Published online Jun 25, 2025. doi: 10.5527/wjn.v14.i2.105815
Revised: March 5, 2025
Accepted: March 21, 2025
Published online: June 25, 2025
Processing time: 61 Days and 7.5 Hours
Renal complications of diabetes mellitus pose a significant public health challenge, contributing to substantial morbidity and mortality globally. Understanding temporal trends and regional disparities in mortality related to diabetic nephropathy is crucial for guiding targeted interventions and policy decisions.
To display the trends and disparities of diabetic nephropathy related mortality.
A retrospective analysis was conducted using death certificate data from the center for disease control and prevention (CDC) wide-ranging online data for epidemiologic research analysis (WONDER) database, spanning from 1999 to 2020, to investigate mortality related to renal complications of diabetes in adults aged 35 or above. Age-adjusted mortality rate (AAMR) per 100000 persons and annual percent change (APC) were computed, with stratification by year, sex, race/ethnicity, and geographic region.
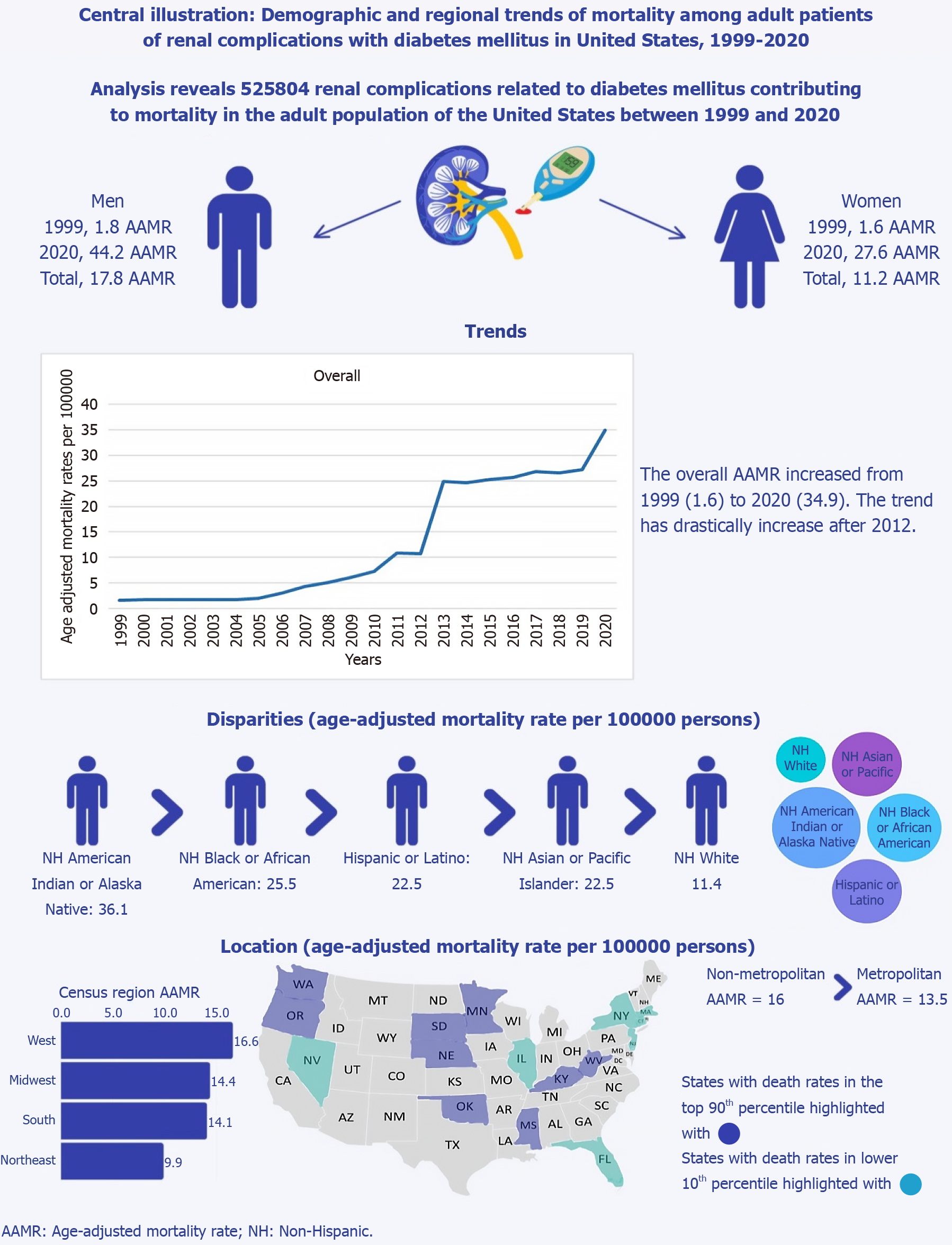
Between 1999 and 2020, a total of 525804 deaths occurred among adults aged 35 to 85+ years due to renal-related issues associated with diabetes. AAMR for renal-related deaths in adult diabetic patients showed a consistent increase from 1.6 in 1999 to 34.9 in 2020 (average APC [AAPC]: 17.23; 95% confidence interval [CI]: 13.35-28.79). Throughout the study period, men consistently had higher AAMR (overall AAMR for men: 17.8; 95%CI: 17.7-17.9). In 1999, the AAMR for men was 1.8, increasing to 44.2 by 2020 (AAPC: 17.54; 95%CI: 13.09-29.53), while for women, it was 1.6 in 1999 and rose to 27.6 by 2020 (AAPC: 15.55; 95%CI: 13.35-21.10). American Indian/Alaska Native adults exhibited the highest overall AAMR (36.1; 95%CI: 35.2-36.9), followed by Black/African American (25.5; 95%CI: 25.3-25.7). The highest mortality was observed in the Western (AAMR: 16.6; 95%CI: 16.5-16.7), followed by the Midwestern region (AAMR: 14.4; 95%CI: 14.314.4). Significant variations in AAMR were observed among different states, with Oklahoma recording the highest (21.2) and Connecticut the lowest (7). The CDC WONDER database could potentially have omissions or inaccuracies. It does not provide data outside of the available variables. Furthermore, dataset after 2020 was not included in this study.
Our findings highlight an alarming rise in mortality related to renal complications of diabetes among United States adults over the past two decades, with concerning disparities across demographic and geographic factors. These results underscore the urgent need for targeted interventions, policies, and protocols to address the growing burden of diabetic nephropathy and substantially reduce mortality rates in the United States. This will help improve the overall health outcome in the United States by identifying communities at risk and implementing tailored assistance to them.
Core Tip: This study focused on investigating renal complications of diabetes mellitus through implementing a large United States database. Our analysis aimed to display mortality rates resulting from renal complications of diabetes on a large scale. The results showed gender, racial, and geographic disparities with higher mortality risk in male patients, native Indian/Alaskan, western states, and nonmetropolitan areas. These disparities emphasized on the importance of involving and encouraging healthcare stakeholders to take further action to improve healthcare specially for vulnerable populations.
- Citation: Muhammad AN, Ahmed F, Eltawansy S, Ali A, Azeem B, Kashan M, Afzaal Z, Ahmed M, Aman K, Amanullah A, Naveed Uz Zafar M, Lajczak P, Obi O. Epidemiological trends in diabetic renal complications in United States adults: A center for disease control and prevention wide-ranging online data for epidemiologic research analysis (1999-2020). World J Nephrol 2025; 14(2): 105815
- URL: https://www.wjgnet.com/2220-6124/full/v14/i2/105815.htm
- DOI: https://dx.doi.org/10.5527/wjn.v14.i2.105815
Diabetes mellitus (DM) is a chronic metabolic disorder associated with elevated blood sugar levels and subsequent insulin dysfunction. DM can damage both macro- and microvasculature, causing complications that impact various body organs, including the kidneys[1]. Diabetic kidney disease (DKD) is a highly prevalent complication of DM, often resulting in chronic kidney disease and end-stage renal disease[2]. The progression of DKD is influenced by factors such as obesity, hypertension, poor glycemic control, and the complex interplay of genetic, environmental, and lifestyle factors[3,4]. In the United States, diabetic nephropathy is the leading cause of renal disease, contributing significantly to morbidity and mortality in the affected individuals, with a prevalence between 20% and 40% among diabetic patients[5].
Approximately 7% of the United States population have DM[6], and renal failure is listed as the cause of death in 6%-12% of adults with DM. The incidence of renal diseases has increased significantly in the diabetic population as compared to the nondiabetic population[7]. The prevalence of diabetic nephropathy remains a strong predictor of morbidity and mortality in diabetic patients[8]; hence, there is an urgent need to understand the trends associated with DKD related mortality in hopes to drastically reduce the mortality rate in the United States population. The existing knowledge gap will be covered by understanding these patterns as we will gain insight into the various aspects that contribute to the increased morbidity and mortality associated with diabetic nephropathy. We will be able to identify high risk regions and direct focused assistance towards these communities to help alleviate the burden of DKD. The present study aimed to examine the United States mortality trends from 1999 to 2020, using advanced statistical methods to assess the link between DM-related renal complications and mortality, while considering the influence of other factors. The results of our study will provide crucial insights for public health interventions and policy-making, guiding targeted interventions to reduce diabetic nephropathy-related mortality among the adult population in the United States.
The center for disease control and prevention (CDC) wide-ranging online data for epidemiologic research analysis (WONDER) database was used to collect data from death certificates[9]. The study analyzed death incidents of adults secondary to diabetes-related renal complications between 1999 and 2020 utilizing the International Classification of Diseases and Related Health Problems 10th version (ICD-10) codes E10.2, E11.2, E12.2, E13.2, and E14.2[10]. The study looked at death records from the Multiple Cause-of-Death Public Use registry to find the incidence of diabetes-related renal complications. Renal complications of diabetes were listed as a contributing factor or as the primary cause of death in these cases. The research did not require permission from a regional institutional review board since it relied on deidentified public use data provided by the government. The STROBE standards for reporting observational research were followed in this study[11].
The population number, year, place of death, demographic features, geographical breakdown, state-specific statistics, and distinction between urban and rural areas are all included in the dataset. The location of death consists of various places, including hospitals, houses, hospices, nursing homes, and long-term care institutions. The term "demographics" refers to information on gender, age, race, and ethnicity. The following are the categories for race and ethnicity: White, Black/African American, Latino, American Indians/Alaska Natives, and Asian/Pacific Islanders.
According to the National Centre for Health Statics Urban-Rural Classification Scheme, the population was divided into urban areas (which included large metropolitan areas with a population of 1 million or more, as well as medium/small metropolitan areas with a population ranging from 50000 to 999999), rural areas (with a population of less than 50000), and other counties in the 2013 United States Census[12]. Additionally, the Northeast, Midwest, South, and West regions are categorized into four distinct geographical groups according to standards set by the United States Census Bureau[13].
We analyzed the mortality rate per 100000 individuals for both unadjusted and age-adjusted data across the period from 1999 to 2020 to investigate regional trends in mortality related to acute renal failure. These rates were divided into categories based on year, gender, race/ethnicity, state, and urban/rural status. The crude mortality rates were determi
Diabetes-related renal complications in adult patients caused a total of 525804 deaths in the United States between 1999 and 2020 (Supplementary Table 1). Of these, 39.78% occurred within medical facilities, 30.42% at home, 20.55% in nursing homes/long-term care facilities, 5.72% in hospices, and 3.42% at other places (Supplementary Table 2).
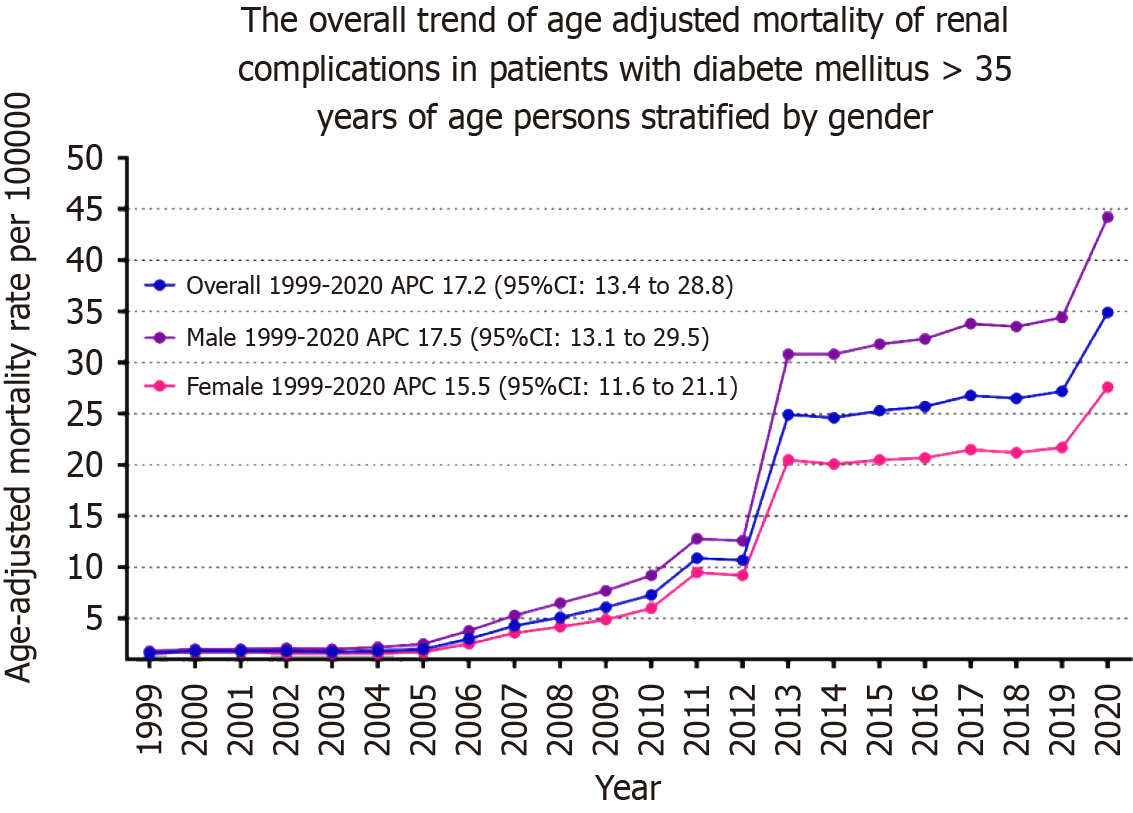
AAMR exhibited a consistent upward trend from 1999 until the study's conclusion in 2020; 1.6 to 34.9, respectively (average APC [AAPC]: 17.23; 95%CI: 13.35-28.79). AAMR slightly increased from 1999 to 2010 (APC: 16.69; 95%CI: -33.79-165.4) followed by a marked increase from 2010 to 2013 (APC: 55.08; 95%CI: 4.27-72.62) and slow increase from 2013 to 2018 (APC: 1.56; 95%CI: -4.03-53.29), and then it again increased dramatically from 2018 to 2020 (APC: 13.71; 95%CI: 3.95-20.90) (Figure 1, Supplementary Tables 3 and 4).
Adult men had consistently higher AAMR than adult women throughout the study period (overall AAMR for men: 17.8, 95%CI: 17.7-17.9; women: 11.2, 95%CI: 11.1-11.2). In 1999, the AAMR for adult men was 1.8, which steadily increased to 9.2 in 2010 (APC: 17.77; 95%CI: -36.74-99.34), followed by a dramatic increase to 30.8 in 2013 (APC: 48.95; 95%CI: -1.52-65.02), and by 2020, it increased to 44.2 (APC: 5.89; 95%CI: 1.02-16.18). For adult women, the AAMR in 1999 was 1.6, which steadily increased to 6 in 2010 (APC: 15.62; 95%CI: -25.63-27.41), followed by a dramatic increase to 20.5 in 2013 (APC: 52.10; 95%CI: 31.49-68.29), and by 2020 it increased to 27.6 (APC: 2.60; 95%CI: 0.27-4.69). The AAMR for adult men and women in 1999 was 1.8 and 1.6, respectively, which increased to 44.2 and 27.6 in 2020 (men: AAPC: 17.54, 95%CI: 13.09-29.53; women: AAPC: 15.55, 95%CI: 13.35-21.10) (Figure 1, Supplementary Tables 3 and 4).
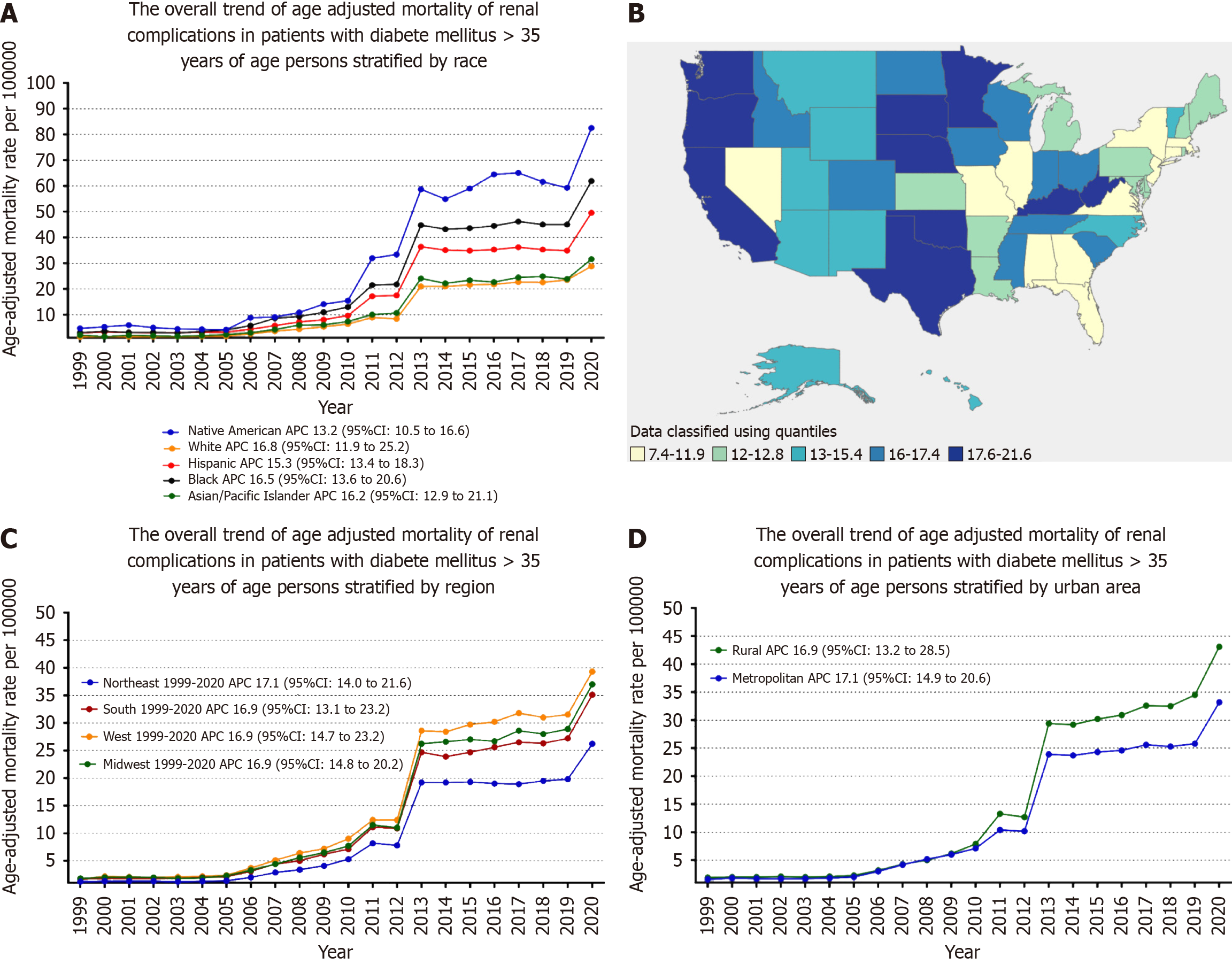
When stratified by race/ethnicity, AAMR was highest among American Indian/Alaska Native, followed by Black/African American, Hispanic, Asian/Pacific Islander, and White populations (overall AAMR for American Indian/Alaska Native: 36.1, 95% CI: 35.2-36.9; Black/African American: 25.5, 95%CI: 25.3-25.7; Hispanic: 22.5, 95%CI: 22.4-22.7; Asian/Pacific Islander: 15.4, 95%CI: 15.2-15.6; White: 11.4, 95% CI: 11.4-11.5).
The AAMR of American Indian/Alaska Natives slightly decreased from 1999 to 2005 (APC: -2.79; 95%CI: -30.45-12.84), followed by a significant increase from 2005 to 2013 (APC: 35.91; 95%CI: 29.51-55.89), and then steadily increased from 2013 to 2020 (APC: 4.73; 95%CI: 1.78-7.60).
The AAMR trend of Black/African American, Asian/Pacific Islander, and White followed the same pattern, progressively increasing from 1999 to 2010 (Black/African American: APC: 17.48; 95%CI: -16.02-26.89; Asian/Pacific Islander: APC: 16.85; 95%CI: -22.56-28.84; White: APC: 16.74; 95%CI: -40.94-35.73), followed by a dramatic increase from 2010 to 2013 (Black/African American: APC: 47.73; 95%CI: 25.1-63.27; Asian/Pacific Islander: APC: 45.58; 95%CI: 2.88-60.93; White: APC: 51.76; 95%CI: 6.27-70.78), and then increased till 2020 (Black/African American: APC: 3.91; 95%CI: 0.26-7; Asian/Pacific Islander: APC: 4.57; 95%CI: 0.79-9.22; White: APC: 4.42; 95%CI: 2.08-7.06).
The AAMR of Hispanics/Latinos steadily increased from 1999 to 2009 (APC: 11.18; 95%CI: -1.32-9.22), followed by a massive increase from 2009 to 2013 (APC: 50.33; 95%CI: 28.09-70.14), then gradually decreased from 2013 to 2018 (APC:
A significant difference in AAMR was observed in different states, with the AAMR ranging from 21.2 (95%CI: 20.8-21.7) in Oklahoma to 7 (95%CI: 6.8-7.3) in Connecticut. States falling into the top 90th percentile were Kentucky, Minnesota, Mississippi, Nebraska, Oklahoma, Oregon, South Dakota, Washington, and West Virginia, which had approximately two times the AAMR compared to states that fell into the lower 10th percentile, namely, Connecticut, Florida, Illinois, Massachusetts, Nevada, New Jersey and New York (Figure 2B, Supplementary Table 6).
On average, throughout the study period, the highest mortality was observed in the Western (AAMR: 16.6; 95%CI: 16.5-16.7), followed by the Midwestern region (AAMR: 14.4; 95%CI: 14.314.4), Southern (AAMR: 14.1; 95%CI: 14-14.2), and Northeastern regions (AAMR: 9.9; 95%CI: 9.8-10) (Figure 2C, Supplementary Table 7).
Nonmetropolitan areas had consistently higher AAMR than metropolitan areas throughout the study period, with an overall AAMR of 16 (95%CI: 15.9-16.1) and 13.5 (95%CI: 13.4-13.5), respectively. AAMR of nonmetropolitan steadily ascended from 1999 to 2009 (APC: 14.80, 95%CI: -5.31-22.87), followed by a dramatic increase from 2009 to 2013 (APC: 47.93, 95%CI: 34.62-67.98), and then slightly increased till 2020 (APC: 5.41, 95%CI: 2.46-8.19). Similarly, the AAMR of metropolitan areas steadily ascended from 1999 to 2010 (APC: 16.23, 95%CI: -34.35-192.1), followed by a dramatic increase from 2010 to 2013 (APC: 54.49, 95%CI: 1.78-72.14), followed by a slight increase from 2013 to 2018(APC: 1.40, 95%CI: -4.32-55.78), and then again significantly increased from 2018 to 2020 (APC: 13.48, 95%CI: 3.47-20.85) (Figure 2D, Supplementary Tables 3 and 8). Figure 3 is a central illustration of the trends in Demographics and Disparities in Renal Complications in our study population (Figure 3).
The results of our study offer valuable insights into the evolving trends of mortality associated with renal complications among diabetic patients in the United States through the past two decades, from 1999 to 2020. Previous research examining mortality trends linked to diabetic nephropathy has similarly pointed towards an increase in mortality rates[19]. We analyzed national mortality data to explain patterns, disparities, and potential contributing factors to guide preventive strategies and improve clinical management for this vulnerable population.
Our analysis showed that men consistently exhibited higher AAMR compared to women throughout the study period. AAMR varied across racial/ethnic groups, with the highest rate among the American Indian/Alaska Native population. Geographically, significant differences in AAMR were evident among states and regions, with Western states and nonmetropolitan areas consistently showing higher AAMR throughout the study period.
The continuous upward trend in AAMR observed over the years likely stems from a complex interplay of factors, including the increasing disease prevalence, longer disease duration, suboptimal glycemic control, presence of comorbidities, healthcare access, and quality issues, as well as environmental and lifestyle factors[20].
The analysis revealed a consistent pattern of higher AAMR among males than females throughout the study period. This gender disparity in the findings may be due to inherent biological elements, behavioral factors, healthcare utilization, and occupational exposure. Additionally, men tend to engage in more hazardous behavior, such as unhealthy dietary habits and higher rates of smoking and alcohol consumption, which can exacerbate the progression of diabetes and its complications[21]. Furthermore, the gender disparity in the frequency of DKD in men and women could be attributed to hormonal differences. Studies have shown a reno-protective effect of estrogen and progesterone[22]. In diabetic nephropathy, depletion of the concentration of plasma estradiol is found with dysfunction of the signaling of the estrogen receptor[23]. Testosterone is associated with exacerbation of diabetic nephropathy[24].
Moreover, significant discrepancies in AAMR were observed across racial and ethnic groups, with American Indian/Alaska Native and white populations having the highest and lowest AAMR, respectively. The average life expectancy in the United States for American Indians is 72.8 years old, which is 6.9 years lower than that for white Americans[25]. These disparities may be influenced by genetic predispositions, cultural factors, and historical trauma, which may affect health behaviors and healthcare utilization, alongside socioeconomic disparities that encompass limited healthcare access and lower socioeconomic status. These factors are linked to a higher prevalence of health issues such as diabetes and a lower likelihood of rehabilitation success[26]. Studies have found that there is a sense of doubt and uncertainty amongst the elderly population of the American Indians particularly regarding the healthcare facilities and costs of healthcare services[27]. Low literacy levels, unemployment, and lack of adequate resources play a part in further promoting healthcare disparities in these populations[28]. Some additional key factors involved in the increased risk for diabetes and kidney disease in the American Indian population include poverty, over nutrition, poor health care, and high intake of sugar[29]. Moreover, higher rates of comorbidities within these communities, such as obesity and hypertension, contribute to the increased burden of diabetic complications. Research consistently shows that American Indian children and adolescents have a higher prevalence of overweight and obesity compared to their white counterparts[30-32]. These high risk communities have an increased risk of metabolic syndrome as well. This, in turn, snowballs and leads to drastic complications associated with diabetes[33].
While exploring the genetic component of the increased mortality in this specific race, a genome wide association study was done in Pima Indians. A variant of 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase isoform 2 (PFKFB2), which is important in glucose metabolism, was identified. This variation appears to increase the risk of diabetic nephropathy[34]. Further aspects of gene expression in American Indians/Alaska Natives remain largely unexplored and efforts are consistently being made. The Traditional Food Project is an initiative to prevent diabetes in American Indian/Alaska Native communities. It recognizes that traditional foods and food sovereignty are important areas to address the public health issues of chronic disease[35].
In addition, we also observed significant geographical variations in AAMR, with the Western region having the highest-burden compared to other United States regions, and it may occur because of macro social determinants of health[36]. The western region of the United States, especially in rural areas, faces significant challenges in accessing quality healthcare[37,38]. These challenges are exacerbated by economic instability, leading to higher poverty rates and an aging population[36]. Factors such as distance to healthcare facilities and overall satisfaction with care can influence the preference for a particular healthcare source[39]. Studies show that older ethnic minorities in rural communities are at increased risk for diabetes complications[40]. Most of these people in western rural United States have low literacy rates, minimal household income, and an inherent inability to afford healthcare services that are not easily accessible to them. All of these factors contribute significantly to the elevated mortality consistent with our findings.
Nonmetropolitan areas consistently recorded higher AAMR than metropolitan areas over the study period. This trend can be attributed to several interrelated factors. First, nonmetropolitan regions often face limited access to healthcare resources, including fewer hospitals, specialists, and medical services[41]. Consequently, residents may experience delays in receiving essential medical treatment, leading to adverse health outcomes and, ultimately, higher mortality rates. Second, nonmetropolitan areas frequently grapple with health disparities stemming from socioeconomic factors such as poverty, lower education levels, and a higher prevalence of unhealthy behaviors like smoking and poor diet choices[42]. These disparities contribute to a higher burden of chronic diseases and overall worse health outcomes in these areas. Additionally, nonmetropolitan populations tend to be older, as younger individuals often migrate to urban centers in search of opportunities[43]. The aging population is with increased risk of age-related health issues, contributing to increased mortality rates. Environmental factors, such as exposure to pollution from agricultural activities or limited access to clean water sources, can also exacerbate health challenges in nonmetropolitan areas[44,45].
Furthermore, the shortage of healthcare providers and lack of infrastructure contribute to inadequate healthcare delivery and management of health conditions. Lastly, unhealthy lifestyle choices such as substance abuse and a sedentary lifestyle are more prevalent in nonmetropolitan regions, further compounding the health risks faced by residents[46,47]. Addressing these complex challenges requires comprehensive strategies that encompass improving healthcare access, socioeconomic conditions, public health interventions, and infrastructure development tailored to the unique needs of the nonmetropolitan communities.
Our study showed significant disparities in AAMR due to renal complications of diabetes across different states, ranging from 7 in Connecticut to 21.2 in Oklahoma. Several factors contribute to these disparities. Access to healthcare stands out as a critical determinant, with states offering better access to specialized clinics and timely screenings likely experiencing lower mortality rates[48]. Socioeconomic factors such as income, education, and insurance coverage further exacerbate these differences, as individuals with higher socioeconomic status often have better access to resources and healthcare services. Cultural values, dietary habits, and lifestyle choices also influence outcomes, with states embracing healthier habits potentially experiencing lower mortality rates[49].
Comorbidities such as obesity have been extensively researched and the results consistently prove that the prevalence of type 2 diabetes and its complications increases exponentially in the presence of these additive factors. This also includes hypertension as diabetes-related kidney disease is significantly higher in hypertensive population[50]. Addi
Our study further cemented the statement that DKD is a significant cause of morbidity and mortality. According to a previous study using population based National Health and Nutrition Examination Survey (NHANES), the ratio of prevalence of DKD adjusted for age, sex, race/ethnicity in 2005-2008 was 1.34 (1.11-1.61) and 0.98 (0.87-1.10) among the United States general population and persons with diabetes, respectively. The trend established for DKD in the general United States population was statistically significant (P = 0.003), but for the diabetic population, it was not statistically significant (P = 0.77)[52]. Hence, it is imperative to establish a trend that is evidence based. Our study validated the previous literature stating that diabetic nephropathy is one of the critical consequences in diabetic population. It further categorizes it and provides vital insight into the patterns and identifies high risk communities.
Addressing these multifaceted elements is imminent when defining guidelines for combatting the increasing prevalence of diabetes-related kidney disease. This can only be achieved through targeted interventions. Focusing on better healthcare access and monitoring the high risk population at regular intervals with a predetermined protocol is crucial. Early screening will play a pivotal role in decreasing the disease burden associated with DKD[33]. These policies are absolutely critical to narrowing the gap in AAMR due to diabetes-related renal complications across regions and improving outcomes among individuals with diabetes nationwide.
The study faced several limitations that merit attention. First, it relied primarily on the CDC WONDER database which records death certificates but may have inaccuracies or omissions. The stratification of years in the database is such that we used 1999-2020 for our study to include two decades worth of data to establish a trend with the most available evidence; hence, any data after 2020 was not included. Additionally, AAMR was calculated using the 2000 United States population as the standard population, and no other standard populations were used. Moreover, the study exclusively focused on individuals aged 35 and above, potentially overlooking variations in younger age groups. Interpretation of trends may have been influenced by unaccounted factors, introducing biases in the conclusions. Furthermore, the lack of clinical data, such as specific biomarkers and clinical parameters, treatment methods, lab results, or therapeutic approa
This analysis of renal complications of diabetes-related mortality data spanning 1999 to 2020 reveals an increasing trend in AAMR at an alarming rate, emphasizing the burden on public health. These findings highlight the significant disparities that exist across racial and ethnic groups and gender. The highest AAMR was observed in American Indian/Alaska Natives and men, respectively. Additionally, geographic differences play an impact, as seen with the consistently higher AAMR observed in nonmetropolitan regions and Western states. The disproportionate impact on these groups demands immediate attention. To combat this escalating threat to public health, it is imperative to implement focused interventions and enhance healthcare accessibility especially in the high risks communities to alleviate the deepening burden of renal complications associated with diabetes. These policies will enhance overall health outcomes nationwide.
| 1. | Prabhakar PK. Pathophysiology of Diabetic Secondary Complication and their Management. Curr Diabetes Rev. 2021;17:395-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | John S. Complication in diabetic nephropathy. Diabetes Metab Syndr. 2016;10:247-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Maric C, Hall JE. Obesity, metabolic syndrome and diabetic nephropathy. Contrib Nephrol. 2011;170:28-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Papadopoulou-Marketou N, Paschou SA, Marketos N, Adamidi S, Adamidis S, Kanaka-Gantenbein C. Diabetic nephropathy in type 1 diabetes. Minerva Med. 2018;109:218-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Kowalski A, Krikorian A, Lerma EV. Diabetes and chronic kidney disease. Dis Mon. 2015;61:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Hahr AJ, Molitch ME. Diabetes, cardiovascular risk and nephropathy. Cardiol Clin. 2010;28:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Samsu N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. Biomed Res Int. 2021;2021:1497449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 494] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 8. | Ajiboye O, Segal JB. National trends in the treatment of diabetic nephropathy in the United States. J Clin Pharm Ther. 2017;42:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Friede A, Reid JA, Ory HW. CDC WONDER: a comprehensive on-line public health information system of the Centers for Disease Control and Prevention. Am J Public Health. 1993;83:1289-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 104] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | ICD-10 Version: 2019. Available from: https://icd.who.int/browse10/2019/en. |
| 11. | Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13:S31-S34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 2045] [Article Influence: 340.8] [Reference Citation Analysis (1)] |
| 12. | Data Access - Urban Rural Classification Scheme for Counties. Available from: https://www.cdc.gov/nchs/data_access/urban_rural.htm. |
| 13. | Census.gov. Available from: https://www.census.gov/. |
| 14. | WISQARS Frequently Asked Questions. Available from: https://www.cdc.gov/injury/wisqars/mapping_help/crude_rate.html. |
| 15. | Missouri Department of Health and Senior Services. Available from: https://health.mo.gov/data/mica/CDP_MICA/AARate.html. |
| 16. | Joinpoint Trend Analysis Software. Available from: https://surveillance.cancer.gov/joinpoint/. |
| 17. | Anderson RN, Rosenberg HM. Age standardization of death rates: implementation of the year 2000 standard. Natl Vital Stat Rep. 1998;47:1-16, 20. [PubMed] |
| 18. | What Is a Two-Tailed Test? Definition and Example, Investopedia. Available from: https://www.investopedia.com/terms/t/two-tailed-test.asp. |
| 19. | Harvey JN. Trends in the prevalence of diabetic nephropathy in type 1 and type 2 diabetes. Curr Opin Nephrol Hypertens. 2003;12:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Shrestha P, Ghimire L. A review about the effect of life style modification on diabetes and quality of life. Glob J Health Sci. 2012;4:185-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Rimm EB, Chan J, Stampfer MJ, Colditz GA, Willett WC. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. BMJ. 1995;310:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 361] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 22. | Giandalia A, Giuffrida AE, Gembillo G, Cucinotta D, Squadrito G, Santoro D, Russo GT. Gender Differences in Diabetic Kidney Disease: Focus on Hormonal, Genetic and Clinical Factors. Int J Mol Sci. 2021;22:5808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 23. | Maric-Bilkan C. Sex Differences in Diabetic Kidney Disease. Mayo Clin Proc. 2020;95:587-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Liu J, Liu Z, Sun W, Luo L, An X, Yu D, Wang W. Role of sex hormones in diabetic nephropathy. Front Endocrinol (Lausanne). 2023;14:1135530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 25. | Dankovchik J, Hoopes MJ, Warren-Mears V, Knaster E. Disparities in life expectancy of pacific northwest American Indians and Alaska natives: analysis of linkage-corrected life tables. Public Health Rep. 2015;130:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Mohammed SA. The dynamic interplay between low socioeconomic status and diabetes for urban American Indians. Fam Community Health. 2011;34:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Jaramillo ET, Willging CE. Producing insecurity: Healthcare access, health insurance, and wellbeing among American Indian elders. Soc Sci Med. 2021;268:113384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Mangla A, Agarwal N. Clinical Practice Issues in American Indians and Alaska Natives. 2023 May 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 29. | Yracheta JM, Lanaspa MA, Le MT, Abdelmalak MF, Alfonso J, Sánchez-Lozada LG, Johnson RJ. Diabetes and Kidney Disease in American Indians: Potential Role of Sugar-Sweetened Beverages. Mayo Clin Proc. 2015;90:813-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Ness M, Barradas DT, Irving J, Manning SE. Correlates of overweight and obesity among American Indian/Alaska Native and Non-Hispanic White children and adolescents: National Survey of Children's Health, 2007. Matern Child Health J. 2012;16 Suppl 2:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Schell LM, Gallo MV. Overweight and obesity among North American Indian infants, children, and youth. Am J Hum Biol. 2012;24:302-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Zephier E, Himes JH, Story M, Zhou X. Increasing prevalences of overweight and obesity in Northern Plains American Indian children. Arch Pediatr Adolesc Med. 2006;160:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Reynolds EL, Akinci G, Banerjee M, Looker HC, Patterson A, Nelson RG, Feldman EL, Callaghan BC. The determinants of complication trajectories in American Indians with type 2 diabetes. JCI Insight. 2021;6:e146849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Muller YL, Piaggi P, Hanson RL, Kobes S, Bhutta S, Abdussamad M, Leak-Johnson T, Kretzler M, Huang K, Weil EJ, Nelson RG, Knowler WC, Bogardus C, Baier LJ. A cis-eQTL in PFKFB2 is associated with diabetic nephropathy, adiposity and insulin secretion in American Indians. Hum Mol Genet. 2015;24:2985-2996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | DeBruyn L, Fullerton L, Satterfield D, Frank M. Integrating Culture and History to Promote Health and Help Prevent Type 2 Diabetes in American Indian/Alaska Native Communities: Traditional Foods Have Become a Way to Talk About Health. Prev Chronic Dis. 2020;17:E12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Borders TF, Aday LA, Xu KT. Factors Associated With Health‐Related Quality of Life Among an Older Population in a Largely Rural Western Region. The Journal of Rural Health. 2004;20:67-75. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Reavy K. The impact of recession on the health care of rural citizens in the northwest United States. Rural Remote Health. 2009;9:1270. [PubMed] |
| 38. | Wilson SL, Kratzke C, Hoxmeier J. Predictors of access to healthcare: what matters to rural Appalachians? Glob J Health Sci. 2012;4:23-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Lucas CA, Rosenthal TC. Access to health care in rural western New York State. N Y State J Med. 1992;92:465-469. [PubMed] |
| 40. | Quandt SA, Bell RA, Snively BM, Smith SL, Stafford JM, Wetmore LK, Arcury TA. Ethnic disparities in glycemic control among rural older adults with type 2 diabetes. Ethn Dis. 2005;15:656-663. [PubMed] |
| 41. | Merwin E, Snyder A, Katz E. Differential access to quality rural healthcare: professional and policy challenges. Fam Community Health. 2006;29:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Fiscella K, Williams DR. Health disparities based on socioeconomic inequities: implications for urban health care. Acad Med. 2004;79:1139-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 43. | Movement of non-metropolitan youth towards the cites. Available from: https://research.acer.edu.au/cgi/viewcontent.cgi?article=1053&context=lsay_research. |
| 44. | Wells EC, Vidmar AM, Webb WA, Ferguson AC, Verbyla ME, de Los Reyes FL 3rd, Zhang Q, Mihelcic JR. Meeting the Water and Sanitation Challenges of Underbounded Communities in the U.S. Environ Sci Technol. 2022;56:11180-11188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Borders TF, Wen H. Illicit Drug and Opioid Use Disorders among Non-Metropolitan Residents Nationally. Lexington, KY: Rural and Underserved Health Research Center, 2018. |
| 46. | Carson V, Iannotti RJ, Pickett W, Janssen I. Urban and rural differences in sedentary behavior among American and Canadian youth. Health Place. 2011;17:920-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Park E. [A comparative study of youth health risk behaviors by region: focused on metropolitan areas, medium sized and small city areas, and rural areas]. J Korean Acad Nurs. 2010;40:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Gong G, Phillips SG, Hudson C, Curti D, Philips BU. Higher US Rural Mortality Rates Linked To Socioeconomic Status, Physician Shortages, And Lack Of Health Insurance. Health Aff (Millwood). 2019;38:2003-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 49. | Montez JK, Mehri N, Monnat SM, Beckfield J, Chapman D, Grumbach JM, Hayward MD, Woolf SH, Zajacova A. U.S. state policy contexts and mortality of working-age adults. PLoS One. 2022;17:e0275466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 50. | Wagnew F, Eshetie S, Kibret GD, Zegeye A, Dessie G, Mulugeta H, Alemu A. Diabetic nephropathy and hypertension in diabetes patients of sub-Saharan countries: a systematic review and meta-analysis. BMC Res Notes. 2018;11:565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 51. | Maqbool M, Cooper ME, Jandeleit-Dahm KAM. Cardiovascular Disease and Diabetic Kidney Disease. Semin Nephrol. 2018;38:217-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 52. | de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 758] [Article Influence: 54.1] [Reference Citation Analysis (0)] |