Revised: January 31, 2013
Accepted: February 5, 2013
Published online: February 6, 2013
Processing time: 104 Days and 13.7 Hours
AIM: To investigate renin expression in pericytes during normal kidney development and after deletion of angiotensinogen, the precursor for all angiotensins.
METHODS: We examined the distribution of renin expressing cells by immunoshistochemistry in the interstitial compartment of wild type (WT) and angiotensinogen deficient (AGT -/-) mice at different developmental stages from embryonic day 18 (E18: WT, n = 4; AGT -/-, n = 5) and at day 1 (P1: WT, n = 5; AGT -/-, n = 5), 5 (P5: WT, n = 7; AGT -/-, n = 8), 10 (P10: WT, n = 3; AGT -/-, n = 5), 21 (P21: WT, n = 7; AGT -/-, n = 5), 45 (P45: WT, n = 3; AGT -/-, n = 3), and 70 (P70: WT, n = 2; AGT -/-, n = 2) of postnatal life. We quantified the number of pericytes positive for renin at all the developmental stages mentioned above and compared the results of AGT -/- mice to their WT counterparts.
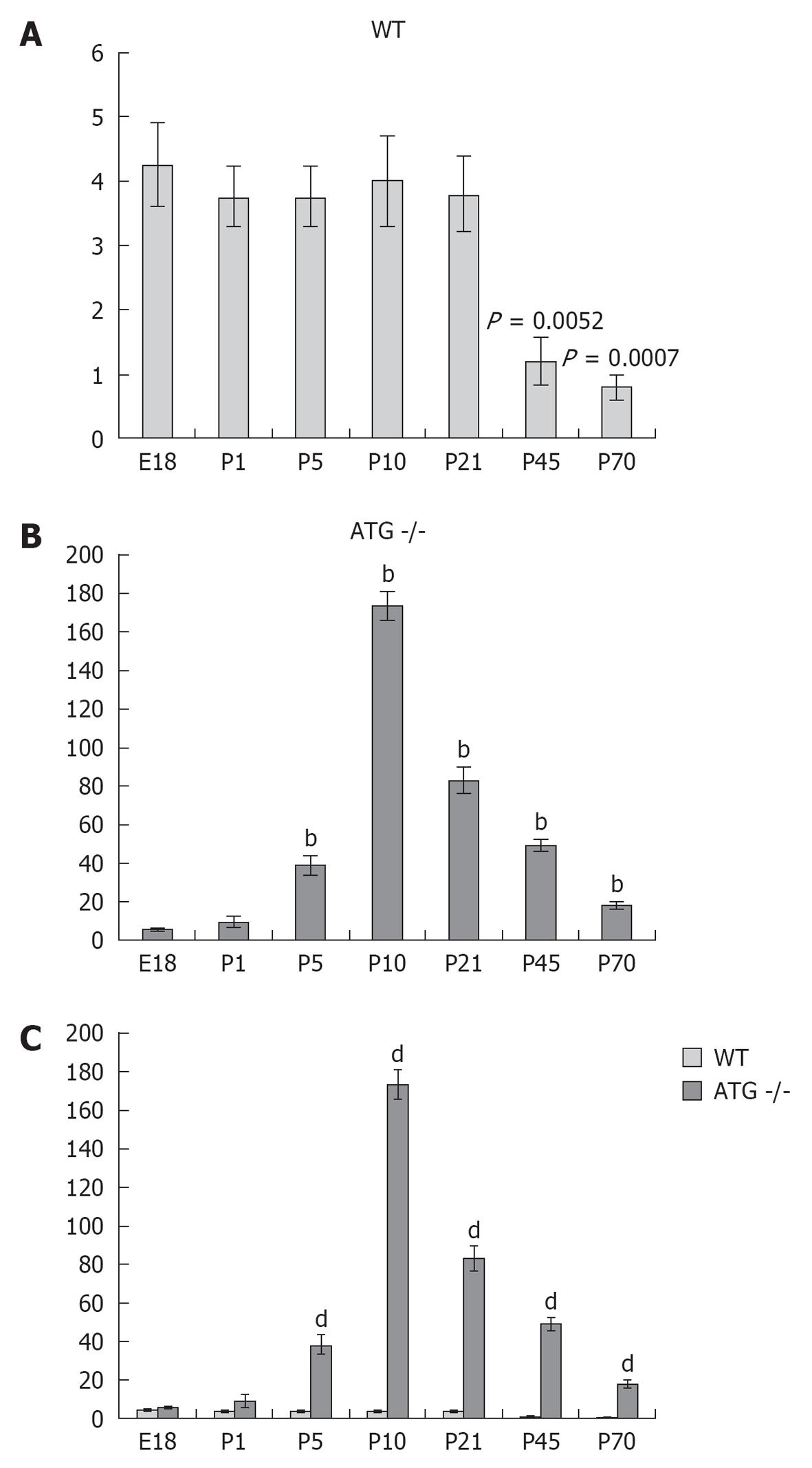
RESULTS: In WT mice, renal interstitial pericytes synthesize renin in early life supporting a lineage relationship with renin cells in the vasculature. The number of pericytes positive for renin per area of 0.32 mm2 (density) in WT mice was maintained from fetal life till weaning age (E18 = 4.25 ± 0.63, P1 = 3.75 ± 0.48, P5 = 3.75 ± 0.48, P10 = 4 ± 0.71, P21 = 3.8 ± 0.58) and markedly decreased in adult life (P45 = 1.2 ± 0.37, P70 = 0.8 ± 0.20). On the other hand, in AGT -/- mice the density of pericytes expressing renin was not significantly different from WT mice at E18 and P1: E18 = 5.75 ± 0.50 vs 4.25 ± 0.63 (P = 0.106), P1 = 9.25 ± 3.50 vs 3.75 ± 0.48 (P = 0.175) but significantly increased from P5 till P70: P5 = 38.25 ± 5 vs 3.75 ± 0.48 (P = 0.0004), P10 = 173 ± 7.50 vs 4 ± 0.70 (P = 5.24567 × 10-7), P21 = 83 ± 6.70 vs 3.8 ± 0.58 (P = 2.97358 × 10-6), P45 = 49 ± 3.50 vs 1.2 ± 0.37 (P = 8.18274 x 10-7) and P70 = 17.8 ± 2.30 vs 0.8 ± 0.20 (P = 3.51151 × 10-5). The AGT -/- mice showed a marked increase in the number of pericytes per field studied starting from P5, reaching its peak at P10, and then a gradually decreasing until P70.
CONCLUSION: Interstitial pericytes synthesize renin during development and the number of renin-expressing pericytes increases in response to a homeostatic threat imposed early in life such as lack of angiotensinogen.
- Citation: Berg AC, Chernavvsky-Sequeira C, Lindsey J, Gomez RA, Sequeira-Lopez MLS. Pericytes synthesize renin. World J Nephrol 2013; 2(1): 11-16
- URL: https://www.wjgnet.com/2220-6124/full/v2/i1/11.htm
- DOI: https://dx.doi.org/10.5527/wjn.v2.i1.11
Renin-expressing cells in the kidney are crucial for the regulation of blood pressure and fluid-electrolyte homeostasis[1-3]. Interestingly, during embryonic and early postnatal life, renin-expressing cells are broadly distributed along large intrarenal arteries, afferent arterioles, and glomeruli[4-7]. With maturation, renin cells along the arterioles and glomeruli differentiate into smooth muscle (SM) and mesangial cells respectively[8]. Thus, renin cells in adults are confined to the juxtaglomerular (JG) portion of the afferent arterioles. However, SM cells and mesangial cells retain the memory to re-express renin in response to a homeostatic threat[8].
Recently, a lineage relationship between renin-expressing cells and pericytes has been suggested[5]. Pericytes are SM like cells, which surround capillaries and contribute to blood vessel development and function[9-12]. In the kidney, at least three cell types can be regarded as pericytes: mesangial cells, interstitial pericytes, and arteriolar SM cells. As mentioned above, although rennin expression has been described in mesangial and arteriolar SM cells, the presence of renin in interstitial pericytes during development or in response to physiological challenges has not been explored. The present study was undertaken to determine whether interstitial pericytes express renin during normal kidney development and whether renin expression in pericytes is influenced by the genetic ablation of the precursor for all angiotensins. For this purpose, we studied angiotensinogen deficient mice because they are well known for their increased renin synthesis and are characterized by the increase of renin producing cells along the afferent arterioles[13,14].
Kidneys from wild type (WT) and angiotensinogen deficient (AGT -/-) mice were processed for renin immunocytochemistry as previously described[6,15-19]. The polyclonal rabbit anti-mouse renin antibody (1:250) developed in our laboratory was previously characterized and found to be highly specific[6,16]. Negative controls included omission of the primary antibody and staining of kidneys from Ren 1c -/- mice, which displayed no immunostaining.
We stained kidneys from mice at 18 d of gestation (E18: WT, n = 4; AGT -/-, n = 5) and at days 1 (P1: WT, n = 5; AGT -/-, n = 5), 5 (P5: WT, n = 7; AGT -/-, n = 8), 10 (P10: WT, n = 3; AGT -/-, n = 5), 21 (P21: WT, n = 7; AGT -/-, n = 5), 45 (P45: WT, n = 3; AGT -/-, n = 3), and 70 (P70: WT, n = 2; AGT -/-, n = 2) of postnatal life.
