Revised: July 31, 2013
Accepted: August 2, 2013
Published online: August 6, 2013
Processing time: 83 Days and 14.3 Hours
Cardiovascular diseases are the major causes of mortality in patients with chronic kidney disease (CKD). The complex process of accelerated athero- and arteriosclerosis in CKD is associated with this phenomenon, where endothelial dysfunction (ED) is one of the initial steps. Hence, the early diagnosis of ED can potentially lead to early interventions which could result in a better outcome for these patients. Several methodologies have been developed for the diagnosis of ED. Laser Doppler flowmetry (LDF) enables us to study the microcirculation continuously in a non-invasive manner. In our review we would like to focus on different tests developed for LDF, like postocclusive reactive hyperaemia, local heating, iontophoresis, microdialysis or analysis of flowmotion. We would also like to summarize the available data in CKD with these methodologies to enlighten their perspectives in the clinical use on this patient population.
Core tip: Atherosclerotic diseases are the leading causes of death in chronic kidney disease (CKD) patients. Endothelial dysfunction is an important initial step of atherosclerotic processes, so with the early diagnosis and treatment of endothelial dysfunction many cardiovascular events can potentially be prevented. Laser Doppler flowmetry (LDF) gives the opportunity of the non-invasive study of microcirculation and endothelial function. This review summarizes the results of different LDF techniques and their usefulness in the diagnosis of endothelial dysfunction in CKD patients.
- Citation: Babos L, Járai Z, Nemcsik J. Evaluation of microvascular reactivity with laser Doppler flowmetry in chronic kidney disease. World J Nephrol 2013; 2(3): 77-83
- URL: https://www.wjgnet.com/2220-6124/full/v2/i3/77.htm
- DOI: https://dx.doi.org/10.5527/wjn.v2.i3.77
Endothelial dysfunction (ED) has been implicated as one of the major pathophysiological mechanisms contributing to the development of atherosclerosis. ED refers to the impairment in the homeostatic properties of the endothelial layer, like endothelium-dependent regulation of vascular tone, hemostasis, permeability, cell adhesion and inflammation. Endothelial dysfunction or more correctly “endothelial activation” is considered as a key initiating step in atherogenesis and also contributes to arterial stiffening. Besides, endothelial dysfunction is predictive of cardiovascular events in patients with established cardiovascular disease[1].
Patients with chronic kidney disease (CKD) are at high risk of cardiovascular disease, and often suffer from accelerated atherosclerosis[2,3]. CKD can progress to end stage renal failure however patients are more likely to die of a cardiovascular disease before reaching the terminal renal stage[4,5]. Although classic cardiovascular risk factors like hypertension, diabetes and obesity are present and frequent in this population, excessive and accelerated cardiovascular diseases cannot be explained by these alone.
There is plenty of evidence indicating that ED has high importance in patients with CKD[5-8]. ED begins early in the progression of CKD, independently of traditional cardiovascular risk factors, and is also observed in children with CKD[9-11]. Amongst the pathophysiological reasons chronic inflammation and excessive oxidative stress seem to be the most important[5]. The causes of inflammation are multiple, including decreased renal function, chronic volume overload, comorbidities, factors associated with the dialysis procedure, and genetic factors. C-reactive protein (CRP) was initially suggested to be merely a biomarker of inflammation, but recent data show that circulating CRP is also a mediator of atherogenesis and inflammation[12-14]. An inverse relationship between CRP and endothelium-dependent vasoreactivity has been described, which suggests that CRP can be a link between inflammation and ED[15].
Nitrogen monoxide (NO) is one of the most important vasodilating substances released by the endothelium. In CKD decreased NO level has often been reported and is associated with impaired endothelial function. The decreased NO may be connected to the reduced endothelial nitric oxide synthase activity which is a consequence of endogenous or exogenous inhibitors. There is some evidence for the accumulation of an endogenous inhibitor of nitric oxide synthase in haemodialysed patients[16]. Asymmetrical dimethyl arginine inhibits NO synthesis, increase vascular resistance and blood pressure[16-18]. There is much evidence which suggest that oxidative stress also plays an important role in decreased bioavailability of NO in CKD. Oxidative stress markers are elevated in CKD and it has been reported that the antioxidant vitamin-C microdialysed into the forearm skin improved the endothelial function[19,20]. These findings suggest that decreased NO availability as a consequence of increased oxidative stress can be another reason of early ED in CKD.
The cutaneous circulation is an easily accessible vascular bed for the assessment of in vivo human microcirculatory function, and may be representative of systemic vascular function[21,22]. Furthermore alterations in microvascular function may occur early in the progression of cardiovascular disease[22,23]. This phenomenon gives the opportunity that with the study of cutaneous microcirculation and early diagnosis of ED quick therapeutical interventions could be performed to avoid the development of severe cardiovascular events.
There are several methods for investigating endothelial dysfunction non-invasively. Evaluation of flow mediated vasodilatation (FMD) by ultrasound is widely used to study ED in coronary artery disease, hyperlipidaemia, hypertension and diabetes[4]. However, it has become more prominent in recent years, that the process is not completely dependent on the release of NO in response to increased shear stress, especially the first phase of the hyperaemia[24]. Another approach is to study forearm blood flow using venous strain-gauge plethysmography. Although this well reproducible method was the gold standard in the assessment of ED for a long time, today its daily use is limited because it requires specially trained investigators and brachial artery catheterization for the administration of the vasoactive drugs[25]. Recently for the assessment of microvascular function the most widely used noninvasive method has been laser Doppler flowmetry (LDF) of the skin.
LDF is based on the reflection of laser beam light. Light undergoes changes in wavelength (Doppler shift) when it is reflected by the moving red blood cells in the microvasculature and a photodiode measures the emerged beam. The magnitude and frequency distribution of these changes in wavelength are related to the number and velocity of moving red blood cells[14,26,27]. Several different signals can be recorded but the red blood cell flux is used the most. LDF enables the evaluation of cutaneous microvascular blood flow over time and its changes. The LD output is semi-quantitative and expressed in perfusion unit (PU) of output voltage (1 PU = 10 mV) in accordance with general consensus (European Laser Doppler Users Groups, London 1992). Many techniques can be associated to LDF such as local heating, post-occlusive reactive hyperaemia, iontophoresis, or microdialysis.
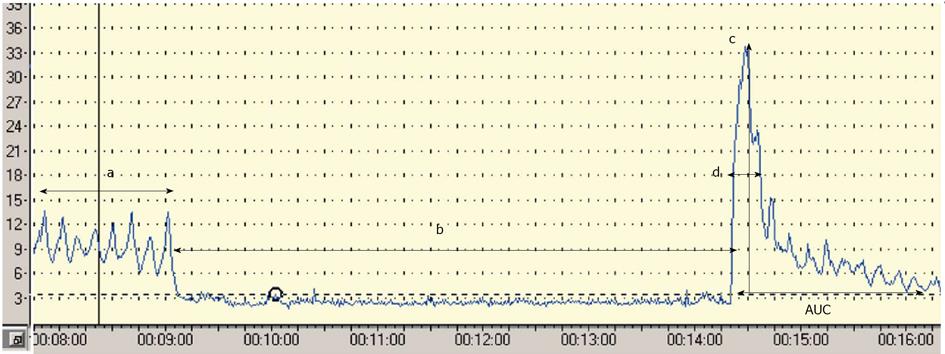
Postocclusive reactive hyperaemia (PORH) refers to the increase in skin blood flow following a brief arterial occlusion. The procedure starts with the occlusion of the brachial artery using a pressure cuff placed around the upper arm and inflated up to 20 mmHg above the systolic blood pressure. The commonly used ischaemic period varies between 3-10 min (unfortunately there is no standardized protocol), and a quick deflation finishes the procedure. A linear correlation between the period of ischaemia and the amplitude of the response has been reported[27,28]; however the length of the ischaemic period is coupled with the increasing pain of the subject. Lots of parameters can be calculated from the response curve. The most widely used as the primary endpoint is the peak hyperaemia after the cuff release. Other parameters used are the area under the hyperaemic curve, the raw value of the peak minus biological zero, the increase in postischaemic flow using area under the curve at baseline and postischaemia[27,29,30], (Figure 1). Four major factors have been thought to play a role in the hyperaemic response: metabolic vasodilators, endothelial vasodilators, myogenic response to shear stress and sensory nerves. Interestingly NO does not play a crucial role in this test, because the response to ischaemia is mostly mediated by prostanoids[31]. In summary, PORH represents a complex microvascular response to an acute period of ischaemia, in which endothelium has a less pronounced role[27]. Some clinical data are available with PORH. Yamamoto et al[32] found decreased microvascular response during PORH test in patients with type 2 diabetes mellitus and similar results have been reported in patients with type 1 diabetes[33]. There are only a few articles in the literature that have used PORH in order to study endothelial function in patients with CKD, and the results are controversial. Stewart et al[29] studied patients with end-stage renal disease (ESRD) with and without diabetes and cardiovascular disease. They found decreased hyperaemic response in patients with renal disease and also with cardiovascular disease and diabetes, but they could not demonstrate difference between patients with ESRD only and healthy controls. Previously we demonstrated decreased peak flow values compared to healthy controls both in patients with hypertension only and in hypertensive patients with ESRD. Among the hypertensive ESRD patients no further decrease of the peak flow was found compared with the hypertensive group[34]. In a follow-up study which is the only one available so far with LDF in CKD, Kruger et al[35] found that although PORH parameters did not correlate with Framingham and Cardiorisk cardiovascular risk scores, but the magnitude of peak flow was associated with the development of cardiovascular disease. According to these results the magnitude of the peak flow can be a useful PORH parameter in the future, but more data are necessary to confirm its value.
Local thermal hyperaemia leads to a temperature dependent increase in skin blood flow and achieves maximal vasodilatation between 42-44ºC. This maximal thermal vasodilatation corresponds to the maximal vasodilator capacity of the vessels[27,36]. Local heating-provoked vasodilatation is mediated by at least two independent mechanisms: the initial peak during the first 10 min relies on local sensory nerves, and is mediated by an axon reflex, which is thought to be dependent on calcitonin gene related peptide and substance P. The plateau phase which occurs after 20-30 min of warming is mediated by NO[37,38]. Previously it was found, that thermal hyperaemia is impaired in type 1 and 2 diabetes and in systemic sclerosis [29,39-41]. In ESRD both DuPont et al[20] and Stewart et al[29] have found decreased thermal peaks and plateau compared to controls. In the follow-up study of Kruger et al[35] it was demonstrated that thermal hyperaemia parameters (first and second peak flow and area under the curve) were associated significantly with the calculated cardiovascular risk using Framingham and Cardiorisk risk scores. They found that both cardiovascular mortality and the development of congestive heart failure were significantly associated with the first thermal peak and the plateau. As this study was performed only on 70 ESRD patients the authors concluded that although abnormal LDF parameters were sensitive indicators of increased cardiovascular risk, but a longitudinal study for further diagnostic and prognostic validation of the method would have been required[35]. Unfortunately no such kind of study was published so far. In summary, the magnitude of the thermal peaks and the plateau are promising parameters of the local thermal hyperaemia test, but a study with higher number of patients would be required to assess further clinical data about its usefulness.
Iontophoresis is based on the principle that a charged drug in solution will migrate across the skin under the influence of a low intensity electric current[42]. The quantity of the penetrating drug depends on the magnitude and duration of the current applied and on the diffusional and electrical characteristics of the skin. When combined with laser Doppler flowmetry this method enables the detection of alterations in cutaneous blood flow in response to the delivery of the vasoactive drug. Usually two types of vasoactive drugs are used; acetylcholine (Ach) and sodium nitroprusside (SNP). Ach and SNP are used to generate endothelium-dependent and independent vasodilatation, respectively[14,27]. A reduction on vascular response to Ach with no concurrent reduction in SNP response indicates endothelial dysfunction. Vascular response during Ach iontophoresis has been found to be impaired in obstructive sleep apnea syndrome, obesity, diabetes, hypercholesterolemia and hypertension[41,43-45]. Data in patients with CKD are controversial. Thang et al[46] found that Ach and SNP iontophoresis provoked vasodilatation was altered in patients with advanced CKD compared to healthy controls. In contrast, Cupisti et al[47] found no alternations in the hyperaemic response after Ach and SNP iontophoresis in patients with CKD compared to healthy controls. Previously we demonstrated decreased SNP and Ach response in hypertensive ESRD patients compared to healthy controls and patients with hypertension without CKD[34]. These data suggest that in conservatively treated CKD patients ED cannot be demonstrated with iontophoresis of Ach and SNP, but in ESRD, where marked microvascular alterations are present this methodology can have clinical implications.
Microdialysis is a technique which enables us to take ions or molecules into the interstitial space of the skin. It can be used to deliver pharmacological agents to a small area of tissue so that no confounding systemic effects occur. In addition, the concentration of substances released in response to the pharmacological action can be measured in the dialysate effluent fluid[27]. This approach has been used to assess the role of NO in postocclusive and thermal hyperaemia and opens a new era in the assessment of human skin microcirculation[37,48]. The major limitation of this technique is its microinvasive approach. There are a very limited number of studies in the literature which used microdialysis in CKD patients in order to evaluate endothelial function. Dupont et al[20] combined local heating with dialysation of Ringer solution, ascorbic acid, L-arginine and nitro-L-arginine methyl ester (L-NAME) in patients with stage 3-4 CKD and age, sex matched controls. They found in the CKD patients impaired cutaneous vasodilation response to local heating, which could have been reversed with the local infusion of ascorbic acid or L-arginine. These findings confirm the results of other workers which suggested that oxidative stress plays important role in the vascular dysfunction in stage 3-4 CKD. According to its microinvasive feature, microdialysis will probably be used in the future only for experimental studies on limited number of patients.
Periodic oscillations of cutaneous blood flow, also called flowmotion, can be quantified by spectral analysis. These periodic oscillations correlate well with endothelial function and spontaneous smooth muscle wall activity. Spectral analysis of laser Doppler signal allows five different frequency intervals to be detected. These oscillations represent the influence of the heartbeat (1 Hz), respiration (0.3 Hz), myogenic activity (0.1 Hz), neurogenic activity (0.04 Hz), and the frequency sub-interval of 0.01 Hz is mostly endothelium dependent[49-51]. The only study which investigated the skin blood flow oscillations during laser Doppler flowmetry in CKD found blunted post ischemic increase of the endothelium dependent skin blood flowmotion sub-interval, which can be considered as an early sign of microvascular endothelial dysfunction in this patient population[52]. So more data are needed with this operator-independent LDF method to discover its usefulness in CKD patients.
One major limitation of LDF is, that it is not possible to measure absolute perfusion values. Measurements in most studies are expressed in arbitrary units called PU and are referred to as flux rather than flow. Moreover, laser Doppler has often considered poorly reproducible, but when the recording site is standardized, and temperature controlled room and heated probes were used, the day to day reproducibility of PORH, thermal hyperaemia and iontophoresis compares well with that of flow mediated dilatation of the brachial artery[27,53]. Besides there are some difficulties in interpreting observational studies of patients with CKD because many diseases that cause CKD (e.g., diabetes mellitus, hypertension) themselves are already associated with endothelial dysfunction. In addition there are no standardized protocols available with the different methods of LDF, which makes it highly difficult to compare data from different studies.
Epidemiological evidence has already established the connection between CKD and cardiovascular disease[35,54]. ED plays a major role in this linkage. Therefore early detection of ED in CKD would be very important. Among other possibilities the measurement of surrogate markers like C-reactive protein, homocysteine, circulating selectins, plasminogen activator inhibitor-1, and asymmetric dimethylarginine[55,56], or semi-invasive techniques such as strain-gauge plethysmography and FMD can be mentioned. An advantage of LDF over these methods is that it gives a good opportunity for the direct, real time assessment of microvascular function in a non invasive manner. Furthermore LDF is an easy and relatively cost effective technique which offers the opportunity of future clinical implications.
But unfortunately we are far from this in CKD. Among the above described LDF methodologies PORH and local thermal hyperaemia have the only evidence in CKD in respect of prediction of cardiovascular events. However, these data originate from only one follow-up study which was performed on a low number of patients. In summary, much more studies are required to assess the predictive value of different parameters evaluated with different tests in CKD, before LDF would gain clinical application.
P- Reviewers Lopez-Hernandez FJ, Olowu WA S- Editor Gou SX L- Editor A E- Editor Lu YJ
| 1. | Martin BJ, Anderson TJ. Risk prediction in cardiovascular disease: the prognostic significance of endothelial dysfunction. Can J Cardiol. 2009;25 Suppl A:15A-20A. [PubMed] |
| 2. | Parfrey PS, Foley RN. The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol. 1999;10:1606-1615. [PubMed] |
| 3. | Wheeler DC. Cardiovascular disease in patients with chronic renal failure. Lancet. 1996;348:1673-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Stoner L, Sabatier MJ. Use of ultrasound for non-invasive assessment of flow-mediated dilation. J Atheroscler Thromb. 2012;19:407-421. [PubMed] |
| 5. | Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1021] [Cited by in RCA: 1129] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 6. | Faure V, Dou L, Sabatier F, Cerini C, Sampol J, Berland Y, Brunet P, Dignat-George F. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J Thromb Haemost. 2006;4:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 250] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 7. | Rabelink TJ, de Boer HC, van Zonneveld AJ. Endothelial activation and circulating markers of endothelial activation in kidney disease. Nat Rev Nephrol. 2010;6:404-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Annuk M, Lind L, Linde T, Fellström B. Impaired endothelium-dependent vasodilatation in renal failure in humans. Nephrol Dial Transplant. 2001;16:302-306. [PubMed] |
| 9. | Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Brunet P. Vascular incompetence in dialysis patients--protein-bound uremic toxins and endothelial dysfunction. Semin Dial. 2011;24:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Lilien MR, Groothoff JW. Cardiovascular disease in children with CKD or ESRD. Nat Rev Nephrol. 2009;5:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Stam F, van Guldener C, Becker A, Dekker JM, Heine RJ, Bouter LM, Stehouwer CD. Endothelial dysfunction contributes to renal function-associated cardiovascular mortality in a population with mild renal insufficiency: the Hoorn study. J Am Soc Nephrol. 2006;17:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 191] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Zhang YX, Cliff WJ, Schoefl GI, Higgins G. Coronary C-reactive protein distribution: its relation to development of atherosclerosis. Atherosclerosis. 1999;145:375-379. [PubMed] |
| 13. | Zoccali C. Endothelial dysfunction and the kidney: emerging risk factors for renal insufficiency and cardiovascular outcomes in essential hypertension. J Am Soc Nephrol. 2006;17:S61-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Hogas SM, Voroneanu L, Serban DN, Segall L, Hogas MM, Serban IL, Covic A. Methods and potential biomarkers for the evaluation of endothelial dysfunction in chronic kidney disease: a critical approach. J Am Soc Hypertens. 2010;4:116-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Cleland SJ, Sattar N, Petrie JR, Forouhi NG, Elliott HL, Connell JM. Endothelial dysfunction as a possible link between C-reactive protein levels and cardiovascular disease. Clin Sci (Lond). 2000;98:531-535. [PubMed] |
| 16. | Bode-Böger SM, Scalera F, Kielstein JT, Martens-Lobenhoffer J, Breithardt G, Fobker M, Reinecke H. Symmetrical dimethylarginine: a new combined parameter for renal function and extent of coronary artery disease. J Am Soc Nephrol. 2006;17:1128-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 225] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 17. | Kielstein JT, Impraim B, Simmel S, Bode-Böger SM, Tsikas D, Frölich JC, Hoeper MM, Haller H, Fliser D. Cardiovascular effects of systemic nitric oxide synthase inhibition with asymmetrical dimethylarginine in humans. Circulation. 2004;109:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 292] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 18. | Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, Vallance P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003;23:1455-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 449] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 19. | Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 547] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 20. | Dupont JJ, Farquhar WB, Townsend RR, Edwards DG. Ascorbic acid or L-arginine improves cutaneous microvascular function in chronic kidney disease. J Appl Physiol. 2011;111:1561-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Abularrage CJ, Sidawy AN, Aidinian G, Singh N, Weiswasser JM, Arora S. Evaluation of the microcirculation in vascular disease. J Vasc Surg. 2005;42:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 169] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol. 2008;105:370-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 336] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 23. | Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: a new target for treatment? Circulation. 2001;104:735-740. [PubMed] |
| 24. | Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Lüscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314-1319. [PubMed] |
| 25. | Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol. 2001;52:631-646. [PubMed] |
| 26. | Lekakis J, Abraham P, Balbarini A, Blann A, Boulanger CM, Cockcroft J, Cosentino F, Deanfield J, Gallino A, Ikonomidis I. Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur J Cardiovasc Prev Rehabil. 2011;18:775-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 221] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 27. | Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci. 2006;27:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 339] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 28. | Tee GB, Rasool AH, Halim AS, Rahman AR. Dependence of human forearm skin postocclusive reactive hyperemia on occlusion time. J Pharmacol Toxicol Methods. 2004;50:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Stewart J, Kohen A, Brouder D, Rahim F, Adler S, Garrick R, Goligorsky MS. Noninvasive interrogation of microvasculature for signs of endothelial dysfunction in patients with chronic renal failure. Am J Physiol Heart Circ Physiol. 2004;287:H2687-H2696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 30. | Ruano J, Lopez-Miranda J, Fuentes F, Moreno JA, Bellido C, Perez-Martinez P, Lozano A, Gómez P, Jiménez Y, Pérez Jiménez F. Phenolic content of virgin olive oil improves ischemic reactive hyperemia in hypercholesterolemic patients. J Am Coll Cardiol. 2005;46:1864-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 160] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Wong BJ, Wilkins BW, Holowatz LA, Minson CT. Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. J Appl Physiol. 2003;95:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Yamamoto-Suganuma R, Aso Y. Relationship between post-occlusive forearm skin reactive hyperaemia and vascular disease in patients with Type 2 diabetes--a novel index for detecting micro- and macrovascular dysfunction using laser Doppler flowmetry. Diabet Med. 2009;26:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Gomes MB, Matheus AS, Tibiriçá E. Evaluation of microvascular endothelial function in patients with type 1 diabetes using laser-Doppler perfusion monitoring: which method to choose? Microvasc Res. 2008;76:132-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Farkas K, Nemcsik J, Kolossváry E, Járai Z, Nádory E, Farsang C, Kiss I. Impairment of skin microvascular reactivity in hypertension and uraemia. Nephrol Dial Transplant. 2005;20:1821-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Kruger A, Stewart J, Sahityani R, O’Riordan E, Thompson C, Adler S, Garrick R, Vallance P, Goligorsky MS. Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: correlation with cardiovascular risk. Kidney Int. 2006;70:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc. 2003;78:603-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 551] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 37. | Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619-1626. [PubMed] |
| 38. | Kellogg DL, Liu Y, Kosiba IF, O’Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86:1185-1190. [PubMed] |
| 39. | Brugler A, Thompson S, Turner S, Ngo B, Rendell M. Skin blood flow abnormalities in diabetic dermopathy. J Am Acad Dermatol. 2011;65:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Boignard A, Salvat-Melis M, Carpentier PH, Minson CT, Grange L, Duc C, Sarrot-Reynauld F, Cracowski JL. Local hyperemia to heating is impaired in secondary Raynaud’s phenomenon. Arthritis Res Ther. 2005;7:R1103-R1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Khan F, Elhadd TA, Greene SA, Belch JJ. Impaired skin microvascular function in children, adolescents, and young adults with type 1 diabetes. Diabetes Care. 2000;23:215-220. [PubMed] |
| 42. | Kalia YN, Naik A, Garrison J, Guy RH. Iontophoretic drug delivery. Adv Drug Deliv Rev. 2004;56:619-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 517] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 43. | Trzepizur W, Gagnadoux F, Abraham P, Rousseau P, Meslier N, Saumet JL, Racineux JL. Microvascular endothelial function in obstructive sleep apnea: Impact of continuous positive airway pressure and mandibular advancement. Sleep Med. 2009;10:746-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Caballero AE, Arora S, Saouaf R, Lim SC, Smakowski P, Park JY, King GL, LoGerfo FW, Horton ES, Veves A. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999;48:1856-1862. [PubMed] |
| 45. | Farkas K, Kolossváry E, Járai Z, Nemcsik J, Farsang C. Non-invasive assessment of microvascular endothelial function by laser Doppler flowmetry in patients with essential hypertension. Atherosclerosis. 2004;173:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | Thang OH, Serné EH, Grooteman MP, Smulders YM, Ter Wee PM, Tangelder GJ, Nubé MJ. Premature aging of the microcirculation in patients with advanced chronic kidney disease. Nephron Extra. 2012;2:283-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Cupisti A, Rossi M, Placidi S, Fabbri A, Morelli E, Vagheggini G, Meola M, Barsotti G. Responses of the skin microcirculation to acetylcholine in patients with essential hypertension and in normotensive patients with chronic renal failure. Nephron. 2000;85:114-119. [PubMed] [DOI] [Full Text] |
| 48. | Wilkins BW, Holowatz LA, Wong BJ, Minson CT. Nitric oxide is not permissive for cutaneous active vasodilatation in humans. J Physiol. 2003;548:963-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Stefanovska A, Bracic M, Kvernmo HD. Wavelet analysis of oscillations in the peripheral blood circulation measured by laser Doppler technique. IEEE Trans Biomed Eng. 1999;46:1230-1239. [PubMed] |
| 50. | Stauss HM, Anderson EA, Haynes WG, Kregel KC. Frequency response characteristics of sympathetically mediated vasomotor waves in humans. Am J Physiol. 1998;274:H1277-H1283. [PubMed] |
| 51. | Kvernmo HD, Stefanovska A, Kirkeboen KA, Kvernebo K. Oscillations in the human cutaneous blood perfusion signal modified by endothelium-dependent and endothelium-independent vasodilators. Microvasc Res. 1999;57:298-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 171] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 52. | Rossi M, Cupisti A, Di Maria C, Galetta F, Barsotti G, Santoro G. Blunted post-ischemic increase of the endothelial skin blood flowmotion component as early sign of endothelial dysfunction in chronic kidney disease patients. Microvasc Res. 2008;75:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Kubli S, Waeber B, Dalle-Ave A, Feihl F. Reproducibility of laser Doppler imaging of skin blood flow as a tool to assess endothelial function. J Cardiovasc Pharmacol. 2000;36:640-648. [PubMed] |
| 54. | Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, Klag MJ, Mailloux LU, Manske CL, Meyer KB. Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis. 1998;32:853-906. [PubMed] |
| 55. | Fliser D. Asymmetric dimethylarginine (ADMA): the silent transition from an ‘uraemic toxin’ to a global cardiovascular risk molecule. Eur J Clin Invest. 2005;35:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Tripepi G, Mallamaci F, Zoccali C. Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: searching for the best risk marker by multivariate modeling. J Am Soc Nephrol. 2005;16 Suppl 1:S83-S88. [PubMed] |