INTRODUCTION
Renal cortical necrosis (RCN) is a potentially fatal variety of kidney disease with adverse and serious outcomes. The total ischemic necrosis of allthe element (glomeruli, blood vessel and tubule) of the affected area of renal cortex is a typical histological feature of RCN. RCN is irreversible lesion leading to total loss of kidney function and end stage kidney failure in complete variety of cortical necrosis. However, recovery of renal function is variable in the incomplete type of cortical necrosis depending upon the amount of necrosed nephron in the kidney. RCN result from severe degree of renal ischemia secondary to significantly reduced renal tissue perfusion usually on account of intravascular coagulation, microvascular injury or extreme vascular spasm. The following two types of cortical necrosis have been identified on the basis of renal histology: (1) diffuse cortical necrosis: Confluent global cortical destruction extends into the columns of bertin. Thin rim of subcapsular and Juxtamedullary tissue is preserved. Irreversible renal failure leading to end stage renal diseases (ESRD) is the final outcome of diffuse cortical necrosis; and (2) patchy cortical necrosis: Contiguous area of cortical necrosis involve up to one-third to half of the entire cortical tissue. Partial recovery of renal function is known to occur in patchy cortical necrosis.
Renal histology of 113 patients with acute renal cortical necrosis (ACN) revealed complete and patchy cortical necrosis in 62.8% and 37.2% patients, respectively[1]. In our series of 57 patient with RCN, diffuse cortical necrosis was observed in 41 (72%) cases while, patchy cortical necrosis was noted in 16 (28%) patients[2]. Complete and patchy cortical necroses were reported in 80% and 20% of cases from another Indian study[3]. Diffuse and patchy cortical necrosis were noted in 84.2% and 15.8% of patient respectively from our centre earlier[4].
EPIDEMIOLOGY OF RCN
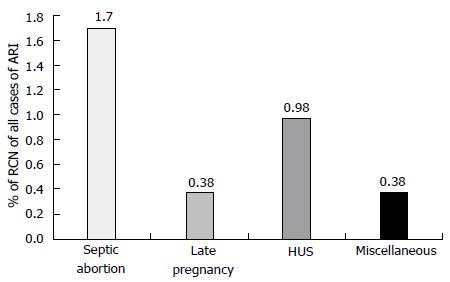
RCN account for less than 2% of all cases of acute renal failure (ARF) in developed country and is rarely reported[5-7]. In contrast to developed country, the incidence of RCN is higher in developing country ranged between 6%-7% of all causes of acute kidney injury (AKI)[4,8-9]. In our previous study the incidence of RCN was 6.3% in patients with AKI[4]. Another Indian study from Chandigarh observed RCN in 7.1% of patients dialyzed for ARF[3,9]. However, decreased incidence of septic abortion related obstetric AKI resulted in reduced incidence of RCN in Indian patients in recent years[2]. To other previous study from India also reported a declining trend (3.8%-4.6%) in the incidence of RCN in patients with AKI similar to our study[1,2,10]. In a series of 1822 patients with ARF, RCN was observed in 57 (3.12%) patients in our most recent study[2]. The overall incidence of RCN decreased to 1.6% (of total cases of acute renal failure) in 1995-2005 from 6.7% in 1984-1994[2]. Septic abortion in obstetric group and haemolytic uremic syndrome (HUS) in non-obstetric group contributed to RCN in 1.7% and 0.98% of cases respectively, of total ARF cases (Figure 1). RCN was reported in one case (0.13%) of 46 patients undergoing kidney biopsy in a series of 748 cases of acute renal failure[11]. Thus, in contrast to developed world (Europe and North America) the incidence of RCN is still high in developing country. The septic complication of pregnancy such as puerperal sepsis and septic abortion is a major cause of higher incidence of RCN in developing countries including India[1,2,4]. The causes of RCN are divided in two group: (1) obstetrical; and (2) non-obstetrical causes. Pregnancy related complications are the most common (50%-70%) causes of RCN and 20%-30% of total cases of RCN are due to non-obstetrical condition. RCN of non-obstetrical origin have higher incidence in male than female[4,6,12]. The various non-obstetrical causes of RCN include; extensive burns, sepsis, HUS, pancreatitis, snake bite, and diabetic ketoacidosis[1,3,13].
Figure 1 Causes of renal cortical necrosis of all cases of acute renal failure (n = 1822); 1984-2005.
Adapted from Prakash et al[2]. HUS: Hemolytic uremic syndrome; RCN: Renal cortical necrosis.
RCN of obstetric origin
Septic abortion, abruptio placentae, puerperal sepsis, eclampsia, obstetric haemorrhage, intrauterine death, and thrombotic microangiopathy of pregnancy (P-TMA) are the causes of RCN in a pregnant women[5,14-17]. Overall, obstetrical causes are the dominant causes of RCN account for 56%-61% of cases[1,2,18,19]. The RCN is reported to occur in 10%-30% of all cases of pregnancy related AKI compared with approximately in 5% of non-pregnant women[20]. We reported RCN in 25% of obstetric ARF in our previous study[19]. Of 57 patients with RCN; pregnancy-associated complication and non-pregnant condition were causative factor for RCN in 32 (52.2%) and 25 (43.8%) respectively[2]. We reported RCN due to pregnancy related complications in 15.2% of obstetric ARF; with higher (11.9%) incidence in post-abortal AKI compare to lower (3.3%) incidence in late pregnancy-AKI[2]. The incidence of RCN was 9% among patient with obstetric AKI in a study from Pakistan[21]. The RCN incidence has decreased from 17% in 1982-1991 to 2.4% in 1992-2002 in obstetric ARF in our recent publication[15]. Thus, the overall incidence of RCN in pregnancy associated AKI has decreased from 20%-30% to 5% in the last two decades in developing countries[2]. The current (2003-2014) incidence of RCN is 1.44% (1/69) in obstetric AKI in our study[22]. Post-abortal sepsis is a common cause of RCN in obstetric AKI in developing countries while, abruptio placentae is responsible for RCN in 50%-60% of case in pregnancy in developed countries[13,23]. Thus, septic abortion is common cause of RCN in developing countries but rarely reported from developed world[14,16,24]. The abortion is commonly conducted by unskilled persons mostly under unhygienic condition which leads to higher incidence of post-abortal sepsis. This possibly may explain higher incidence of RCN in post-abortal AKI in developing world. It is postulated that endothelial injury due to endotoxin may cause endovascular damage and vascular thrombosis with consequent renal ischemia in patient with sepsis and septic abortion.
RCN of non-obstetric origin
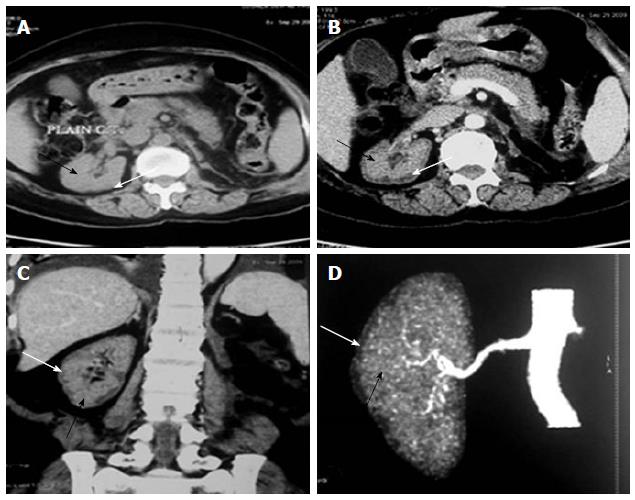
The various non-obstetrical causes of RCN include; extensive burns, snake bite, sepsis, pancreatitis, HUS, infancy and childhood dehydration, malaria and drugs and toxin[1,2,25-29]. Non-pregnancy associated complication accounted for RCN in 34.8% of total ARF cases in our earlier study[16]. RCN was due to pregnancy and non-pregnancy related complications, in 56.2% and 43.8% cases respectively in our recent publication[2]. HUS was the most common cause 18/25 (72%) of cortical necrosis in the non-obstetrical group[2]. Severe sepsis, extensive burns (80%), massive gastrointestinal haemorrhage, acute pancreatitis and diarrhoea associated shock are other causes of RCN in non-pregnant group[2]. The changing clinical feature of RCN was analysed and compared in 28 patients in English literature before and after 1980 from two countries; France (F) and India (I). This analysis revealed that pregnancy related cortical necrosis decrease to 28% after 1980 from 68% (F) and 71% (I) before 1980, while non-pregnancy related cortical necrosis increased to 72% after 1980 from 32% (F) and 29 (I) before 1980. The RCN was due to sepsis in 4/12 (F) and snake bite 6/14 (I) cases before 1980 but drug associated cortical necrosis was observed in 4/21 patient after1980 among the non-obstetrical causes of cortical necrosis[30]. RCN was reported in 19.9% of patient among 131 cases with post-surgical ARF from Japan in an autopsy study[31]. Despite a bit increasing trends in non-obstetrical cause of RCN, obstetrical complication is still remains the dominant cause of RCN in developing country. The development of RCN in live kidney donor and malaria was noted in Indian literature[32,33]. Figure 2 shows the RCN in live kidney donor. Donor was on maintenance haemodialysis for 4 mo and she eventually died of severe sepsis related to pneumonia[33]. RCN developed in a congenital solitary kidney following Road Traffic accident (Figure 3) in a child aged 11 years.
Figure 2 Renal cortical necrosis in a living kidney donor.
Adapted from Prakash et al[32]. Non-contrast (A) computed tomography (CT) scan of abdomen at the level of right hilum showing hypoattenuating peripheral cortical rim (white arrow) as compared to the inner isoattenuating parenchyma (black arrow), On contrast-enhanced axial (B) and coronal (C) scans the central viable parenchyma enhances (black arrow) while the peripheral necrosed cortex does not show any significant enhancement (white arrow). Overall picture is suggesting renal cortical necrosis. The corresponding appearance is also well noted CT Angiogram (D) showing absent uniform nephrogram (white arrow).
Figure 3 Contrast enhanced computed tomography showing diffuse cortical necrosis in solitary left kidney after road traffic accident in a child aged 11 years.
This image shows isoattenuated peripheral cortical rim (white arrow) and hypoattenuated parenchyma (red arrow).
PATHOGENETIC MECHANISMS OF RCN
The pathophysiological events leading to RCN are poorly understood. However, significantly diminished renal arterial perfusion is the final common pathway resulting in ischemic necrosis of renal cortex. The exact pathogenetic mechanism of RCN is not completely known. The vasospasm of small vessel and liberation of toxin with consequent endothelial injury seems to be initiating event in the process of cortical necrosis[34,35]. The vasculature in pregnancy is more sensitive to vasoconstrictors, possibly related to sex hormone[35]. ACN and the generalised Schwartzmann reaction induced by endotoxin in rabbit have similar clinical feature[36-38]. Two small doses of endotoxin given at interval of 24 h may cause generalised Schwartzmann reaction in non-pregnant animal while only one dose is sufficient to produce this phenomenon in pregnant rabbits[37]. Intravascular coagulation was considered as the initial event in pathogenesis of RCN. However, available evidences does not support the role of intravascular coagulation in the genesis of RCN[6].
The role of endothelium-derived vasoactive substance particularly endothelin-1 has been suggested in the pathogenesis of ischemic ARF. The endothelin-1 is one of the most potent vasoconstrictor substance known[39], and renal vasculature appears to be 10 times more sensitive to this effect of endothelin-1 compared to all other vascular organ[40]. It is suggested that endotoxin initiate endothelial injury with subsequent development of intravascular thrombosis and reduce renal perfusion causing cortical necrosis in patient with sepsis. The endothelial injury seems to be a primary event in development of TMA[41]. In patient with HELLP syndrome, endothelial damage may progress to endovascular thrombosis leading to lumen occlusion, hypoperfusion and ischemic necrosis of renal cortex[42]. Two possible pathogenetic factors may contribute the development of RCN: (1) renal hypo-perfusion resulting from blood loss or hypotension such as in postpartum haemorrhage; and (2) vascular endothelial injury either through direct mechanism (HUS, eclampsia and snake bite) or indirect mechanism via release of circulating substances (sepsis, pancreatitis and intravascular haemolysis). It is postulated that endothelin may act as final common factor leading to renal damage and subsequent RCN, because both renal hypo-perfusion and endothelial injury stimulate release of endothelin from vascular endothelial cell. However, further detailed studies are required to established the possible role of endothelin in the pathogenesis of RCN.
CLINICAL AND DIAGNOSTIC FEATURES OF RCN
RCN is a rare but catastrophic cause of AKI. Absolute anuria (urine output nil in 24 h) or anuria (urine output < 100 mL/24 h) are the usual presenting symptoms of acute RCN. Because of the systemic nature of illness causing RCN, the lesion is usually bilateral. Prolonged anuria (> 4 wk) in clinical setting of haemorrhage, sepsis, shock or disseminated intravascular coagulation suggests the clinical diagnosis of RCN. The mean duration of absolute anuria from the onset till death or partial recovery of renal function while on dialysis was 24.5 ± 26.2 (range 6-100) d in our study[2,4]. Hematuria (microscopic or gross) can be seen in patients with ACN. The renal biopsy is the gold standard to confirm the diagnosis of RCN. The typical histological feature of RCN is ischemic necrosis of all elements of renal parenchyma of cortical region (Figure 4). RCN was diagnosed on kidney biopsy specimen within the first week of the onset of disease. However, contrast enhanced computed tomography (CECT) scan is a suitable non-invasive modality for the early diagnosis of RCN[43]. The presence of hypoattenuated subcapsular rim of renal cortex on CECT scan is atypical radiological abnormalities in patient with RCN (Figure 3). In addition, a non-contrast CT scan (NCCT) is more sensitive in picking up cortical calcification. However, renal cortical calcification develop too late and also not observed in all patients with RCN[20,44,45] (Figure 5). Thus, demonstration of cortical calcification on NCCT scan is not useful in early diagnosis of RCN. Other less invasive method of diagnosis like MRI scan are useful alternative[46]. Recently, contrast enhanced ultrasound scan is found to another non-invasive modality for early diagnosis of RCN[47].
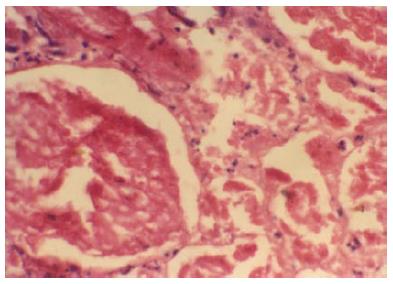
Figure 4 Glomeruli and neighbouring tubules are showing coagulative necrosis.
There is complete loss of nuclei in glomeruli and tubules, but connective tissue framework is preserved. Neutrophilic infiltrations are seen around necrosed glomeruli and in interstitium (HE × 250). Typical histologic feature of renal cortical necrosis.
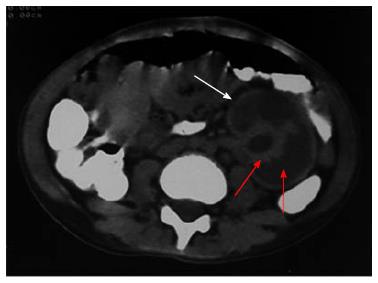
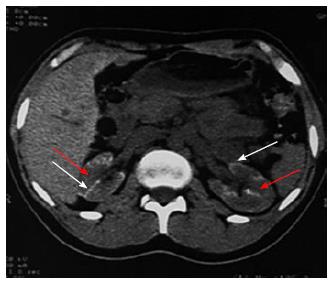
Figure 5 Non contrast computed tomography showing bilateral cortical calcification in a patient with renal cortical necrosis 72 d after acute pancreatitis.
Non contrast computed tomography scan of abdomen at the level of renal hila showing linear hyperattenuation along the renal cortical rim (white arrow) with hypoattenuating medulla (red arrow) in bilateral kidney.
CLINICAL COURSE AND OUTCOMES OF RCN
The clinical course of patients with RCN can be divided into five broad groups: (1) death in uraemia during the acute phase; (2) survival without dialysis; (3) late return to dialysis/transplant; (4) survival only with chronic maintenance dialysis/transplant; and (5) late resumption of sufficient renal function to become dialysis independent. The mortality was 87% during acute phase of illness in our previous study[4]. However, mortality decreased to 19% in 1995-2005 from 72% in 1984-1994[2]. The maternal mortality in obstetric RCN was 72.7% in 1982-1991. Mortality was observed in 1 of 3 patients in 1992-2002 and no mortality in the last decades. Thus, maternal mortality reduced to zero in 2003-2014 from 72.7% in 1982-1991[22]. The causes of death during acute phase of illness are; severe uraemia, sepsis, pulmonary oedema, gastrointestinal haemorrhage and hyperkalemia including multiorgan failure[2]. Thus, the majority of deaths are due to sepsis and uremic complication in those who could not afford dialysis. However, the prognosis and survival of patients with RCN has improved markedly in our recent publication due to availability of renal replacement therapy and overall improved medical care[2,15,22]. Survival without dialysis is possible in patients with patchy cortical necrosis because surviving nephrons carry the function of the remaining kidney. In certain patients, there may be slow rise in creatinine clearance and a gradual gain in renal function over one to two years, so that the glomerular filtration rate may reach a final plateau level of approximately 20-24 mL/min[48,49]. It is assumed that juxtamedullary glomeruli (which comprise 15%-20% of total) escape destruction, even in the complete cortical necrosis and that early functional return is due to recovery of these nephron segment. The deterioration in renal function had been reported several years (1-10 years) after the acute cortical necrosis in a significant number of patients. Factors causing these late functional downturn are not clear but may include pyelonephritis, hypertension and shrinkage of the kidney due to progressive fibrosis and/or calcification[50,51].
The fate and outcome of RCN has changed in developing countries mainly due to decreasing incidence of RCN in patients with acute renal failure. We observed the incidence of RCN decreased to 1.6% in 1995-2005 from 6.7% in 1984-1994[2]. The incidence of RCN in obstetric AKI has decreased to 2.4% in 1992-2002 from 17% in 1982-1991 in our previous publication[15]. The most recent incidence of RCN in obstetric AKI was 1.44% in our study[22]. This changing picture of RCN is mainly due to a decrease in incidence of poost-abortal sepsis. Public awareness, legalization of abortion law, and overall improved health services are other reasons for such improvement in the prognosis and outcome of cortical necrosis at our centre.
CONCLUSION
The renal prognosis of RCN has improved. The partial recovery of renal function had increased to 33.3% in 1995-2005 from 11% in 1984-1994. The mortality of patients with RCN had markedly reduced to 19% in 1995-2005 from higher mortality of 72% in 1984-1994 due to wider availability of dialysis and overall improvement in health care facilities. Because of decreased mortality, higher (47.6%) proportion of patients with cortical necrosis had progressed to ESRD in 1995-2005, compared to lower (16.6%) number of patients in 1984-1994. Thus, both increased number of patient survival and better renal outcome contributed to improved prognosis of RCN in recent years. In addition to improved prognosis of RCN, overall incidence of RCN has decreased to 3% of total cases of ARF. The current incidence of RCN in obstetric AKI is 1.44% at our centre.
P- Reviewer: Nakhoul FM, Nechifor G, Olowu WA, Su MM S- Editor: Tian YL L- Editor: A E- Editor: Wu HL