Kidney sections were viewed using a DM 5500 B microscope (Leica Camera, Solms, Germany) and pictures were taken using a DFC310 FX camera (Leica Camera, Solms, Germany) attached to the microscope.
Pericytes positive for renin were counted in images taken at the same magnification from 0.32 mm2 areas with the highest density of pericytes positive for renin (2-3 images per animal, 2 animals per age group and 2 animals per genotype).
All animals were studied in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the University of Virginia Animal Care Committee.
All data are presented as mean ± SE. To assess whether the overall density of pericytes expressing renin in AGT -/- mouse kidneys differs from WT kidneys throughout development the Mann-Whitney U test was performed. To compare the presence of pericytes expressing renin in AGT -/- mouse kidneys from their WT counterparts at each individual age studied the statistical significance was calculated using two tailed unpaired Student’s t test. P≤ 0.05 was considered significant.
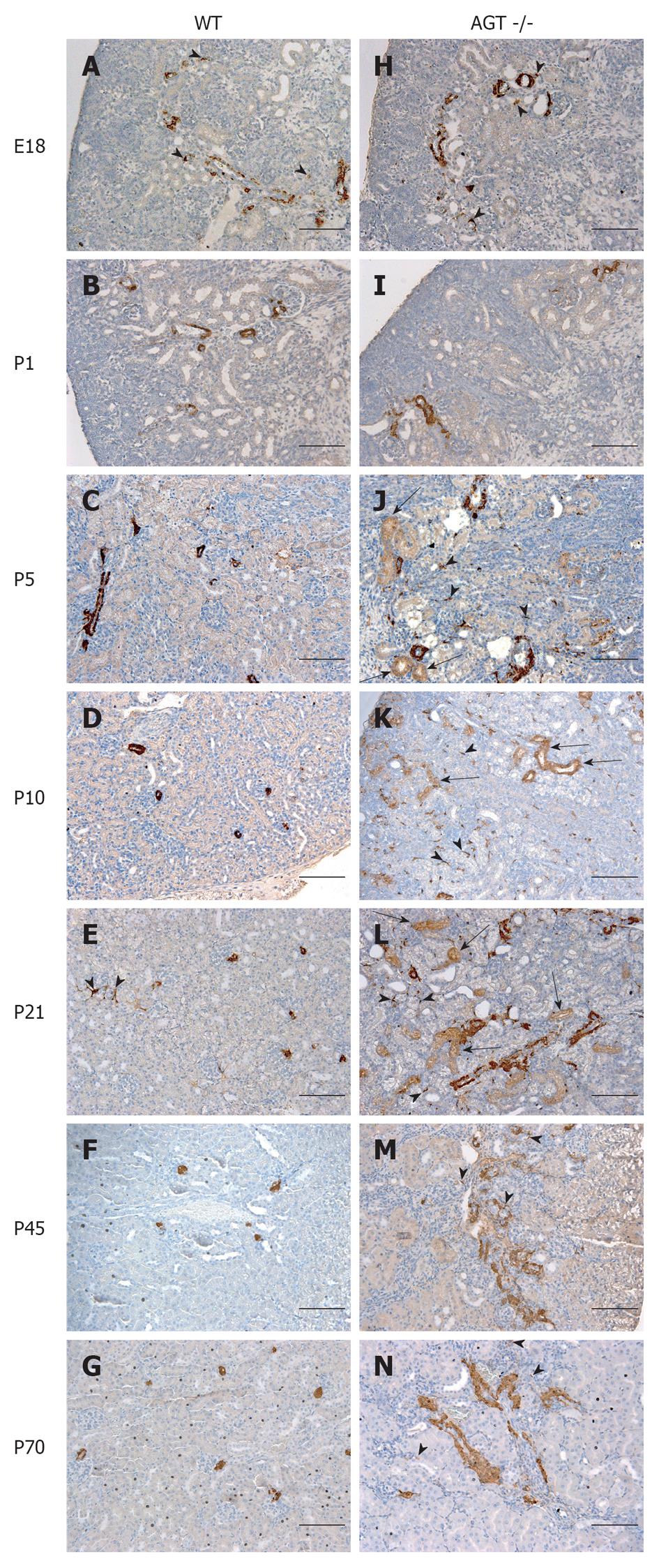
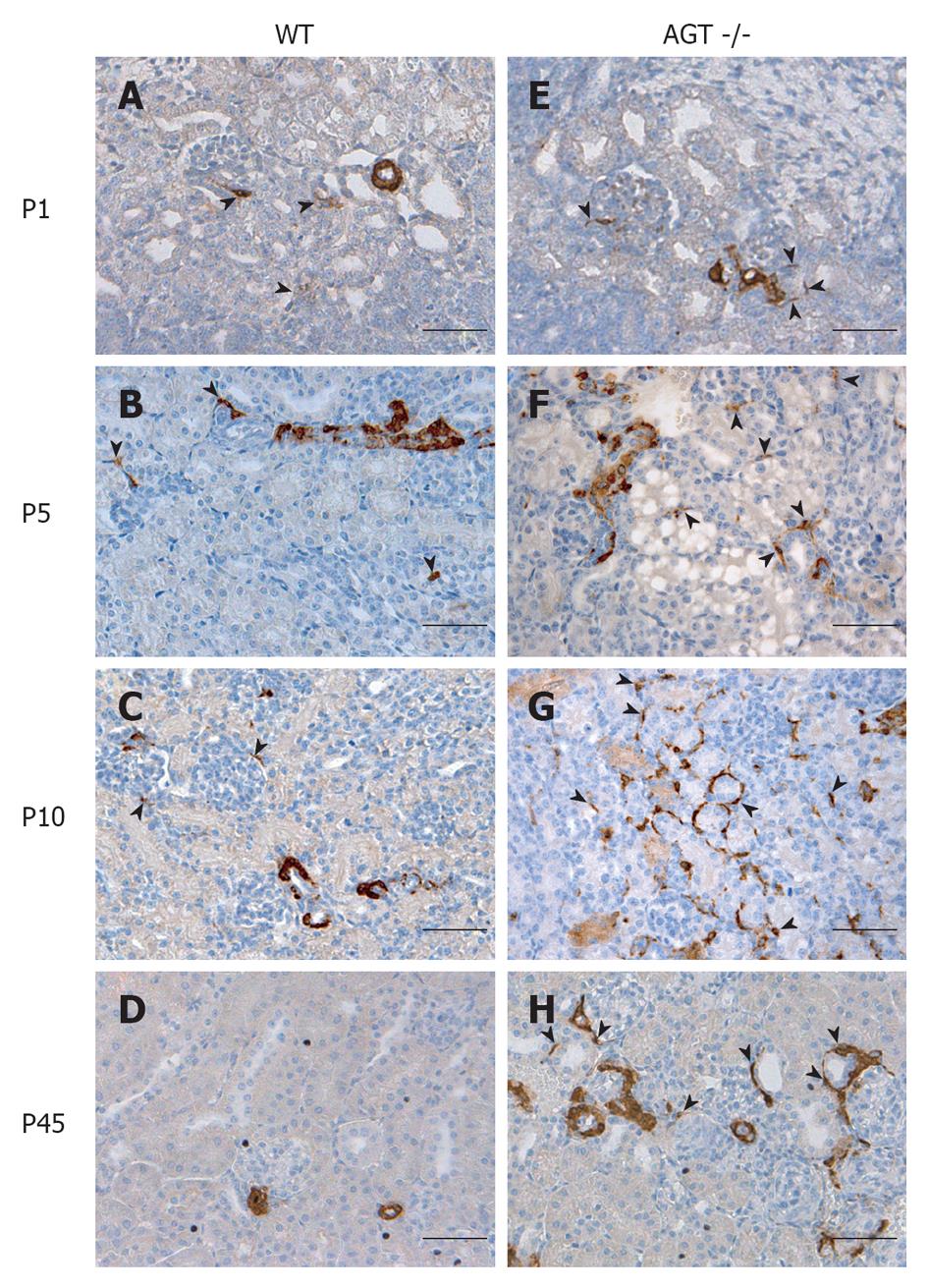
The distribution of renin in the kidneys of AGT -/- mice was compared to that found in WT mice in Figures 1 and 2. In E18 WT mice, renin expression was not limited to arterioles and JG areas, but was also detected in some pericytes, which were located in the interstitium in between tubules, arterioles, and glomeruli (Figure 1A, arrowheads). Almost all of the pericytes expressing renin were found in the cortical interstitium. Staining in tubules was also observed, although it was faint at this age. At P1 and P5 renin was found in pericytes and faint in tubules (Figures 1B, C and 2A, B, arrowheads). By P10, renin immunostaining diminished in the vasculature but was still present in pericytes (Figures 1D and 2C, arrowheads) and by P21 it was found in pericytes in less than half of the animals studied (Figure 1E, arrowheads). By P45 renin was evident only in arterioles mainly in a JG position (Figures 1F and 2D). At 70 d of postnatal life renin staining was frankly diminished and encountered solely in a JG distribution (Figure 1G).
The distribution of renin in the kidneys of AGT -/- mice was significantly different from the one found in WT mice. At E18 renin was expressed in the JG area, arterioles, glomeruli, tubules, and pericytes (Figure 1H) not different from WT mice at the same age. At P1 expression of tubular and pericyte renin was also similar to the P1 WT (Figures 1I and 2E). At P5 renin was found in JG cells, arterioles, pericytes, and tubules (Figures 1J and 2F). At this stage, renin-expressing pericytes were arranged in two configurations: encircling tubules, and scattered throughout the interstitium alongside capillaries. At P10, renin continued to be located in tubules, arterioles, and JG cells (Figures 1K and 2G). Renin was also found in vessels and within some glomeruli (mesangium). Pericyte renin was more pronounced in the medullary interstitium. The renin-expressing pericytes had a spider-like shape and were located between tubules and around endothelial cells (Figure 2G). At P21 renin expression in AGT -/- kidneys was increased overall compared to previous ages. Renin was evident in the tubules, arterioles, vessels, JG area, and in fewer pericytes (Figure 1L). At P45 the extension of renin immunostaining in AGT -/- kidneys was markedly increased and renin was found in the JG areas, thick arteries, arterioles, and in pericytes surrounding them (Figures 1M and 2H). Whereas vascular and pericyte renin was very prominent in P45 AGT -/- animals, little tubular renin was present at this age. At 70 d of postnatal life renin staining in AGT -/- mice was still present throughout the very abnormal thick arteries and arterioles and in some interstitial pericytes (Figure 1N).
Next, we quantified the number of pericytes positive for renin and normalized them per surface area (0.32 mm2) at every developmental stage described above (E18-P70). Whereas the median density of pericytes expressing renin in the WT group was 3 per area (range 0-6) in the AGT -/- group it was significantly increased to 37.5 (range 4-185) (P < 0.0005, two tailed test).
Figure 3 shows the quantification and distribution of the density of pericytes positive for renin at different stages during development in kidneys of WT and ATG -/- mice. Whereas the density of pericytes per area in WT mice was maintained from fetal life until weaning age (E18 = 4.25 ± 0.63, P1 = 3.75 ± 0.48, P5 = 3.75 ± 0.48, P10 = 4 ± 0.71, P21 = 3.8 ± 0.58, P1 vs E18, P = 0.55; P5 vs E18, P = 0.55; P10 vs E18, P = 0.80; P21 vs E18, P = 0.618) it markedly decreased in adult life (P45 = 1.2 ± 0.37, P70 = 0.8 ± 0.20, P45 vs E18, P = 0.0032; P70 vs E18, P = 0.0007).
On the other hand, the AGT -/- mice showed a marked increase starting from P5, reaching its peak at P10, and then gradually decreasing until P70. When each age group was compared to their WT counterpart we found that in AGT -/- mice the density of pericytes expressing renin was not significantly different from WT mice at E18 and P1 (E18 = 5.75 ± 0.50 vs 4.25 ± 0.63, P = 0.106; P1 = 9.25 ± 3.50 vs 3.75 ± 0.48, P = 0.175) but significantly increased from P5 till P70 (P5 = 38.25 ± 5 vs 3.75 ± 0.48, P = 0.0004; P10 = 173 ± 7.50 vs 4 ± 0.70, P = 5.24567 × 10-7; P21 = 83 ± 6.70 vs 3.8 ± 0.58, P = 2.97358 × 10-6; P45 = 49 ± 3.50 vs 1.2 ± 0.37, P = 8.18274 × 10-7; and P70 = 17.8 ± 2.30 vs 0.8 ± 0.20, P = 3.51151 × 10-5).
The synthesis of renin by pericytes has not been documented. The present studies show that in the kidney, interstitial pericytes synthesize renin during development. The number of pericytes expressing renin markedly increases in response to lack of angiotensins, a homeostatic threat imposed early in life.
During embryonic and fetal kidney development, renin is broadly distributed along the pre-glomerular arteries and inside glomeruli[4-7]. With maturation, renin is restricted to a JG position as seen in the adult animal[8]. We show here that renin is also expressed in pericytes during normal fetal and early postnatal life. In correspondence with the restriction of vascular renin with increased age, the expression of renin in pericytes markedly decreases after 21 d of postnatal life, suggesting that common factors regulate renin in arterioles and pericytes[8]. The nature of those factors remains to be identified, but it is tempting to speculate that an increase in the ability to conserve sodium with age may play a significant role[20].
The results also show that in mice genetically incapable of making angiotensin peptides, the extent of renin expression in pericytes is significantly enhanced. AGT-/- mice are hypotensive and devoid of the negative feedback loop that suppresses renin[13,14]. Thus, two potent regulatory mechanisms combine to augment renin production in a failed attempt to maintain homeostasis[21]. As a result, renin expression increases not only in the vasculature but also in interstitial pericytes, indicating that these cells are subjected to angiotensin-dependent renin inhibition. AGT -/- mice continued to produce renin suggesting initially a persistence of the fetal pattern of renin expression.
Equally interesting is that at 10 d of postnatal life, renin expression in pericytes increases dramatically: new or preexisting pericytes adopted the renin phenotype in an attempt to reestablish homeostasis. The reason(s) for this recruitment is unknown, but it should be noted that pericytes are in close proximity to numerous other cells including tubular, interstitial and vascular cells and are likely to receive and respond to the same signals that convey the physiological imbalance caused by the lack of angiotensin II[9]. Regional tissue ischemia caused by the combination of hypotension and the well-known vascular abnormalities described in AGT -/- animals may be a potent factor inducing the recruitment of additional renin-synthesizing cells[22].
The question is why pericytes, and not other cells, in the interstitial compartment are capable of making renin. Lineage tracing studies from our laboratory demonstrated that renin cells are precursors for other cell types in the kidney, including SM cells of the renal arterioles[8]. We further showed that the descendants from those precursors are capable of re-expressing renin under homeostatic threat. More recently, we found that renin cells are related to pericytes and both, in turn, descend from the same precursor[5,23]. Thus, pericytes possess all the machinery and the epigenetic memory for renin synthesis if required to maintain homeostasis.
We also noted the presence of renin in distal tubules, mostly in the renal medulla of embryonic and young postnatal WT mice. These results are in agreement with previous studies that demonstrated the presence of renin in connecting tubules of adult animals subjected to acute sodium depletion, a situation resembling that of the growing animal[20,24-26].
Similar to the situation in pericytes and arterioles of AGT -/- mice, tubular renin persisted throughout life suggesting that in response to this homeostatic threat, a common signal regulates renin expression in these compartments. The nature of this signal remains to be identified.
Renin is the key regulated enzyme of the renin angiotensin system, and plays a crucial role in the maintenance of blood pressure and fluid electrolyte homeostasis. Whereas in the normal adult kidney renin expression is restricted to a juxtaglomerular localization, in response to a threat to homeostasis or to the pharmacological inhibition of the renin angiotensin system there is an increase in the number of renin expressing cells that resembles a fetal pattern. Renin cells have been associated to the development and branching of the renal arterial tree but its relationship with the microcirculation has not been explored.
Pericytes are smooth muscle-like interstitial cells in close contact with capillaries crucial for the maintenance of a normal capillary network crucial for organ function. Recent studies have shown that in response to injury this population of cells within the kidney expand and differentiate to myofibroblasts, leading to the development of renal interstitial fibrosis. Understanding the origin and characteristics of this cell population has been recently identified as an important area of research.
Recent studies have identified the renal pericytes as the source of cells that contribute to the fibrotic tissue in response to injury. Expression of renin protein in pericytes during normal development and in response to a genetic manipulation that results in a lack of inhibition of the renin angiotensin system has not been previously reported.
In this study authors show that in mice genetically incapable of making angiotensin peptides, the extent of renin expression in pericytes is significantly enhanced indicating that these cells are subjected to angiotensin-dependent renin inhibition. Pharmacological inhibition of the renin angiotensin system may result in increase in the expression of renin in pericytes. Functional studies will be necessary to determine whether pericytes expressing renin lose their normal contractility and contribute to ischemia affect renal blood flow and contribute to the development of renal microvascular disease and progression of chronic kidney disease.
This interesting study demonstrates that the number of renin-expressing pericytes diminishes with age. Renal pericytes are involved in the pathogenesis of kidney disease and its association with the renin angiotensin system may result in a potential therapeutic target.
P-Reviewers Morimoto S, Arici M, Futrakul P, Wu-Wong JR S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Lopez ML, Gomez RA. The renin phenotype: roles and regulation in the kidney. Curr Opin Nephrol Hypertens. 2010;19:366-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Sequeira Lopez ML, Gomez RA. Novel mechanisms for the control of renin synthesis and release. Curr Hypertens Rep. 2010;12:26-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Keeton TK, Campbell WB. The pharmacologic alteration of renin release. Pharmacol Rev. 1980;32:81-227. [PubMed] |
| 4. | Gomez RA, Lynch KR, Chevalier RL, Wilfong N, Everett A, Carey RM, Peach MJ. Renin and angiotensinogen gene expression in maturing rat kidney. Am J Physiol. 1988;254:F582-F587. [PubMed] |
| 5. | Brunskill EW, Sequeira-Lopez ML, Pentz ES, Lin E, Yu J, Aronow BJ, Potter SS, Gomez RA. Genes that confer the identity of the renin cell. J Am Soc Nephrol. 2011;22:2213-2225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Sequeira Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA. Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol. 2001;281:F345-F356. [PubMed] |
| 7. | Gomez RA, Lynch KR, Sturgill BC, Elwood JP, Chevalier RL, Carey RM, Peach MJ. Distribution of renin mRNA and its protein in the developing kidney. Am J Physiol. 1989;257:F850-F858. [PubMed] |
| 8. | Sequeira López ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell. 2004;6:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 221] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 9. | Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1119] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 10. | Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol. 2011;55:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 297] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 11. | Smith SW, Chand S, Savage CO. Biology of the renal pericyte. Nephrol Dial Transplant. 2012;27:2149-2155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Gaengel K, Genové G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 662] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 13. | Kim HS, Maeda N, Oh GT, Fernandez LG, Gomez RA, Smithies O. Homeostasis in mice with genetically decreased angiotensinogen is primarily by an increased number of renin-producing cells. J Biol Chem. 1999;274:14210-14217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Nagata M, Tanimoto K, Fukamizu A, Kon Y, Sugiyama F, Yagami K, Murakami K, Watanabe T. Nephrogenesis and renovascular development in angiotensinogen-deficient mice. Lab Invest. 1996;75:745-753. [PubMed] |
| 15. | Pentz ES, Lopez ML, Kim HS, Carretero O, Smithies O, Gomez RA. Ren1d and Ren2 cooperate to preserve homeostasis: evidence from mice expressing GFP in place of Ren1d. Physiol Genomics. 2001;6:45-55. [PubMed] |
| 16. | Gomez RA, Pentz ES, Jin X, Cordaillat M, Sequeira Lopez ML. CBP and p300 are essential for renin cell identity and morphological integrity of the kidney. Am J Physiol Heart Circ Physiol. 2009;296:H1255-H1262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Sequeira-Lopez ML, Weatherford ET, Borges GR, Monteagudo MC, Pentz ES, Harfe BD, Carretero O, Sigmund CD, Gomez RA. The microRNA-processing enzyme dicer maintains juxtaglomerular cells. J Am Soc Nephrol. 2010;21:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Pentz ES, Moyano MA, Thornhill BA, Sequeira Lopez ML, Gomez RA. Ablation of renin-expressing juxtaglomerular cells results in a distinct kidney phenotype. Am J Physiol Regul Integr Comp Physiol. 2004;286:R474-R483. [PubMed] |
| 19. | Reddi V, Zaglul A, Pentz ES, Gomez RA. Renin-expressing cells are associated with branching of the developing kidney vasculature. J Am Soc Nephrol. 1998;9:63-71. [PubMed] |
| 20. | Spitzer A. The role of the kidney in sodium homeostasis during maturation. Kidney Int. 1982;21:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 50] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Sequeira Lopez ML, Gomez RA. The role of angiotensin II in kidney embryogenesis and kidney abnormalities. Curr Opin Nephrol Hypertens. 2004;13:117-122. [PubMed] |
| 22. | Kim HS, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, Jennette JC, Coffman TM, Maeda N, Smithies O. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci USA. 1995;92:2735-2739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 474] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 23. | Sequeira Lopez ML, Gomez RA. Development of the renal arterioles. J Am Soc Nephrol. 2011;22:2156-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Rohrwasser A, Ishigami T, Gociman B, Lantelme P, Morgan T, Cheng T, Hillas E, Zhang S, Ward K, Bloch-Faure M. Renin and kallikrein in connecting tubule of mouse. Kidney Int. 2003;64:2155-2162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Gonzalez AA, Liu L, Lara LS, Seth DM, Navar LG, Prieto MC. Angiotensin II stimulates renin in inner medullary collecting duct cells via protein kinase C and independent of epithelial sodium channel and mineralocorticoid receptor activity. Hypertension. 2011;57:594-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265-1274. [PubMed] |