Published online Mar 6, 2016. doi: 10.5527/wjn.v5.i2.195
Peer-review started: October 6, 2015
First decision: December 4, 2015
Revised: December 30, 2015
Accepted: January 27, 2016
Article in press: January 29, 2016
Published online: March 6, 2016
Processing time: 158 Days and 9.6 Hours
Intradetrusor injections of botulinum toxin are the cornerstone of medical treatment of neurogenic detrusor overactivity. The primary aim of this treatment is to ensure a low pressure regimen in the urinary bladder, but the mechanisms leading to long-term protection of the urinary tract remain poorly understood. In this paper, we highlight the potential benefits of intradetrusor injections of botulinum toxin regarding local effects on the bladder structures, urinary tract infections, stone disease, vesico ureteral reflux, hydronephrosis, renal function based on a comprehensive literature review.
Core tip: Intradetrusor injection of botulinum toxin prevent damage of the upper urinary tract via several potential mechanisms including reduction of bladder pressure, urothelium and suburothelium modifications, sensory receptors expression, and hypoxia reduction. These data could explain the favourable effects of intradetrusor injection of botulinum toxin on urinary tract infections, stone disease, vesico ureteral reflux, hydronephrosis, renal function.
- Citation: Baron M, Grise P, Cornu JN. How botulinum toxin in neurogenic detrusor overactivity can reduce upper urinary tract damage? World J Nephrol 2016; 5(2): 195-203
- URL: https://www.wjgnet.com/2220-6124/full/v5/i2/195.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i2.195
Neurogenic lower urinary tract dysfunction is a highly prevalent disease, impairing significantly patient’s quality of life, and results in a huge medico-economic burden[1]. In particular, neurogenic detrusor overactivity (NDO) is a common feature in the context of neurological diseases, resulting most of the time in urgency, increased frequency and urge urinary incontinence (UUI)[2]. NDO (which can be associated with sphincter dyssynergia) is also at high risk of upper urinary tract deterioration in the long term, because of high bladder pressure, low BC and low bladder capacity. A bladder pressure > 40 cmH2O has indeed been stated as the major urodynamic warning for upper urinary tract deterioration[3]. This increase in bladder pressure can be due to NDO but also to detrusor sphincter dyssynergie in itself, that may need a specific treatment. The current review will only deal with the effect of botulinum toxin injection in the detrusor to treat NDO.
The management of NDO has two aims. While patients often focus on symptom relief (especially UUI), another objective is the long term protection of the upper urinary tract, in order to preserve renal function. To achieve these goals, NDO management must restore a continent, low pressure reservoir without vesico ureteral reflux (VUR), along with an adequate capacity and a good compliance. The first line treatment of NDO is antimuscarinics but many patients do not respond to antimuscarinics therapy, and require further treatment[4].
Intradetrusor injections of botulinum toxin are one of the options available in patients who do not respond to medical therapy. This approach has now been approved and is extensively used for NDO management[5]. Two types of botulinum toxin are available on the market: Abobotulinum toxin-A (Dysport) and onabotulinum toxin-A (Botox). Whilst the clinical efficacy of the treatment has been assessed in well-designed prospective studies and meta-analyses, the long term effect on prevention of upper urinary tract disease such as urinary tract infections (UTIs), VUR, hydronephrosis, stones, and chronic kidney disease is not fully understood yet.
The aim of this review was to summarize available evidence about how botulinum toxin can prevent upper urinary tract disease in a context of NDO.
Urodynamic data: In addition to relief of lower urinary tract symptoms, intradetrusor injections of botulinum toxin have been shown to substantially modify the results of urodynamic studies in patients with NDO. Schurch et al[6] in 2000, for the first time, injected 300 UI of botulinum-A toxin (Botox) in the detrusor of 21 patients with spinal cord injury (SCI) who had UUI by DO refractory to antimuscarinics. At 6 wk, 17 out of 19 patients (89.4%) had complete continence. The overall mean reflex volume (RV) significantly increased from 215 ± 90.4 mL before the injections to 415.76 ± 211.1 (P = 0.016) after the injections. The mean maximum cystometric bladder capacity (MCC) significantly increased from 296 ± 145.2 to 480 ± 134.1 (P = 0.016), respectively. There was also a significant decrease after treatment in mean maximum detrusor voiding pressure (MDP) from 65.6 ± 29.2 cm water to 35 ± 32.1 (P = 0.016). Mean post-void residual urine volume (PVR) catheterized at the end of the urodynamic examination increased significantly from a mean of 261.8 ± 241.3 mL to 490 ± 204.8 (P = 0.016).
Following this first proof of concept, a number of other trials have investigated the changes in UDS after Intradetrusor injections of botulinum toxin. Through another prospective randomized, placebo controlled, double-blind, multi-center in 2005, Schurch et al[7] have found no significant difference between 200UI and 300UI of onabotulinum toxin A injections. However this comparative study was focused on incontinence episodes as a primary endpoint and do not allow conclusion about the comparison of urodynamic features. Reitz et al[8] in a prospective, open labeled study, used 300 U of Botox in the detrusor of 231 neurologic patients [167 SCI, 11 MS, 22 myelomeningocele (MMC)]. The evaluation was made at 12 and 36 wk. At 12 wk, the mean MCC increased significantly from 272 to 420 mL (P < 0.0001) while the mean MDP decreased from 61 cmH2O to 30 cmH2O (P < 0.0001). The mean bladder compliance (BC) also increased significantly from 32 mL/cmH2O to 72 mL/cmH2O and the mean PVR increased from 236 to 387 mL (P < 0.0001). At 36 wk, the results were quite similar although the mean BC was not significantly different from baseline (32 mL/cmH2O to 51 mL/cmH2O). These urodynamic data were correlated with a rate of 132 (73.3%) patients fully continent at 12 wk.
In a long term follow-up of 17 SCI patients at 6 years after 300 UI botulinum-toxin A injections, Giannantoni et al[9] in 2009 showed persistent urodynamics modifications. The unhibited detrusor contraction (UDC) first volume increased significantly from 213 ± 40.8 at baseline to 344 ± 32.6 at 4 mo, 365.4 ± 49.7 at 1 year, 410.8 ± 60.2 at 3 year, 413.7 ± 58.9 at 6 years (P < 0.001 between baseline and 6 years follow-up). Correspondingly the UDC maximum pressure decreased from 97.6 ± 32.4 to 23.8 ± 10.8 at 6 years (P < 0.01) whereas maximum cystometric capacity increased from 243 ± 64.7 to 420.8 ± 55.7 (P < 0.001).
The results are quite similar with Abobotulinum toxin A (Dysport). Del Popolo et al[10] in 2008 have retrospectively evaluated three Dysport doses (500 U, 750 U and 1000 U) in 199 patients with SCI and NDO refractory to antimuscarinics. The evaluation was made at 3, 6 and 12 mo. The mean MCC increased significantly from 226 to 407 mL after the first injection and was still at 380 after seven injections while the mean BC significantly increased from 27 mL/cmH2O to 41 mL/cmH2O after one and seven injections. There was no significant difference between all doses. In 2010, our team has reported the comparison of two Dysport doses (500 U and 750 U) in a prospective, double-blind, randomized, comparative trial[11]. Seventy-seven patients were included, 49 had SCI, 18 had MS and 11 had other neurological causes. At four weeks, the mean MCC increased from 242 to 434 mL with Dysport 500 U and from 180 to 423 mL with Dysport 750 U. The BC also increased from 32 to 37 mL/cmH2O and from 23 to 59 mL/cmH2O for Dysport 500 U and 750 U respectively. There were no significant differences between two groups.
These studies highlight the important urodynamic modifications induced by intradetrusor botulinum toxin injections.
Pathophysiology: Botulinum toxin act at the neuromuscular junction level by temporarily blocking acetylcholine (Ach) presynaptic release from parasympathetic nerves. It induces a paralysis of the detrusor smooth muscle that induce urodynamic changes and symptoms relief.
Serotype type A cleaves the SNAP-25 protein complex which plays an important role in the fusion of neurotransmitter-filled transmitter vesicles with the plasma membrane and their release during exocytosis. This induces an highly specific blockage of acetylcholine release at the neuromuscular junction of somatic and autonomic presynaptic nerve terminals[12].
Those fragments of SANP-25 protein complex are detectable in the bladder for longer periods that would be expected in striated muscle[13]. However, this motor effect does not entirely explain all the bladder changes. In fact, at the bladder level, BoNT/A seems to have a role in modulating both efferent and afferent neurologic activity, i.e., both motor and sensitive fibers[14].
Apostolidis et al[15] showed that BoNT/A injections for human DO decrease sensory receptors P2X3 and TRPV1 levels in suburothelial nerve fibers. Those sensory receptors are overexpressed in neurological bladder suburothelium and are believed to play a role in sensory signal transduction in normal animal bladder[16]. At 4 and 16 wk after BoNT/A intradetrusor injections in 38 patients (22 with neurologic DO and 16 with idiopathic DO, there was a significant decrease in P2X3-immunoreactive and TRPV1-immunoreactive (-IR) (P < 0.0004 and P < 0.0008, respectively), when significant improvements were observed in clinical and urodynamic parameters. P2X3-IR and TRPV1-IR fibers decrease were significantly correlated with reduction of urgency episodes at 4 and 16 wk (P < 0.0013 at 4 wk and P < 0.02 at 16 wk), but not maximum cystometric capacity or detrusor pressures.
Conte et al[17] also showed that, after BoNT/A injections for detrusor overactivity, patients with Parkinson disease or SCI, significantly reduced at MCC, the expected soleus Hoffman reflex (H reflex) inhibition, whereas in those with SCI, it turned the H reflex facilitation into a slight inhibition. This reflex (basically defined as a reflectory contraction of muscle after stimulation of the related sensory fibers) tests the afferent information from the bladder (C and Aδ fibers) that modulates the spinal motoneuron excitability. Those results highlight the fact that BoNT/A might influences H reflex modulation at MCC by reducing bladder afferent signalling.
However, motor effect seems to play a major role in increasing MCC and BC and decreasing MDP significantly. It creates a low pressure bladder during filling and storage phases. The ureteral outlet may be affected by a bladder pressure over 40 cmH2O or by a BC under 10 mL/cmH2O leading to upper urinary tract functional obstruction[3]. Prolonged periods of elevated detrusor pressure during bladder filling or voiding have been found to put the upper urinary tract at risk[18]. Primary aim of therapy in patients with such problems is conversion to a low pressure bladder during filling even if this leads to incomplete emptying and the need to supplement emptying with catheterization.
Clinical results: The impact of intra-detrusor botulinum toxin injections on UTIs has been investigated in various clinical trials. Gamé et al[19] in 2008 has evaluated the impact of BoNTA 300 U on symptomatic UTIs (sUTIs). sUTIs were defined by the association of bacteriological criteria and symptoms such as fever, intensification of spasticity, intensification of autonomic hyperreflexia, pain and worsening of the neurological status. Of the thirty patients, 15 had SCI, 14 had MS and 1 had myelitis. All had at least one episode of sUTIs during the 6 mo prior to the injection (mean number 1.79 ± 0.39 per patient). At 6 mo, the number of sUTIs decreased significantly (0.2 ± 0.41) (P = 0.003) with only three patients having sUTIs (one pyelonephritis, one prostatitis, one orchitis). Of those three patients, two had SCI and one suffered from MS and they were those in whom BoNTA injections had the least effect on urodynamic changes. The overall incidence of bacteriuria was 43%.
In 2009, GIannantoni et al[9], at 6 years follow-up of 300 U botulinum toxinA injections, in 17 SCI patients, reported a decreased in UTIs episodes from 6.7 ± 2.1 per year at baseline to 1.6 ± 1.3 at 4 mo, 3.3 ± 2.1 at one year, 1.7 ± 2.0 at 3 years and 1.8 ± 0.5 at 6 years (P = 0.001 between baseline and 6 years). However the definition of symptomatic UTI used in the trial is not specified.
Cruz et al[20], evaluated in 2011 the safety of onabotulinumtoxinA, in a randomized, double-blind, controlled study vs placebo. 275 patients (121 SCI, 154 MS) were randomized in three groups (92 to placebo, 92 to onabotulinumtoxinA 200 U, and 91 to onabotulinumtoxinA 300 U). The mean rate of UTI was similar between all treatment groups, including placebo, in the SCI population (50%, 52%, 56.4% for placebo, 200 U and 300 U groups) whereas in the MS population, it was higher in the onabotulinumtoxinA groups compared with placebo (32%, 58%, 70% for placebo, 200 U and 300 U groups). Twelve percent, 30%, and 42% of patients in the placebo, onabotulinumtoxinA 200-U, and 300-U groups respectively, initiated CIC after the first injection. However, this level 1 study presented a major pitfall, that is the absence of clear definition of UTIs. Indeed the authors confused symptomatic UTIs (with clinical signs, including fever, and a positive urine culture) and asymptomatic bacteriuria (colonization), that is obviously increased by the high rate of self catheterization. In another level 1 study, Ginsberg et al[21] evaluated the safety of BoNTA in a randomized, double blind, controlled placebo trial in 416 patients (227 MS, 189 SCI). Two doses of Botox were used (200 U and 300 U). At 12 wk evaluation, the most frequent adverse effects reported were UTI and urinary retention. In MS population, the rate of UTI was higher after BoNTA than placebo (51% and 50% in 300 U and 200 U groups vs 28% for placebo) while it was similar in all groups in patients with SCI (42%, 48%, 50% in placebo, 200 U, 300 U groups respectively). But again, the authors disclosed that there was no clear definition between symptomatic and asymptomatic UTIs, so these studies cannot results in valuable hypotheses.
In a more focused study, Kuo et al[22] reported in 2011, among 132 onabotulinumA 200 U injections in 33 SCI patients, nine episodes of febrile UTIs (6.8%) and 37 (28%) episodes of asymptomatic UTI. Herschorn et al[23] in 38 patients with SCI and 19 with MS found a similar rate of UTI between placebo and 300 UI of onabotulinum A: 55 and 57% respectively. However, he didn’t separate MS and SCI patients. Jia et al[24] in 2013 found similar results in men with SCI receiving 300 U of botox. The mean number of sUTIs prior to surgery was 1.49 ± 1.43 per patient over 6 mo and decreased to 0.78 ± 0.96 (P = 0.023) at 6 mo post-operatively. However, the overall sUTIs frequency had the tendency to decrease in patients who developed two or more UTIs before injection and to increased in patients who presented one or zero UTI before injection. The sUTIs included two acute epidymitis episodes. The others were acute pyelonephritis.
Physiopathology: UTIs are a major cause of morbidity and one of the main reason for hospitalization in neurologic patients[25]. It must be distinguished from asymptomatic bacteriuria, which is not threatening for the patient. One important confounder in clinical studies about intradetrusor injections of botulinum toxin is that treated patients often practice self catheterization, that increases the risk of asymptomatic bacteriuria. But the overall rate of symptomatic UTIs is thought to be decreased.
In the neurogenic patient, there are some structural and physiological factors that can be related to an increased risk of UTIs including: Over-distention of the bladder, vesicoureteral reflux, high pressure voiding, large post-void residuals, presence of stones in the urinary tract, and outlet obstruction (detrusor- sphincter dyssynergia, urethral stricture, enlarged prostate)[26]. The method of bladder drainage has also a strong influence on UTI. The use of clean intermittent catheterization (CIC) has permitted to significantly overall decrease the mean rate of UTI in patients with neurological disorders[26], despite the fact that CIC are associated with asymptomatic bacteriuria[27].
Botulinum toxin injections and CIC (when needed) result in both a low pressure bladder regimen and minimal post-void residual, that are two conditions lowering the risk of symptomatic UTIs. Indeed, the major factor of UTI is DO (eventually combined with outflow obstruction) which induce maximum detrusor pressure[28], resulting in reduced blood flow as shown by animal models[29]. Focal bladder hypoxia is associated with further deterioration of the detrusor function and fibrosis[30,31], and has been postulated to favor adherence of bacteria to the urothelium[32].
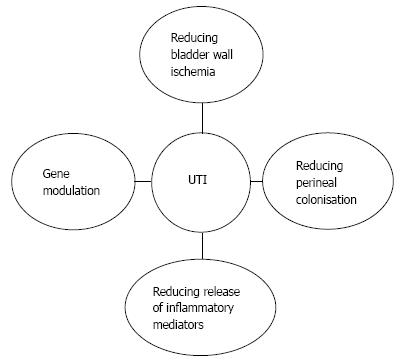
Many other mechanisms have been proposed as key factors influencing occurrence of UTIs (Figure 1). Wöllner et al[33] have shown that BoNT/A had a no direct antibacterial effect. Thirunavukkarasusx et al[34] demonstrated a high modulation of genes and pathway involved in neuroinflammatory, focal adhesion, cell adhesion molecules and gap junctions genes in intestinal epithelial cells lines treated with botulinumtoxinA. Although it has not been studied, there might be the same effects in the urothelium that could decrease bacterial adhesion.
Moreover, the symptoms of UTI presented by neurological patients may be induced by a local inflammation arising from the local release of inflammatory mediators such as substance P (SP), neurokinin A, glutamate and calcitonin gene-related peptide (CGRP) from afferent nerves. Bacteria could cause a direct stimulation of afferent A-delta and C-fibres with an increased release of those neurotransmitters inducing dysuria, urgency, frequency and general symptoms such as malaise, fever and increased spasticity. In vitro[35] and in vivo[36] analysis have shown an effect of botulinum toxin in reducing glutamate release and decreasing pain. This might alleviate bladder symptoms and the awareness of sUTI by the patients. Furthermore, CGRP is a potent vasodilator, and SP enhances vascular permeability. These substances are involved in the physiological control of blood flow. The potential effect of botulinum toxin on modulation of inflammation and sensory pathways and its potential influence on UTIs occurrence remains to be elucidated.
VUR causes UTI, hydronephrosis and alters the upper urinary tract by mechanically delivering infected urine to the renal pelvis. BoNTA injections have been postulated as having a positive influence on VUR through various ways.
Clinical data: Very few studies have evaluated the impact of BoNTA on VUR and renal pelvis dilatation. To our knowledge, no studies have ever reported on VUR nor renal pelvis dilation induced by botulinum toxin as a primary outcome. Classically, trigonal injections are avoided owing to the potential risk of precipitating VUR from inhibition of the active trigonal antireflux mechanism. Nevertheless, according to the literature review by Davis et al[37] in 2015, no study have shown new onset of RVU nor worsening of preexisting RVU, induced by trigonal injections.
In the opposite, RVU treated by BoNTA injections has been described. In a randomized, single-blinded study, Abedl-Meduig[38] compared initial detrusor vs combined detrusor-trigone 300 IU BTX-A injections in 38 adults with SCI and refractory neurogenic urinary incontinence due to NDO. At baseline 2 patients in the detrusor arm had unilateral grade 2 and 3 VUR while 2 in the combined arm had unilateral grade 1 and 3 VUR, respectively. At week 8 no patient had new onset VUR or upgrading of preexisting VUR. Mascarenhas et al[39] in 2008, performed trigonal injections in 21 neurological patients (12 SCI, 8 viral myelitis, 1 MS), 20 had no VUR previous to the injection and 1 had VUR grade II unilateral. At 8 wk evaluation, no cases of de novo VUR were detected and the patient with preinjection VUR had complete resolution of the reflux. For Gamé et al[19] who used 300 U of BOTOX in 30 neurologic patients, 6 patients had VRR previously to the injections, and only 2 had one reflux remaining after the injections. But the difference was not significant. None of them had infections after treatment. Giannantoni et al[9] in 2009 studied 17 SCI patients with DO. Three had VUR of grade III prior to treatment. At one year post injection, no one had persistent VUR.
Arrabal-Polo et al[40] in 2012 have presented the case of a children non neurologic who presented a primary reflux and was successfully treated with botulinum toxin after failure of endoscopic treatment (Deflux and Macroplastique).
Giannantoni et al[9], detected on kidney and bladder ultrasound, bilateral and monolateral renal pelvis dilatation in six and five patients, respectively, before the injection. At for weeks after the 300 U Botox injection, the dilatation disappear in all patients. Those results were maintened at 3 and 6 years follow up. In Mascarenhas study[39], of 21 neurological patients, four (19.0%) had mild hydronephrosis and one (4.8%) had moderate hydronephrosis at baseline. Postoperative ultrasound after 8 wk of BoNTA injections, showed no hydronephrosis in 20 (95.2%) patients and mild hydronephrosis in 1 (4.8%, P = 0.125).
Pathophysiology: The occurrence of VUR result from different mechanisms which defines whether reflux is considered as primary or as secondary. In general, VUR is considered primary if there is a deficiency of the uretero-vesical junction (UVJ). Secondary reflux is caused by overwhelming of the normal function of the UVJ. Bladder neurological dysfunction is often the root cause of secondary reflux[41]. Chronic increases in intravesical pressure resulting from bladder outlet obstruction or detrusor overactivity can distort bladder architecture and UVJ. It can cause herniation of the bladder mucosa through the weakest point of the hiatus above the ureter and produce a “Hutch diverticulum” and secondary reflux[42].
Uretero-hydronephrosis is also induced by high bladder pressure. Thus, reducing bladder pressure by botulinum toxin may improve VUR and hydronephrosis. Although low bladder pressure is achieved, a major deterioration of the UVJ might lead to persistent VUR. Indeed, increase wall tension in the ureter might lead to a significant decrease in smooth muscle perfusion and cause ischaemic lesion in the ureter[43].
This emphasizes the importance to control the bladder pressure at the initial stage of an overactive neurogenic bladder in order to avoid secondary damages on bladder and upper urinary tract. These data also point out the potential interest, notably in children, of an urodynamic evaluation as primary VUR can be due to an anatomical defect but also a severe voiding dysfunction, especially if bilateral.
Clinical data: No study has evaluated the relationship between botulinum toxin and renal stones. Only Wefer et al[44] in 2010 reported less than 6 patients out of 214 (2.8%) presenting bladder stones. However he was not able to determine wether these disorders was BoNTA treatment related. Ginsberg et al[21] reported in 2012, out of 416 patients, only one case of bladder stone formation after 300 U botox injection.
Pathogenesis: Renal and bladder calculi are an important source of morbidity for patients with neurogenic bladder. The incidence of renal stones in neurogenic patient is about 6.8%[45] higher than in the common population. Old series have reported that most of the calculi were of struvite, induced by UTI[46]. However, more recent trials have established that stones may also be of metabolic origin. For instance, Matlaga et al[47] in 2005 has evaluated 32 renal calculi in a population of MMC and SCI, and found only 37.5% of struvite calculi and 62.5% of metabolic calculi. This modification of the origin of the stones might be due to a decrease in UTI in neurological population over the years, due to improvement of the urinary conditions in those patients.
By decreasing the mean rate of UTI and UUT dilatation, BoNTA injections may lead to decrease the incidence of struvite calculi but further studies are warranted.
Patients with neurogenic bladder are at increased risk of bladder stone formation. According to Chen et al[45], within 10 years after SCI, 15% to 30% of patients will have formed at least one stone. The risk of forming a subsequent stone quadruples when a patient has already formed one stone[48]. Furthermore, the manner in which the bladder is managed in SCI appears to have a significant impact on the risk of stone formation. One large study of over 450 patients noted that the use of CIC was associated with a significant reduction in the risk of bladder stone formation, with an annual risk of 0.2%, compared with 4% in those patients managed by a chronic indwelling catheter[48].
CIC in patients treated by BoNTA might be beneficial for decreasing bladder stones formation. In the opposite, in patients who were not using CIC previously to the injections, there might be and increase risk of developing bladder calculi. However, this remains hypothetical and needs to be further established by dedicated, well performed clinical trials.
Clinical data: In a long term follow-up of 17 patients during 6 years after 300 U of botulinum injections, Giannantoni et al[9] didn’t show any impairment of renal function. Kuo et al[21] evaluated the impact of botulinum toxin 200 U on renal function in 33 patients with supra sacral SCI. Videourodynamic and 99mTc-DTPA renal scanning for glomerular filtration rate (GFR) were performed at screening and every 3 mo during 24 mo of assessment. Onabotulinum toxin injections were repeated every 6 mo. GFR significantly decreased throughout the treatment course (96.27 ± 22.50 at baseline vs 83.51 ± 23.96 at 24 mo, P = 0.028). There was no significant change in mean serum Cr levels during the same period (0.623 ± 0.183 vs 0.675 ± 0.175, P = 0.802).
In 2014, the same team[49] evaluated the effect of 300 U vs 200 U of onabotulinum toxinA on renal function in 72 SCI patients. During the follow-up period, the changes in GFR from baseline to all time points did not differ significantly within each group or between the two groups. At baseline, the GFR was 94.2 ± 22.1 mL/min and 84.2 ± 19.6 mL/min in 200-U and 300-U groups, respectively. At the end-point, the GFR was 90.5 ± 24.2 mL/min and 88.0 ± 28.2 mL/min in the 200-U and 300-U groups, respectively.
There were no significant difference between 300 U group and 200 U group (P = 0.197) neither between group with compliance > 30 and group with low compliance (< 30).
Four patients had improved their renal function (2 in 200 U and 2 in 300 U group) at the end of the study. Inhibited detrusor contracture decreased significantly after the second detrusor injection of 300-U of onabotulinumtoxinA compared to that in the 200-U group.
Pathophysiology: The ultimate consequence of all upper urinary tract complications in neurological patients is the impairment of renal function. Although bladder management methods have evolved in recent decades, chronic renal insufficiency remains a significant cause of morbidity and it is one of the major concern to have in mind when treating those patients[50].
In urodynamics studies, a bladder pressure > 40 cmH2O mostly due to detrusor hyperreflexia and low BC are the major risk factors for renal damage in SCI patients[3]. However, CIC, antimuscarinic therapy, and regular urodynamic monitoring have been reported to reduce the risk of renal failure[51].
These studies show that renal function remains stable when patient have urodynamics modifications after botulinum toxin injections but without significant improvement (Table 1). However, the median term in follow-up of these series may be a limit for renal function study. The neurological disorder in also an important point to consider and SCI patients are more at risk of renal deterioration than multiple sclerosis patients. It highlights the fact that patients must be followed carefully on long term after botulinum injections.
| Ref. | Type of toxin | Patients | sUTI before injections | sUTI after injection | P | Bacteriuria % (n) | Symptomatic and asymptomatic UTI after injections | P |
| Gamé et al[19], 2008 | Botox | 30 | 1.79/pp/6 mo | 0.2/pp/6 mo | 0.003 | 43 | ||
| 300 UI | 15 MS | |||||||
| 14 SCI | ||||||||
| 1 Myelitis | ||||||||
| Giannantoni et al[9], | Botox | 17 SCI | 6.7/pp/yr | 1.8/pp/yr | 0.001 | |||
| 2009 | 300 UI | |||||||
| Cruz et al[20], 2011 | Placebo | 154 MS | Placeb: 32% | P < 0.05 | ||||
| Botox | 200 UI: 58% | (vs placebo) | ||||||
| 200 UI | 300 UI: 70% | |||||||
| 300 UI | ||||||||
| Cruz et al[20], 2011 | Placebo | 121 SCI | Placebo: 50% | |||||
| Botox | 200 UI: 52.6% | |||||||
| 200 UI | 300 UI: 56.4% | |||||||
| 300 UI | ||||||||
| Kuo et al[22], 2011 | Botox | 33 SCI | 6.80% | 28 (37) | ||||
| 200 UI | ||||||||
| Herschorn et al[23], 2011 | Placebo | 57 | Placebo: 55% | |||||
| Botox | 38 SCI | 300 UI: 57% | ||||||
| 300 UI | 19 MS | |||||||
| Ginsberg et al[21], | Placebo | 227 MS | Placebo: 28% | |||||
| 2012 | Botox | 200 UI: 51% | ||||||
| 200 UI | 300 UI: 50% | |||||||
| 300 UI | ||||||||
| Ginsberg et al[21], | Placebo | 189 SCI | Placebo: 42% | |||||
| 2012 | Botox | 200 UI: 48% | ||||||
| 200 UI | 300 UI: 50% | |||||||
| 300 UI | ||||||||
| Jia et al[24], 2013 | Botox | SCI 41 | 1.49/pp/6 mo | 0.78/pp/6 mo | ||||
| 300 UI |
Early and repeated detrusor onabotulinumtoxinA injections could therefore be beneficial to SCI patients before upper urinary tract deterioration.
An explanation why detrusor botulinum injection may not improve renal function is that anatomical renal damages may be irreversible, and also that renal deterioration may be caused by other factors. In particular, SCI patients are at higher risk to develop cardiovascular disease than others[52]. Many other confounding factors in neurological patients can induce renal impairment such as, diabetes, obesity, lipid disorders, metabolic syndrome, and disturbances of the autonomous nervous system, which may result in blood pressure abnormalities, arrhythmias and cardiac disease[51,53].
All these factors have to be taken into account when evaluating the long-term impact of on kidney function in the neurological patients. For the moment, this has not been correctly assessed and BoNTA are postulated as protective for the urinary tract in the long term, mainly through indirect benefits.
Botulinum toxin injections regulate urodynamic parameters in a context of neurogenic OAB. It furthermore may have a positive effect on UTIs, but this has to be put in perspective with the increased use of CIC. There is also an anticipated positive effect of BoNTA injections on hydronephrosis, VUR and stone disease, but with a weaker level of evidence. Long term effects on renal function are also probably positive, but this parameter remains multifactorial.
P- Reviewer: Apostolidis A, Hillelsohn J S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Panicker JN, Fowler CJ, Kessler TM. Lower urinary tract dysfunction in the neurological patient: clinical assessment and management. Lancet Neurol. 2015;14:720-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 297] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 2. | King A, Quirouet A, Moore CK. Urologic applications of botulinum toxin. Cleve Clin J Med. 2015;82:456-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | McGuire EJ, Woodside JR, Borden TA, Weiss RM. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981;126:205-209. [PubMed] |
| 4. | Madhuvrata P, Singh M, Hasafa Z, Abdel-Fattah M. Anticholinergic drugs for adult neurogenic detrusor overactivity: a systematic review and meta-analysis. Eur Urol. 2012;62:816-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Groen J, Pannek J, Castro Diaz D, Del Popolo G, Gross T, Hamid R, Karsenty G, Kessler TM, Schneider M, ‘t Hoen L. Summary of European Association of Urology (EAU) Guidelines on Neuro-Urology. Eur Urol. 2016;69:324-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 329] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 6. | Schurch B, Stöhrer M, Kramer G, Schmid DM, Gaul G, Hauri D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol. 2000;164:692-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 447] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 7. | Schurch B, de Sèze M, Denys P, Chartier-Kastler E, Haab F, Everaert K, Plante P, Perrouin-Verbe B, Kumar C, Fraczek S, Brin MF; Botox Detrusor Hyperreflexia Study Team. Botulinum toxin type a is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 393] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 8. | Reitz A, Stöhrer M, Kramer G, Del Popolo G, Chartier-Kastler E, Pannek J, Burgdörfer H, Göcking K, Madersbacher H, Schumacher S. European experience of 200 cases treated with botulinum-A toxin injections into the detrusor muscle for urinary incontinence due to neurogenic detrusor overactivity. Eur Urol. 2004;45:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 289] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 9. | Giannantoni A, Mearini E, Del Zingaro M, Porena M. Six-year follow-up of botulinum toxin A intradetrusorial injections in patients with refractory neurogenic detrusor overactivity: clinical and urodynamic results. Eur Urol. 2009;55:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Del Popolo G, Filocamo MT, Li Marzi V, Macchiarella A, Cecconi F, Lombardi G, Nicita G. Neurogenic detrusor overactivity treated with english botulinum toxin a: 8-year experience of one single centre. Eur Urol. 2008;53:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Grise P, Ruffion A, Denys P, Egon G, Chartier Kastler E. Efficacy and tolerability of botulinum toxin type A in patients with neurogenic detrusor overactivity and without concomitant anticholinergic therapy: comparison of two doses. Eur Urol. 2010;58:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Coelho A, Dinis P, Pinto R, Gorgal T, Silva C, Silva A, Silva J, Cruz CD, Cruz F, Avelino A. Distribution of the high-affinity binding site and intracellular target of botulinum toxin type A in the human bladder. Eur Urol. 2010;57:884-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Schulte-Baukloh H, Zurawski TH, Knispel HH, Miller K, Haferkamp A, Dolly JO. Persistence of the synaptosomal-associated protein-25 cleavage product after intradetrusor botulinum toxin A injections in patients with myelomeningocele showing an inadequate response to treatment. BJU Int. 2007;100:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Apostolidis A, Dasgupta P, Fowler CJ. Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol. 2006;49:644-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 262] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 15. | Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, Dasgupta P, Fowler CJ, Anand P. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005;174:977-982; discussion 982-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 365] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 16. | Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 524] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 17. | Conte A, Giannantoni A, Proietti S, Giovannozzi S, Fabbrini G, Rossi A, Porena M, Berardelli A. Botulinum toxin A modulates afferent fibers in neurogenic detrusor overactivity. Eur J Neurol. 2012;19:725-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Kurzrock EA, Polse S. Renal deterioration in myelodysplastic children: urodynamic evaluation and clinical correlates. J Urol. 1998;159:1657-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Gamé X, Castel-Lacanal E, Bentaleb Y, Thiry-Escudié I, De Boissezon X, Malavaud B, Marque P, Rischmann P. Botulinum toxin A detrusor injections in patients with neurogenic detrusor overactivity significantly decrease the incidence of symptomatic urinary tract infections. Eur Urol. 2008;53:613-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Cruz F, Herschorn S, Aliotta P, Brin M, Thompson C, Lam W, Daniell G, Heesakkers J, Haag-Molkenteller C. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: a randomised, double-blind, placebo-controlled trial. Eur Urol. 2011;60:742-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 344] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 21. | Ginsberg D, Gousse A, Keppenne V, Sievert KD, Thompson C, Lam W, Brin MF, Jenkins B, Haag-Molkenteller C. Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J Urol. 2012;187:2131-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 22. | Kuo HC, Liu SH. Effect of repeated detrusor onabotulinumtoxinA injections on bladder and renal function in patients with chronic spinal cord injuries. Neurourol Urodyn. 2011;30:1541-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Herschorn S, Gajewski J, Ethans K, Corcos J, Carlson K, Bailly G, Bard R, Valiquette L, Baverstock R, Carr L. Efficacy of botulinum toxin A injection for neurogenic detrusor overactivity and urinary incontinence: a randomized, double-blind trial. J Urol. 2011;185:2229-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 24. | Jia C, Liao LM, Chen G, Sui Y. Detrusor botulinum toxin A injection significantly decreased urinary tract infection in patients with traumatic spinal cord injury. Spinal Cord. 2013;51:487-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Cardenas DD, Hoffman JM, Kirshblum S, McKinley W. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil. 2004;85:1757-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 326] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 26. | The prevention and management of urinary tract infections among people with spinal cord injuries. National Institute on Disability and Rehabilitation Research Consensus Statement. January 27-29, 1992. J Am Paraplegia Soc. 1992;15:194-204. [PubMed] |
| 27. | Schlager TA, Dilks S, Trudell J, Whittam TS, Hendley JO. Bacteriuria in children with neurogenic bladder treated with intermittent catheterization: natural history. J Pediatr. 1995;126:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Esclarín De Ruz A, García Leoni E, Herruzo Cabrera R. Epidemiology and risk factors for urinary tract infection in patients with spinal cord injury. J Urol. 2000;164:1285-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 190] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Saito M, Wein AJ, Levin RM. Effect of partial outlet obstruction on contractility: comparison between severe and mild obstruction. Neurourol Urodyn. 1993;12:573-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Ghafar MA, Shabsigh A, Chichester P, Anastasiadis AG, Borow A, Levin RM, Buttyan R. Effects of chronic partial outlet obstruction on blood flow and oxygenation of the rat bladder. J Urol. 2002;167:1508-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Azadzoi KM, Pontari M, Vlachiotis J, Siroky MB. Canine bladder blood flow and oxygenation: changes induced by filling, contraction and outlet obstruction. J Urol. 1996;155:1459-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Siroky MB. Pathogenesis of bacteriuria and infection in the spinal cord injured patient. Am J Med. 2002;113 Suppl 1A:67S-79S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Wöllner J, Schmidig K, Gregorini F, Kessler TM, Zbinden R, Mehnert U. Is there a direct antimicrobial effect of botulinum neurotoxin type A? BJU Int. 2012;110:E886-E890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Thirunavukkarasusx N, Ghosal KJ, Kukreja R, Zhou Y, Dombkowski A, Cai S, Singh BR. Microarray analysis of differentially regulated genes in human neuronal and epithelial cell lines upon exposure to type A botulinum neurotoxin. Biochem Biophys Res Commun. 2011;405:684-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Cui M, Khanijou S, Rubino J, Aoki KR. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain. 2004;107:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 453] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 36. | da Silva LB, Kulas D, Karshenas A, Cairns BE, Bach FW, Arendt-Nielsen L, Gazerani P. Time course analysis of the effects of botulinum neurotoxin type A on pain and vasomotor responses evoked by glutamate injection into human temporalis muscles. Toxins (Basel). 2014;6:592-607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Davis NF, Burke JP, Redmond EJ, Elamin S, Brady CM, Flood HD. Trigonal versus extratrigonal botulinum toxin-A: a systematic review and meta-analysis of efficacy and adverse events. Int Urogynecol J. 2015;26:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Abdel-Meguid TA. Botulinum toxin-A injections into neurogenic overactive bladder--to include or exclude the trigone? A prospective, randomized, controlled trial. J Urol. 2010;184:2423-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Mascarenhas F, Cocuzza M, Gomes CM, Leão N. Trigonal injection of botulinum toxin-A does not cause vesicoureteral reflux in neurogenic patients. Neurourol Urodyn. 2008;27:311-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Arrabal-Polo MA, Nogueras-Ocaña M, Jiménez-Pacheco A, Palao-Yago F, Tinaut-Ranera J, López-León V, Zuluaga-Gómez A. Vesicoureteral refulx in overactive bladder: medical resolution through botulin toxin injection. Arch Esp Urol. 2012;65:844-848. [PubMed] |
| 41. | McDougal WS, Wein AJ, Kavoussi LR, Novick AC. Campbell Walsh-Urology.10th ed. Holland: Elsevier Science Health Science 2011; 3271-3272. |
| 42. | Hutch JA. Saccule formation at the ureterovesical junction in smooth walled bladders. J Urol. 1961;86:390-399. [PubMed] |
| 43. | Dinlenc CZ, Liatsikos EN, Smith AD. Ureteral ischemia model: an explanation of ureteral dysfunction after chronic obstruction. J Endourol. 2001;15:213-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 44. | Wefer B, Ehlken B, Bremer J, Burgdörfer H, Domurath B, Hampel C, Kutzenberger J, Seif C, Sievert KD, Berger K. Treatment outcomes and resource use of patients with neurogenic detrusor overactivity receiving botulinum toxin A (BOTOX) therapy in Germany. World J Urol. 2010;28:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Chen Y, DeVivo MJ, Roseman JM. Current trend and risk factors for kidney stones in persons with spinal cord injury: a longitudinal study. Spinal Cord. 2000;38:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Burr RG. Urinary calculi composition in patients with spinal cord lesions. Arch Phys Med Rehabil. 1978;59:84-88. [PubMed] |
| 47. | Matlaga BR, Kim SC, Watkins SL, Kuo RL, Munch LC, Lingeman JE. Changing composition of renal calculi in patients with neurogenic bladder. J Urol. 2006;175:1716-1719; discussion 1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Ord J, Lunn D, Reynard J. Bladder management and risk of bladder stone formation in spinal cord injured patients. J Urol. 2003;170:1734-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Chen YC, Kuo HC. The therapeutic effects of repeated detrusor injections between 200 or 300 units of onabotulinumtoxinA in chronic spinal cord injured patients. Neurourol Urodyn. 2014;33:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Greenwell MW, Mangold TM, Tolley EA, Wall BM. Kidney disease as a predictor of mortality in chronic spinal cord injury. Am J Kidney Dis. 2007;49:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Kuhlemeier KV, Lloyd LK, Stover SL. Long-term followup of renal function after spinal cord injury. J Urol. 1985;134:510-513. [PubMed] |
| 52. | Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86:142-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 421] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 53. | Kappus N, Weinstock-Guttman B, Hagemeier J, Kennedy C, Melia R, Carl E, Ramasamy DP, Cherneva M, Durfee J, Bergsland N. Cardiovascular risk factors are associated with increased lesion burden and brain atrophy in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |