Published online Aug 6, 2014. doi: 10.5527/wjn.v3.i3.107
Revised: June 25, 2014
Accepted: July 27, 2014
Published online: August 6, 2014
Processing time: 198 Days and 9.3 Hours
AIM: To study the long-term outcome of ketoconazole and tacrolimus combination in kidney transplant recipients.
METHODS: From 2006 to 2010, ketoconazole was given in 199 patients and was continued for at least 1 year or until graft failure (Group 1), while 149 patients did not receive any ketoconazole (Group 2). A combination of tacrolimus, mycophenolate and steroid was used as maintenance therapy. High risk patients received basiliximab induction.
RESULTS: Basic demographic data was similar between the 2 groups. The 5-year cumulative incidence of biopsy-confirmed and clinically-treated acute rejection was significantly higher in Group 1 than in Group 2 (34% vs 18%, P = 0.01). The 5-year Kaplan-Meier estimated graft survival (74.3% vs 76.4%, P = 0.58) and patient survival (87.8% vs 87.5%, P = 0.93) were not different between the 2 groups. Multivariable analyses identified ketoconazole usage as an independent risk of acute rejection (HR = 2.33, 95%CI: 1.33-4.07; P = 0.003) while tacrolimus dose in the 2nd month was protective (HR = 0.89, 95%CI: 0.75-0.96; P = 0.041).
CONCLUSION: Co-administration of ketoconazole and tacrolimus is associated with significantly higher incidence of acute rejection in kidney transplant recipients.
Core tip: Tacrolimus is mainly metabolized by cytochrome P450 enzymes and ketoconazole is a potent inhibitor of P450. Transplant programs often use ketoconazole to reduce the tacrolimus dose and financial cost. Small short-term studies had previously supported such practice, but the long-term outcome are still lacking. We hereby report our center’s experience of this combination in kidney transplant recipients. Our study suggests that co-administration of ketoconazole and tacrolimus is associated with significantly higher incidence of acute rejection in kidney transplant recipients.
- Citation: Khan E, Killackey M, Kumbala D, LaGuardia H, Liu YJ, Qin HZ, Alper B, Paramesh A, Buell J, Zhang R. Long-term outcome of ketoconazole and tacrolimus co-administration in kidney transplant patients. World J Nephrol 2014; 3(3): 107-113
- URL: https://www.wjgnet.com/2220-6124/full/v3/i3/107.htm
- DOI: https://dx.doi.org/10.5527/wjn.v3.i3.107
Tacrolimus is a macrolide antibiotic produced from Streptomyces tsukubaensis. It binds to FK506-binding protein to form a complex that inhibits calcineurin phosphatase in Tlymphocytes [commonly referred as calcineurin inhibitor (CNI)]. Tacrolimus has been widely used as the primary immunosuppressive agent in kidney transplant patients[1]. Due to its narrow therapeutic index, optimal dosing with therapeutic monitoring is necessary. Tacrolimus is mainly metabolized by cytochrome P450-3A in the liver and a substrate of P-glycoprotein, an efflux pump, in both liver and intestine[2,3]. The inhibition of P450-3A and P-glycoprotein can slow down its metabolism and ketoconazole is such a potent inhibitor. Transplant programs often use ketoconazole to reduce the tacrolimus dose and cost. The financial benefit and short-term safety of such a practice had been reported previously by a few small studies in Egypt[4,5]. But the long-term outcomes of such a practice are still lacking.
Our interest in examining this issue comes from our participation in the National Institutes of Health funded pilot study of solid organ transplants in human immunodeficiency virus (HIV) infected patients[6-8]. There were unexpectedly higher incidences of acute rejection (by a factor of 2 to 3) in both kidney and liver recipients. About half of the rejections were early and aggressive[6,7]. In these patients, the targeted trough levels of CNIs were consistent with those in non-HIV recipients, but their required doses of CNI were significantly lower due to the necessary treatment with anti-retroviral protease inhibitor, which also inhibits P450-3A and P-glycoprotein. Due to the altered pharmacokinetics, their total exposure to CNI [area under curve (AUC)] likely was considerably low. This may be more plausible than other proposed viral and/or immunological factors to explain the higher-than-expected rejection rates in HIV infected patients[9-11]. In the current study, we analyze the long-term outcome of patients who received co-administration of ketoconazole and tacrolimus in non-HIV infected kidney transplant recipients.
This is a retrospective review, and patients were identified using the transplant center database at Tulane University Hospital and Clinic. During the period between 2006 and 2010, there were consecutive 450 non-HIV infected adult patients who underwent a primary kidney transplant at our center. Among them, ketoconazole was given in 199 patients after transplant surgery and was continued for at least 1 year or until graft failure (Group 1), while 149 patients did not receive any ketoconazole (Group 2). A combination of tacrolimus, mycophenolate and steroid was used as maintenance therapy in all patients. Factors for excluding patients from this study included primary graft non-function (n = 3), death in first week of transplant surgery (n = 2), incomplete data due to lost fellow-up (n = 19), different combination of maintenance immunosuppressives (n = 48) or usage of other drug that inhibits (such as diltiazem, amiodarone, etc.) or reduces (such as phenytoin, isoniazid, etc.) P450 enzymes (n = 9). The patients, whose ketoconazole was stopped before 1 year of kidney transplant (n = 17) or before the graft failure (n = 4) were also excluded from analysis. All patients were followed up closely at our center, including scheduled routine labs and clinic visits. Patients with acute illness were usually directly admitted or transferred to our center, and the outside events were recorded in a timely manner.
High risk patients, defined as having peak panel reactive antibody > 10%, human leukocyte antigen mismatch > 4, donor cold ischemia time > 18 h or expanded criteria donor kidneys, received induction therapy with 2 doses of basiliximab (20 mg per dose). Corticosteroids were administrated as methylprednisolone 500 mg IV intraoperatively, then tapered on postoperative days 1 to 3, and changed to oral prednisone 60 mg on postoperative day 4. The patients typically continued oral prednisone 20 mg daily for the first month, then 10 mg daily for the 2nd month and 5 mg daily thereafter. Each patient was started mycophenolate, either mycophenolate mofetil at 1 g or enteric-coated sodium mycophenolate at 720 mg, twice daily after transplant. Oral tacrolimus was started immediately after transplant, and doses were adjusted to keep the 12-h trough levels at 8 to 12 ng/mL for the first year. The target of tacrolimus trough levels was then maintained at 4 to 6 ng/mL after the first year. Whole blood tacrolimus concentrations were measured with liquid chromatography-tandem mass spectrometry in our hospital. The decision of adding ketoconazole was typically made within the first week of transplant surgery in order for patients to achieve the targeted trough levels prior to discharge. The dose of ketoconazole was started at100mg per day in all patients, and further increased to 200 mg per day in 4 patients.
All patients received one tablet of sulfamethoxazole/trimethoprim DS three times per week for the first year as prophylaxis for pneumocystis pneumonia and bacterial infections. Three-month antifungal prophylaxis with oral nystatin was given to patients who did not receive ketoconazole. Cytomegalovirus (CMV) prophylaxis was given to CMV seronegative recipients who received organs from a CMV seropositive donor. The regimen included IV ganciclovir during the transplant hospitalization followed by oral ganciclovir or valganciclovir for 3 mo.
Acute rejection was presumed when patients had a sudden increase of serum creatinine that could not be explained by other clinical causes. Kidney biopsy was performed before the treatment. The severity of rejection was defined according to Banff criteria. Acute cellular rejection of grade 1 or below was initially treated with IV methylprednisolone for 3 d. Thymoglobulin was used for steroid resistant cellular rejection, or as the initial therapy for rejection of Banff grade 2 or higher. Acute antibody mediated rejection (AMR) was diagnosed with positive C4d staining in the peritubular capillaries and/or demonstration of donor specific antibody. AMR was treated with a course of 5 to 7 daily plasmapheresis and intravenous immunoglobulin (IVIG) (150 mg/kg) in addition to IV methylprednisolone and thymoglobulin.
The outcome measures included: (1) incidence of biopsy-confirmed and clinically-treated acute rejection; (2) patient and kidney graft survival; (3) quality of graft function; and (4) incidence of clinically treated infections. Statistical analyses were performed using Statistics Analysis System (SAS) version 9.3 software (SAS Institute Inc, Cary, NC, United Statas). Chi-squared or Fisher exact test was used for count data, t test was used for continuous measures. Product-limit estimates of survival curves were generated by the Kaplan-Meier method and the survival difference was analyzed by log-rank test. Multivariable logistic regression analysis with a stepwise variable selection was used for examining risk factors of acute rejection. A P value < 0.05 was considered statistically significant. If there was no data at or around the particular time point, the previous or next available measure was used for analysis.
From 2006 to 2010, a total of 450 adult patients received a primary kidney transplant in our center. All of them were transplanted 3 to 7.5 years ago as of July 31, 2013, which is the end of the study period. Table 1 summarizes the demographic characteristics at the time of kidney transplants, and shows that there was no significant difference between the 2 groups. The total daily tacrolimus dose, 12-h trough level and graft function (serum creatinine) are summarized in Table 2. Both groups achieved similar targeted trough levels at all times according to our immunosuppressive protocol. Compared to Group 2, Group 1 initially required higher dose of tacrolimus during the first week of transplant. With administration of ketoconazole, their daily tacrolimus dose decreased. Subsequently, Group 1 required significantly lower dose of tacrolimus in the first month and in all times after that. The graft function remained comparable between the 2 groups.
| Group 1(n = 199) | Group 2(n = 149) | P value | |
| Age, mean ± SD (yr) | 47.2 ± 13.2 | 48.8 ± 14.4 | 0.21 |
| Gender (%) | |||
| Male | 56 | 61 | 0.47 |
| Female | 44 | 39 | |
| Race (%) | |||
| Black | 64 | 55 | 0.19 |
| Non-black | 36 | 45 | |
| BMI (kg/m2) | 28.3 ± 5.4 | 27.4 ± 5.7 | 0.34 |
| Peak PRA (%) | 15.5 ± 25.3 | 13.8 ± 27.0 | 0.27 |
| HLA mismatch | 4.1 ± 1.4 | 3.9 ± 1.6 | 0.52 |
| Causes of ESRD (%) | 0.75 | ||
| Diabetes | 25 | 31 | |
| Hypertension | 38 | 35 | |
| Nephritis | 19 | 15 | |
| PCKD | 8 | 6 | |
| Others | 10 | 13 | |
| Induction (%) | 55 | 51 | 0.51 |
| Donors (%) | 0.63 | ||
| Living | 26 | 29 | |
| Deceased | 74 | 71 | |
| CIT (h) | 17.8 ± 7.2 | 18.5 ± 6.4 | 0.24 |
| 1 wk | 1 mo | 2 mo | 1 yr | 3 yr | 5 yr | |
| Tacrolimus dose (mg/d) | ||||||
| Group 1 | 10.9 ± 5.6 | 7.5 ± 4.8 | 6.0 ± 3.6 | 5.6 ± 3.8 | 5.3 ± 3.1 | 4.9 ± 2.8 |
| Group 2 | 8.6 ± 4.1 | 8.3 ± 3.7 | 8.1 ± 3.2 | 7.8 ± 3.0 | 7.0 ± 2.2 | 6.2 ± 2.5 |
| P value | 0.004 | 0.03 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Tacrolimus trough level (× 10 mg/L) | ||||||
| Group 1 | 11.3 ± 2.1 | 9.7 ± 1.9 | 9.3 ± 1.7 | 8.6 ± 2.1 | 6.4 ± 1.9 | 5.3 ± 1.4 |
| Group 2 | 10.7 ± 1.8 | 10.2 ± 2.0 | 9.5 ± 1.6 | 9.0 ± 1.8 | 5.8 ± 1.7 | 4.8 ± 1.5 |
| Serum Cr (× 10 mg/L) | ||||||
| Group 1 | 2.2 ± 1.3 | 1.7 ± 1.1 | 1.5 ± 0.9 | 1.6 ± 1.2 | 1.6 ± 0.8 | 1.7 ± 0.9 |
| Group 2 | 1.9 ± 1.1 | 1.7 ± 0.9 | 1.6 ± 0.7 | 1.5 ± 0.8 | 1.5 ± 0.7 | 1.6 ± 0.8 |
The key post transplant events are summarized in Table 3. The delayed graft function (DGF) was defined by an inadequate renal function that required dialysis support in the first week after transplant. In both groups, the percentage of patients who had DGF was similar. Acute rejections were the biopsy-confirmed and clinically-treated ones. The 5-year cumulative incidence of acute rejection was significantly higher in Group 1 than Group 2, but the types of rejection were not different. There was no significant difference in the incidence of CNI toxicity or infectious disease between the 2 groups. Here, the CNI toxicity was the renal toxicity confirmed by kidney biopsy and required CNI dose reduction.
| Group 1(n = 199) | Group 2(n = 149) | P value | |
| Posttransplant events, n (%) | |||
| Delayed graft function | 56 (28) | 39 (26) | 0.77 |
| Acute rejection | 68 (34) | 27 (18) | 0.01 |
| Type of rejection | 0.49 | ||
| Cellular rejection | 49 | 17 | |
| Antibody rejection | 14 | 6 | |
| Both rejections | 5 | 4 | |
| CNI toxicity | 8 (4) | 15 (10) | 0.09 |
| Infectious diseases | 63 (32) | 54 (36) | 0.37 |
| Type of infection | 0.67 | ||
| CMV | 32 | 22 | |
| BKV | 14 | 13 | |
| HSV | 5 | 6 | |
| Bacteria | 7 | 10 | |
| Fungus | 5 | 3 | |
| Total graft loss, n (%) | 52 (26) | 35 (23) | 0.57 |
| Causes of graft loss | 0.88 | ||
| DWFG | 22 | 16 | |
| CAN | 17 | 10 | |
| Rejection | 9 | 5 | |
| Infection | 2 | 3 | |
| Others | 2 | 1 | |
| Total patient death, n (%) | 27 (14) | 18 (12) | 0.68 |
| Causes of death | 0.88 | ||
| CVD | 14 | 10 | |
| Infections | 6 | 5 | |
| Malignancy | 2 | 2 | |
| Others | 3 | 1 |
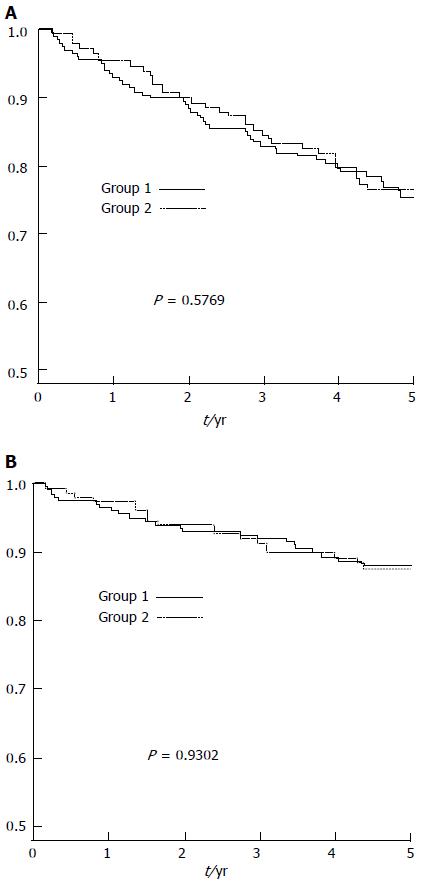
There was no statistical difference in graft survival by Kaplan-Meier analysis between the two groups (Figure 1A). The estimated graft survivals at 1, 3 and 5 years were 92.4%, 82.4% and 74.3% in Group 1, and 94.6%, 83.8% and 76.4% in Group 2 (Log-Rank P = 0.58). There was no difference in patient survival between the 2 groups (Figure 1B). The Kaplan-Meier estimated 1, 3, and 5 years patient survivals were 96%, 91.4% and 87.8% in Group 1, and 96.6%, 90.5%, 87.5% in Group 2 (Log-Rank P = 0.93). The causes of graft loss and death were listed in Table 3. There was no statistical difference in the overall causes of graft loss or patient death between the 2 groups.
The risk factors for acute rejection were examined by multivariable logistic regression analyses. The identified significant factors are listed in Table 4. We found that ketoconazole usage was an independent risk of acute rejection ((HR = 2.33, 95%CI: 1.33-4.07; P = 0.003). Tacrolimus dose at each time point was also tested. The daily dose of tacrolimus in the 2nd month after transplant was a significant factor in determining the risk of rejection (HR = 0.89, 95%CI: 0.75-0.96; P = 0.041), i.e., the higher the daily dose, the lower the risk of rejection. Other commonly described risk factors in literatures, such as black ethnicity, DGF and infectious complications were also demonstrated in our study, while live donor kidneys were associated with lower risk of rejection compared to the deceased donor kidneys.
| Hazard ratio | 95%CI | P value | |
| Race (black vs non-black ) | 2.68 | 1.67-6.73 | 0.032 |
| Donor (living vs deceased) | 0.32 | 0.11-0.94 | 0.038 |
| Ketoconazole (yes vs no) | 2.33 | 1.33-4.07 | 0.003 |
| Delayed graft function (yes vs no) | 2.14 | 1.22-3.73 | 0.008 |
| Infection (yes vs no) | 1.89 | 1.04-3.48 | 0.038 |
| Tacrolimus dose (mg/d) in 2nd month | 0.89 | 0.75-0.96 | 0.041 |
Tacrolimus remains a backbone of modern immunosuppressive therapy in solid organ transplants. Due to the numerous adverse effects, narrow safety margin and large intra- and inter-individual variability in pharmacokinetics, therapeutic monitoring is mandatory[1-3]. The normal pharmacokinetic curve of tacrolimus has a peak-and-trough pattern. A rapid peak phase reflects the absorption after an oral dose while a slow slope towards trough level reflects the drug metabolism. Ideally, tacrolimus dosing should be based on a 12 h area under the curve (AUC) that indicates the extent of systemic exposure. In clinical practice, oral doing is usually guided by monitoring 12 h trough levels, because of the convenience for blood sampling and the assumed correlation between trough level and AUC[2,3,12,13]. However, this correlation varies considerably and the best sampling time for a spot tacrolimus level to predict its total body exposure remains controversial[2,3,12-14]. The advance in pharmacogenetics has led the discovery of several gene polymorphisms in P450 family, which explains the inter-individual variability of tacrolimus metabolism[13,15,16]. Transplant patients expressing P450-3A5 (expressers) were shown to need higher doses of tacrolimus than non-expressers to reach similar trough levels[13,16].
Our study suggests that co-administration of ketoconazole with tacrolimus increase the risk of acute rejection. The 5-year cumulative incidence of acute rejection was significantly higher in Group 1 (34%) than Group 2 (18%), although the types of rejection were not different. Both groups achieved similar targeted trough levels at all time-points according to protocol. Compared to Group 2, Group 1 initially required higher dose of tacrolimus in the first week of transplant. As expected, their daily tacrolimus dose decreased with addition of ketoconazole. Subsequently, group 1 had significantly lower dose of tacrolimus in the first month and at all times after that. The risk factors for acute rejection were examined and we found that the use of ketoconazole was an independent risk of acute rejection (OR = 2.33, 95%CI: 1.33-4.07; P = 0.003). The daily dose of tacrolimus in the 2nd month after transplant was protective from rejection (HR = 0.89, 95%CI: 0.75-0.96; P = 0.041), i.e., the higher the daily dose, the lower the risk of rejection. This suggests that higher incidence of rejection may be directly related to the reduced dose of tacrolimus from the co-administration of ketoconazole.
Previous studies from Egypt reported long-term safety and financial savings of coadministration of ketoconazole with cyclosporine in 51 patients after living-related kidney transplants[17,18]. The same group further studied the coadministration of ketoconazole with tacrolimus[4,5]. A total of 70 live donor kidney transplant recipients were randomized into ketoconazole group (100 mg/d) and control group (without ketoconazole). By six months, ketoconazole group experienced significant reduction of tacrolimus dose (by 58.7%) and cost (by 56.9%)[4]. After 2 years, ketoconazole group still had a remarkable reduction of tacrolimus dose (by 53.8%) and financial cost (by 52.9%). There was no adverse effect of ketoconazole throughout the 2 years[5]. None of the Egyptian studies noted higher incidence of acute rejection with ketoconazole. Our current study is different from theirs in many aspects. In addition to a larger population and longer follow-up, our transplant recipients were more heterogeneous where the majority of patients were African Americans (64% in Group 1). Deceased donor kidneys (74%) rather than living-donor kidneys were the dominant allografts and about 30% of patients experienced DGF after transplants. Many patients were also highly sensitized and/or poorly matched with donors. Therefore, our patients would be considered to have higher risk for acute rejection[19,20]. This may explain the difference in the results. Similar to their studies, we did not found any toxic side effect of using low dose of ketoconazole in kidney transplant patients.
In HIV-infected transplant patients, we have experienced difficulties in dosing CNI. As reported previously, the acute rejection rates were unexpectedly high (31% at 1 year, and 41% at 3 years) in HIV-infected kidney recipients despite the fact that their targeted trough levels of CNI were similar to non-HIV patients. More than half (52%) of acute rejection episodes did not respond to steroid therapy[6]. Even in HIV-infected liver transplant recipients, about half of the acute rejections occurred within the first 3 wk of transplant[7]. The protease inhibitor (also a potent inhibitor of P450-3A and P-glycoprotein) used to control HIV infection in these patients likely changed the normal pharmacokinetic curve of CNI. Jain et al[11] found that the pharmacokinetic curves of tacrolimus in these patients did not show a normal peak-and-trough pattern, but rather resembled a flat line. Recently, van Maarseveen et al[21] studied the pharmacokinetics of tacrolimus in patients receiving ritonavir. It was found that their pharmacokinetic curves lacked an absorption peak every 12 h. When similar trough level was targeted, their mean 12-h AUC was approximately 44% lower than the AUC in HIV-negative recipients. Therefore, the authors suggested that the trough levels of tacrolimus in the HIV-positive patients receiving ritonavir should be approximately 40% higher compared to HIV-negative recipients in order to achieve an equivalent exposure (AUCs) of tacrolimus. Indeed, the previous study also noted that a higher tacrolimus trough level was associated with a decreased risk of first rejection (HR = 0.90; 95%CI: 0.81-1.00; P = 0.04) in HIV-infected transplant patients[6].
Interestingly, a recent study found that HIV could infect the transplanted renal allografts despite undetectable viremia. The reinfection of HIV in tubular cells was hypothesized to stimulate immune responses and increase the risk of rejection[9]. A dysregulated immune response in HIV-infected host was also proposed by others[6,7]. However, a French report showed similar rejection rates (15%) after kidney transplants in HIV-infected patients vs non-infected patients[22]. They attributed the lower rejection rate to the use of raltegravir (an integrase inhibitor)-based antiviral therapy, which does not inhibit P450 system. Subsequently, their patients had “normal” exposures to CNI. Lack of higher incidence of rejection in the French study does not support the hypothesis of either viral infection or dysregulated immune response as the predominant mechanism for the higher rejection rates observed in the United States study. Taken together, a more plausible explanation appears to be lower exposure to CNI due to co-administration of protease inhibitor in these HIV-infected recipients.
We speculate that similarly altered pharmacokinetic phenomenon would exist in our patients, which could explain our result. Co-administration of ketoconazole lowered the dose of tacrolimus and flatted the normal peak-and-trough curve, therefore, decreased the AUC of tacrolimus and increased the risk of acute rejection.
Our study is limited by its retrospective nature, single center data, and lack of peak level monitoring and AUC data of tacrolimus. Nevertheless, it is the first report of high risk of rejection associated with coadministration of ketoconazole with tacrolimus in HIV-negative transplant recipients. This is consistent with the results in HIV-infected transplant patients. It is an important issue for caring those patients with financial difficulty. This issue may be particularly relevant in the developing countries where co-administration of an inexpensive P450-3A inhibitor is a common practice to cut costs associated with expensive CNI. Our data suggests that high vigilance and careful monitoring is necessary, especially if other risk factors of rejection are present. Clearly, a prospective, randomized or a self-controlled study is needed to characterize the pharmacokinetic curves of tacrolimus with ketoconazole, so that a higher trough level can be proposed for clinical practice.
Tacrolimus is mainly metabolized by cytochrome P450 enzymes and ketoconazole is a potent inhibitor of P450. Transplant programs often use ketoconazole to reduce the tacrolimus dose and financial cost. But the long-term safety of this combination in kidney transplant recipients remains to be studied.
This experience in human immunodeficiency virus (HIV) infected patients has noted unexpected high incidences of acute rejection after kidney and liver transplants. In these patients, the targeted trough levels of calcineurin inhibitors (CNIs) were consistent with those in non-HIV recipients, but their required doses of CNI were significantly lower due to the necessary treatment with anti-retroviral protease inhibitor, which inhibits P450 enzymes.
Due to the altered pharmacokinetics of CNI by P450 inhibition from protease inhibitor, the total exposure to CNI (area under curve) is considerably low. This may explain the higher-than-expected rejection rates in HIV infected patients. In the current study, the authors have reported similar outcome from the coadministration of ketoconazole with tacrolimus in non-HIV infected kidney transplant recipients.
Coadministration of a P450 inhibitor to cut the dose and cost of tacrolimus may increase the risk of graft rejection. High vigilance and careful monitoring is necessary. A prospective, randomized or a self-controlled study is needed to characterize the pharmacokinetic curves of tacrolimus with ketoconazole, so that a higher trough level can be proposed for clinical practice.
Kidney transplant: remove a kidney from a donor and put it into a patient with kidney failure; Rejection: recipient body attacks donor kidney as a foreign object; tacrolimus: a key drug to prevent rejection; ketoconazole: a cheap antibiotics that inhibits the breakdown of tacrolimus, therefore, saves the dose and cost of tacrolimus.
This is a needed study exploring the outcome of attempting to reduce the dose of tacrolimus by adding ketoconazole to the regimen in first kidney transplant recipients to reduce the cost of immunosuppressive drugs. The interest of this work lies primarily in the observation of the authors regarding the five-year cumulative incidence of acute rejection in the group with ketoconazole and the consequently conclusion that the use of ketoconazole was an independent risk of acute rejection. The overall study is correct and the literature is to date.
P- Reviewer: Cantarovich F, Friedman EA, Wagner KD S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Zhang R. Modern Immunosuppressive therapy in kidney transplantation. Open J Organ Transpl Surg. 2013;3:22-31. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Lemahieu WP, Maes BD, Verbeke K, Vanrenterghem Y. CYP3A4 and P-glycoprotein activity in healthy controls and transplant patients on cyclosporin vs. tacrolimus vs. sirolimus. Am J Transplant. 2004;4:1514-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Scholten EM, Cremers SC, Schoemaker RC, Rowshani AT, van Kan EJ, den Hartigh J, Paul LC, de Fijter JW. AUC-guided dosing of tacrolimus prevents progressive systemic overexposure in renal transplant recipients. Kidney Int. 2005;67:2440-2447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | el-Dahshan KF, Bakr MA, Donia AF, Badr Ael-S, Sobh MA. Co-administration of ketoconazole to tacrolimus-treated kidney transplant recipients: a prospective randomized study. Nephrol Dial Transplant. 2004;19:1613-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | El-Dahshan KF, Bakr MA, Donia AF, Badr Ael-S, Sobh MA. Ketoconazole-tacrolimus coadministration in kidney transplant recipients: two-year results of a prospective randomized study. Am J Nephrol. 2006;26:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Stock PG, Barin B, Murphy B, Hanto D, Diego JM, Light J, Davis C, Blumberg E, Simon D, Subramanian A. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med. 2010;363:2004-2014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 442] [Cited by in RCA: 393] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 7. | Terrault NA, Roland ME, Schiano T, Dove L, Wong MT, Poordad F, Ragni MV, Barin B, Simon D, Olthoff KM. Outcomes of liver transplant recipients with hepatitis C and human immunodeficiency virus coinfection. Liver Transpl. 2012;18:716-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 8. | Hayes K, Van Sickels N, Buell J, Killackey M, Zhang R, Slakey D, Lukitsch I, Alper A, Mushatt D, Asad S. Successful transplantation of HIV patients: the Louisiana experience. J La State Med Soc. 2012;164:191-193. [PubMed] |
| 9. | Canaud G, Dejucq-Rainsford N, Avettand-Fenoël V, Viard JP, Anglicheau D, Bienaimé F, Muorah M, Galmiche L, Gribouval O, Noël LH. The kidney as a reservoir for HIV-1 after renal transplantation. J Am Soc Nephrol. 2014;25:407-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Frassetto LA, Browne M, Cheng A, Wolfe AR, Roland ME, Stock PG, Carlson L, Benet LZ. Immunosuppressant pharmacokinetics and dosing modifications in HIV-1 infected liver and kidney transplant recipients. Am J Transplant. 2007;7:2816-2820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Jain AK, Venkataramanan R, Shapiro R, Scantlebury VP, Potdar S, Bonham CA, Pokharna R, Rohal S, Ragni M, Fung JJ. Interaction between tacrolimus and antiretroviral agents in human immunodeficiency virus-positive liver and kidney transplantation patients. Transplant Proc. 2002;34:1540-1541. [PubMed] |
| 12. | Naik P, Madhavarapu M, Mayur P, Nayak KS, Sritharan V. Pharmacokinetics of tacrolimus in adult renal transplant recipients. Drug Metabol Drug Interact. 2012;27:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Chen SY, Li JL, Meng FH, Wang XD, Liu T, Li J, Liu LS, Fu Q, Huang M, Wang CX. Individualization of tacrolimus dosage basing on cytochrome P450 3A5 polymorphism--a prospective, randomized, controlled study. Clin Transplant. 2013;27:E272-E281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 14. | Frassetto LA, Tan-Tam CC, Barin B, Browne M, Wolfe AR, Stock PG, Roland M, Benet LZ. Best single time point correlations with AUC for cyclosporine and tacrolimus in HIV-infected kidney and liver transplant recipients. Transplantation. 2014;97:702-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Yee ML, Tan HH, Sia WJ, Yau WP. Influences of Donor and Recipient Gene Polymorphisms on Tacrolimus Dosing and Pharmacokinetics in Asian Liver Transplant Patients. Open J Organ Transpl Surg. 2013;3:53-61. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Tang HL, Xie HG, Yao Y, Hu YF. Lower tacrolimus daily dose requirements and acute rejection rates in the CYP3A5 nonexpressers than expressers. Pharmacogenet Genomics. 2011;21:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Sobh MA, Hamdy AF, El Agroudy AE, El Sayed K, El-Diasty T, Bakr MA, Ghoneim MA. Coadministration of ketoconazole and cyclosporine for kidney transplant recipients: long-term follow-up and study of metabolic consequences. Am J Kidney Dis. 2001;37:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | el-Agroudy AE, Sobh MA, Hamdy AF, Ghoneim MA. A prospective, randomized study of coadministration of ketoconazole and cyclosporine a in kidney transplant recipients: ten-year follow-up. Transplantation. 2004;77:1371-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Quiroga I, McShane P, Koo DD, Gray D, Friend PJ, Fuggle S, Darby C. Major effects of delayed graft function and cold ischaemia time on renal allograft survival. Nephrol Dial Transplant. 2006;21:1689-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 20. | Chouhan KK, Zhang R. Antibody induction therapy in adult kidney transplantation: A controversy continues. World J Transplant. 2012;2:19-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | van Maarseveen EM, Crommelin HA, Mudrikova T, van den Broek MP, van Zuilen AD. Pretransplantation pharmacokinetic curves of tacrolimus in HIV-infected patients on ritonavir-containing cART: a pilot study. Transplantation. 2013;95:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Touzot M, Pillebout E, Matignon M, Tricot L, Viard JP, Rondeau E, Legendre C, Glotz D, Delahousse M, Lang P. Renal transplantation in HIV-infected patients: the Paris experience. Am J Transplant. 2010;10:2263-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |