying causes of kidney disease and slow the progression of kidney failure and those that support the patient and prevent complications arising from advanced kidney disease. The Academy of Nutrition and Dietetics, through their Nutrition Care Process advises using a nutrition diagnosis and potential disease etiology to develop an appropriate nutrition prescription for renal patients[54]. Assessment tools that are validated for CKD include the Subjective Global Assessment (SGA) and Malnutrition Inflammation Score (MIS)[54]. Nutritional interventions strive to address the comorbid conditions in CKD, such as hypertension, glucose and lipid homeostasis, inflammation and increased oxidative stress. Malnutrition in CKD (as assessed by the SGA or MIS) results in poor clinical outcomes and increased mortality rates in CKD patients[54,55]. Protein energy wasting (PEW) is also common in CKD and can be distinguished from malnutrition (inadequate nutrient intake) since CKD-related factors may also contribute to PEW[56]. Since low protein and low phosphorus diets have been shown to be effective in slowing the progression of kidney disease, careful consideration is required to design dietary interventions that maintain total energy intake while maintaining adequate but not excessive protein and phosphorus intake[54].
The Dietary Approaches to Stop Hypertension diet is useful in reducing blood pressure[57]; however, it may only be useful in very early stages of CKD since it recommends 4.5 g/d potassium, 1.7 g/d phosphorus and 1.4 g/kg per day protein which may be problematic in later stages of CKD[58]. The International Study of Macronutrients and Blood Pressure study demonstrated that more vegetable protein, but not animal protein, was associated with lower blood pressure[59] indicating that the source of protein may be important in renal diets. In the Nurses’ Health Study, Knight et al[60] showed that high non-dairy animal protein intake appeared to accelerate renal function decline in women with renal insufficiency, but not in women with normal kidney function. More attention has focused on vegetarian diets in the field of renal nutrition in general due to properties that ameliorate the factors contributing to renal dysfunction[54,61-64]. Specifically, foods of plant origin tend to have higher energy/protein and energy/phosphorus ratios (i.e., can satisfy energy requirements while maintaining a relatively low nitrogen and phosphorus intake), are low in saturated fats[54,65], tend to improve glycemic control compared to omnivorous diets[66] and may reduce oxidative stress and inflammation[67]. A number of human studies have demonstrated renal benefits of dietary vegetable protein[68-73].
Only a limited number of dietary intervention studies have been conducted in PKD patients. The Modification of Diet in Renal Disease Study showed no benefit over 18-45 mo follow-up (mean = 2.2 years) of protein restriction or improved blood pressure control on the rate of decline in GFR in ADPKD subjects with moderate renal insufficiency (n = 141; GFR 25 to 55 mL/min per 1.73 m2; mean GFR = 37.8 mL/min per 1.73 m2)[74]. And, in some cases, deterioration of renal function actually seemed to be exacerbated by these interventions[74]. A very low protein (0.28 g/kg per day)/low phosphorus (4-9 mg/kg per day) diet with keto acid-amino acid supplementation did however demonstrate limited efficacy (P = 0.06) in slowing disease progression in ADPKD patients with greater renal insufficiency (n = 59; GFR 13 to 24 mL/min per 1.73 m2; mean GFR = 17.4 mL/min per 1.73 m2). A key limitation of the study was that subjects may have already reached advanced stages of ADPKD such that maximal benefits of such interventions may not have been achievable[74]. Retrospective analysis conducted by Choukroun et al[75] of data from a hemodialytic ADPKD population (n = 109) during follow-up care (average 6.7 ± 0.3 years) at Necker Hospital in Paris, France revealed a relationship between mean arterial pressure (MAP) and change in creatinine clearance (r = 0.226; P = 0.01) but observed no significant effect of protein intake (r = 0.109; P = 0.33). Despite the importance of nutrition in renal disease, there is a paucity of human clinical data in this area[54]. Soybean protein has perhaps been the most extensively studied plant-based protein in subjects with kidney disease and has demonstrated renal protective properties in a number of clinical studies[76,77]. Dietary intervention studies with soy protein have yet to be conducted in PKD patients.
The soybean consists of 36% (wt/wt dry matter) protein which can be extracted using differential processing techniques to yield a variety of soy protein ingredients such as soy flour, soy protein concentrate and soy protein isolates that can be incorporated into a multitude of food forms[78,79]. Soy protein is the only high quality plant-based protein that is widely available. Soy protein is a complete protein (provides adequate levels of all of the essential amino acids to support human nutritional needs) and is comparable in quality to milk, meat, and eggs as measured by the protein digestibility-corrected amino acid score, which is the globally recognized method for determining protein quality[80]. Soy intake has been and continues to be greater in Eastern Asian rather than Western countries with tofu, natto and miso being the most common representatives/examples of traditional soy foods[81]. Therefore, these populations have frequently been utilized to evaluate the health effects of soy consumption as part of the diet over the course of the entire lifespan.
Soybeans also contain mono/oligosaccharides (15% wt/wt dry matter), fiber (15% wt/wt dry matter), oil (18% wt/wt dry matter) and relatively small amounts of phytic acid, tocopherols, phytosterols, saponins and isoflavones[78,79]. Soy isoflavones (genistein, daidzein and glycitein) are present in soybeans and in most soy protein products primarily as b-glycosides and their associated acetyl- and malonyl-ester forms and very little is present as non-conjugated isoflavones (aglycone form)[82,83]. The aglycone form of isoflavones exhibit structural similarity to estrogen and these compounds are sometimes referred to as phytoestrogens, but have a limited ability to bind estrogen receptors, demonstrating a greater affinity for estrogen receptor β (ER-β) rather than estrogen receptor α (ER-α) and therefore may be better considered as selective estrogen receptor modulators[84]. It should be pointed out that isoflavones are efficiently metabolized, once absorbed by the gut, to glucuronide and sulfate conjugates in humans and these conjugates account for 90% or more of circulating isoflavones in human plasma[84,85]. Daidzein is converted to equol [(3S)-3-(4-hydroxyphenyl)-7-chromanol)] and/or O-demethylangolensin through the actions of certain commensal intestinal bacteria in some but not all human subjects[63,81,86,87] and these metabolites are also present in plasma primarily as glucuronide conjugates[88]. Conjugated isoflavones are generally very weak estrogen receptor ligands and may have as yet unidentified biologic activities in vivo[89]. Finally, soy protein consumption leads to different target gene expression than is observed with estradiol treatment in animal models, confirming that the metabolic effects of soy protein cannot be equated with the hormonal effects of estrogen per se[90].
Plasma and urinary concentrations of isoflavones increase somewhat proportionally with the consumption of soy foods in both animals and humans[63]. Therefore, these parameters are frequently utilized as biomarkers of subject compliance in dietary intervention studies that include soy despite significant individual to individual variability which is also influenced by kidney function status, which will be discussed later.
A brief summary of human studies assessing the role of soy protein in renal function follows. Studies published in English were included if the main dietary intervention or subject of study was clearly identified as soy protein and was ingested as whole soy or as soy products.
Searches of the PubMed and SciFinder® database studies in English identified three single arm intervention studies (Table 1) and thirty-two placebo-controlled chronic interventions studies (Table 2) assessing the effects of soy protein on renal measures in subjects with varying degrees of renal dysfunction. Three studies studied the metabolism of soy isoflavones in subjects with ESRD or in renal transplant patients (Table 3). Two studies looked at the effects of soy protein consumption on renal calcium metabolism in healthy, normal subjects (Table 4). Eight studies assessed the renal response to single meal ingestions of soy protein in comparison to other proteins in healthy and Type 2 diabetic subjects with some renal dysfunction (Table 5). Citations in the Tables are listed in approximate order of severity of renal disease of study subjects.
| Ref. | Studydesign | Kidney function | Subjects/group | Amount of soy protein used | Control/comparator protein | Duration of intervention | Outcomes | Notes |
| Cupisti et al[93] | Single arm dietary intervention study | Renal transplant patients with moderate HC | 13 subjects completed study (7M, 6F) | Goal was to replace 25 g/d animal protein with soy protein (dietary counseling only) | Animal protein (baseline) | 5 wk on soy diet | Significant decrease in urinary creatinine after 5 wk on soy protein compared to baseline (P < 0.05) | |
| Soy protein resulted in significant decrease in TC (P < 0.05) and LDL-C (P < 0.01) after 5 wk compared to baseline; no change in HDL-C | ||||||||
| Cupisti et al[94] | Single arm dietary intervention | Renal transplant patients and and age, sex-matched healthy controls (latter for vascular measure comparisons only) | 20 per group (12M, 8F) | Goal was to replace 25 g/d animal protein with soy protein (dietary counseling only) | Animal protein (baseline and WO) | 5 wk on soy diet followed by 5 wk WO | Renal transplant patients had significantly reduced FMD compared to age- and sex-matched control subject (P < 0.001) with no differences between groups in non-endothelium-mediated vasodilation | First study to show improvement in endothelial function in brachial arteries of renal transplant patients when animal protein substituted with soy protein |
| Soy diet did not change total dietary protein intake, BW, renal function, urinary protein excretion, serum Ca or P | ||||||||
| Soy diet reduced TC and LDL-C and LOOH (P < 0.01) compared to baseline diet | ||||||||
| Soy diet resulted in improvement in FMD (P = 0.003) compared to baseline while reactive hyperemia and endothelium-independent vasodilation was unchanged; FMD returned to baseline after WO | ||||||||
| Increase in FMD correlated to increase in L-arg/ADMA ratio (P < 0.05) with soy diet | ||||||||
| D’Amico et al[95,96] | Single arm dietary intervention | Nephrotic patients with proteinuria > 1-5 g/24 h over 25 mo and HL | 20 subjects (13M, 7F) | 0.7-0.8 g/kg per day mostly from soy protein in test diet; test diet also contained vegetable oils and no cholesterol | 0.7–0.8 g/kg per day animal protein (baseline and WO) | 8 wk baseline diet followed by 8 wk soy diet and then 8 wk WO | TC, LDL-C, HDL-C, apoAI and apoB decreased on soy diet compared to baseline diet (P < 0.001); no change in TG; lipids tended to revert to baseline during WO | Fibre, type of fat and no cholesterol were also other components of the soy protein arm that were different from the control diet; there was a modest but significant decrease in BW on the soy protein diet (no change in BMI) |
| Urinary protein, urea, Na and P excretion were reduced significantly from baseline during the soy diet (P < 0.001) | ||||||||
| Soy diet results in significant decrease in CrCl with no change in serum creatinine; this persisted during WO | ||||||||
| BP did not change |
| Ref. | Study design | Kidney function | Subjects/group | Amount of soy protein used | Control/comparator protein | Duration of intervention | Outcomes | Notes |
| Liu et al[128] | RC | Pre-hypertensive PM women | 90 subjects/group (85, 87 and 81 completed study in the soy, daidzein and placebo groups, respectively) | 40 g soy flour/d, 12.8 g soy protein/d | 40 g lowfat milk powder (placebo) or 40 g lowfat milk powder with 63 mg/d daidzein | 6 mo | No significant changes in most renal parameters were observed between groups | All subjects were equol producers |
| Soy flour intake resulted in less decrease in eGFRCockcroft (P = 0.044) and % change in eGFR (P = 0.031) after 6 mo compared to the milk placebo group (P = 0.044) | ||||||||
| Effect of soy flour consumption to increase eGFR was greater in women with higher initial plasma cystatin C concentrations (Cys-C > 1.14 mg/L) (P = 0.001 for eGFRCockcroft) compared to milk placebo | ||||||||
| Ahmed et al[131] | RC | Glomerulo-pathy with proteinuria (non-diabetic) | 9 subjects/group, | 0.8 g/kg per day soy protein | 0.8 g/kg per day an animal protein or 0.8 g/kg per day a soy protein + fiber | 8 wk | No significant changes in anthropometric measures, serum lipids or proteinuria between diet groups | Significant decreases from baseline in overall energy and protein intake in all groups confounds end of study comparisons |
| total 27 subjects (4M, 23F) | ||||||||
| Soroka et al[129] | RC | Non-diabetic, non-nephrotic CRF patients (urinary protein excretion < 3 g/d) | 9 completed study (5M, 4F) | 0.71 g/kg BW protein, mostly soy protein with egg (VPD) | 0.85 g/kg per day APD (1:1, animal sources:grains) | 6 mo | No difference in renal function between groups seen; both groups saw reduction in rate of GFR decline | High dropout and small number of subjects |
| BUN, Urinary N excretion, PCR, 24 h urinary creatinine and phosphate were lower in VPD group | Differences in total energy and protein intake in VPD and APD | |||||||
| D’Amico et al[102], Gentile et al[103] | RC | Non-diabetic, nephrotic patients with proteinuria > 2.5 g/24 h for a mean of 24 mo and HC | 20 subjects (9M, 11F) | Protein intake at end of study was calculated from urinary urea excretion to be 1.16 ± 0.04 g/kg per day (98% of this estimated to be soy protein) | Soy protein used in both experimental arms of study; baseline diet was comparator | 8 wk for each arm (baseline diet, soy ± 5 g/d fish oil in random order) followed by WO for 3 mo on baseline diet) | Soy diet significantly reduced TC, LDL-C, HDL-C, apoB (P < 0.0001) and apoAI (P < 0.01) compared to baseline; TGs were unaffected; lipids tended towards baseline values after WO | Both diet interventions resulted in modest decrease in BW and BMI (-4%) which was significantly different from baseline; both values tended towards baseline during WO |
| Addition of 5 g/d fish oil to soy diet resulted in significant elevation of TC and apoB compared to soy diet alone (P < 0.01) | ||||||||
| Urinary protein, urea, P, Na and creatinine excretion was significantly decreased by both diet interventions (P < 0.01); measures tended towards baseline after WO | ||||||||
| Blood glucose was significantly reduced by both diet interventions (P < 0.01) however soy diet alone reduced blood glucose more than soy diet and fish oil (P < 0.01) | ||||||||
| Anderson et al[97] | RC | T2D with proteinuria, obese, and HTN | 8 M | 1.0 g/kg per day protein, 50% soy protein in soy test diet | 1.0 g/kg per day protein, 50% ground beef in animal test diet | 8 wk, 4 wk WO | TC and TG decreased by soy diet (P < 0.05) vs animal protein diet | Low number of subjects |
| SUN sig decreased by soy protein (P < 0.05) | ||||||||
| Change in GFR similar with both diets | ||||||||
| Urine protein excretion increased by soy vs animal protein diet (P = 0.028) | ||||||||
| Azadbakht et al[98] | RP | T2D subjects with nephropathy, proteinuria, HTN | 41 subjects: 18 M, 23 F | 0.8 g/kg per day protein, 35% soy protein (textured soy protein), 35% animal protein, 30% vegetable protein | 0.8 g/kg per day protein, 70% animal and 30% vegetable protein | 4 yr | Decreased FPG in soy group (P = 0.03 | |
| Soy protein group decreased TC (P < 0.01), LDL-C (P = 0.01) and TG (P = 0.01 | ||||||||
| Serum CRP decreased (P = 0.02) on soy protein diet | ||||||||
| Soy protein diet reduced proteinuria (P = 0.001) and urinary creatinine (P = 0.01) | ||||||||
| Miraghajani et al[108,113] | RC | T2D subjects with nephropathy | 25 subjects completed the study (10 M, 15 F) | 2.5 g soy protein (240 mL soymilk/d) | 3.3 g cow milk protein (240 mL milk/d) | 4 wk interventions with 2 wk WO | Soy protein consumption resulted in a significant difference in % change of fibrin D-dimer concentrations compared to milk protein (P = 0.04); there were no differences in % changes in TNFα, IL-6, CRP, MDA or fibrinogen concentrations between groups | Amount of soy protein used in diet intervention was low |
| Soy protein consumption resulted in significant decrease in systolic BP compared to cow milk protein (-4.50% vs + 5.89%, P = 0.02) | ||||||||
| Teixeira et al[106] | RC | T2D subjects with nephropathy | 14 male subjects | 0.5 g/kg per day soy protein (Approximately equal 50% of total daily intake) | 0.5 g/kg per day casein | 8 wk interventions with 4 wk WO | Urinary albumin-creatinine ratio was significantly reduced by ISP (P < 0.0001) and increased by casein (P = 0.002) | |
| Change in urinary albumin-creatinine ratio correlated inversely with plasma isoflavone levels (P = 0.012) | ||||||||
| CrCl did not change (GFR) with either diet | ||||||||
| HDL-C was increased after ISP (P = 0.0041) while it tended to be lower after casein (P = 0.0847) | ||||||||
| TC and LDL-C not changed by either diet | ||||||||
| Total and glycated hemoglobin did not change in either group | ||||||||
| No differences in BP between groups; however soy diet resulted in higher plasma arg/lys ratios (P = 0.0097) which persisted after fasting | ||||||||
| Stephens et al[104] | RC | T1D subjects with hyper-filtration GFR > 120 mL/min/1.73 m2 | 12 subjects completed study (6 M, 6 F 0) | 45-55 g/d soy protein to substitute for animal protein in control diet | 45-55 g/d animal protein | 8 wk interventions; no WO | GFR sig lower in soy group vs control group (P = 0.02) | No washout between interventions |
| Excretion of urinary creatinine, urea and Na not diff between groups | ||||||||
| Microalbuminuria within normal ranges and unaffected by diet | ||||||||
| TC and LDL-C significantly reduced in soy group (P < 0.02, 0.05, respectively) whereas TG and HDL-C not diff between groups | ||||||||
| Serum glucose was not affected by soy protein diet but was significantly increased on the control diet (P < 0.05) compared to baseline | ||||||||
| Serum albumin did not change but total serum protein decreased in soy group (P < 0.05) | ||||||||
| Chen et al[101] | RP | Nondiabetic hemodialysis patients | Soy group: 10 HL (7 F, 3 M) and 8 NL (6 F, 2 M) | 30 g/d soy protein | 30 g/d milk protein | 12 wk | No significant differences between groups in serum nutritional parameters or hemodialysis adequacy | Test proteins consumed on top of usual hemodialysis diet |
| TC and TG decreased in HL subjects consuming soy vs milk protein over time (P < 0.05 at 12 wk) | ||||||||
| Control group: 9 HL (7 F, 2 M) and 10 NL (7 F, 3 M) | Non-HDL-C, apoB, TC/HDL-C ratio and insulin decreased in HL subjects consuming soy vs milk protein at 12 wk (P < 0.05) | |||||||
| Non-significant differences between protein groups in NL subjects | ||||||||
| Soy protein resulted in significant decrease in fasting insulin in NL group at 12 wk compared to values at baseline (P < 0.05) | ||||||||
| Chen et al[100] | RP | Non-diabetic hemodialysis patients with HC | Soy group: 13 (9 M, 4 F) | 30 g/d soy protein | 30 g/d milk protein | 12 wk | No significant differences between groups in serum nutritional parameters or hemodialysis adequacy | Not clear if some of the subject are the same as reported in Chen et al[101] as study protocols are the same |
| Milk group: 13 (10 M, 3 F) | TC, non-HDL-C, apoB, TC/HDL-C and LDL-C/HDL-C ratios decreased in subjects consuming soy vs milk protein at 12 wk (P < 0.05) | |||||||
| No differences in TG between soy and milk groups | ||||||||
| Soy protein resulted in significant decrease in fasting insulin at 12 wk compared to milk protein group (P < 0.05) | ||||||||
| Imani et al[110] | RP | PD patients | 18 subjects | 14 g soy protein at dinner each day | Meat instead of soy protein at dinner | 8 wk | Soy protein diet resulted in significant 17% reduction in plasma coagulation factor IX activity compared to control group (P < 0.05) | Study was not blinded |
| Soy group (9 M, 9 F), | No significant changes in oxLDL, P, fibrinogen or activities of coagulation factors VII and X between groups | Mean energy and protein intakes were less than recommended amounts (30 kcal/kg per day and 1.2 g/kg per day, respectively) which is common among PD patients | ||||||
| Control group (9 M, 9 F) | ||||||||
| Fanti et al[112] | RP | ESRD patients on chronic HD with elevated CRP (> 10 mg/L) | Soy group = 15; control milk group = 10 | 25 g/d | 25 g/d milk protein | 8 wk | 5 to 10-fold increase in mean serum IF concentration in soy group at end of study (P < 0.001) | Small number of subjects |
| No significant change in CRP between groups, however, significant inverse correlation of CRP with IF concentration | Test proteins provided as beverages, a cereal-type product and as snack bar | |||||||
| Significant positive correlation of serum IF concentration and serum albumin and IGF-1 | ||||||||
| Siefker et al[109] | RP | HD patients | 17 subjects | 25 g soy protein (4 times per week) | Whey protein (exact amount not specified); provided 4 times per week | 4 wk | No difference between groups on serum markers of renal function except creatinine; whey protein showed a significant decrease in creatinine from baseline (P < 0.05) whereas there was no change in the soy protein group from baseline | Small number of subjects |
| 8 subjects on soy protein diet; 9 on whey protein | oxLDL was significantly decreased after soy protein consumption (P < 0.05) compared to baseline; the % change in oxLDL compared to the whey group was significantly different (P < 0.05) | |||||||
| No differences in plasma concentrations of 8-iso-PGF2α, TNFα, or CRP between diet groups | ||||||||
| Tomayko et al[114] | RP | MHD patients | Soy group = 12 | 27 g/d soy protein | 27 g whey protein or noncaloric placebo powder (2 g Crystal Light) | 6 mo | A significant time x treatment effect for IL-6 levels (P = 0.036) with both whey and soy protein groups decreasing compared to control group | First study to observe improvements in inflammation and physical function after intradialytic nutritional support in MHD patients with serum albumin ≥ 3.9 g/dL (i.e., not malnourished) |
| Whey group = 11 | Soy diet resulted in a significant decrease in neutrophil-lymphocyte ratio (systemic inflammation marker) compared to control or whey diet (P = 0.02) | |||||||
| Placebo control = 15 | Alkaline phosphatase, a marker of bone turnover, was increased in the control diet compared with both protein diet groups (P = 0.04) | |||||||
| A significant time by treatment interaction was seen for gait speed when all 3 groups analyzed (P = 0.048); both soy and whey groups indicated improved gait speed while control diet had a decline | ||||||||
| Shuttle walk test time was significantly improved in the whey group (P < 0.05) and when protein groups were combined (P < 0.05) versus the control group; shuttle walk test time was increased in the soy group but was not significant compared to the control group (which had decreased test times) |
| Ref. | Study design | Kidney function | Subjects/group | Amount of soy protein used | Control/comparator protein | Duration of intervention | Outcomes | Notes |
| Fanti et al[115], Franke et al[192] | 3 separate protocols: | ESRD patients on HD and normal healthy subjects | 23 HD subjects and 10 healthy subjects for baseline IF measures | 20 g soy protein | Baseline diet is self-selected standard renal diet | Single meal interventions | 55%-65% of HD patients had undetectable serum IFs and 35%-45% had concentrations > 200 nM on standard renal diet | First study to report blood levels of genistein and daidzein in ESRD patients |
| Assessment of baseline serum concentrations of IFs | 7 HD patients and 8 healthy subjects for meal intervention study (8 h only); 2 healthy subjects and 3 HD subjects had multiple serum and urine timepts collected | Serum concentrations of IFs greater post-soy protein ingestion compared to baseline for both groups (P < 0.001); concentrations in HD subjects after 8 h of soy protein consumption were greater than those in healthy subjects (P < 0.05) | Daidzein metabolites equol and O-DMA were not detected in sera of any of the subjects | |||||
| Post-ingestion concentrations of IFs | 5 HD patients for pre- and post-dialysis IF measures | Half-lives of genistein and daidzein averaged 3.5 and 6 h in healthy subjects, respectively but were increased to an average of 47 and 58 h in HD patients | ||||||
| Effects of hemodialysis on IF concentrations | HD did not effectively remove IFs from serum since (due to higher molecular weight of conjugates and large proportion of unconjugated IFs are bound to albumin) | |||||||
| Fanti et al[116] | Observational | Randomly selected HD patients residing in the United States, Japan or Thailand | Subjects from: | Habitual dietary intake of soy was assessed by questionnaires developed by their renal replacement therapy programme dieticians | Study aim was to compare habitual dietary intake of soy in 3 countries | N/A | Serum IF concentrations significantly higher in HD patients from Japan compared to United States or Thailand (P < 0.0001) | |
| United States = 20 | Significant correlation between soya intake and genistein (P < 0.0001), daidzein (P < 0.0001), glycitein (P < 0.001) and O-DMA (P < 0.01) in subjects from all 3 countries | |||||||
| Japan = 20 | ESRD HD patients displayed consistently higher concentrations of daidzein compared to genistein, while the reverse occurs in healthy subjects | |||||||
| Thailand = 17 | Concentrations of sulphated and unconjugated compounds in HD subjects (Japan only studied) are comparable to those detected in healthy subjects | |||||||
| Locati et al[117] | Single arm intervention study | Renal transplant patients | 16 subjects (11 M, 5 F) | 25 g soy protein substituted for 25 g animal protein | 25 g animal protein (as habitual diet) | 5 wk | Serum IFs were measured and 5 different groups were identified on the basis of the IF profiles: (1) 4 subjects had no detectable IFs; (2) only genistein was quantifiable in 7 patients; (3) 3 patients had only detectable genistein and daidzein; (4) 2 subjects only had detectable genistein and equol; and (5) 1 subject had the highest observed genistein and daidzein with detectable dihydrogenistein and equol | Concentrations of serum IFs in the renal transplant patients were similar to those observed in healthy subjects |
| Ref. | Study design | Kidney function | Subjects/group | Amount of soy protein used | Control/comparator protein | Duration of intervention | Outcomes | Notes |
| Breslau et al[119] | RC | Normal | 15 subjects completed animal and ovo-vegetarian diet phases; 10 completed all 3 phases (including vegetarian) | Soy protein accounted for most of the 75 g protein/d in vegetarian phase; accounted for an unspecified but lower amount in ovo-vegetarian phase | Animal protein accounted for most of the 75 g per day in the animal protein phase; consisted of dairy, beef, chicken and fish | 12 d No WO | Serum uric acid concentrations were significantly lower with the vegetarian and ovo-vegetarian diets compared to the animal protein diet (P < 0.01); urinary uric acid excretion was significantly lower in ovo-vegetarian diet vs animal diet only (P < 0.02) | Diets were constant for Ca, P, Na and total protein |
| Urinary Ca and P were significantly lower in vegetarian diet compared to beef diet (P < 0.02); urinary oxalate was significantly higher in vegetarian vs beef diet (P < 0.02) | ||||||||
| Animal protein diet resulted in lower PTH level vs vegetarian diet (P < 0.05) | ||||||||
| Serum 1,25-(OH)2D was higher in the vegetarian vs animal protein diet(P < 0.01) | ||||||||
| Roughead et al[120] | RC | Normal PM women | 13 female subjects | 25 g soy protein substituted for 25 g meat protein | 25 g meat protein in control diet | 7 wk | Ca retention was not affected by substituting soy protein for meat protein | |
| Urinary pH was higher on the soy diet compared to the control diet (P < 0.0001); renal acid excretion was lower during soy diet (P = 0.0001) however urinary Ca excretion was similar between soy and meat diets | ||||||||
| Substitution of soy protein for meat protein did not affect bone metabolism as indicated by no differences between diets in a number of specific bone biomarkers | ||||||||
| No differences between soy and meat protein diets in plasma lipid or hemostatic measures |
| Ref. | Study design | Kidney Function | Subjects/group | Amount of soy protein used | Control/comparator protein | Duration of intervention | Outcomes | Notes |
| Bilo et al[122] | Single meal intervention study (crossover) | Normal healthy subjects | 6 normal subjects; 5 M, 1 F | Studies in normal subjects only: 80 g soy protein in single oral administration | Studies in normal subjects only: 80 g lactoprotein or beef protein or 36 g amino acids | Normal subjects: 8 individual renal function tests run on separate days | Soy protein ingestion induced significantly lower rises in GFR and ERPF compared to beef protein but not compared to lactoprotein or 36 g amino acid ingestion | Subjects with chronic renal insufficiency (PKD, NS, or MGP) were studied in a separate series of experiments in this publication, but were not used to evaluate soy protein |
| Buzio et al[127] | Single meal intervention study (crossover) | Normal healthy subjects | 7 (gender not specified) | 80 g (0.9-1.3 g.kg BW) | 80 g red meat or 80 g dairy (cheese) | Single meal interventions conducted 1 wk apart | CrCl and urinary protein were not different between protein loads | Publication describes 2 separate experiments; soy protein effects on renal function only assessed in second experimental protocol |
| UAp was significantly lower after soy protein meal versus red meat or cheese meals (P < 0.01) (samples taken 4 h post-meal) | ||||||||
| Water excretion rate was higher after soy protein load versus meat (P < 0.05) or cheese (P < 0.01) | ||||||||
| Serum total protein was lower after soy protein load compared to meat (P < 0.01) or cheese (P < 0.01) loads | ||||||||
| Deibert et al[126] | Single meal intervention study (crossover) | Normal healthy and metabolic syndrome subjects; all with normal kidney function | 10 subjects per group (All males) | 1st intervention: 1 g/kg/BW soy protein: Milk protein (83% soy protein); | N/A | Single meal intervention in normal healthy subjects; 2 meal interventions in subjects with metabolic syndrome (1 wk apart) | Patients with metabolic syndrome had significantly elevated baseline GFR and ERPF compared to healthy subjects (P = 0.02) | 0.3 g/kg/BW is amount of protein used in meal replacement therapy |
| 2nd intervention same protein source at 0.3 g/kg BW | After ingestion of 1 g/kg/BW protein, GFR and ERPF increased in both groups however the subjects with metabolic syndrome had significantly higher increases in GFR (P < 0.002) and ERPF (P < 0.02) compared to normal subjects; no significant effect of ingestion of 0.3 g/kg per BW protein on renal parameters in subjects with metabolic syndrome | |||||||
| Howe et al[118] | Single meal intervention study (Latin square crossover) | Healthy PM women | 8 F subjects | 45 g soy protein | 0 g protein, 45 g beef or dairy protein (cottage cheese) | Single meal intervention; 6 meal interventions (1 wk apart) | Urinary Ca excretion was significantly greater after 45 g protein meal for all proteins compared to basal (0 g protein) meal (P < 0.05) | |
| % Ca resorbed by the kidney was significantly reduced after the dairy and soy protein meals (P < 0.05) | ||||||||
| Serum ionized Ca was unaffected, however, serum P was significantly lowered by all protein meals (P < 0.05) compared to 0 g protein meal | ||||||||
| Soy protein meal significantly reduced calcitonin versus baseline (P < 0.05) however, all protein means tended to lower calcitonin compared to baseline | ||||||||
| Dairy protein significantly increased PTH (P < 0.05) compared to baseline, however all protein meals tended to elevate PTH compared to baseline | ||||||||
| Serum insulin was significantly increased by all protein meals (over time) compared 0 g protein meal (P < 0.05) | ||||||||
| Kontessis et al[70] | Single meal intervention study (crossover) | Normal healthy subjects | 7 M subjects | 80 g soy protein | 80 g lean beef | 2 separate single meal interventions | GFR and ERPF increased significantly after acute beef protein load (P < 0.005 compared to baseline) but did not increase with soy protein load | Amount of soy protein in vegetable protein diet in the reported chronic study was not specified so is therefore not summarized |
| Renal vascular resistance fell significantly after beef load (P < 0.05) but was unchanged after soy protein load; plasma 6-keto-PGF1α rose significantly after meat load (P < 0.05) but not after soy protein load | ||||||||
| Fractional albumin and IgG clearance rose after beef load (P < 0.05 and P < 0.001, respectively) but did not change significantly after soy protein load; plasma protein concentrations were not different between different protein loads; UAp was not different between groups | ||||||||
| Plasma glucagon increase was higher after meat load (P < 0.05) compared to soy protein load; no differences were seen between proteins on plasma insulin or growth hormone | ||||||||
| Nakamura et al[123] | Single meal intervention study (crossover) | Healthy and T2D subjects (T2D divided into 3 groups based on AER: Group A ≤ 20 μg/min (Normal); B = 20-200 μg/min; C ≥ 200 μg/min | 11 healthy subjects (8M, 3F); 20 T2D patients (10 M, 10 F) | 1g/kg soy protein (as bean curd) | 1 g/kg tuna fish protein | Meals fed on separate days | In healthy subjects, eGFR increased (P < 0.01) after tuna meal but no significant difference after soybean curd meal | |
| In Grp A, eGFR increased with tuna meal (P < 0.01) but not after soybean curd | ||||||||
| In Group B there was no difference in GFR with either protein | ||||||||
| In group C, GFR sig decreased after tuna meal (P < 0.05) but not with soy protein | ||||||||
| No changes in AER with any protein in any group | ||||||||
| Nakamura et al[124] | Single meal intervention study (crossover) | Healthy and T2D subjects | 10 healthy subjects and 6 T2D subjects | 0.7 g/kg soy protein (as bean curd) | 0.7 g/kg tuna fish protein or egg white protein or dairy protein (cheese) | Meals fed on separate days | eGFR was only significantly increased after ingestion of tuna fish protein (P < 0.001) and not after consumption of soy, egg white or dairy proteins | |
| Orita et al[125] | Single meal intervention study (crossover) | Healthy subjects | 6 male subjects | 86.9 g soy protein | 86.9 g beef protein or fasting (0 g protein) | Meals fed 1 wk apart | Inulin clearance (GFR) was significantly increased over baseline at 2 h post beef or soy protein compared to fasting (P < 0.005 and P < 0.05, respectively) | First study to show an increase in GFR after a soy protein load in healthy subjects |
| Creatinine clearance (GFR) was significantly increased by both beef and soy proteins at 2 and 3 h post-ingestion compared to fasting (P < 0.01) | ||||||||
| Plasma glucagon was significantly increased at 1 to 3 h post-ingestion by both beef and soy protein compared to fasting (P < 0.01) |
As previously mentioned, lipid management is particularly important in patients with renal disease as many patients present with hyperlipidemia regardless of the etiology of their kidney dysfunction. Soy protein lowers plasma cholesterol[91] and, as of 2015, has an approved health claim based on this property in 13 countries[92]. Three single arm dietary intervention studies (Table 1) demonstrated that consumption of 25 g or more of soy protein/day resulted in a significant lowering of total and LDL cholesterol (LDL-C) in renal transplant patients[93,94] and in nephrotic patients with proteinuria[95,96] when compared to their baseline diets. Soy protein consumption lowered plasma apoB concentrations in the nephrotic patients[95,96]. Eight placebo-controlled chronic intervention studies in subjects with various degrees of renal dysfunction demonstrated that soy protein consumption resulted in significant lowering of plasma total cholesterol compared to the control diets[97-104]. Five of these studies showed that soy protein diets resulted in significant lowering of LDL-C[98,99,102-104], four demonstrated a reduction in plasma apoB[100-103] and two studies reported a significant lowering of non-HDL-C[100,101], with the latter being considered a more important prognostic biomarker for cardiovascular disease than LDL-C[105]. Addition of 5 g/d fish oil to the soy diet in one study[103] did not improve any of the lipid parameters, and, in fact, tended to raise LDL-C and apoB concentrations. Soy protein consumption tends to reduce plasma cholesterol more in renal patients with elevated rather than normal cholesterol concentrations[101] which is not unlike that observed in many other intervention trials with soy protein[91]. Several studies summarized in Table 2 also noted that soy protein consumption tended to lower plasma triglycerides[97-99,101] whereas other studies reported no significant change[100,103,104]. Teixeira et al[106] observed a significant increase in HDL-C after soy consumption in Type 2 diabetic subjects with nephropathy, while Stephenson et al[104] reported no change in Type 1 diabetic subjects with early stage renal dysfunction. A decrease in apoAI and HDL-C was reported by Gentile et al[103] in non-diabetic nephrotic patients with renal dysfunction after soy protein consumption for 8 wk. While Tokede et al[91] concluded that soy protein consumption in mixed populations was associated with modest beneficial effects on HDL-C concentrations, the studies cited above on renal compromised subjects suggest that there may be differences in the ability of soy protein to modulate absolute concentrations of HDL-C depending on the nature of renal dysfunction. It should be also noted that absolute concentrations of HDL-C are probably less important than the ability of HDL-C particles to mediate cholesterol efflux from cholesterol-laden cells in the body[107], so future studies on soy consumption should focus on this property of HDL particles rather than HDL-C concentrations alone.
Soy protein consumption was also associated with improvements in plasma glucose metabolism in several chronic intervention studies. Gentile et al[103] noted that fasting blood glucose concentrations were reduced significantly in non-diabetic subjects while on the soy diet alone compared to those values at baseline or on the soy protein plus fish oil diet. Azadbakht et al[98] also noted a significant decrease in fasting blood glucose concentrations in Type 2 diabetic subjects who had been on a soy diet for 4 years compared to the control (animal protein) diet. Teixeira et al[106] did not observe any improvements in blood glucose control following an 8 wk intervention with soy protein in Type 2 diabetic subjects in contrast to Stephenson et al[104] who noted that soy protein consumption in Type 1 diabetic subjects did not alter fasting glucose concentrations compared to the baseline diet but that the animal protein diet resulted in a significant elevation. Chen et al[101] noted that soy protein consumption decreased fasting insulin concentrations in hyperlipidemic subjects compared to milk protein consumption; in normolipidemic subjects soy protein consumption resulted in decreased plasma insulin concentrations compared to the baseline diet. The same authors also observed that serum insulin levels were significantly decreased by soy protein vs milk protein consumption in hypercholesterolemic subjects while plasma glucose levels were equivalent in both groups, suggesting an insulin sensitizing effect of soy protein consumption[100].
Hypertension contributes to deterioration of renal function as mentioned earlier, however, only a few soy protein intervention studies have evaluated soy protein’s effects on vascular function or blood pressure in patients with renal dysfunction (Tables 1 and 2). Cupisti et al[94] were the first to show that soy protein consumption resulted in improvements in endothelial function in the brachial arteries of renal transplant patients compared to baseline diets. Increased flow mediated dilation after soy consumption correlated to increases in the plasma arginine/asymmetric dimethyl arginine (ADMA) ratios in the subjects[94]. Arginine is the substrate for and ADMA is the endogenous inhibitor of endothelial nitric oxide synthase, so increases in this ratio would be expected to increase endothelial-dependent vasodilation. Interestingly, Teixeira et al[106] also showed increases in plasma arginine/lysine ratios after soy vs casein diet, however, no differences in blood pressure between groups was noted. Compared to baseline diet, soy protein consumption by nephrotic patients resulted in significant decreases in blood pressure in a study by D’Amico et al[95,96]. In a randomized crossover study by Miraghajani et al[108], soy milk vs cow milk consumption for four weeks also significantly reduced blood pressure in Type 2 diabetics (n = 25) with nephropathy. However, no significant differences in renal function as assessed by proteinuria, blood urea nitrogen (BUN), serum creatinine and eGFR, were observed[108].
