Published online May 6, 2015. doi: 10.5527/wjn.v4.i2.245
Peer-review started: June 21, 2014
First decision: August 14, 2014
Revised: December 27, 2014
Accepted: January 15, 2015
Article in press: January 19, 2015
Published online: May 6, 2015
Processing time: 322 Days and 18.3 Hours
The aim of this study is to review four case-based scenarios regarding the treatment of symptomatic hypogonadism in men. The article is designed as a review of published literature. We conducted a PubMed literature search for the time period of 1989-2014, concentrating on 26 studies investigating the efficacy of various therapeutic options on semen analysis, pregnancy outcomes, time to recovery of spermatogenesis, as well as serum and intratesticular testosterone levels. Our results demonstrated that exogenous testosterone suppresses intratesticular testosterone production, which is an absolute prerequisite for normal spermatogenesis. Cessation of exogenous testosterone should be recommended for men desiring to maintain their fertility. Therapies that protect the testis involve human chorionic gonadotropin (hCG) therapy or selective estrogen receptor modulators (SERMs), but may also include low dose hCG with exogenous testosterone. Off-label use of SERMs, such as clomiphene citrate, are effective for maintaining testosterone production long-term and offer the convenience of representing a safe, oral therapy. At present, routine use of aromatase inhibitors is not recommended based on a lack of long-term data. We concluded that exogenous testosterone supplementation decreases sperm production. It was determined that clomiphene citrate is a safe and effective therapy for men who desire to maintain fertility. Although less frequently used in the general population, hCG therapy with or without testosterone supplementation represents an alternative treatment.
Core tip: Symptomatic hypogonadism is both a common and growing health issue. Our four case-based scenarios assess different treatment options for hypogonadotropic male hypogonadism such as clomiphene citrate, human chorionic gonadotropin, and anastrozole. Furthermore, we provide clinical recommendations that can help physicians when confronted with situations such as the ones presented in this article.
- Citation: Crosnoe-Shipley LE, Elkelany OO, Rahnema CD, Kim ED. Treatment of hypogonadotropic male hypogonadism: Case-based scenarios. World J Nephrol 2015; 4(2): 245-253
- URL: https://www.wjgnet.com/2220-6124/full/v4/i2/245.htm
- DOI: https://dx.doi.org/10.5527/wjn.v4.i2.245
According to a recent study by Mulligan et al[1], symptomatic hypogonadism affects approximately 40% of men aged 45 years or older. With the maturation of the Baby Boomer population, it is anticipated that there may be a significant increase in men desiring children at an older age. Testosterone therapies have been increasingly used in aging men, as well as men of reproductive age. A study by Samplaski et al[2] showed that 88.4% of men were azoospermic while on exogenous testosterone. In addition, this study demonstrated that cessation of therapy led to recovery of spermatogenesis in most infertile males. More startlingly, an estimated 6.5 million men in the United States will have hypogonadism by 2025[3]. Out of concern for this growing epidemic, this article will review four case-based scenarios concerning the treatment of hypogonadism in men. These case studies will include an assessment of the efficacy of potential treatment options as well as provide clinical recommendations for physicians.
In our first case-based scenario, a 38-year-old hypogonadal male patient has severely abnormal semen analyses as a result of use of testosterone therapy. His baseline Testosterone (T) level is 260 ng/dL. This male’s baseline semen analysis prior to T therapy is 50 million/mL at 60% motility and now presents with 8 million sperm/mL with motility of 40%. This patient has been on a topical T therapy for 1 year and his luteinizing hormone (LH) is 4 mIU/L and follicle-stimulating hormone (FSH) level is 4 mIU/mL. This male commonly presents with infertility after being treated with testosterone therapy for symptomatic hypogonadism. Severe oligozoospermia and azoospermia be seen. Often times, the prescribing physician has not asked the patient about interest in fertility or has expressed unawareness of the detrimental effects of testosterone therapy on spermatogenesis.
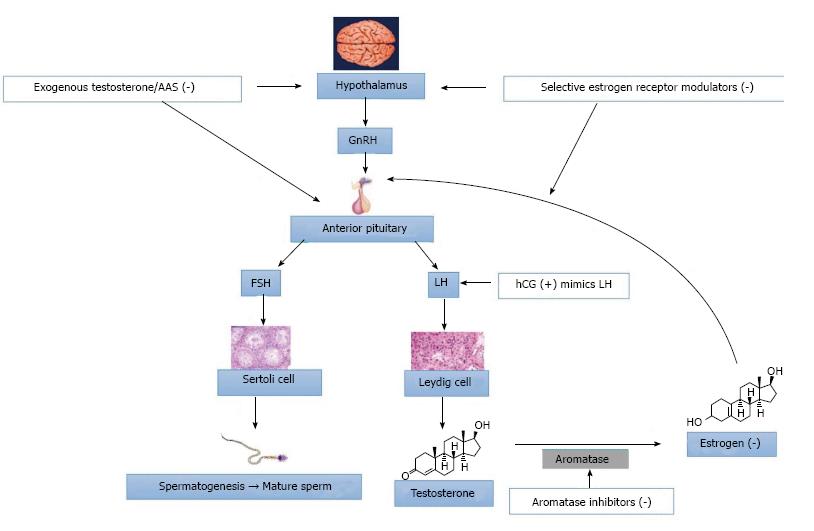
Exogenous testosterone is detrimental for spermatogenesis: Exogenous testosterone’s mechanism creates a negative feedback on the hypothalamic-pituitary axis. This effectively decreases the production of gonadotropin and gonadotropin-releasing hormone. Consequently, the secretion of FSH and LH are also inhibited. These impairments on hormones result in overall decreases in intratesticular testosterone levels (ITT) as well as testosterone production. Typically, ITT concentrations are roughly fifty to one hundred times serum levels. Exogenous testosterone treatment can suppress ITT production to such an extent that spermatogenesis can be dramatically compromised at ITT concentrations to less than 20 ng/mL[4]. ITT is an absolute requirement for normal spermatogenesis. Without ITT, one can develop azoospermia[5,6]. However, the rates of success in recovering spermatogenesis after use of exogenous T are generally quite favorable.
Contraceptive studies using testosterone demonstrate that spermatogenesis may return after cessation of testosterone therapy: In one investigation, Gu et al[7] from China administered 500 mg of testosterone undecanoate monthly for 30 mo. The study used a primary outcome of pregnancy rate. In more than 1500 person-years of exposure in the 24-mo efficacy phase, only nine pregnancies were reported (855 men) resulting in a failure rate of 1.1 per 100 men. Forty-three men (4.8%) did not attain azoospermia or severe oligozoospermia (< 1 × 106 sperm/mL). One hundred and eight days was the median time to the onset of azoospermia or severe oligozoospermia. Only two participants did not return to a normal fertility range of spermatogenesis. Spermatogenesis recovered at a median time of 196 d which was calculated from the beginning of the recovery phase. Recovery of sperm concentrations to baseline values was 182 d and to normal sperm output (> 20 × 106/mL) was 230 d. Most notably, all but 17 participants who completed the 12-mo recovery period returned to normal levels of spermatogenesis. Furthermore, 15 of the 17 patients who did not recover returned to normal reference levels after an additional 3-mo follow-up. Although this study had a follow-up period of 2.5 years, it is important to note that longer-term data are not available in the published literature.
Recovery of spermatogenesis may be prolonged for some men, and may vary based on ethnicity: In a separate investigation, Liu et al[8] performed an integrated, multivariate analysis of 30 studies published between 1990-2005, in which semen analyses were recorded each month until recovery to a threshold of 20 million/mL. One thousand five hundred and forty-nine healthy eugonadal men aged between 18 and 51 years were treated with either androgens or androgens plus progestagens. The strength of this large meta-analysis was > 1200 man-years of treatment and > 700 man-years of post-treatment recovery. It required median times of 3.4, 3.0 and 2.5 mo for sperm to recover to thresholds of 20, 10, and 3 million per mL, respectively. Shorter treatment duration, shorter-acting testosterone preparations, older age, higher sperm concentrations at baseline, Asian origin, faster suppression of spermatogenesis, and lower LH levels at baseline identified with higher rates of recovery. As this contraceptive trial was performed in men of Chinese ethnicity, similar trials with men of other ethnicities might not be reliable.
It should be advised that recovery of spermatogenesis might be prolonged for a small number of men, which may be of a larger concern with advanced maternal age. This study concluded that hormonal male contraceptive regimens demonstrate complete reversibility within an anticipated time course. However, the absence of pregnancy outcome data is a noteworthy limitation of the published literature. In addition, it is critical to stress that none of the literature measures time to fecundity and pregnancy outcomes do not correlate with semen analysis data.
Low-dose human chorionic gonadotropin maintains ITT in normal men with testosterone-induced hypogonadism: Low dose human chorionic gonadotropin (hCG) with intramuscular testosterone enanthate (200 mg/wk) can also maintain ITT and serum testosterone levels[9]. Some men are reluctant to stop testosterone therapy due to the symptomatic benefit, despite understanding the fertility risk. Use of hCG with testosterone may be a viable alternative for this select group of men. The use of hCG with intramuscular testosterone was initially studied for the development of a male contraceptive agent. Coviello et al[9] administered low doses of hCG (0, 125, 250, or 500 IU every other day) to normal men during this 3 wk study and measured serum and ITT levels. While the administration of testosterone alone resulted in profound decreases in ITT concentrations (94% from baseline in the TE and placebo hCG group), the addition of low dose hCG resulted in maintenance of the ITT levels. Although serum T increased from baseline in all groups, ITT remained significantly higher than serum T in all four groups after treatment. Despite supraphysiologic doses of exogenous testosterone, high levels of ITT can be maintained with the low-dose hCG.
Prevention of azoospermia and maintenance of fertility in hypogonadal men on TRT with low dose hCG: Hsieh et al[10] also studied the effect of hCG administration with testosterone replacement therapy on spermatogenesis. In this small series, ten men received short-acting testosterone preparations in addition to low doses of hCG. The key finding of this study was that spermatogenesis was maintained. Although there was a relatively small decrease in sperm density, no men became azoospermic.
Discontinuing testosterone therapy alone may be adequate to return spermatogenesis to baseline levels as suggested by the hormonal contraception trials. However, men studied in these contraceptive trials most commonly had normal baseline T levels and semen analyses, and may not reflect men presenting with low T or infertility. In our experience symptomatic hypogonadal men may often be reluctant as their symptoms return. These men would especially benefit from medical therapy.
The second case-based scenario is one of a 33-year-old subfertile male who has been treated with testosterone therapy to improve fertility potential. He had a sperm concentration of 12 million sperm/mL and motility of 45% prior to T therapy. His baseline T level was 400 ng/dL. His baseline serum T level was 270 ng/dL (normal is 300-800 ng/dL). The LH level was low normal at 3 mIU/L and the serum FSH was 5 mIU/mL. indicative of mid-normal range. Upon presentation, this male has seen his physician for infertility and has been found to have impaired semen quality. His physician prescribed an intramuscular T preparation 6 mo ago, not recognized the potential for a detrimental effect on spermatogenesis. At present, his sperm density is 2 million sperm/mL and the motility is 40%. The serum T level on therapy is 600 ng/dL. Upon physical examination, the testes are normal in size and there is no sign of varicocele. Otherwise, this patient is healthy and does not use any illicit drugs. His prolactin and estradiol was normal. This gentleman has an abnormal semen analysis and low T with an unspecified cause.
Testosterone therapy does not improve spermatogenesis and should not used by men of reproductive age: This practice is not unusual. Ko et al[11] conducted a recent survey of United States urologists. The survey observed that up to 25% of these urologists have used testosterone therapy in an effort to improve spermatogenesis. However, this thought process is incorrect. As discussed before, testosterone therapy results in a mechanism that impairs spermatogenesis. Furthermore, in a recent study, testosterone use has increased greatly from 2000 to 2011 in the United States, specifically since 2008. This can be attributed to novel types of testosterone therapy, a greater awareness of symptoms associated with below normal testosterone levels, and consumer marketing by pharmaceutical companies. This investigation revealed that 12.4% of men in the United States who began testosterone therapy were between 18 and 39 years of age with 74% being between 40 and 64 years of age[12]. This is of upmost concern since it suggests that testosterone is being given to men that could be in their reproductive years.
Clomiphene citrate may improve serum testosterone levels: Clomiphene citrate (CC) can be a fairly effective treatment option in increasing serum testosterone levels[13,14]. Clomiphene is a selective estrogen receptor modulator (SERM)[15]. This class of medications competitively binds to estrogen receptors on the hypothalamus and the pituitary gland (Figure 1). Accordingly, the pituitary gland recognizes less estrogen and creates more LH, which escalates overall testosterone production by the testes. CC is administrated orally with a common dosing that starts at 25 mg every other day with an upward titration to 50 mg daily, as needed. When LH and FSH levels are already high, it is not as effective in raising serum testosterone levels. Currently, use of hormonal dynamic testings, such as clomiphene or hCG stimulation testing, are not well-defined or commonly used. Potential side effects include gynecomastia, hypertension, cataracts, weight gain, and acne.
Clomiphene citrate as a treatment option for patients with hypogonadism: In a recent study by Da Ros et al[16], clomiphene citrate was tested for effectiveness in restoring endogenous testosterone production. In these trials, 125 men with an average age of 62 years were given clomiphene citrate (25 mg daily). Before treatment, all men had either below normal or low normal testosterone levels. Moreover, all patients complained about decreases in libido. The average follow up was 6 mo. Post-treatment testosterone levels increased by an average of 115%. The study concluded that clomiphene citrate should be considered as a therapy for male patients with hypogonadism.
Clomiphene citrate is an effective and less expensive treatment option: Taylor et al[17] conducted a study in which CC gave rise to significant increases in testosterone levels from baseline values. This was similar to increases made in testosterone gel replacement therapy (TGRT). One hundred and four men began CC (50 mg every other day) or TGRT (5 gm of 1% gel). The average follow up was 23 mo for CC or 46 mo for TGRT. Average post-treatment testosterone levels were 573 ng/dL (average baseline 277 ng/dL) in the CC group and 553 ng/dL (average baseline 221 ng/dL) in the TGRT group. The authors observed that the cost per month of CC was about $190 less than the cost of Testim® 1% (5 gm daily) at $270 and Androgel® 1% (5 gm daily) at $265. Compared with TGRT, CC demonstrates a less expensive option for men with hypogonadism, representing efficacy with minor side effects.
Long-term use of Clomiphene citrate is a safe way to improve serum testosterone levels: A similar study on clomiphene was performed by Moskovic et al[18] where forty-six hypogonadal males with an average age of 44 years were treated with clomiphene citrate for more than 12 mo. The main outcome measures were long-term results of clomiphene treatment on hypogonadal males and predictors of response. Average baseline serum T levels were 228 ng/dL. Post-treatment serum T levels were 612 ng/dL, 562 ng/dL, and 582 ng/dL after 1, 2, and 3 years, respectively. Patients were also given the Androgen Deficiency in Aging Males (ADAM) questionnaire. The average pre-treatment ADAM score was 7 as compared to an ADAM score of 3 after 1 year of treatment. This investigation concluded that CC is effective as a long-term therapy for men with symptomatic hypogonadism. In addition, CC can improve many of the ADAM symptoms.
In an earlier study from the same institution, Katz et al[3] concluded that long-term use of CC improved serum testosterone levels to normal in a safe and effective manner. In this analysis, eighty-six men between 22 and 37 years old with hypogonadism (T levels < 300 ng/dL) were assessed and treated for an average of 19 mo. The participating men started with 25 mg of CC every other day. They were then titrated to 50 mg every other day. 550 ng/dL was the goal testosterone level. Once preferred testosterone levels were reached, testosterone/onadotropin levels were measured biannually. With regards to questions on the Androgen Deficiency in Aging Males (ADAM) questionnaire, advances were noted in each area excluding loss of height. Five of the ten variables saw significant improvement including feeling sad/grumpy, lack of energy, decreased life enjoyment, decreased libido, and decreased sports performance. This study demonstrates that CC is both an effective and safe testosterone therapy substitute in hypogonadal men.
A randomized, prospective trial of CC for men with hypogonadism with normal semen parameters is vital to confirm the recommendation for the use of SERMs for fertility preservation. It is essential that this study show that semen profiles are not negatively affected. A purified androgenic isomer of generic clomiphene is presently completing phase III clinical trials in the United States[19]. The anticipated patient is the overweight, hypogonadal male interested in maintaining fertility potential.
Oral enclomiphene citrate initiates production of serum testosterone and sperm in men with low testosterone: Androxal®, or enclomiphene citrate, is the trans-isomer of clomiphene citrate[20]. Enclomiphene citrate is currently completing phase III clinical trials in the United States and may in the future be another alternative treatment to testosterone therapies. In a randomized study by Kaminetsky et al[21], the investigators compared levels of testosterone, FSH, and LH after hypogonadal males used either oral enclomiphene citrate or testosterone gel. Twelve male subjects were assigned to either of the two treatments. At baseline, the average testosterone level for all patients was 165 ± 66 pg/dL. Treatment with both enclomiphene and testosterone gel raised serum testosterone levels back to the normal range. Both groups had about the same serum T levels after 3 mo and 6 mo. After 6 mo, serum T levels were 525 ± 256 pg/dL for enclomiphene and 545 ± 268 pg/dL for testosterone gel. The distinguishing factors between these two treatments are their FSH and LH levels as well as their sperm counts. Only enclomiphene citrate was associated with rises in FSH and LH as well as sperm counts. All of the enclomiphene citrate subjects had sperm counts above 75 million/mL, with an average sperm count of 176 million/mL. In contrast, the testosterone gel subjects did not surpass sperm counts of more than 12 million/mL. These findings were also evident throughout the follow-up period. This study suggests that enclomiphene citrate may prove to be a superior treatment as it is effective in increasing testosterone as well as sperm counts. The rise in FSH and LH levels could also point towards a shift back to normal endogenous testosterone production.
Wiehle et al[22] carried out another study for enclomiphene citrate. This randomized study also compared the effects of enclomiphene (Androxal®) vs AndroGel®, a transdermal testosterone. Enclomiphene citrate was given in three different doses: 6.25 mg, 12.5 mg and 25 mg Androxal®. Forty-four men with testosterone levels less than 350 ng/dL at baseline were included in the study. Their average age was 53 years. After six weeks of treatment, patients who took 25 mg enclomiphene had an average testosterone level of 604 ± 160 ng/dL while patients on the transdermal testosterone had an average testosterone level of 500 ± 278 ng/dL. While these results were almost equivalent, AndroGel® patients saw a decrease in FSH and LH levels whereas enclomiphene patients saw an increase. These outcomes correlate with the results of the aforementioned study. This study concluded that enclomiphene citrate was capable of increasing serum T and LH levels.
Repros Therapeutics Inc[19] observed the effect of 12 d of use of clomiphene citrate, enclomiphene, and zuclomiphene in baboons. All of the animal subjects were administered 1.5 mg of one treatment per day. Zuclomiphene did have much of a significant effect on increasing testosterone levels from baseline levels of 170 ng/dL. Enclomiphene had a much greater effect (8-fold increase to 1144 ng/dL) than clomiphene citrate (5-fold increase to 559 ng/dL). However, neither clomiphene nor enclomiphene demonstrated any effect on FSH or LH levels. This could be due to a flaw in the study.
Similar to the first case study, testosterone (T) therapy should be stopped, and treatment with clomiphene should begin. Cessation of T therapy should be the first treatment concern for nearly all men who are interested in preserving their fertility. Longer durations of T therapy are likely to have more significant effect on the return of testosterone but undoubtedly the amount of T would be expected to have an effect on return of spermatogenesis. Clomiphene would only be expected to benefit men with secondary hypogonadism based on its mechanism of action. It is important to assess serum LH levels prior to therapy to determine that these levels are low or normal.
In the third case-based scenario, a 42-year-old male patient with symptomatic hypogonadism has a desire to father children at an unspecified future time. Upon presentation, this male has symptomatic hypogonadism without a specific underlying cause. While he knows he wants to have children in the future, he does not have a clear idea regarding timeframe. He is not married and does not have any children. This male’s baseline T is 220 ng/dL. His LH is 4 mIU/L and FSH level is 4 mIU/mL. Semen analysis is 26 million sperm/mL with motility of 70%. He is healthy, has a normal physical exam and is currently not on any therapy.
Clomiphene citrate results in similar satisfaction and efficacy to testosterone therapy: There has been concern that clomiphene citrate may not result in as much symptomatic improvement compared to testosterone therapies. There are no prospective, controlled trials to confirm or refute this concern. In a recent retrospective, age-matched comparison, Ramasamy et al[23] assessed their results using the ADAM questionnaire and serum T levels in 31 men on topical testosterone, 31 men on injectable testosterone and 31 men on clomiphene. Clomiphene-treated men had similar total testosterone levels to topical testosterone-treated males. Men on injectable testosterone had the highest serum T levels. Similar ADAM questionnaire satisfaction was noted between treatment groups. The authors concluded that testosterone supplementation regimens and clomiphene citrate are efficacious for improving serum total testosterone. No difference in overall hypogonadal symptoms was noted among men on any testosterone supplementation therapy. Despite lower serum total testosterone, men on clomiphene citrate and testosterone gels reported satisfaction similar to that of men treated with testosterone injections.
Exogenous hCG increases serum testosterone levels thus increasing ITT concentrations: Although most men taking testosterone for contraceptive use trials recover their baseline spermatogenesis, this recovery could take up to 18 mo and may not always happen. Use of clomiphene is generally effective, but off-label. High quality safety studies of greater than 18 to 24 mo of use are lacking. Another treatment option could be hCG.
hCG is an LH analog that stimulates Leydig cell to produce more testosterone. hCG stimulates testosterone production by the Leydig cells by functioning as an LH analogue. hCG can be extracted from urine as well as other recombinant sources. Exogenous hCG increases serum testosterone levels and ITT concentrations. hCG alone can only maintain spermatogenesis for a short period of time. In a small case series directed by Depenbusch et al[24], thirteen azoospermic men with hypogonadotropic hypogonadism were initially administered hCG and human menopausal gonadotropin (hMG) to induce spermatogenesis. hCG was then administered 500-2500 IU hCG subcutaneously biweekly alone for up to two years (range 3-24 mo). After 12 mo of treatment, sperm counts decreased gradually but remained present in all patients, except for one who became azoospermic. The declining sperm counts demonstrate that FSH is crucial for the continuation of normal spermatogenesis.
Low levels of hCG increase ITT concentrations and serum testosterone levels: Treating patients with a high dose of hCG is not necessary with regards to the upkeep of spermatogenesis. In a study conducted by Roth et al[25], 37 normal patients became experimentally gonadotropin deficient through the use of GnRH antagonists. The patients were then randomized and treated with 0, 15, 60, or 125 IU SC hCG every other day or 7.5 g testosterone gel daily for a duration of 10 d. Steroid measurements at baseline and endpoint were taken after obtaining testicular fluid by percutaneous aspiration. ITT concentrations increased proportionally to the dose from 77 nmol/L to 923 nmol/L in the 0- and 125-IU treatment groups, respectively (P < 0.001). Furthermore, significant correlation (P < 0.01) existed between serum hCG and both ITT and serum T levels. The study established that low dose hCG treatment dose-dependently increases ITT concentrations in normal men brought about with gonadotropin deficiency. Even though hCG can be advantageous in increasing serum T levels, this treatment can be very costly. In addition, the fact that this medication is applied through an injection can deter potential users.
Aromatase inhibitors as a treatment option: Another therapeutic target is the aromatase enzyme, which converts testosterone to estradiol. It is mainly located in the testes, brain, adipose tissue, and liver. Estradiol inhibits the secretion of gonadotropins, which may affect the production of ITT. The purpose of aromatase inhibitors is to inhibit the process of converting androgens to estrogen, effectively increasing T, LH, and FSH levels and having effects similar to those of anti-estrogens. This type of treatment has been employed to stimulate spermatogenesis and bring about improvements to male fertility. Aromatase inhibitors may be more beneficial than antiestrogens in male patients with low serum T to estradiol ratios (< 10) and who are obese.
Typically, aromatase inhibitors have been classified into two types: steroidal or non-steroidal. Anastrozole and letrozole are examples of non-steroidal aromatase inhibitors. Typically, adrenal steroid supplementation is not required. Although aromatase inhibition by these two medications is almost 100%, their administration does not completely suppress estradiol levels in men and actually decreases the plasma T to estradiol ratio by 77%. This incomplete suppression may be linked to the high levels of circulating testosterone in men and may provide an advantage by limiting the adverse side effect profile.
Men with conditions including idiopathic male infertility, primarily men with lower serum testosterone to estradiol ratios (< 10), and men with hypogonadism, often related to obesity have been treated with aromatase inhibitors. They have also been used in men with Klinefelter’s syndrome in order to stabilize serum T levels prior to testicular sperm extraction.
Testolactone or anastrozole may increase sperm quality and concentrations: Raman et al[26] conducted a study in order to detect a difference between treatment with testolactone or anastrozole. The male patients in this study had T/E2 ratios that were less than 10. The patients were treated with either testolactone or anastrozole. Both treatments resulted in significant improvements in sperm concentrations, morphology, motility, and T/E2 ratios. With regards to the small group of 25 infertile, oligospermic men treated with anastrozole, sperm concentration increased substantially from 5.5 to 15.6 million per mL, and the total motile sperm concentration per ejaculate increased from 833 to 2931 million (P < 0.005). The study reported no changes in the azoospermic group treated with anastrozole. In addition, pregnancy rates were not reported for any of the patients regardless of improvements in the semen parameter.
Options for therapy include long-term CC with or without drug holidays, testosterone with low dose hCG, testosterone with or without drug holidays, or alternating combinations of the prior options (Table 1). Katz et al[3] conducted a study demonstrating the effectiveness of treating 86 hypogonadal men with low-dose clomiphene over the course of 19 mo. No major side effects were reported and improvements were made in more than three items on the ADAM questionnaire for 60% of the patients. Long term studies with anastrozole are lacking.
| Name of medication | Dosing and administration | Side effects | Anticipated results |
| Clomiphene | Oral: 25 mg every other day with max of 50 mg daily | Gynecomastia, weight gain, hypertension, cataracts, and acne | Recovery of spermatogenetic function, increases in testosterone levels |
| hCG | Intramuscular injection; 125-500 IU every other day | Headache, restlessness, tiredness, swelling of the ankles/feet, mental/mood changes, pain/swelling of the breast | Recovery of spermatogenetic function, increases in testosterone levels |
| hMG | Intramuscular injection; 75 IU three times a week | Gynecomastia, dizziness, fainting, headache, loss of appetite, irregular heartbeat | Recovery of spermatogenetic function |
| Anastrozole | Oral; either 0.5 or 1 mg | Blood pressure increase, anorexia, malaise, rash, peripheral edema, aches glossitis, paresthesias vomiting/nausea | Improvements in T/E2 ratio, increases in sperm concentration |
Lastly, the fourth case-based scenario is centered on a 30-year-old male with symptomatic hypogonadism due to chronic anabolic steroid abuse. He completed 3 cycles of nandrolone over the last two years. These men are typically using anabolic steroids to improve muscle mass and body image. Long-term use in cycles are commonly observed. Sophisticated hormonal regimens that are self-prescribed have evolved. Severe impairments in spermatogenesis may be seen and may be permanent depending on the duration and potency of agents used. He has been off anabolic steroids for 3 mo. At present time his T level is 170 ng/dL and his LH and FSH are low at 1.5 (UNITS). His semen analysis is 1 million sperm/mL with 10% motility. Upon physical examination, the testes have mild atrophy and his overall physique is still muscular.
Chronic anabolic steroid use is detrimental to spermatogenesis: Anabolic-androgenic steroids use is currently widespread as they are now readily available in over-the-counter medicines. According to recent evidence, illegal use of anabolic steroids may be the most prevalent cause of symptomatic hypogonadism in young men[27]. Other side effects of anabolic steroid use include hepatotoxicity, cholestasis, renal failure, gynecomastia, and infertility[28]. Furthermore, a meta-analysis study comprised of 197 studies was done on the epidemiology of anabolic steroids use[29]. The study resulted in a global lifetime prevalence rate of 3.3%. This data demonstrates the prevalence of this problem, which is connected to symptomatic hypogonadism.
Men using anabolic steroids may be deficient in ITT concentrations required to sustain spermatogenesis even though they may have serum androgen concentrations that are between normal and high. Many males who use anabolic steroids acquire hypogonadotropic hypogonadism along with an ensuing atrophy of the testes. The user of anabolic steroids often has azoospermia or oligozoospermia as well as irregularities of sperm morphology and motility[30,31]. For men with hypogonadotropic hypogonadism from anabolic steroid abuse, administration of intramuscular injections of hCG at doses of 2000 to 3000 units 2 to 3 times per week for 4 mo can initiate spermatogenesis[32,33].
Cessation of anabolic steroid abuse may reverse suppression of spermatogenesis: Mills et al[34] tested the recovery of spermatogenesis after exogenous testosterone administration in 26 men with a recent history of anabolic steroid use. In this relatively small study, all men discontinued exogenous testosterone usage and began treatment with human chorionic gonadotropin (hCG) 3000 units IM every other day. The treatment lasted for at least 3 mo. In regards to the two men who remained azoospermic, one had insufficient follow-up and the other was suspected of continued anabolic steroid use. Men who were using intramuscular testosterone (hCG) at the time of presentation recovered spermatogenesis in an average of 3.1 mo. However, men receiving transdermal testosterone supplementation at the time of presentation took an average of 7.4 mo to recover. Mills et al[34] concluded that impairment of fertility following testosterone replacement therapy suppression is reversible and that the rate of sperm may be related to the delivery system.
hCG with or without human menopausal gonadotropin has been used most commonly to restore fertility. hCG may be considered, but it requires frequent injections and can produce side effects (Table 1). Occasionally, hCG can exacerbate depression and irritability in hypogonadal men. Cessation of the anabolic steroids with use of clomiphene may be the most beneficial. Non-responders to hCG will require the addition of human menopausal gonadotropin with a daily injection of 75 IU[34].
Men wishing for future fertility should refrain from utilizing exogenous testosterone due to the potential for long-term detrimental effects on spermatogenesis. In a minority of cases, spermatogenesis is not recovered, although it is difficult to say whether this is due to testosterone treatment or to the natural evolution of the condition. Clomiphene citrate, an oral selective estrogen receptor modulator, is an off-label yet innocuous and potent therapy for men who wish to retain future potential fertility. hCG therapy, although less used, with or without testosterone supplementation represents an alternative treatment. Currently, it is not recommended to repeatedly use aromatase inhibitors due to a paucity of long-standing data.
P- Reviewer: Grossberg G, Mas M, Rey R, Yu HP S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
| 1. | Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60:762-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 485] [Cited by in RCA: 492] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 2. | Samplaski MK, Loai Y, Wong K, Lo KC, Grober ED, Jarvi KA. Testosterone use in the male infertility population: prescribing patterns and effects on semen and hormonal parameters. Fertil Steril. 2014;101:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 3. | Katz DJ, Nabulsi O, Tal R, Mulhall JP. Outcomes of clomiphene citrate treatment in young hypogonadal men. BJU Int. 2012;110:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Zirkin BR, Santulli R, Awoniyi CA, Ewing LL. Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology. 1989;124:3043-3049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 178] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | McLachlan RI, O’Donnell L, Meachem SJ, Stanton PG, de K, Pratis K, Robertson DM. Hormonal regulation of spermatogenesis in primates and man: insights for development of the male hormonal contraceptive. J Androl. 2002;23:149-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 6. | Weinbauer GF, Nieschlag E. Gonadotrophin-releasing hormone analogue-induced manipulation of testicular function in the monkey. Hum Reprod. 1993;8 Suppl 2:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Gu Y, Liang X, Wu W, Liu M, Song S, Cheng L, Bo L, Xiong C, Wang X, Liu X. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J Clin Endocrinol Metab. 2009;94:1910-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Liu PY, Swerdloff RS, Christenson PD, Handelsman DJ, Wang C. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. Lancet. 2006;367:1412-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 168] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Coviello AD, Matsumoto AM, Bremner WJ, Herbst KL, Amory JK, Anawalt BD, Sutton PR, Wright WW, Brown TR, Yan X. Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression. J Clin Endocrinol Metab. 2005;90:2595-2602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Hsieh TC, Pastuszak AW, Hwang K, Lipshultz LI. Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy. J Urol. 2013;189:647-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Ko EY, Siddiqi K, Brannigan RE, Sabanegh ES. Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. J Urol. 2012;187:973-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Layton JB, Li D, Meier CR, Sharpless JL, Stürmer T, Jick SS, Brookhart MA. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99:835-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 13. | Tenover JS, Bremner WJ. The effects of normal aging on the response of the pituitary-gonadal axis to chronic clomiphene administration in men. J Androl. 1991;12:258-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Guay AT, Bansal S, Heatley GJ. Effect of raising endogenous testosterone levels in impotent men with secondary hypogonadism: double blind placebo-controlled trial with clomiphene citrate. J Clin Endocrinol Metab. 1995;80:3546-3552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Shelly W, Draper MW, Krishnan V, Wong M, Jaffe RB. Selective estrogen receptor modulators: an update on recent clinical findings. Obstet Gynecol Surv. 2008;63:163-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Da Ros CT, Averbeck MA. Twenty-five milligrams of clomiphene citrate presents positive effect on treatment of male testosterone deficiency - a prospective study. Int Braz J Urol. 2012;38:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Taylor F, Levine L. Clomiphene citrate and testosterone gel replacement therapy for male hypogonadism: efficacy and treatment cost. J Sex Med. 2010;7:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Moskovic DJ, Katz DJ, Akhavan A, Park K, Mulhall JP. Clomiphene citrate is safe and effective for long-term management of hypogonadism. BJU Int. 2012;110:1524-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Repros Therapeutics Inc. A Randomized, Double Blind, Placebo-Controlled, Multi-Center Phase III Study in Men With Acquired Hypogonadotropic Hypogonadism to Compare Changes in Testosterone and Sperm Concentration Following Treatment With 12.5 mg or 25 mg Androxal or AndroGel 1.62%. Bethesda (MD): National Library of Medicine (US). Available from: http: //clinicaltrials.gov/show/NCT01993225 NLM Identifier: NCT01993225. |
| 20. | Kaminetsky J, Hemani ML. Clomiphene citrate and enclomiphene for the treatment of hypogonadal androgen deficiency. Expert Opin Investig Drugs. 2009;18:1947-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Kaminetsky J, Werner M, Fontenot G, Wiehle RD. Oral enclomiphene citrate stimulates the endogenous production of testosterone and sperm counts in men with low testosterone: comparison with testosterone gel. J Sex Med. 2013;10:1628-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 22. | Wiehle R, Cunningham GR, Pitteloud N, Wike J, Hsu K, Fontenot GK, Rosner M, Dwyer A, Podolski J. Testosterone Restoration by Enclomiphene Citrate in Men with Secondary Hypogonadism: Pharmacodynamics and Pharmacokinetics. BJU Int. 2013;Jul 12; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Ramasamy R, Scovell JM, Kovac JR, Lipshultz LI. Testosterone supplementation versus clomiphene citrate for hypogonadism: an age matched comparison of satisfaction and efficacy. J Urol. 2014;192:875-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Depenbusch M, von Eckardstein S, Simoni M, Nieschlag E. Maintenance of spermatogenesis in hypogonadotropic hypogonadal men with human chorionic gonadotropin alone. Eur J Endocrinol. 2002;147:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Roth MY, Page ST, Lin K, Anawalt BD, Matsumoto AM, Snyder CN, Marck BT, Bremner WJ, Amory JK. Dose-dependent increase in intratesticular testosterone by very low-dose human chorionic gonadotropin in normal men with experimental gonadotropin deficiency. J Clin Endocrinol Metab. 2010;95:3806-3813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Raman JD, Schlegel PN. Aromatase inhibitors for male infertility. J Urol. 2002;167:624-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 148] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Coward RM, Rajanahally S, Kovac JR, Smith RP, Pastuszak AW, Lipshultz LI. Anabolic steroid induced hypogonadism in young men. J Urol. 2013;190:2200-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Rahnema CD, Lipshultz LI, Crosnoe LE, Kovac JR, Kim ED. Anabolic steroid-induced hypogonadism: diagnosis and treatment. Fertil Steril. 2014;101:1271-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 29. | Sagoe D, Molde H, Andreassen CS, Torsheim T, Pallesen S. The global epidemiology of anabolic-androgenic steroid use: a meta-analysis and meta-regression analysis. Ann Epidemiol. 2014;24:383-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 359] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 30. | Basaria S. Androgen abuse in athletes: detection and consequences. J Clin Endocrinol Metab. 2010;95:1533-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 31. | Fronczak CM, Kim ED, Barqawi AB. The insults of illicit drug use on male fertility. J Androl. 2012;33:515-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 32. | Turek PJ, Williams RH, Gilbaugh JH, Lipshultz LI. The reversibility of anabolic steroid-induced azoospermia. J Urol. 1995;153:1628-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Menon DK. Successful treatment of anabolic steroid-induced azoospermia with human chorionic gonadotropin and human menopausal gonadotropin. Fertil Steril. 2003;79 Suppl 3:1659-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Mills JN, Grober ED, Khera M, Fenig DM, Sathymoorthy K, Lipshultz LI. Recovery of Spermatogenesis After Exogenous Testosterone Administration. J Urol. 2008;179:656-657. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |