Published online Jul 6, 2015. doi: 10.5527/wjn.v4.i3.415
Peer-review started: December 23, 2014
First decision: January 8, 2015
Revised: March 9, 2015
Accepted: April 10, 2015
Article in press: April 14, 2015
Published online: July 6, 2015
Processing time: 196 Days and 17.2 Hours
AIM: To investigate the predictive value of low freeT3 for long-term mortality in chronic hemodialysis (HD) patients and explore a possible causative role of chronic inflammation.
METHODS: One hundred fourteen HD patients (84 males) consecutively entered the study and were assessed for thyroid function and two established markers of inflammation, high sensitivity C-reactive protein (hsCRP) and interleukin-6 (IL-6). Monthly blood samples were obtained from all patients for three consecutive months during the observation period for evaluation of thyroid function and measurement of inflammatory markers. The patients were then divided in two groups based on the cut-off value of 1.8 pg/mL for mean plasma freeT3, and were prospectively studied for a mean of 50.3 ± 30.8 mo regarding cumulative survival. The prognostic power of low serum fT3 levels for mortality was assessed using the Kaplan-Meier method and univariate and multivariate regression analysis.
RESULTS: Kaplan-Meier survival curve showed a negative predictive power for low freeT3. In Cox regression analysis low freeT3 remained a significant predictor of mortality after adjustment for age, diabetes mellitus, hypertension, hsCRP, serum creatinine and albumin. Regarding the possible association with inflammation, freeT3 was correlated with hsCRP, but not IL-6, and only at the first month of the study.
CONCLUSION: In chronic hemodialysis patients, low plasma freeT3 is a significant predictor of all-cause mortality. Further studies are required to identify the underlying mechanisms of this association.
Core tip: Monthly blood samples were obtained from 114 patients for three consecutive months during the observation period for evaluation of thyroid function and measurement of inflammatory markers high sensitivity C-reactive protein (hsCRP) and interleukin-6 (IL-6). Patients were then followed-up for 7-years. Low mean freeT3 (< 1.8 pg/mL) emerged as a significant predictor of all-cause mortality after adjustment for age, diabetes mellitus, hypertension, hsCRP, serum creatinine and albumin. However, freeT3 was correlated with hsCRP, but not IL-6, and only at the first month suggesting that further studies are required to identify the underlying pathogenetic mechanisms of the association between thyroid function and survival.
- Citation: Fragidis S, Sombolos K, Thodis E, Panagoutsos S, Mourvati E, Pikilidou M, Papagianni A, Pasadakis P, Vargemezis V. Low T3 syndrome and long-term mortality in chronic hemodialysis patients. World J Nephrol 2015; 4(3): 415-422
- URL: https://www.wjgnet.com/2220-6124/full/v4/i3/415.htm
- DOI: https://dx.doi.org/10.5527/wjn.v4.i3.415
Despite the significant advances in hemodialysis (HD) techniques during the last decades, morbidity and mortality of HD patients remains unacceptably high and it is attributed mainly to the excess prevalence of cardiovascular disease (CVD)[1]. Beyond traditional risk factors (Framingham risk factors), which are highly prevalent in end-stage renal disease (ESRD) patients, patients on renal replacement therapy appear to be subject to the deleterious effect of a number of other harmful factors, including uremic milieu, anemia, bone-mineral disorders and hyperhomocysteinemia[2]. Moreover, recent studies suggested that oxidative stress, endothelial dysfunction and inflammation, also exacerbate cardiovascular disease[3,4]. So far, there is a lack of a strong marker of disease severity in ESRD, easy to perform and with high availability and low cost, that could guide treatment or even serve as a prognostic indicator of mortality[5]. Presumably, a multifactorial approach of traditional markers combined with novel biomarkers could fulfill this necessity, though this hypothesis needs to be confirmed in large prospective trials[6,7].
Low free triiodothyronin (freeT3) has emerged as a potent biomarker in ESRD in several studies and represents the main finding of non thyroidal illness syndrome (NTIS) in renal disease[2,8-10]. This syndrome, has been a debate for several years, as changes in hormone levels have been considered either a laboratory pitfall or an adoptive response to chronic stress aimed to spare calories[11,12]. It is important to emphasize the significance of values range, especially in differentiating incidents of subclinical hypothyroidism that could be mistaken as low T3 syndrome cases[13]. More recently, a narrower range of TSH values was proposed, although not widely accepted[14,15].
The exact pathogenetic mechanisms of NTIS are not yet fully elucidated but iodine retention, alterations to protein binding, derangements to deiodinases activity and dysregulation at the hypothalamic level appear to play a major role[16]. In addition, some studies demonstrated a significant negative correlation between interleukin-6 (IL-6) and serum T3 levels and suggested that NTIS is an acute phase response, yielded by activation of a cytokine network[17,18]. However, a causal role of chronic inflammation in the development of low T3 syndrome remains to be identified.
The aim of this prospective study was to investigate the predictive power of low freeT3 for long-term mortality in chronic hemodialysis patients; moreover the correlation between thyroid function and inflammation, assessed by measurements of high sensitivity CRP (hsCRP) and IL-6, was studied.
Prospective, observational study in 118 clinically stable chronic hemodialysis patients.
One hundred eighteen chronic hemodialysis patients from two large dialysis centers in Northern Greece, the Renal Unit at “G. Papanikolaou” General Hospital of Thessaloniki and the Department of Nephrology at University Hospital of Alexandroupolis, consecutively entered the study. Both centers had similar methods regarding hemodialysis and treatment strategies in ESRD, which are in accordance with the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines[19]. All patients had been stabilized on renal replacement therapy for > 3 mo prior to enrollment (mean HD duration 113.4 ± 65.1 mo, range 15-427 mo) and were clinically stable and free of active infection. Patients with known history of thyroid disorders or taking medications with possible effect in thyroid hormone values, like amiodarone or lithium, were excluded from the study[20]. None of the patients was receiving antibiotics at the time of the study or had required hospitalization up to 3 mo prior to study entry. All patients were on a 4hr three times weekly hemodialysis schedule. The dialysis modalities were on-line hemodiafiltration (OL-HDF) or conventional hemodialysis (C-HD) with high-flux or low-flux dialyzer membranes respectively. Both dialysis centers used semisynthetic membranes (polyamide or polysulphone) with surface ranging from 1.6-2.1 m2. The dialysis solution consisted of standard bicarbonate preparations (HCO3-: 32-35 mmol/L, Na: 138 mmol/L, K: 1-3 mmol/L, Mg: 0.5-0.75 mmol/L, Ca: 1.25-1.75 mmol/L). Low-molecular-weight or unfractionated heparin were used as standard anticoagulation. Dialysis prescription was guided by a goal of achieving a value of ≥ 0.65 for the urea reduction ratio and a value of Kt/V ≥ 1.2. The above indices of adequacy of dialysis were calculated by the formula [(pre-dialysis urea)-(post-dialysis urea)/predialysis urea], and by the second generation Daugirdas equation, respectively.
Body mass index (BMI) was calculated by dividing the dry weight in kilograms by the square of the height in meters. Blood pressure (BP) was measured in a supine position after 15 min of recumbency before a routine midweek dialysis session with standard mercury sphygmomanometers. Patients were considered hypertensive if they had pre-dialysis BP ≥ 160/90 mmHg or if they were receiving one or more anti-hypertensive drugs at the time of the study. History of cardiovascular disease was defined as history of myocardial infarction, coronary artery bypass or clinical signs of angina pectoris, stroke or transient ischemic attack or peripheral vascular disease.
The study protocol was conformed to the ethical guidelines of the Declaration of Helsinki and was approved both by the institutional review board and the ethics committee of each participating centre. Furthermore, all patients gave informed consent for the legal use of their blood samples and handling of their personal data before their enrollment.
Monthly blood samples were taken before a midweek routine hemodialysis session, for three consecutive months. Blood was drawn from the “arterial” lumen of fistula needle or the arterial port of the central hemodialysis catheter after extracting heparin lock in order to avoid artifactual alterations of measured freeT3 and the other studied parameters[21]. All samples were centrifuged for 15 min at 3500 rpm and the supernatant was immediately separated into vials (SST II BD Vacutainer). Blood samples from Alexandroupoli were transferred to General Hospital “G. Papanikolaou” in Thessaloniki in dry ice. We were tied in with all appropriate scientific measures and legislation about transportation of biologic fluids. Samples from both institutions were eventually gathered and stored at G.H. “G. Papanikolaou” at -70 °C.
All laboratory parameters were measured in the Central Biochemical Department of General Hospital “G. Papanikolaou”. Complete blood cell count, urea, creatinine, total cholesterol, triglyceride, total protein and albumin, were determined by routine techniques using an automated analyser. Serum thyroid parameters (freeT3, freeT4, TSH), were measured by chemiluminescent, immunometric assay, according to the routine laboratory methods, using an IMMULITE-2000 analyzer, Laboratory reference values were 0.4 to 4.0 mIU/mL for TSH, 1.8 to 4.2 pg/mL for freeT3 and 0.89 to 1.76 ng/dL for freeT4.
Serum CRP levels were measured by high sensitivity nephelometrry (“Beckman Coulter” Ireland Inc). The detection limit was 0.1 mg/dL, with intra-assay and inter-assay coefficient of variation of 5% and 6.5%, respectively. Values above the threshold of 0.15 mg/dL were considered to be abnormal.
Serum IL-6 concentrations were measured by sandwich ELISA immunoassay using commercially available standard kits (AMS Biotechnology, United Kingdom). The concentrations of IL-6 were calculated by reference to standard curves performed with the corresponding recombinant molecule. All serum samples were tested in duplicate. The detection limit was 0.92 pg/mL, with intra-assay and inter-assay coefficient of variation of 3.4% and 5.2%, respectively.
After the initial assessment and the determination of laboratory parameters for three consecutive months, all patients were followed-up for up to 7 years. During follow-up deaths were recording accurately by reviewing patient’s hospital records.
Normality of variable distribution was tested using Kolmogorov-Smirnov test. Data are reported as mean ± SD (normally distributed data), median and interquartile range (non-normally distributed data) or as percentage frequency, as appropriate. Friedman’s test was used to detect significant variations between non-parametric variables during the three month blood sampling period. According to the conducted power analysis each group had to include a minimum of 30 subjects in order to test differences in survival after a 7 year follow-up period. The significance of differences in means between the two groups was assessed by Student’s t test or Mann-Whitney test. Differences in proportions were tested with the use of χ2 test. Correlations were tested by Pearson’s r or Spearman test for parametric and non-paramertric data analysis respectively. Non-normally distributed variables were log-transformed before entering regression analysis. For the survival analyses, patients were divided in low and normal freeT3 groups according to mean 3 mo freeT3. Patients included in the low freeT3 group had mean 3 mo values below the lower laboratory’s normal range (1.8 pg/mL). Time was calculated from the end of the 3 mo observation and blood sampling period until death or censoring. The data were censored if a patient underwent renal transplantation, switched from hemodialysis to peritoneal dialysis during the study period, died and at the end of follow-up. Furthermore, patients were censored if they were diagnosed with thyroid disease, primary or as a complication of treatment. Survival curves according to mean freeT3 values were calculated using the Kaplan-Meier method. Differences in survival were assessed with the use of log-rank test. Survival analyses were also made with the Cox proportional hazards model. The relative risks for mortality were determined by univariate and multivariate Cox regression analysis and presented as hazard ratio (HR; 95%CI). Covariates tested in the Cox model were age, diabetes mellitus, hypertension, hsCRP, creatinine, history of CVD, duration of hemodialysis and hemoglobin. Variables were included in the multivariate analyses if they had a P value < 0.05 in the univariate analysis or if they were clinically important confounders. The calculations were performed using SPSS 19.0 (Statistical Package for Social Sciences, IBM SPSS, Chicago, Ill). Statistical review of the study was performed by a biomedical statistician. A two-tailed P value < 0.05 was considered to be statistically significant.
The statistical review of the study was performed by a biomedical statistician.
During the blood sampling period four patients that required hospitalization or died were excluded from the analyses. Thus finally 114 patients (84 males) consecutively entered the study and were followed-up for 7 years (mean ± SD: 55.3 ± 2.9 mo). Mean age was 62.3 ± 14.3 years and mean HD duration 114.3 ± 91 mo (range 15-427 mo). Primary disease was diabetic nephropathy in 23.9% of the patients, glomerulonephritis in 19.7% and unknown in 27.4%. Three-month mean freeT3 was 2.17 ± 1.25 pg/mL, freeT4 was 0.956 ± 0.19 ng/dL, TSH was 1.94 ± 0.65 mIU/mL, hsCRP was 1.57 ± 2.98 mg/dL and IL-6 was 14.12 ± 11.52 pg/mL. Patients were divided into two groups based on the mean freeT3 values; the low freeT3 group consisted of thirty five patients (30.7%) with freeT3 below the lower normal laboratory range (1.8 pg/mL) and the normal freeT3 group consisted of 79 patients with freeT3 ≥ 1.8 pg/mL).
Baseline (at the beginning of follow-up period) demographic, hemodynamic and clinical characteristics of low and normal freeT3 groups are shown in Table 1 and laboratory parameters in Table 2. Compared with patients that had normal freeT3, patients with low freeT3 were significantly older (P = 0.019) and had higher prevalence of diabetes mellitus (P = 0.017) and history of cardiovascular disease (P = 0.048). Gender, HD duration and morning shift, dialysis modality, Kt/V, type of vascular access, prevalence of smoking and hypertension and systolic and diastolic blood pressure did not differ significantly between the two groups (Table 1). Moreover, compared with patients with normal freeT3, patients with low freeT3 had at baseline significantly lower serum creatinine (P = 0.041) and albumin (P = 0.002). Low freeT3 group had also higher hsCRP and above the upper normal range (1.5 mg/dL) but the difference failed to reach statistical significance (P = 0.156). Hemoglobin and hematocrit, white blood cell count, blood urea, lipid levels, IL-6, freeT4 and TSH did not show significant differences in the two patient groups (Table 2).
| Variable | Normal freeT3(n = 79) | Low freeT3(n = 35) | P value |
| Age | 59.96 ± 14.74 | 66.84 ± 13.11 | 0.019 |
| Female gender | 18 (22.78%) | 12 (34.28%) | 0.237 |
| BMI (kg/m2) | 25.65 ± 2.92 | 26.49 ± 4.02 | 0.622 |
| HD duration (mo) | 66.42 ± 59.69 | 56.49 ± 41.08 | 0.302 |
| HD morning shift | 75 (94.93%) | 32 (91.42%) | 0.418 |
| OL-HDF | 22 (27.84%) | 11 (31.42%) | 0.484 |
| CVC access | 10 (12.65%) | 9 (25.71%) | 0.160 |
| Kt/V | 1.27 ± 0.39 | 1.29 ± 0.42 | 0.822 |
| Smoking | 16 (20.25%) | 9 (25.71%) | 0.606 |
| History of CVD | 24 (30.37%) | 17(48.57%) | 0.048 |
| Diabetes | 16 (20.25%) | 16 (45.71%) | 0.017 |
| Hypertension | 60 (75.94%) | 23 (65.71%) | 0.488 |
| SBP (mmHg) | 131.06 ± 19.94 | 130.50 ± 21.94 | 0.819 |
| DBP (mmHg) | 68.42 ± 13.87 | 71.22 ± 14.47 | 0.357 |
| Variable | Normal freeT3(n = 79) | Low freeT3(n = 35) | P value |
| Creatinine (mg/dL) | 9.50 ± 2.75 | 8.44 ± 2.35 | 0.041 |
| Urea (mg/dL) | 146.59 ± 36.73 | 140.33 ± 51.06 | 0.556 |
| Cholesterol (mg/dL) | 180.67 ± 47.3 | 175.8 ± 52.52 | 0.176 |
| Triglycerides (mg/dL) | 183.86 ± 94.69 | 171.20 ± 130.36 | 0.215 |
| Hematocrit (%) | 37.41 ± 3.74 | 36.63 ± 3.86 | 0.860 |
| Hemoglobin (g/dL) | 12.04 ± 1.33 | 11.52 ± 1.31 | 0.262 |
| WBC (/mm3) | 7.33 ± 2.20 | 7.61 ± 2.18 | 0.406 |
| Albumin (g/dL) | 3.99 ± 0.34 | 3.74 ± 0.497 | 0.002 |
| TSH (μIU/mL) | 2.77 ± 8.45 | 1.87 ± 1.02 | 0.354 |
| FreeT4 (ng/dL) | 1.13 ± 0.20 | 1.11 ± 0.3 | 0.170 |
| IL-6 (pg/mL) | 13.73 ± 13.29 | 16.49 ± 16.85 | 0.948 |
| hsCRP (mg/dL) | 1.56 ± 3.37 | 1.99 ± 2.73 | 0.156 |
No statistically significant variation was observed in hsCRP or IL-6 values within the 3 monthly samples (Friedman test, P = 0.088 and P = 0.168 respectively). In contrast, freeT3 levels showed a statistical significant variation during the three months (P < 0.001).
A linear statistically significant association was noted between age and mean freeT3, which resulted in the following algorithm: Mean freeT3 = 2.80-0.01*Age. In addition, serum albumin showed a strong correlation with freeT3 in all three consecutive measurements (meanAlb - meanfreeT3: Spearman’s rho = 0.318, P = 0.001). No statistically significant correlations were observed between hsCRP or IL-6 with the other studied clinical parameters.
In the first month, significant correlations were found between TSH and freeT4 (Spearman’s rho = -0.259, P = 0.010), hsCPR and freeT3 (Spearman’s rho = -0.323, P = 0.001) and hsCRP and IL-6 (Spearman’s rho = 0.312, P = 0.001). These associations remained significant in the following 2 mo except from the correlation between hsCRP and freeT3.
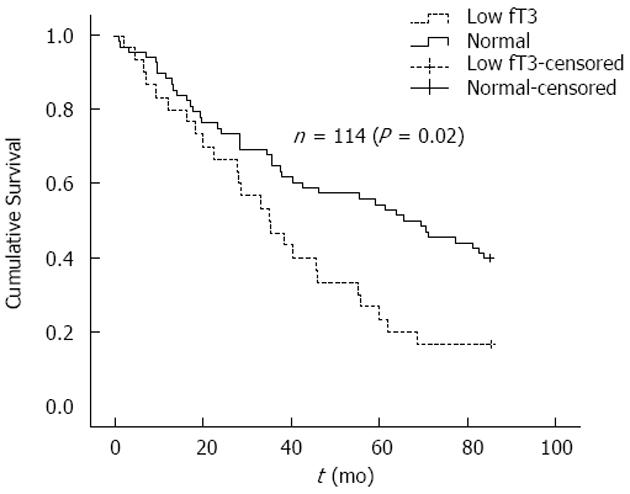
During the 7 year follow-up period, 69 patients (60.5%) died. In addition, 11 patients were transplanted, one switched from hemodialysis to peritoneal dialysis and three patients were lost to follow-up. During follow-up 41 deaths (59.4%) were recorded in the normal freeT3 group and 25 deaths (83.3%) in the low freeT3 group (P = 0.036). Also, compared with patients that had normal freeT3 levels, patients with low freeT3 had a lower survival (54.9 ± 3.4 mo vs 39.8 ± 2.7 mo, P = 0.019). Furthermore, Kaplan-Meier survival curves differed significantly between patients with low and normal freeT3 levels [Log Rank (Mantel-cox), P = 0.02] (Figure 1).
In univariate (unadjusted) Cox regression analysis low freeT3 was a significant predictor of mortality [HR = 1.89 (1.146-3.124), P = 0.011]. Other parameters associated significantly with mortality were age, HD duration, history of CVD, hypertension, hemoglobin and hematocrit, serum creatinine and hsCRP. Moreover, diabetes mellitus showed a correlation of borderline significance with mortality (Table 3). In contrast, no association was observed between serum albumin and survival (P = 0.178). In addition, in a Cox Regression model, low freeT3 remained a significant independent predictor of mortality after adjustment for age, diabetes, hypertension, hsCRP, serum creatinine and albumin. However, in a multivariate stepwise Cox Regression analysis, including also hemoglobin, dialysis vintage and history of CVD, plasma freeT3 values failed to retain their predictive power for all-cause mortality. In the fully adjusted model, factors independently associated with mortality were age (3.2% higher odds of death for every year) and history of CVD (83.5% likelihood of death). Moreover, for every 1 g/dL of raise in hemoglobin levels there was a risk reduction for mortality of 43%. Diabetes mellitus and hsCRP values were not also associated with mortality in the fully adjusted model (Table 4).
| Variable | Spearman’s rho | P value |
| Age (yr) | 0.345 | < 0.001 |
| Diabetes mellitus (yes/no) | 0.199 | 0.050 |
| Hemodialysis vintage (mo) | -0.435 | 0.017 |
| Hemoglobin (g/dL) | -0.329 | 0.001 |
| Hematocrit (%) | -0.270 | 0.007 |
| Hypertension (yes/no) | 0.275 | 0.006 |
| Free T3 (pg/mL) | -0.257 | 0.011 |
| Previous CVD events (yes/no) | 0.305 | 0.002 |
| hsCRP (mg/dL) | 0.240 | 0.017 |
| Creatinine (mg/dL) | -0.400 | < 0.001 |
| Variables | All cause mortality relative risks,(95%CI), P values | |
| Model 1(unadjusted) | Model 2(fully adjusted) | |
| LowT3_1.8 (pg/mL) | 1.89 (1.146-3.124) P = 0.013 | 1.61 (0.88-2.92) P = 0.115 |
| Age (1 yr) | - | 1.031 (1.00-1.05) P = 0.008 |
| DM (yes) | - | 1.22 (0.68-2.18) P = 0.50 |
| History of CVD (yes) | - | 1.878 (1.09-3.21) P = 0.021 |
| Mean_Hb (1 g/dL) | - | 0.561 (0.426-0.73) P = 0.000 |
| hsCRP (0.1 mg/dL) | - | 1.076 (0.91-1.27) P = 0.388 |
| HD duration (1 mo) | - | 0.990 (0.98-0.99) P = 0.004 |
Chronic uremia, similar to other chronic illnesses, may cause a variety of nonspecific wasting syndromes including protein loss, accumulation of fat stores, hyperglycemia and insulin resistance, hypoproteinemia, and hypertriglyceridemia[22]. Thyroid abnormalities are also very often in ESRD patients, with low freeT3 levels observed in the majority of cases[23,24]. The latter represents the main finding of euthyroid sick syndrome or non thyroidal illness syndrome which has been a debate for several year. Interestingly, recent studies in other models of metabolic derangements, investigated the causal link between circadian misalignment and metabolic homeostasis using a controlled simulation of “shift-work” in the clinical laboratory[25]. Circadian dysregulation caused decreased leptin levels and resulted in hyperglycemia and hyperinsulinemia. Moreover, daily cortisol excretion was reversed, arterial pressure was increased and sleep efficiency decreased[26]. Thus, it was hypothesized that neuroendocrine derangements should be crucial in ESRD patients and might play an important role in NTIS while also explain the possible association with inflammation and cardiometabolic syndrome[27].
In the present study, the prognostic value of NTIS for long-term mortality in chronic hemodialysis patients and its probable association with low-grade inflammation, a common feature of chronic uremia, was investigated[5]. Our results showed no association of freeT4 or TSH with either hsCRP or IL-6. Moreover, freeT3 had significant negative correlation with hsCRP but only at baseline. Some studies previously examined the latter association but the number is limited and their results are rather inconsistent. Zoccali et al[8] first reported that freeT3 had a significant negative correlation with CRP and IL-6. Meuwese et al[9] investigated the trimestal variation of thyroid hormones and IL-6; in their study, IL-6 was positively associated with TSH and negatively with T3 whereas CRP levels were positively associated with T4 but only at baseline. Finally, and in accordance with our results, in the study of Carrero et al[28], only T3 and not freeT3 showed a correlation with CRP and IL-6. The above discrepant results could be, at least partially, explained by differences in patient’s characteristics, exclusion criteria and laboratory methods used. It should be noted that in our study patients with recent infection or chronic inflammatory diseases were carefully excluded as well as patients who presented with infection during the 3 mo observation-sampling period. However, based on the above it appears reasonable to conclude that in HD patients, chronic low grade inflammation does not play a major role in the development of NTIS.
During the 7 year follow-up period, 60.5% of patients died, mostly from cardiovascular causes. Compared with patients who remained alive, patients who died had significantly lower freeT3 values. Moreover, Kaplan Meier survival curves differed significantly between patients with low and normal freeT3. In univariate Cox regression analysis survival was also associated with freeT3. Other factors significantly affecting outcome were age, HD duration, diabetes mellitus, history of CVD, presence of hypertension, hemoglobin, serum creatinine and hsCRP. In contrast, no association was observed between serum albumin and survival either in univariate or multivariate analysis. However, it should be mentioned that although the low T3 group had significantly lower serum albumin compared to normal T3 group, only 8 patients (14%) of the former group had serum albumin below the lower laboratory normal range and none had albumin lower than 3 g/dL. Given the well known association between malnutrition, inflammation and atherosclerosis in uremia, the above findings are consistent with the presence of a very low grade of inflammation in our patient population and relatively good nutritional status. Interestingly, in multivariate Cox Regression analyses, low freeT3 remained a significant independent predictor of mortality after adjustment for some traditional and uremia-related risk factors including age, diabetes, hypertension, hsCRP, serum creatinine and albumin. Of note, compared with patients with normal freeT3, those with low freeT3 had increased incidence of fatal and non-fatal CVD events during follow up (data not shown). However, in the fully adjusted model including also mean hemoglobin, history of CVD event, and HD duration, low freeT3 lost its power as a predictor of mortality. Several previous studies have also suggested a correlation of NTIS with mortality although they have important differences with our report. Zoccali et al[10] observed an independent association between freeT3 and mortality during an average follow-up of 42 mo; however, they included both incident and prevalent HD patients whereas freeT3 values were significantly higher compared to those in our patients. Horáček et al[29] in unselected HD patients found that survival curves differed between patients with low and normal freeT3 values during a five-year follow-up; however, more detailed survival analyses are not reported. In their study in incident dialysis patients, Carrero et al[28] found an independent association between only T3, but not freeT3, with survival during a 20 mo median follow-up. Fernández-Reyes et al[30] were unable to detect any association between freeT3 and mortality in patients who survived at least 12 mo on dialysis during an almost 34 mo follow-up. Finally, in the study of Ozen et al[31], in chronic HD patients, freeT3 was also found to be correlated with survival only in unadjusted analysis. Taken together, the above studies argue for a role of thyroid function in the outcome of hemodialysis patients although in some cases the above association could be confounded by other factors which are commonly present in uremia, including inflammation and malnutrition. Nevertheless, further studies are needed to identify the exact nature of the association between low freeT3 and mortality in ESRD patients. Moreover, it remains to be investigated whether or not routine measurement of freeT3 in hemodialysis would add significantly greater predictive power for mortality to models based on traditional and uremia-related risk factors including inflammation. Nevertheless, although the use of thyroid hormone therapy in NTIS is controversial, design of interventional studies in hemodialysis patients aimed to investigate whether normalization of T3 values would actually reduce mortality is a tempting idea[32-34]. However, a study in ESRD patients with NTIS, showed that administration of T3 resulted in excess protein turnover, therefore increasing the need for dialysis[35].
Our study has some strengths. Firstly, only one previous study examined thyroid hormone variation. Secondly, patients were carefully examined to rule out any occult infection before study entry and moreover, patients were excluded if they presented with an acute infection during the three month observation-sampling period. Finally, the present report has the longest follow-up. However, to properly address the implications of the present study some limitations should be considered. It is known that thyroid hormone production follows a circadian rhythm and blood samples were taken at two different time points according to the patient’s shifts. However, only a very small percentage of the patients (6%) underwent hemodialysis in the evening and moreover, no association was observed between HD shift and either freeT3 or freeT4 values. Nevertheless, we believe that circadian rhythms may present alterations in patients on hemodialysis similar to other incidents of biological clock disarrangements like shift workers, although time of sampling was not an issue in relevant studies[29]. These metabolic changes could actually be interpreted as an adaptive mechanism to the high energy expenditure during hemodialysis treatment. In addition, our study included only chronic hemodialysis (prevalent) patients and the results would not necessarily apply to incident dialysis patients.
In conclusion, in this prospective study in chronic hemodialysis patients, low freeT3 levels had emerged as a significant predictor of mortality independently of traditional and some uremia-related risk factors including age, diabetes mellitus, hypertension, serum albumin and hsCRP. However, an association between NTIS and inflammation could not be documented and the exact mechanisms underlying the above association remain to be identified in future mechanistic and interventional trials.
Non thyroidal illness syndrome (NTIS) has been associated with several chronic severe illnesses. This syndrome has been a debate for several years, as alterations in thyroid hormone levels have been considered either a laboratory pitfall or an adoptive response to chronic stress. In end stage renal disease the main finding of the syndrome is low free triiodothyronin (freeT3) which recently has emerged as a potent biomarker of cardiovascular risk and a predictor of mortality.
Previous studies have suggested a probable association between low freeT3 and inflammation in end stage renal disease and a link between NTIS and cardiorenal syndrome. However, the predictive power of NTIS for long-term mortality and the mechanisms underlying the above associations remain unclear.
In the present study, the predictive value of low NTIS for long-term mortality in chronic hemodialysis patients and its link with low-grade inflammation was investigated. Our results showed that low freeT3 was a significant predictor of mortality independently of traditional and some uremia-related risk factors including inflammatory markers. However, an association between NTIS and inflammation was not documented and the exact mechanisms underlying the above association remained unclear. In this regard, there is a limited number of previous relevant studies and their results are rather inconsistent. This study has several strengths. Firstly, only one previous study examined trimestral thyroid hormone variation. Secondly, patients were carefully examined to rule out any occult infection before their enrollment and moreover, patients were excluded if they presented with an acute infection during the three month observation-sampling period. Finally, the present report has the longest follow-up.
So far, there is a lack of a strong marker of cardiovascular disease in end-stage renal disease, easy to perform and with high availability and low cost, that could guide treatment or even serve as a predictor of mortality. Presumably, a multifactorial approach of traditional markers combined with novel biomarkers could fulfill this necessity. Among the latter, low freeT3 has emerged as a potent predictor of adverse clinical outcome, which is easy to perform, inexpensive and widely available.
Euthyroid sick syndrome or non thyroidal illness syndrome refers to patients with severe chronic illnesses like starvation, sepsis, end stage renal disease, myocardial infarction and others, in whom a decrease in serum thyroid hormone levels is observed without any identifiable primary thyroid disease.
It is a very good study well conducted.
P- Reviewer: Bernieh B, Hammes M, Trimarchi H S- Editor: Ji FF L- Editor: A E- Editor: Yan JL
| 1. | Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, Johansen K, Kasiske BL, Kutner N, Liu J. US Renal Data System 2013 Annual Data Report. Am J Kidney Dis. 2014;63:A7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 516] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 2. | Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimbürger O, Massy Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol. 2008;3:505-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 418] [Article Influence: 24.6] [Reference Citation Analysis (1)] |
| 3. | Honda H, Qureshi AR, Heimbürger O, Barany P, Wang K, Pecoits-Filho R, Stenvinkel P, Lindholm B. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis. 2006;47:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 372] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 4. | Carrero JJ, Stenvinkel P. Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: a hypothesis proposal. Clin J Am Soc Nephrol. 2009;4 Suppl 1:S49-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | Ortiz A, Massy ZA, Fliser D, Lindholm B, Wiecek A, Martínez-Castelao A, Covic A, Goldsmith D, Süleymanlar G, London GM. Clinical usefulness of novel prognostic biomarkers in patients on hemodialysis. Nat Rev Nephrol. 2012;8:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Lameire N, Van Biesen W, Vanholder R. Did 20 years of technological innovations in hemodialysis contribute to better patient outcomes? Clin J Am Soc Nephrol. 2009;4 Suppl 1:S30-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Tripepi G, Mallamaci F, Zoccali C. Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: searching for the best risk marker by multivariate modeling. J Am Soc Nephrol. 2005;16 Suppl 1:S83-S88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Zoccali C, Tripepi G, Cutrupi S, Pizzini P, Mallamaci F. Low triiodothyronine: a new facet of inflammation in end-stage renal disease. J Am Soc Nephrol. 2005;16:2789-2795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Meuwese CL, Dekker FW, Lindholm B, Qureshi AR, Heimburger O, Barany P, Stenvinkel P, Carrero JJ. Baseline levels and trimestral variation of triiodothyronine and thyroxine and their association with mortality in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2012;7:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Zoccali C, Mallamaci F, Tripepi G, Cutrupi S, Pizzini P. Low triiodothyronine and survival in end-stage renal disease. Kidney Int. 2006;70:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome: an update. J Endocrinol. 2010;205:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 12. | Chopra IJ, Hershman JM, Pardridge WM, Nicoloff JT. Thyroid function in nonthyroidal illnesses. Ann Intern Med. 1983;98:946-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 180] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Dietrich JW, Stachon A, Antic B, Klein HH, Hering S. The AQUA-FONTIS study: protocol of a multidisciplinary, cross-sectional and prospective longitudinal study for developing standardized diagnostics and classification of non-thyroidal illness syndrome. BMC Endocr Disord. 2008;8:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab. 2005;90:5483-5488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 264] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 15. | Biondi B. The normal TSH reference range: what has changed in the last decade? J Clin Endocrinol Metab. 2013;98:3584-3587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | St Germain DL, Galton VA, Hernandez A. Minireview: Defining the roles of the iodothyronine deiodinases: current concepts and challenges. Endocrinology. 2009;150:1097-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 200] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 17. | Boelen A, Platvoet-Ter Schiphorst MC, Wiersinga WM. Association between serum interleukin-6 and serum 3,5,3’-triiodothyronine in nonthyroidal illness. J Clin Endocrinol Metab. 1993;77:1695-1699. [PubMed] |
| 18. | Rozing MP, Westendorp RG, Maier AB, Wijsman CA, Frölich M, de Craen AJ, van Heemst D. Serum triiodothyronine levels and inflammatory cytokine production capacity. Age (Dordr). 2012;34:195-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Hemodialysis Adequacy 2006 Work Group. Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48 Suppl 1:S2-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 324] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 20. | Trainer TD, Howard PL. Thyroid function tests in thyroid and nonthyroid disease. Crit Rev Clin Lab Sci. 1983;19:135-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Jaume JC, Mendel CM, Frost PH, Greenspan FS, Laughton CW. Extremely low doses of heparin release lipase activity into the plasma and can thereby cause artifactual elevations in the serum-free thyroxine concentration as measured by equilibrium dialysis. Thyroid. 1996;6:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | De Groot LJ. Dangerous dogmas in medicine: the nonthyroidal illness syndrome. J Clin Endocrinol Metab. 1999;84:151-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 225] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Lim VS. Thyroid function in patients with chronic renal failure. Am J Kidney Dis. 2001;38:S80-S84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Song SH, Kwak IS, Lee DW, Kang YH, Seong EY, Park JS. The prevalence of low triiodothyronine according to the stage of chronic kidney disease in subjects with a normal thyroid-stimulating hormone. Nephrol Dial Transplant. 2009;24:1534-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res. 2010;106:447-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 358] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 26. | Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453-4458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1765] [Cited by in RCA: 1586] [Article Influence: 99.1] [Reference Citation Analysis (1)] |
| 27. | Van den Berghe G. The neuroendocrine response to stress is a dynamic process. Best Pract Res Clin Endocrinol Metab. 2001;15:405-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Carrero JJ, Qureshi AR, Axelsson J, Yilmaz MI, Rehnmark S, Witt MR, Bárány P, Heimbürger O, Suliman ME, Alvestrand A. Clinical and biochemical implications of low thyroid hormone levels (total and free forms) in euthyroid patients with chronic kidney disease. J Intern Med. 2007;262:690-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Horáček J, Dusilová Sulková S, Kubišová M, Safránek R, Malířová E, Kalousová M, Svilias I, Malý J, Sobotka L, Zák P. Thyroid hormone abnormalities in hemodialyzed patients: low triiodothyronine as well as high reverse triiodothyronine are associated with increased mortality. Physiol Res. 2012;61:495-501. [PubMed] |
| 30. | Fernández-Reyes MJ, Diez JJ, Collado A, Iglesias P, Bajo MA, Estrada P, Del Peso G, Heras M, Molina A, Selgas R. Are low concentrations of serum triiodothyronine a good marker for long-term mortality in hemodialysis patients? Clin Nephrol. 2010;73:238-240. [PubMed] |
| 31. | Ozen KP, Asci G, Gungor O, Carrero JJ, Kircelli F, Tatar E, Sevinc Ok E, Ozkahya M, Toz H, Cirit M. Nutritional state alters the association between free triiodothyronine levels and mortality in hemodialysis patients. Am J Nephrol. 2011;33:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Bartalena L. The dilemma of non-thyroidal illness syndrome: to treat or not to treat? J Endocrinol Invest. 2003;26:1162. [PubMed] |
| 33. | Brent GA, Hershman JM. Thyroxine therapy in patients with severe nonthyroidal illnesses and low serum thyroxine concentration. J Clin Endocrinol Metab. 1986;63:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 202] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Pingitore A, Galli E, Barison A, Iervasi A, Scarlattini M, Nucci D, L’abbate A, Mariotti R, Iervasi G. Acute effects of triiodothyronine (T3) replacement therapy in patients with chronic heart failure and low-T3 syndrome: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2008;93:1351-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 35. | Lim VS, Tsalikian E, Flanigan MJ. Augmentation of protein degradation by L-triiodothyronine in uremia. Metabolism. 1989;38:1210-1215. [PubMed] |