Several chronic intervention studies showed that soy protein consumption was associated with decreases in measures of systemic oxidative processes (Tables 1 and 2). Cupusti et al[94] showed that soy protein consumption was associated with a reduction in plasma lipid peroxides in renal transplant patients compared to baseline diet. Siefker et al[109] observed that oxidized LDL concentrations were significantly reduced in hemodialysis patients after soy protein consumption (25 g/d for 4 day/wk for 4 wk) compared to a dairy protein control. Imani et al[110] failed to see any difference in oxidized LDL concentrations in peritoneal dialysis patients after 8 wk on a soy vs meat protein intervention (14 g/d).
Inflammation is associated with increased morbidity and mortality in patients with advanced kidney disease[111] and some intervention studies have indicated that soy protein may have anti-inflammatory properties. Azadbakht et al[98] observed a significant decrease in serum C-reactive protein (CRP) after 4 years of a soy protein vs animal protein diet. Fanti et al[112] did not see any significant decrease in CRP in ESRD patients after 8 wk on a soy vs milk protein supplement, however, this group did observe a significant inverse correlation between serum isoflavone and CRP concentrations. Serum isoflavone concentrations were also positively correlated with serum albumin and insulin-like growth factor concentrations which are markers of positive nutritional status. Miraghajani et al[113], on the other hand, did not see any reductions in inflammatory markers CRP, TNFα or interleukin 6 (IL-6) in Type 2 diabetic subjects with nephropathy after 4 wk of a low dose soy protein diet (< 5 g/d). Similarly, Siefker et al[109], while observing a significant decrease in oxidized LDL concentrations after 4 wk of soy protein intake in hemodialysis patients, did not see decreases in CRP, TNFα or 8-iso-prostaglandin F2α. Miaghajani et al[113] and Imani et al[110] both reported effects of soy protein consumption on reducing markers of coagulation. Miraghajani et al[113] observed reductions in D-dimer (fibrin degradation products that have been found to be correlated with renal dysfunction) after soy milk vs cow milk consumption in Type 2 diabetics with nephropathy, with no differences in fibrinogen levels between groups. Imani et al[110] reported significant decreases in plasma coagulation factor IX activity in peritoneal dialysis patients after soy vs meat protein consumption, but no changes in fibrinogen or Factor VII or X activities. In a more recent study, Tomayko et al[114] reported that 27 g/d soy vs whey protein for 6 mo resulted in a significant decrease in neutrophil-lymphocyte ratio, a marker of systemic inflammation, in adequately nourished maintenance hemodialysis patients.
It is probably of importance to researchers to note that the metabolism and excretion of isoflavones, derived predominantly from soy in the diet, are mediated by the kidneys. Three studies have investigated the metabolism of soy isoflavones in subjects with renal disease (Table 3). Fanti et al[115] noted that 55%-65% of hemodialysis patients in the United States had undetectable concentrations of serum isoflavones when they were on a standard renal diet. However, after a single soy meal ingestion, these levels were significantly increased in healthy subjects, but in ESRD patients on hemodialysis, serum isoflavone increases were on average increased three to four times higher than seen in the healthy subjects[115]. Half-lives of genistein and daidzein averaged 3.5 and 6 h, respectively in healthy subjects but were increased to 47 and 58 h in hemodialysis patients. Fanti et al[116] also observed that hemodialysis patients in Asian countries have significantly higher serum isoflavone concentrations than that seen in the United States. Hemodialysis does not effectively remove glucuronide-conjugated isoflavones due to their relatively high molecular weight[115]. Concentrations of sulfated and unconjugated isoflavones, however, tend to be similar between hemodialysis patients and healthy subjects[116]. Fanti et al[116] also, not surprisingly, observed significant correlations between dietary intake of soy foods and overall serum concentrations of isoflavones. Ratios of daidzein and genistein in the serum also tend to be different between ESRD and healthy subject indicating the alterations in normal tubular excretion properties in the ESRD patients[116]. It appears that renal patients who have undergone renal transplantation exhibit concentrations of serum isoflavones comparable to those observed in healthy subjects[117].
A study by Howe et al[118] cited in Table 4 evaluated the postprandial responses of calcium metabolism to single meal loads of varying protein sources in healthy postmenopausal women. High protein intake has been shown to increase urinary calcium excretion[118]. Protein loads of 45 g of soy, beef or cottage cheese protein (but not 15 g) resulted in significant increases in urinary calcium excretion up to 3 h postprandially and the percent of calcium resorbed by the kidney was significantly reduced after the dairy and soy protein meals[118]. Serum ionized (free) calcium was unaffected but serum phosphorus was significantly lowered by all protein meals compared to the non-protein meal[118]. Soy protein significantly reduced calcitonin levels vs baseline and dairy protein significantly increased parathyroid hormone (PTH), however, all proteins tended to lower calcitonin and raise PTH[118]. Thus, there may be subtle effects of protein source on calcium metabolism in the acute setting that involves renal metabolism. Table 4 summarizes two chronic intervention studies with soy protein that assessed its effect on calcium metabolism. Breslau et al[119] reported that serum uric acid concentrations were significantly lower after 12 d intervention on a largely soy-based vegetarian and ovo-vegetarian diet compared to the animal protein diet. The animal protein diet exhibited a significantly higher urinary uric acid excretion compared to that observed with the ovo-vegetarian diet and is likely explained by the high purine content in animal foods[119]. Excretion of calcium and phosphorus were significantly lower and urinary oxalate higher in the vegetarian diet compared to the beef diet[119]. Breslau et al[119] reported lower PTH levels in the animal vs vegetarian diet group and serum 1,25-(OH)2D was higher in the vegetarian compared to animal diet group[119]. Roughead et al[120] did not observe any difference in urinary calcium excretion between soy and meat protein diets after 7 wk of intervention in healthy postmenopausal women, despite a significantly higher urinary pH in the soy group. Overall, no differences in measures of bone metabolism or body calcium retention were seen when women were ingesting the soy or meat protein diets[120]. A recent study of maintenance hemodialysis patients (cited in Table 2) measured alkaline phosphatase (ALP), a measure of bone turnover and an independent predictor of mortality in these patients[114]. The investigators noted that both soy and whey proteins gave rise to lower serum ALP compared to a non-protein placebo supplement[114] which indicates that both proteins may be useful in improving health in these severely renal-compromised patients. This is further supported by the same authors’ measures of physical function in these patients; both protein groups showed benefits in gait speed and shuttle walk test times on the protein vs placebo diets[114]. In this study, subjects were not considered malnourished (mean serum albumin > 3.9 mg/dL) and yet, still sustained benefit from protein supplementation suggesting that there is the potential for enhanced effects in ESRD patients with nutritional deficiencies.
Table 5 summarizes studies conducted to evaluate the effects of single meal interventions with soy vs other proteins on renal function. It is well established that acute protein ingestion or infusion of amino acids results in a transient increase in GFR, or “hyperfiltration”[121]. While this mechanism may be the normal response to protein ingestion, in patients with CKD, the hyperfiltration induced by a high protein diet is believed to contribute to the decline in renal function that deteriorates the undamaged nephron function[121]. Acute ingestion of > 50 g soy protein has been shown to result in significantly lower increases in GFR compared to equal amounts of meat or fish protein, but not egg white, dairy protein or amino acid ingestion, in several studies[70,122-124] while one study showed an increase in GFR after soy protein consumption that was equivalent to that induced by beef protein[125]. Kontessis et al[70] determined that plasma glucagon was higher after a meat meal compared to a soy meal and plasma glucagon levels correlate with GFR[121]. However, Orita et al[125] noted that both beef and soy elicited similar increases in plasma glucagon. Differences between these two studies may be in the way the proteins were provided to the subjects since the protein loads were similar; the subjects in the Kontessis et al[70] study consumed soy as a powder dissolved in flavored water while the soy protein consumed by subjects in the Orita et al[125] study consumed the protein as a fried paste. The degree of renal function in subjects also affected the response to soy protein ingestion. Diebert et al[126] observed that subjects with metabolic syndrome had higher baseline GFR compared to healthy subjects and that there was a significantly greater increase in GFR in the subjects with metabolic syndrome after ingesting 1 g/kg body weight soy protein compared to healthy subjects. Notably, no differences from baseline GFR responses between subjects with metabolic syndrome and healthy subjects were seen when the protein load was reduced to 0.3 g/kg body weight which is the amount of protein commonly used in meal replacement products for weight management[126]. Nakamura et al[123] also noted that when GFR data from subjects with Type 2 diabetes was divided by degree of urinary albumin excretion, that GFR was lower in the soy (bean curd) vs tuna protein meal only in the subjects with mild proteinuria and was not significantly different between proteins in the group with mid-range proteinuria. In subjects with significant proteinuria, the tuna protein meal caused a decrease in GFR but there was no significant change after intake of the soy protein[123]. However, no differences in AER were observed between groups. The authors concluded that the amino acid compositions of the two proteins may be exerting differential effects on GFR based on elevated levels of circulating alanine, glycine and arginine observed after tuna fish meal consumption vs bean curd[123].
In the acute protein loading study in normal subjects reported by Kontessis et al[70], fractional albumin and immunoglobulin G clearance (excretion divided by GFR) increased after a beef protein load but did not change after soy protein consumption. Urinary urea was increased similarly in both groups[70]. In contrast, Buzio et al[127] reported significantly lower urinary urea appearance after soy compared to red meat or cheese consumption suggesting differences in soy protein digestion or absorption. Differences between these studies may again be related to the form of soy protein provided; in the study by Buzio et al[127] the soy protein was provided as soybeans and tofu and not as protein powder in the Kontessis et al[70] study.
Soy protein consumption was shown to result in beneficial reductions in baseline GFR in chronic intervention studies in Type 1 diabetics with hyperfiltration compared to an animal protein diet[104] and in pre-hypertensive postmenopausal women compared to a dairy protein supplement[128] (Table 2). In the former study, the effect of 45-55 g/d soy protein to significantly reduce GFR in the subjects with demonstrated renal dysfunction compared to the same subjects on animal-based diet could be seen in 8 wk of intervention[104]. In the latter study, the postmenopausal women subjects had minimally compromised or normal renal function as assessed by GFR values, but after 6 mo on a soy protein diet (12.8 g/d) there was less of a decrease in eGFR than observed in subjects consuming the milk protein placebo[128]. The effect of soy protein consumption to reduce eGFR was greater in women with higher initial cystatin C concentrations (indicating poorer renal function at study initiation)[128]. No significant changes were observed in any other renal parameter in that study. Soroka et al[129] also evaluated GFR after a 6 mo intervention with a vegetable protein (mostly soy) or animal protein in non-diabetic, non-nephrotic chronic renal disease patients. No differences in GFR between groups were observed at the end of the study but both groups demonstrated reductions in the rate of decline of their renal disease[129]. The high dropout rate and small number of subjects that completed the study as well as differences in the total energy and protein intakes between the diets makes it difficult to make any conclusions as to any benefits of soy protein on renal function. In another small study comparing 0.5 g/kg per day of soy or beef protein consumed for 8 wk by Type 2 diabetic subjects with proteinuria, Anderson et al[97] observed that both interventions resulted in similar decreases in GFR. Only the soy protein group demonstrated a significant decrease in serum urea nitrogen and an increase in urinary protein excretion[97]. No other study has reported an increase in urinary protein excretion after a soy protein intervention. Other studies cited in Table 2 have demonstrated a reduction in proteinuria after soy protein interventions[98,103,106,130]. Teixeira et al[106] also noted that changes in urinary albumin-creatinine ratios correlated inversely with plasma isoflavone concentrations. It is interesting to speculate that in subjects with more progressed renal dysfunction, elevated isoflavones resulting from inefficient renal clearance may help to protect the kidney from further damage.
Taken together, the data suggests that both short- (weeks) and long-term (years) consumption of soy-based diets is renoprotective in both healthy and renal compromised individuals. While this effect has not been seen in all studies[97,108,131], the majority of studies show no negative consequences of soy protein consumption on renal function. A renoprotective effect of soy protein is supported by a recent meta-analysis of nine clinical trials comprised of 197 subjects, concluding that soy protein intake reduced serum creatinine in patients with pre-dialysis CKD[77].
The renoprotective effects of a soy-based diet were demonstrated 25 years ago using the male Fischer 344 rat model of chronic, progressive nephropathy. In 1988, Kalu et al[132] demonstrated that life-long feeding of a soy-based diet (21 g soy/100 g diet) attenuated the late-life (21 mo and older) increase in serum creatinine[132]. Interestingly, the soy-fed rats had similar renal function as the life-long caloric restriction - the gold standard for protection against age-related nephropathy. Iwasaki et al[133] used a similar protocol and demonstrated that median life span of the control rats was 730 d, compared to 844 in the soy-fed rats[133]. In the control group, 41% of the rats that spontaneously died exhibited end-stage chronic nephropathy, which was reduced to 7% by the soy diet. A soy protein diet was shown to improve longevity in male Fischer 344 rats similar to life-long caloric restriction[134]. This positive effect on survival was also seen in a study feeding male Wistar rats soy milk and normal rat chow beginning at 3 mo; the percentage of surviving animals at 18 mo increased from 55% to 87%[135]. Importantly, the effect of a soy diet is not due simply to protein restriction as 40% diet restriction without restricting protein intake is highly effective at attenuating nephropathy[136].
There is an extensive body of literature on the positive effect of soy on PKD in animal models. In Pcy mice, a model of PKD, a soy-based diet, both high and low protein diet (17.4 or 6 g/100 g diet, respectively, casein protein as control) for 13 wk reduced cyst size in low protein diets[137]. The protective effect in this model was reproduced in another study[138]. Han:SPRD-Cy rats, a PKD model, were fed 20% soy or casein diets ad libitum for 8 wk after weaning; the soy diet decreased serum creatinine and reduced cysts[139]. In the same model, animals were fed 20% soy protein for 1 or 3 wk (casein protein as control) and at 3 wk, cyst area was reduced, and creatinine clearance improved[140]. The positive effect of soy on creatinine clearance has also been observed in this model using heat-treated soy protein isolate[141]. Interestingly, a soy diet 2 wk before mating - the diet is discontinued during pregnancy and lactation, afforded renoprotection in this model as assessed by proteinuria, but not serum creatinine or creatinine clearance[142].
The renoprotective effects of soy protein have also been shown in metabolic models of renal dysfunction, including Zucker Diabetic Fatty (ZDF) and high-fructose fed rats[143-145]. A 20% soy diet (casein control) for 160 d reduced proteinuria, as well as glomerulosclerosis and tubulointerstitial fibrosis, in obese Zucker rats[146]. In female obese Zucker rats, a soy protein diet decreased proteinuria and glomerular damage, but did not affect creatinine clearance[147]. In the male obese Zucker, a soy protein isolate diet (23.1 g /100 g diet) was begun 10 d after unilateral nephrectomy and renal damage was further induced by deoxycorticosterone acetate (DOCA). Proteinuria and urinary N-acetyl-beta-D-glucosaminidase (NAG) were reduced by soy at 1 and 2 wk after DOCA[148].
The renoprotective effects of soy have also been observed in other models of renal dysfunction. A soy protein diet (24.5%) reduced proteinuria in the male Imai rat model of spontaneous focal segmental glomerulosclerosis[149]. However, when the control casein diet was supplemented with soy, protection was not seen. A soy diet also attenuated BUN and improved creatinine clearance in this model[149]. However, soy has not been shown to be renoprotective in all studies. A 20% soy protein diet did not reduce cyst volume in Han:SPRD-Cy rats[150]. In female PCK rats fed soy protein isolate 200g/kg diet (casein control) beginning at 5 wk and maintained on the diet for 12 wk, no positive effect on cyst size, inflammation, fibrosis, or BUN was seen[151]. In a comparison of whey protein (13.8 g/100 g diet) and soy protein (13.1 g/100 g diet), Wistar rats were fed diets for 12 wk; no differences in renal morphology or function (albuminuria or BUN) were observed[152]. In obese Zucker rats, a soy protein diet attenuated glomerular damage, but not proteinuria[153]. Soy protein did not have a protective effect on GFR (assessed by creatinine clearance) in a canine model of nephropathy[154]. The MRL/Mp-lpr/lpr mice are an autoimmune model of lupus; weanling female mice were fed soy (20% soy protein and 5% soybean oil) for 4-16 wk; in this model, soy increased proteinuria, reduced creatinine clearance, and increased serum creatinine[155].
Given the positive effects of soy in several animal models, and limited clinical studies, efforts have been made to identify the mechanism of the soy-based protection - both the specific soy constituent and the molecular/cellular pathways affected by soy. Beneficial effects have been seen in several of the animal models using a low-isoflavone diet[156,157], suggesting that the isoflavones are not responsible for renoprotection. This is supported by the finding that genistein did not have a protective effect in the Pcy mouse model[138] and that isoflavone-enriched soy protein diet did not enhance the effect of soy on decreasing cysts[158]. However, genistein alone did reduce inflammation, oxidative stress and albuminuria and increased creatinine clearance in a high fructose model of renal dysfunction[144,159]. In addition, in male obese ZDF × Spontaneously Hypertensive Hyperlipidemic rats, a high isoflavone, but not a low-isoflavone, diet attenuated BUN[143]. In recent studies, β-conglycinin has been shown to have a positive effect on blood pressure, nephrin expression, proteinuria and lipid peroxidation in diabetic nephropathy in Spontaneously Hypertensive rats[160].
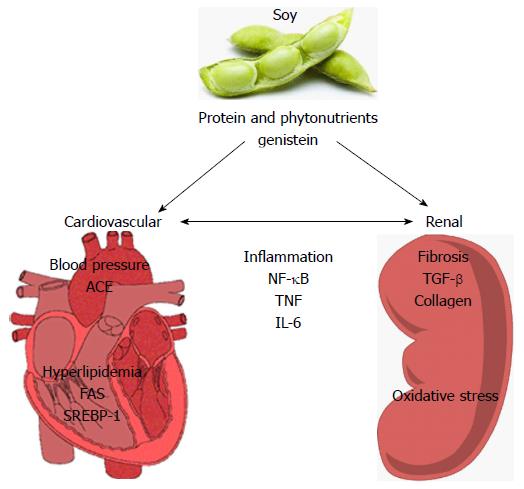
Limited progress has been made in determining the cellular and molecular targets of soy in relation to renoprotection (Figure 1). Systemically, soy has been shown to have blood pressure lowering effects and positive effects on hyperlipidemia. Soy can reduce plasma[161,162] and renal ACE activity[163]. In 5/6 nephrectomized rats, Yang et al[164] showed that substitution of casein for 14 wk with either pepsin-hydrolyzed or intact soy protein in the diet ameliorated increases in systolic and diastolic blood pressure coincident with significant decreases in plasma and renal ACE activities, kidney TNFα levels, proteinuria and plasma insulin concentrations[164]. Yang et al[165] also evaluated the effects of 6 wk consumption of pepsin-hydrolyzed soy protein in a rat model of L-NAME (L-NG-Nitro-L-arginine methyl ester)-induced hypertension, observing dose-dependent reductions in blood pressure, BUN, ACE activities and renal vascular damage[165]. There is also data suggesting that soy may have beneficial effects on lipid profiles that correlate with renoprotection. In a puromycin-induced model of nephrotic syndrome, soy protein (20%) reduced both hypercholesterolemia, hypertriglyceridemia and proteinuria[166]. In addition, the soy diet reduced sterol regulatory element-binding protein 1 and fatty acid synthase expression. In aging Wistar rats (18 mo), soy milk decreased total serum cholesterol, LDL-C and serum triglycerides and reduced renal lipid peroxidation[135]. Soybean β-conglycinin has been shown to reduce cholesterol and improve renal function (albuminuria) in a streptozotocin-induced diabetes[163].
Soy may also target the kidney itself to improve renal function, specifically via anti-inflammatory, antioxidant, or anti-fibrotic mechanisms. A reduction in renal NF-κB has been observed in experimental studies[145]. TNF-α and IL-6 are decreased by soy[145,164,165]. Chemokine (C-C Motif) receptor 2 (CCR2) is a chemokine receptor that is implicated in nephropathy[167]; soy attenuates CCR2 expression[168]. In a rat PKD model, a soy protein diet also decreases cyclooxygenase -1 and -2 activities[169]. ROS are generated by normal cellular metabolic processes and include superoxide O2-, hydrogen peroxide (H2O2) and the hydroxyl radical (OH-)[170,171]. Reactive nitrogen species, such as peroxynitrite (ONOO-), are formed through reaction of O2- and NO[172]. As reviewed by Tucker et al[173], markers of oxidative stress have been shown to be significantly elevated in CKD patients and to be inversely correlated with eGFR[173-176]. There is evidence that soy can reduce bromate- and iron-induced H2O2 levels in the kidney, which corresponds with decreased lipid peroxidation[177,178]. A soy diet (14.1% total energy) supplemented with genistein (40 mg/kg per day) has been shown to increase antioxidant capacity, including increased catalase, and decrease lipid peroxidation in a doxorubicin-induced renal dysfunction[179]. Mechanistically, however, other studies have shown that soy did not affect superoxide dismutase, catalase, or glutathione-peroxidase activity, but did reduce nitrotyrosine levels[180]. Thus, while soy may have antioxidant properties, the underlying mechanism has not been elucidated. There is data supporting the hypothesis that soy may inhibit the development of fibrosis, the common pathway in the development of CKD. Soy has been shown to reduce fibrosis, most notably in PKD[181] and genistein reduces fibrosis in a high-fructose model[145]. There is also evidence that soy decreases collagen expression[145,182]. TGF-β is a potent pro-fibrotic mediator in the kidney[183]; several studies have shown that soy attenuates renal TGF-β expression[160,166,184]. In human HK-2 cells, parathyroid hormone-induced epithelial-to-mesenchymal transition alpha smooth muscle actin expression is attenuated by genistein (25-100 μmol/L); in addition, there is reduced expression of pro-fibrotic connective tissue growth factor expression[185]. Reviewed previously, Wnt/β-catenin signaling is strongly implicated in renal fibrosis through its downstream induction of profibrotic gene expression[186] as well as cyst formation in PKD[187]. The effects of soy protein intake on Wnt signaling have only recently begun to be explored. Several studies conducted in rodent models of dyslipidemia have demonstrated effects of soy protein consumption on hepatic gene expression of Wnt pathway intermediates[188-190]. Further investigation is needed to understand soy’s impact on the Wnt/β-catenin pathway in the kidney and how this may function in renoprotection.
Soy protein consumption has benefits in patients at risk for and who have demonstrated renal dysfunction and symptoms of early kidney disease. Soy protein can improve the dyslipidemia that contributes to and results from renal disease. In addition, soy protein has been shown to reduce blood pressure and improve vascular health in subjects with renal disease and this may be related to its ability to reduce markers of oxidative stress and inflammation. Studies have shown that in the long term soy protein consumption can reduce deterioration of glomerular function and proteinuria, albeit in small-scale clinical studies. In acute studies, soy protein meals tend to increase GFR less than animal-derived protein (but not dairy) meals. The reasons for this remain to be elucidated and may be related to elevations of select amino acid profiles and/or micronutrients associated with soy vs animal derived proteins. Animal studies have begun to identify possible mechanisms of action of soy protein and its components in slowing the onset and progression of kidney dysfunction and more research, both human and animal, is needed to elucidate the mechanism of soy protein’s renoprotective effects. Furthermore, studies, both preclinical and clinical, can further contribute to our knowledge of the role of dietary soy protein on renal health by more careful design and reporting of the interventions and outcomes as prescribed by Klein et al[191]. A large-scale clinical trial including detailed information on the soy source, analysis and reporting of analytical methodology used to determine bioactive constituents, both in the diet as well as in biological assessment following diets, and identifying dietary constituents that may interact with soy in the diet, is warranted based on the promising results summarized in this paper.
P- Reviewer: Gheith O, Lehtonen SH, Pedersen EB, Trkulja B S- Editor: Qiu S L- Editor: A E- Editor: Jiao XK
| 1. | National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-266. [PubMed] |
| 2. | Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Inter. 2013;3:1-150. |
| 3. | Eboh C, Chowdhury TA. Management of diabetic renal disease. Ann Transl Med. 2015;3:154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 4. | Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3153] [Cited by in RCA: 2863] [Article Influence: 238.6] [Reference Citation Analysis (0)] |
| 5. | Mora-Fernández C, Domínguez-Pimentel V, de Fuentes MM, Górriz JL, Martínez-Castelao A, Navarro-González JF. Diabetic kidney disease: from physiology to therapeutics. J Physiol. 2014;592:3997-4012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 6. | Su SL, Lin C, Kao S, Wu CC, Lu KC, Lai CH, Yang HY, Chiu YL, Chen JS, Sung FC. Risk factors and their interaction on chronic kidney disease: A multi-centre case control study in Taiwan. BMC Nephrol. 2015;16:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Wouters OJ, O’Donoghue DJ, Ritchie J, Kanavos PG, Narva AS. Early chronic kidney disease: diagnosis, management and models of care. Nat Rev Nephrol. 2015;11:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Kodner C. Nephrotic syndrome in adults: diagnosis and management. Am Fam Physician. 2009;80:1129-1134. [PubMed] |
| 9. | James MT, Hemmelgarn BR, Tonelli M. Early recognition and prevention of chronic kidney disease. Lancet. 2010;375:1296-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 423] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 10. | United States Renal Data System (USRDS). USRDS 2014 Annual Data Report. [accessed 2015 Sept 1]. Available from: http//www.usds.orf/adr/aspx. |
| 11. | Hoerger TJ, Simpson SA, Yarnoff BO, Pavkov ME, Ríos Burrows N, Saydah SH, Williams DE, Zhuo X. The future burden of CKD in the United States: a simulation model for the CDC CKD Initiative. Am J Kidney Dis. 2015;65:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 12. | Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1187] [Cited by in RCA: 1307] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 13. | Gorodetskaya I, Zenios S, McCulloch CE, Bostrom A, Hsu CY, Bindman AB, Go AS, Chertow GM. Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int. 2005;68:2801-2808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 217] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Pagels AA, Söderkvist BK, Medin C, Hylander B, Heiwe S. Health-related quality of life in different stages of chronic kidney disease and at initiation of dialysis treatment. Health Qual Life Outcomes. 2012;10:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 15. | Palmer S, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, Pellegrini F, Saglimbene V, Logroscino G, Fishbane S. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. 2013;84:179-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 545] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 16. | Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8787] [Cited by in RCA: 8985] [Article Influence: 408.4] [Reference Citation Analysis (0)] |
| 17. | James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5429] [Cited by in RCA: 5503] [Article Influence: 500.3] [Reference Citation Analysis (0)] |
| 18. | Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, O’Connor A, Perumal K, Rahman M, Steigerwalt S. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2010;55:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 300] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 19. | Townsend RR, Anderson AH, Chen J, Gadebegku CA, Feldman HI, Fink JC, Go AS, Joffe M, Nessel LA, Ojo A. Metabolic syndrome, components, and cardiovascular disease prevalence in chronic kidney disease: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Nephrol. 2011;33:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Townsend RR, Taler SJ. Management of hypertension in chronic kidney disease. Nat Rev Nephrol. 2015;11:555-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Maditz KH, Gigliotti JC, Tou JC. Evidence for a role of proteins, lipids, and phytochemicals in the prevention of polycystic kidney disease progression and severity. Nutr Rev. 2013;71:802-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Tan YC, Blumenfeld JD, Anghel R, Donahue S, Belenkaya R, Balina M, Parker T, Levine D, Leonard DG, Rennert H. Novel method for genomic analysis of PKD1 and PKD2 mutations in autosomal dominant polycystic kidney disease. Hum Mutat. 2009;30:264-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Hewitson TD. Renal tubulointerstitial fibrosis: common but never simple. Am J Physiol Renal Physiol. 2009;296:F1239-F1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 24. | Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1019] [Cited by in RCA: 1147] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 25. | Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol. 2010;6:643-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 487] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 26. | Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010;21:1819-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 611] [Cited by in RCA: 698] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 27. | Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond). 2013;124:139-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 583] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 28. | Filiopoulos V, Vlassopoulos D. Inflammatory syndrome in chronic kidney disease: pathogenesis and influence on outcomes. Inflamm Allergy Drug Targets. 2009;8:369-382. [PubMed] |
| 29. | Sanz AB, Sanchez-Niño MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A. NF-kappaB in renal inflammation. J Am Soc Nephrol. 2010;21:1254-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 456] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 30. | Lin H, Hou CC, Cheng CF, Chiu TH, Hsu YH, Sue YM, Chen TH, Hou HH, Chao YC, Cheng TH. Peroxisomal proliferator-activated receptor-alpha protects renal tubular cells from doxorubicin-induced apoptosis. Mol Pharmacol. 2007;72:1238-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Esteban V, Lorenzo O, Rupérez M, Suzuki Y, Mezzano S, Blanco J, Kretzler M, Sugaya T, Egido J, Ruiz-Ortega M. Angiotensin II, via AT1 and AT2 receptors and NF-kappaB pathway, regulates the inflammatory response in unilateral ureteral obstruction. J Am Soc Nephrol. 2004;15:1514-1529. [PubMed] |
| 32. | Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl. 2005;S57-S65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 349] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 33. | Vielhauer V, Mayadas TN. Functions of TNF and its receptors in renal disease: distinct roles in inflammatory tissue injury and immune regulation. Semin Nephrol. 2007;27:286-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Ruggenenti P, Mise N, Pisoni R, Arnoldi F, Pezzotta A, Perna A, Cattaneo D, Remuzzi G. Diverse effects of increasing lisinopril doses on lipid abnormalities in chronic nephropathies. Circulation. 2003;107:586-592. [PubMed] |
| 35. | Charlesworth JA, Gracey DM, Pussell BA. Adult nephrotic syndrome: non-specific strategies for treatment. Nephrology (Carlton). 2008;13:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Athyros VG, Tziomalos K, Karagiannis A. Treatment options for dyslipidemia in chronic kidney disease and for protection from contrast-induced nephropathy. Expert Rev Cardiovasc Ther. 2015;13:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Pandya V, Rao A, Chaudhary K. Lipid abnormalities in kidney disease and management strategies. World J Nephrol. 2015;4:83-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Sarnak MJ, Bloom R, Muntner P, Rahman M, Saland JM, Wilson PW, Fried L. KDOQI US commentary on the 2013 KDIGO Clinical Practice Guideline for Lipid Management in CKD. Am J Kidney Dis. 2015;65:354-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 39. | Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res. 2014;55:561-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 468] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 40. | Moorhead JF, Chan MK, El-Nahas M, Varghese Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet. 1982;2:1309-1311. [PubMed] |
| 41. | Rosario RF, Prabhakar S. Lipids and diabetic nephropathy. Curr Diab Rep. 2006;6:455-462. [PubMed] |
| 42. | Srivastava SP, Shi S, Koya D, Kanasaki K. Lipid mediators in diabetic nephropathy. Fibrogenesis Tissue Repair. 2014;7:12. [PubMed] |
| 43. | Hirano T. Abnormal lipoprotein metabolism in diabetic nephropathy. Clin Exp Nephrol. 2014;18:206-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Holzer M, Schilcher G, Curcic S, Trieb M, Ljubojevic S, Stojakovic T, Scharnagl H, Kopecky CM, Rosenkranz AR, Heinemann A. Dialysis Modalities and HDL Composition and Function. J Am Soc Nephrol. 2015;26:2267-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 45. | Yamamoto S, Kon V. Chronic kidney disease induced dysfunction of high density lipoprotein. Clin Exp Nephrol. 2014;18:251-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Nikodimopoulou M, Liakos S. Secondary hyperparathyroidism and target organs in chronic kidney disease. Hippokratia. 2011;15:33-38. [PubMed] |
| 47. | Clement LC, Macé C, Avila-Casado C, Joles JA, Kersten S, Chugh SS. Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat Med. 2014;20:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 48. | Palmer SC, Craig JC, Jones A, Higgins G, Willis N, Strippoli GF. Celebrating 20 years of evidence from the Cochrane Collaboration: what has been the impact of systematic reviews on nephrology? Nephrol Dial Transplant. 2015;30:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Scarpioni R, Ricardi M, Albertazzi V, Melfa L. Treatment of dyslipidemia in chronic kidney disease: Effectiveness and safety of statins. World J Nephrol. 2012;1:184-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181-2192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1946] [Cited by in RCA: 1758] [Article Influence: 125.6] [Reference Citation Analysis (0)] |
| 51. | Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Grönhagen-Riska C. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1474] [Cited by in RCA: 1474] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 52. | Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1926] [Cited by in RCA: 1860] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 53. | Silbernagel G, Baumgartner I, Wanner C, März W. Toward individualized cholesterol-lowering treatment in end-stage renal disease. J Ren Nutr. 2014;24:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Steiber AL. Chronic kidney disease: considerations for nutrition interventions. JPEN J Parenter Enteral Nutr. 2014;38:418-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Amparo FC, Kamimura MA, Molnar MZ, Cuppari L, Lindholm B, Amodeo C, Carrero JJ, Cordeiro AC. Diagnostic validation and prognostic significance of the Malnutrition-Inflammation Score in nondialyzed chronic kidney disease patients. Nephrol Dial Transplant. 2015;30:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | Obi Y, Qader H, Kovesdy CP, Kalantar-Zadeh K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care. 2015;18:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 57. | Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3558] [Cited by in RCA: 3441] [Article Influence: 143.4] [Reference Citation Analysis (0)] |
| 58. | Tyson CC, Nwankwo C, Lin PH, Svetkey LP. The Dietary Approaches to Stop Hypertension (DASH) eating pattern in special populations. Curr Hypertens Rep. 2012;14:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | Elliott P, Stamler J, Dyer AR, Appel L, Dennis B, Kesteloot H, Ueshima H, Okayama A, Chan Q, Garside DB. Association between protein intake and blood pressure: the INTERMAP Study. Arch Intern Med. 2006;166:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 60. | Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003;138:460-467. [PubMed] |
| 61. | Goraya N, Wesson DE. Dietary interventions to improve outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens. 2015;24:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 62. | Piccoli GB, Attini R, Vasario E, Gaglioti P, Piccoli E, Consiglio V, Deagostini C, Oberto M, Todros T. Vegetarian supplemented low-protein diets. A safe option for pregnant CKD patients: report of 12 pregnancies in 11 patients. Nephrol Dial Transplant. 2011;26:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Ranich T, Bhathena SJ, Velasquez MT. Protective effects of dietary phytoestrogens in chronic renal disease. J Ren Nutr. 2001;11:183-193. [PubMed] |
| 65. | Filipowicz R, Beddhu S. Optimal nutrition for predialysis chronic kidney disease. Adv Chronic Kidney Dis. 2013;20:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Yokoyama Y, Barnard ND, Levin SM, Watanabe M. Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovasc Diagn Ther. 2014;4:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 89] [Reference Citation Analysis (0)] |
| 67. | Kuhlmann MK, Levin NW. Interaction between nutrition and inflammation in hemodialysis patients. Contrib Nephrol. 2005;149:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 68. | Jibani MM, Bloodworth LL, Foden E, Griffiths KD, Galpin OP. Predominantly vegetarian diet in patients with incipient and early clinical diabetic nephropathy: effects on albumin excretion rate and nutritional status. Diabet Med. 1991;8:949-953. [PubMed] |
| 69. | Kitazato H, Fujita H, Shimotomai T, Kagaya E, Narita T, Kakei M, Ito S. Effects of chronic intake of vegetable protein added to animal or fish protein on renal hemodynamics. Nephron. 2002;90:31-36. [PubMed] |
| 70. | Kontessis P, Jones S, Dodds R, Trevisan R, Nosadini R, Fioretto P, Borsato M, Sacerdoti D, Viberti G. Renal, metabolic and hormonal responses to ingestion of animal and vegetable proteins. Kidney Int. 1990;38:136-144. [PubMed] |
| 71. | Kontessis PA, Bossinakou I, Sarika L, Iliopoulou E, Papantoniou A, Trevisan R, Roussi D, Stipsanelli K, Grigorakis S, Souvatzoglou A. Renal, metabolic, and hormonal responses to proteins of different origin in normotensive, nonproteinuric type I diabetic patients. Diabetes Care. 1995;18:1233. [PubMed] |
| 72. | Moe SM, Zidehsarai MP, Chambers MA, Jackman LA, Radcliffe JS, Trevino LL, Donahue SE, Asplin JR. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 365] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 73. | Wiseman MJ, Hunt R, Goodwin A, Gross JL, Keen H, Viberti GC. Dietary composition and renal function in healthy subjects. Nephron. 1987;46:37-42. [PubMed] |
| 74. | Klahr S, Breyer JA, Beck GJ, Dennis VW, Hartman JA, Roth D, Steinman TI, Wang SR, Yamamoto ME. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. Modification of Diet in Renal Disease Study Group. J Am Soc Nephrol. 1995;5:2037-2047. [PubMed] |
| 75. | Choukroun G, Itakura Y, Albouze G, Christophe JL, Man NK, Grünfeld JP, Jungers P. Factors influencing progression of renal failure in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995;6:1634-1642. [PubMed] |
| 76. | Anderson JJ, Anthony MS, Cline JM, Washburn SA, Garner SC. Health potential of soy isoflavones for menopausal women. Public Health Nutr. 1999;2:489-504. [PubMed] |
| 77. | Zhang J, Liu J, Su J, Tian F. The effects of soy protein on chronic kidney disease: a meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2014;68:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Kawamura S. Quantitive paper chromatography of sugars of the cotyledon, hull, and hypocotyl of soybeans of selected varieties. Kagawa Univ Fac Tech Bull. 1967;18:117-131. |
| 79. | Medic J, Atkinson C, Hurburgh Jr CR. Current Knowledge in Soybean Composition. J Am Oil Chem Soc. 2014;91:363-384. [RCA] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 80. | Hughes GJ, Ryan DJ, Mukherjea R, Schasteen CS. Protein digestibility-corrected amino acid scores (PDCAAS) for soy protein isolates and concentrate: criteria for evaluation. J Agric Food Chem. 2011;59:12707-12712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 81. | Messina M, Messina V. The role of soy in vegetarian diets. Nutrients. 2010;2:855-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 82. | Murphy PA, Barua K, Hauck CC. Solvent extraction selection in the determination of isoflavones in soy foods. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:129-138. [PubMed] |
| 83. | Setchell KD, Cole SJ. Variations in isoflavone levels in soy foods and soy protein isolates and issues related to isoflavone databases and food labeling. J Agric Food Chem. 2003;51:4146-4155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 84. | Setchell KD, Brown NM, Zhao X, Lindley SL, Heubi JE, King EC, Messina MJ. Soy isoflavone phase II metabolism differs between rodents and humans: implications for the effect on breast cancer risk. Am J Clin Nutr. 2011;94:1284-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 85. | Shelnutt SR, Cimino CO, Wiggins PA, Ronis MJ, Badger TM. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am J Clin Nutr. 2002;76:588-594. [PubMed] |
| 86. | Rafii F. The role of colonic bacteria in the metabolism of the natural isoflavone daidzin to equol. Metabolites. 2015;5:56-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 87. | Yuan JP, Wang JH, Liu X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora--implications for health. Mol Nutr Food Res. 2007;51:765-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 224] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 88. | Legette LL, Prasain J, King J, Arabshahi A, Barnes S, Weaver CM. Pharmacokinetics of equol, a soy isoflavone metabolite, changes with the form of equol (dietary versus intestinal production) in ovariectomized rats. J Agric Food Chem. 2014;62:1294-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 89. | Zhang Y, Song TT, Cunnick JE, Murphy PA, Hendrich S. Daidzein and genistein glucuronides in vitro are weakly estrogenic and activate human natural killer cells at nutritionally relevant concentrations. J Nutr. 1999;129:399-405. [PubMed] |
| 90. | Singhal R, Shankar K, Badger TM, Ronis MJ. Hepatic gene expression following consumption of soy protein isolate in female Sprague-Dawley rats differs from that produced by 17{beta}-estradiol treatment. J Endocrinol. 2009;202:141-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 91. | Tokede OA, Onabanjo TA, Yansane A, Gaziano JM, Djoussé L. Soya products and serum lipids: a meta-analysis of randomised controlled trials. Br J Nutr. 2015;114:831-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 92. | Food and Drug Administration. Soy Protein and Heart Disease Health Claim. [accessed 2015 Sept 28]. Available from: http//www.soyfoods.org/nutrition-health/soy-for-healthy-living/soy-for-heart-disease/soy-protein-and-heart-disease-health-claim. |
| 93. | Cupisti A, D’Alessandro C, Ghiadoni L, Morelli E, Panichi V, Barsotti G. Effect of a soy protein diet on serum lipids of renal transplant patients. J Ren Nutr. 2004;14:31-35. [PubMed] |
| 94. | Cupisti A, Ghiadoni L, D’Alessandro C, Kardasz I, Morelli E, Panichi V, Locati D, Morandi S, Saba A, Barsotti G. Soy protein diet improves endothelial dysfunction in renal transplant patients. Nephrol Dial Transplant. 2007;22:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 95. | D’Amico G, Gentile MG. Effect of dietary manipulation on the lipid abnormalities and urinary protein loss in nephrotic patients. Miner Electrolyte Metab. 1992;18:203-206. [PubMed] |
| 96. | D’Amico G, Gentile MG, Manna G, Fellin G, Ciceri R, Cofano F, Petrini C, Lavarda F, Perolini S, Porrini M. Effect of vegetarian soy diet on hyperlipidaemia in nephrotic syndrome. Lancet. 1992;339:1131-1134. [PubMed] |
| 97. | Anderson JW, Blake JE, Turner J, Smith BM. Effects of soy protein on renal function and proteinuria in patients with type 2 diabetes. Am J Clin Nutr. 1998;68:1347S-1353S. [PubMed] |
| 98. | Azadbakht L, Atabak S, Esmaillzadeh A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with nephropathy: a longitudinal randomized clinical trial. Diabetes Care. 2008;31:648-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 99. | Azadbakht L, Shakerhosseini R, Atabak S, Jamshidian M, Mehrabi Y, Esmaill-Zadeh A. Beneficiary effect of dietary soy protein on lowering plasma levels of lipid and improving kidney function in type II diabetes with nephropathy. Eur J Clin Nutr. 2003;57:1292-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 100. | Chen ST, Chen JR, Yang CS, Peng SJ, Ferng SH. Effect of soya protein on serum lipid profile and lipoprotein concentrations in patients undergoing hypercholesterolaemic haemodialysis. Br J Nutr. 2006;95:366-371. [PubMed] |
| 101. | Chen ST, Ferng SH, Yang CS, Peng SJ, Lee HR, Chen JR. Variable effects of soy protein on plasma lipids in hyperlipidemic and normolipidemic hemodialysis patients. Am J Kidney Dis. 2005;46:1099-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 102. | D’Amico G, Gentile MG. Influence of diet on lipid abnormalities in human renal disease. Am J Kidney Dis. 1993;22:151-157. [PubMed] |
| 103. | Gentile MG, Fellin G, Cofano F, Delle Fave A, Manna G, Ciceri R, Petrini C, Lavarda F, Pozzi F, D’Amico G. Treatment of proteinuric patients with a vegetarian soy diet and fish oil. Clin Nephrol. 1993;40:315-320. [PubMed] |
| 104. | Stephenson TJ, Setchell KD, Kendall CW, Jenkins DJ, Anderson JW, Fanti P. Effect of soy protein-rich diet on renal function in young adults with insulin-dependent diabetes mellitus. Clin Nephrol. 2005;64:1-11. [PubMed] |
| 105. | Boekholdt SM, Arsenault BJ, Mora S, Pedersen TR, LaRosa JC, Nestel PJ, Simes RJ, Durrington P, Hitman GA, Welch KM. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA. 2012;307:1302-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 571] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 106. | Teixeira SR, Tappenden KA, Carson L, Jones R, Prabhudesai M, Marshall WP, Erdman JW. Isolated soy protein consumption reduces urinary albumin excretion and improves the serum lipid profile in men with type 2 diabetes mellitus and nephropathy. J Nutr. 2004;134:1874-1880. [PubMed] |
| 107. | Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 392] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 108. | Miraghajani MS, Najafabadi MM, Surkan PJ, Esmaillzadeh A, Mirlohi M, Azadbakht L. Soy milk consumption and blood pressure among type 2 diabetic patients with nephropathy. J Ren Nutr. 2013;23:277-282.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 109. | Siefker K, DiSilvestro RA. Safety and antioxidant effects of a modest soy protein intervention in hemodialysis patients. J Med Food. 2006;9:368-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 110. | Imani H, Tabibi H, Atabak S, Rahmani L, Ahmadinejad M, Hedayati M. Effects of soy consumption on oxidative stress, blood homocysteine, coagulation factors, and phosphorus in peritoneal dialysis patients. J Ren Nutr. 2009;19:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimbürger O, Massy Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol. 2008;3:505-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 418] [Article Influence: 24.6] [Reference Citation Analysis (1)] |
| 112. | Fanti P, Asmis R, Stephenson TJ, Sawaya BP, Franke AA. Positive effect of dietary soy in ESRD patients with systemic inflammation--correlation between blood levels of the soy isoflavones and the acute-phase reactants. Nephrol Dial Transplant. 2006;21:2239-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 113. | Miraghajani MS, Esmaillzadeh A, Najafabadi MM, Mirlohi M, Azadbakht L. Soy milk consumption, inflammation, coagulation, and oxidative stress among type 2 diabetic patients with nephropathy. Diabetes Care. 2012;35:1981-1985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 114. | Tomayko EJ, Kistler BM, Fitschen PJ, Wilund KR. Intradialytic protein supplementation reduces inflammation and improves physical function in maintenance hemodialysis patients. J Ren Nutr. 2015;25:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 115. | Fanti P, Sawaya BP, Custer LJ, Franke AA. Serum levels and metabolic clearance of the isoflavones genistein and daidzein in hemodialysis patients. J Am Soc Nephrol. 1999;10:864-871. [PubMed] |
| 116. | Fanti P, Stephenson TJ, Kaariainen IM, Rezkalla B, Tsukamoto Y, Morishita T, Nomura M, Kitiyakara C, Custer LJ, Franke AA. Serum isoflavones and soya food intake in Japanese, Thai and American end-stage renal disease patients on chronic haemodialysis. Nephrol Dial Transplant. 2003;18:1862-1868. [PubMed] |
| 117. | Locati D, Morandi S, Cupisti A, Ghiadoni L, Arnoldi A. Characterization and quantification of soy isoflavone metabolites in serum of renal transplanted patients by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:3473-3481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 118. | Howe JC. Postprandial response of calcium metabolism in postmenopausal women to meals varying in protein level/source. Metabolism. 1990;39:1246-1252. [PubMed] |
| 119. | Breslau NA, Brinkley L, Hill KD, Pak CY. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab. 1988;66:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 274] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 120. | Roughead ZK, Hunt JR, Johnson LK, Badger TM, Lykken GI. Controlled substitution of soy protein for meat protein: effects on calcium retention, bone, and cardiovascular health indices in postmenopausal women. J Clin Endocrinol Metab. 2005;90:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 121. | Bankir L, Roussel R, Bouby N. Protein- and diabetes-induced glomerular hyperfiltration: role of glucagon, vasopressin, and urea. Am J Physiol Renal Physiol. 2015;309:F2-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 122. | Bilo HJ, Schaap GH, Blaak E, Gans RO, Oe PL, Donker AJ. Effects of chronic and acute protein administration on renal function in patients with chronic renal insufficiency. Nephron. 1989;53:181-187. [PubMed] |
| 123. | Nakamura H, Takasawa M, Kashara S, Tsuda A, Momotsu T, Ito S, Shibata A. Effects of acute protein loads of different sources on renal function of patients with diabetic nephropathy. Tohoku J Exp Med. 1989;159:153-162. [PubMed] |
| 124. | Nakamura H, Yamazaki M, Chiba Y, Tani N, Momotsu T, Kamoi K, Ito S, Yamaji T, Shibata A. Acute loading with proteins from different sources in healthy volunteers and diabetic patients. J Diabet Complications. 1991;5:140-142. [PubMed] |
| 125. | Orita Y, Okada M, Harada S, Horio M. Skim soy protein enhances GFR as much as beefsteak protein in healthy human subjects. Clin Exp Nephrol. 2004;8:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 126. | Deibert P, Lutz L, Konig D, Zitta S, Meinitzer A, Vitolins MZ, Becker G, Berg A. Acute effect of a soy protein-rich meal-replacement application on renal parameters in patients with the metabolic syndrome. Asia Pac J Clin Nutr. 2011;20:527-534. [PubMed] |
| 127. | Buzio C, Mutti A, Perazzoli F, Alinovi R, Arisi L, Negro A. Protein-induced changes in kidney function depend on the time of administration but not on the dietary source. Nephron. 1990;56:234-240. [PubMed] |
| 128. | Liu ZM, Ho SC, Chen YM, Tang N, Woo J. Effect of whole soy and purified isoflavone daidzein on renal function--a 6-month randomized controlled trial in equol-producing postmenopausal women with prehypertension. Clin Biochem. 2014;47:1250-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 129. | Soroka N, Silverberg DS, Greemland M, Birk Y, Blum M, Peer G, Iaina A. Comparison of a vegetable-based (soya) and an animal-based low-protein diet in predialysis chronic renal failure patients. Nephron. 1998;79:173-180. [PubMed] |
| 130. | Azadbakht L, Esmaillzadeh A. Soy-protein consumption and kidney-related biomarkers among type 2 diabetics: a crossover, randomized clinical trial. J Ren Nutr. 2009;19:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 131. | Ahmed MS, Calabria AC, Kirsztajn GM. Short-term effects of soy protein diet in patients with proteinuric glomerulopathies. J Bras Nefrol. 2011;33:150-159. [PubMed] |
| 132. | Kalu DN, Masoro EJ, Yu BP, Hardin RR, Hollis BW. Modulation of age-related hyperparathyroidism and senile bone loss in Fischer rats by soy protein and food restriction. Endocrinology. 1988;122:1847-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 133. | Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP. The influence of dietary protein source on longevity and age-related disease processes of Fischer rats. J Gerontol. 1988;43:B5-12. [PubMed] |
| 134. | Shimokawa I, Higami Y, Hubbard GB, McMahan CA, Masoro EJ, Yu BP. Diet and the suitability of the male Fischer 344 rat as a model for aging research. J Gerontol. 1993;48:B27-B32. [PubMed] |
| 135. | Fontenla M, Prchal A, Cena AM, Albarracín AL, Pintos S, Benvenuto S, Sosa ML, Fontenla de Petrino S. Effects of soy milk as a dietary complement during the natural aging process. Nutr Hosp. 2008;23:607-613. [PubMed] |
| 136. | Masoro EJ, Iwasaki K, Gleiser CA, McMahan CA, Seo EJ, Yu BP. Dietary modulation of the progression of nephropathy in aging rats: an evaluation of the importance of protein. Am J Clin Nutr. 1989;49:1217-1227. [PubMed] |
| 137. | Aukema HM, Housini I, Rawling JM. Dietary soy protein effects on inherited polycystic kidney disease are influenced by gender and protein level. J Am Soc Nephrol. 1999;10:300-308. [PubMed] |
| 138. | Tomobe K, Philbrick DJ, Ogborn MR, Takahashi H, Holub BJ. Effect of dietary soy protein and genistein on disease progression in mice with polycystic kidney disease. Am J Kidney Dis. 1998;31:55-61. [PubMed] |
| 139. | Ogborn MR, Bankovic-Calic N, Shoesmith C, Buist R, Peeling J. Soy protein modification of rat polycystic kidney disease. Am J Physiol. 1998;274:F541-F549. [PubMed] |
| 140. | Fair DE, Ogborn MR, Weiler HA, Bankovic-Calic N, Nitschmann EP, Fitzpatrick-Wong SC, Aukema HM. Dietary soy protein attenuates renal disease progression after 1 and 3 weeks in Han: SPRD-cy weanling rats. J Nutr. 2004;134:1504-1507. [PubMed] |
| 141. | Aukema HM, Housini I. Dietary soy protein effects on disease and IGF-I in male and female Han: SPRD-cy rats. Kidney Int. 2001;59:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 142. | Cahill LE, Peng CY, Bankovic-Calic N, Sankaran D, Ogborn MR, Aukema HM. Dietary soya protein during pregnancy and lactation in rats with hereditary kidney disease attenuates disease progression in offspring. Br J Nutr. 2007;97:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 143. | Davis J, Iqbal MJ, Steinle J, Oitker J, Higginbotham DA, Peterson RG, Banz WJ. Soy protein influences the development of the metabolic syndrome in male obese ZDFxSHHF rats. Horm Metab Res. 2005;37:316-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 144. | Palanisamy N, Kannappan S, Anuradha CV. Genistein modulates NF-κB-associated renal inflammation, fibrosis and podocyte abnormalities in fructose-fed rats. Eur J Pharmacol. 2011;667:355-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 145. | Palanisamy N, Venkataraman Anuradha C. Soy protein prevents renal damage in a fructose-induced model of metabolic syndrome via inhibition of NF-kB in male rats. Pediatr Nephrol. 2011;26:1809-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 146. | Trujillo J, Ramírez V, Pérez J, Torre-Villalvazo I, Torres N, Tovar AR, Muñoz RM, Uribe N, Gamba G, Bobadilla NA. Renal protection by a soy diet in obese Zucker rats is associated with restoration of nitric oxide generation. Am J Physiol Renal Physiol. 2005;288:F108-F116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 147. | Maddox DA, Alavi FK, Silbernick EM, Zawada ET. Protective effects of a soy diet in preventing obesity-linked renal disease. Kidney Int. 2002;61:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 148. | Asanoma M, Tachibana N, Hirotsuka M, Kohno M, Watanabe Y. Effects of soy protein isolate feeding on severe kidney damage in DOCA salt-treated obese Zucker rats. J Agric Food Chem. 2012;60:5367-5372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 149. | Sakemi T, Ikeda Y, Shimazu K. Effect of soy protein added to casein diet on the development of glomerular injury in spontaneous hypercholesterolemic male Imai rats. Am J Nephrol. 2002;22:548-554. [PubMed] |
| 150. | Sankaran D, Bankovic-Calic N, Cahill L, Yu-Chen Peng C, Ogborn MR, Aukema HM. Late dietary intervention limits benefits of soy protein or flax oil in experimental polycystic kidney disease. Nephron Exp Nephrol. 2007;106:e122-e128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 151. | Maditz KH, Oldaker C, Nanda N, Benedito V, Livengood R, Tou JC. Dietary n-3 polyunsaturated fatty acids or soy protein isolate did not attenuate disease progression in a female rat model of autosomal recessive polycystic kidney disease. Nutr Res. 2014;34:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 152. | Aparicio VA, Nebot E, Tassi M, Camiletti-Moirón D, Sanchez-Gonzalez C, Porres JM, Aranda P. Whey versus soy protein diets and renal status in rats. J Med Food. 2014;17:1011-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 153. | Hwang SY, Taylor CG, Zahradka P, Bankovic-Calic N, Ogborn MR, Aukema HM. Dietary soy protein reduces early renal disease progression and alters prostanoid production in obese fa/fa Zucker rats. J Nutr Biochem. 2008;19:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 154. | Finco DR, Cooper TL. Soy protein increases glomerular filtration rate in dogs with normal or reduced renal function. J Nutr. 2000;130:745-748. [PubMed] |
| 155. | Zhao JH, Sun SJ, Horiguchi H, Arao Y, Kanamori N, Kikuchi A, Oguma E, Kayama F. A soy diet accelerates renal damage in autoimmune MRL/Mp-lpr/lpr mice. Int Immunopharmacol. 2005;5:1601-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 156. | Davis J, Higginbotham A, O’Connor T, Moustaid-Moussa N, Tebbe A, Kim YC, Cho KW, Shay N, Adler S, Peterson R. Soy protein and isoflavones influence adiposity and development of metabolic syndrome in the obese male ZDF rat. Ann Nutr Metab. 2007;51:42-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 157. | Ogborn MR, Nitschmann E, Bankovic-Calic N, Weiler HA, Aukema HM. Dietary soy protein benefit in experimental kidney disease is preserved after isoflavone depletion of diet. Exp Biol Med (Maywood). 2010;235:1315-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 158. | Philbrick DJ, Bureau DP, Collins FW, Holub BJ. Evidence that soyasaponin Bb retards disease progression in a murine model of polycystic kidney disease. Kidney Int. 2003;63:1230-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 159. | Palanisamy N, Viswanathan P, Anuradha CV. Effect of genistein, a soy isoflavone, on whole body insulin sensitivity and renal damage induced by a high-fructose diet. Ren Fail. 2008;30:645-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 160. | Yang HY, Wu LY, Yeh WJ, Chen JR. Beneficial effects of β-conglycinin on renal function and nephrin expression in early streptozotocin-induced diabetic nephropathy rats. Br J Nutr. 2014;111:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 161. | Palanisamy N, Viswanathan P, Ravichandran MK, Anuradha CV. Renoprotective and blood pressure-lowering effect of dietary soy protein via protein kinase C beta II inhibition in a rat model of metabolic syndrome. Can J Physiol Pharmacol. 2010;88:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 162. | Yang HY, Yang SC, Chen JR, Tzeng YH, Han BC. Soyabean protein hydrolysate prevents the development of hypertension in spontaneously hypertensive rats. Br J Nutr. 2004;92:507-512. [PubMed] |
| 163. | Yeh WJ, Yang HY, Chen JR. Soy β-conglycinin retards progression of diabetic nephropathy via modulating the insulin sensitivity and angiotensin-converting enzyme activity in rats fed with high salt diet. Food Funct. 2014;5:2898-2904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 164. | Yang HY, Chen JR, Chang LS. Effects of soy protein hydrolysate on blood pressure and angiotensin-converting enzyme activity in rats with chronic renal failure. Hypertens Res. 2008;31:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 165. | Yang HY, Chen JR. Renoprotective effects of soy protein hydrolysates in N(omega)-nitro-L-arginine methyl ester hydrochloride-induced hypertensive rats. Hypertens Res. 2008;31:1477-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 166. | Tovar AR, Murguía F, Cruz C, Hernández-Pando R, Aguilar-Salinas CA, Pedraza-Chaverri J, Correa-Rotter R, Torres N. A soy protein diet alters hepatic lipid metabolism gene expression and reduces serum lipids and renal fibrogenic cytokines in rats with chronic nephrotic syndrome. J Nutr. 2002;132:2562-2569. [PubMed] |
| 167. | Seok SJ, Lee ES, Kim GT, Hyun M, Lee JH, Chen S, Choi R, Kim HM, Lee EY, Chung CH. Blockade of CCL2/CCR2 signalling ameliorates diabetic nephropathy in db/db mice. Nephrol Dial Transplant. 2013;28:1700-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 168. | Aukema HM, Gauthier J, Roy M, Jia Y, Li H, Aluko RE. Distinctive effects of plant protein sources on renal disease progression and associated cardiac hypertrophy in experimental kidney disease. Mol Nutr Food Res. 2011;55:1044-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 169. | Peng CY, Sankaran D, Ogborn MR, Aukema HM. Dietary soy protein selectively reduces renal prostanoids and cyclooxygenases in polycystic kidney disease. Exp Biol Med (Maywood). 2009;234:737-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 170. | Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology (Carlton). 2012;17:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 362] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 171. | Small DM, Gobe GC. Oxidative stress and antioxidant therapy in chronic kidney and cardiovascular disease. In: Oxidative Stress and Chronic Degenerative Diseases - A Role for Antioxidants. Edited by Morales-Gonzalez JA 2013; . |
| 172. | Wardle EN. Cellular oxidative processes in relation to renal disease. Am J Nephrol. 2005;25:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 173. | Tucker PS, Scanlan AT, Dalbo VJ. Chronic kidney disease influences multiple systems: describing the relationship between oxidative stress, inflammation, kidney damage, and concomitant disease. Oxid Med Cell Longev. 2015;2015:806358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 174. | Cottone S, Lorito MC, Riccobene R, Nardi E, Mulè G, Buscemi S, Geraci C, Guarneri M, Arsena R, Cerasola G. Oxidative stress, inflammation and cardiovascular disease in chronic renal failure. J Nephrol. 2008;21:175-179. [PubMed] |
| 175. | Dounousi E, Papavasiliou E, Makedou A, Ioannou K, Katopodis KP, Tselepis A, Siamopoulos KC, Tsakiris D. Oxidative stress is progressively enhanced with advancing stages of CKD. Am J Kidney Dis. 2006;48:752-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 304] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 176. | Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 547] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 177. | Khan N, Sultana S. Abrogation of potassium bromate-induced renal oxidative stress and subsequent cell proliferation response by soy isoflavones in Wistar rats. Toxicology. 2004;201:173-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 178. | Khan N, Sultana S. Induction of renal oxidative stress and cell proliferation response by ferric nitrilotriacetate (Fe-NTA): diminution by soy isoflavones. Chem Biol Interact. 2004;149:23-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 179. | Javanbakht MH, Sadria R, Djalali M, Derakhshanian H, Hosseinzadeh P, Zarei M, Azizi G, Sedaghat R, Mirshafiey A. Soy protein and genistein improves renal antioxidant status in experimental nephrotic syndrome. Nefrologia. 2014;34:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 180. | Pedraza-Chaverrí J, Barrera D, Hernández-Pando R, Medina-Campos ON, Cruz C, Murguía F, Juárez-Nicolás C, Correa-Rotter R, Torres N, Tovar AR. Soy protein diet ameliorates renal nitrotyrosine formation and chronic nephropathy induced by puromycin aminonucleoside. Life Sci. 2004;74:987-999. [PubMed] |
| 181. | Ibrahim NH, Jia Y, Devassy JG, Yamaguchi T, Aukema HM. Renal cyclooxygenase and lipoxygenase products are altered in polycystic kidneys and by dietary soy protein and fish oil treatment in the Han: SPRD-Cy rat. Mol Nutr Food Res. 2014;58:768-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 182. | Palanisamy N, Anuradha CV. Soy protein preserves basement membrane integrity through a synergistic effect on nephrin, matrix metalloproteinase and vascular endothelial growth factor. Am J Nephrol. 2011;34:529-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 183. | Meng XM, Tang PM, Li J, Lan HY. TGF-β/Smad signaling in renal fibrosis. Front Physiol. 2015;6:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 376] [Cited by in RCA: 546] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 184. | Trujillo J, Cruz C, Tovar A, Vaidya V, Zambrano E, Bonventre JV, Gamba G, Torres N, Bobadilla NA. Renoprotective mechanisms of soy protein intake in the obese Zucker rat. Am J Physiol Renal Physiol. 2008;295:F1574-F1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 185. | Guo Y, Zhang A, Ding Y, Wang Y, Yuan W. Genistein ameliorates parathyroid hormone-induced epithelial-to-mesenchymal transition and inhibits expression of connective tissue growth factor in human renal proximal tubular cells. Arch Med Sci. 2013;9:724-730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 186. | Tan RJ, Zhou D, Zhou L, Liu Y. Wnt/β-catenin signaling and kidney fibrosis. Kidney Int Suppl (2011). 2014;4:84-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 227] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 187. | Lancaster MA, Gleeson JG. Cystic kidney disease: the role of Wnt signaling. Trends Mol Med. 2010;16:349-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 188. | Butteiger DN, Hibberd AA, McGraw NJ, Napawan N, Hall-Porter JM, Krul ES. Soy Protein Compared with Milk Protein in a Western Diet Increases Gut Microbial Diversity and Reduces Serum Lipids in Golden Syrian Hamsters. J Nutr. 2016;146:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 189. | Cain J, Banz WJ, Butteiger D, Davis JE. Soy protein isolate modified metabolic phenotype and hepatic Wnt signaling in obese Zucker rats. Horm Metab Res. 2011;43:774-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 190. | Zhou D, Lezmi S, Wang H, Davis J, Banz W, Chen H. Fat accumulation in the liver of obese rats is alleviated by soy protein isolate through β-catenin signaling. Obesity (Silver Spring). 2014;22:151-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 191. | Klein MA, Nahin RL, Messina MJ, Rader JI, Thompson LU, Badger TM, Dwyer JT, Kim YS, Pontzer CH, Starke-Reed PE. Guidance from an NIH workshop on designing, implementing, and reporting clinical studies of soy interventions. J Nutr. 2010;140:1192S-1204S. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 192. | Franke AA, Yu MC, Maskarinec G, Fanti P, Zheng W, Custer LJ. Phytoestrogens in human biomatrices including breast milk. Biochem Soc Trans. 1999;27:308-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |