Published online Jun 25, 2025. doi: 10.5527/wjn.v14.i2.102713
Revised: February 25, 2025
Accepted: March 4, 2025
Published online: June 25, 2025
Processing time: 165 Days and 2.4 Hours
Proliferative lupus nephritis (PLN) is the most severe form of lupus nephritis (LN). There are limited data available on renal outcomes of PLN from developing countries.
To determine the clinicopathological characteristics and long-term outcomes in terms of remission, requirement of kidney replacement therapy (KRT), and pa
A retrospective analysis was conducted on biopsy-proven focal or diffuse PLN cases diagnosed between 1998 and 2019 at the Sindh Institute of Urology and Transplantation and followed up at the renal clinic for a minimum of 5 years. All patients were induced with a combination of intravenous cyclophosphamide and corticosteroids for 6 months, followed by maintenance treatment with azathio
The mean age at the onset of systemic lupus erythematosus was 24.12 years ± 8.89 years, and at LN onset, 26.63 years ± 8.61 years. There was a female predominance of 184 (88.9%) cases. Among baseline characteristics, reduced estimated glome
Baseline renal functions, requirement of KRT, and diffuse proliferative disease were the most relevant prognostic factors for kidney survival among this cohort. Short-term renal outcomes were good. Long-term outcomes were poorer with AZA-based maintenance therapy than with MMF, with more ESKD and mortality.
Core Tip: Lupus nephritis (LN) presents significant variability in clinical manifestations and treatment response. Although current treatments have markedly improved outcomes for patients with proliferative LN (PLN), a significant number of patients still gradually progress to end-stage kidney disease. There is still a lack of understanding about the factors that affect therapy non-response and the survival rates of patients with PLN, particularly from developing countries. This study aims to bridge these gaps, enhancing understanding of outcome disparities between developed and developing countries.
- Citation: Ahmed S, Elahi T, Mubarak M, Ahmed E. Clinicopathological characteristics and long-term outcomes of adult patients with proliferative lupus nephritis. World J Nephrol 2025; 14(2): 102713
- URL: https://www.wjgnet.com/2220-6124/full/v14/i2/102713.htm
- DOI: https://dx.doi.org/10.5527/wjn.v14.i2.102713
One of the gravest forms of systemic lupus erythematosus (SLE) is lupus nephritis (LN), which affects the kidneys. About 38% of individuals with SLE experience renal complications, with a prevalence ranging from 12% to 69%, influenced by factors such as ethnicity, sex, and age of onset[1]. Among Black individuals, a higher frequency, earlier onset, and poorer prognosis have been documented[2,3]. Conversely, renal involvement in European populations is reportedly 27.9%[4]. There is significant variability among Asian countries, with rates ranging from 18% to 100% but predominantly exceeding 50%[5].
LN is currently classified histopathologically into classes I to VI, based on the 2003 classification system by the International Society of Nephrology/Renal Pathology Society (ISN/RPS)[6], over half of the patients are affected by proliferative LN (PLN)[6], which includes either class III or class IV, either alone or in combination with class V. This condition includes both focal and diffuse disease, leading to a higher risk of mortality and affecting both short- and long-term renal survival. Consequently, for patients with PLN, aggressive immunosuppressive therapy is suggested to enhance renal outcomes. This approach has resulted in a global 10-year renal survival rate of nearly 90%[7]. Caucasians generally show a better response and more favorable long-term outcomes compared to more ethnically diverse populations in the United States and Asia[8,9].
Patients with LN exhibit a wide spectrum of clinical presentations, from subtle urinary irregularities to severe, symptomatic cases of nephritic syndrome or swiftly advancing renal failure[10]. It is an important cause of chronic kidney disease (CKD) and mortality[11,12]. Despite advancements in treatment strategies and improved patient survival over recent decades, 10%-20% of patients still progress to end-stage kidney disease(ESKD) within the first 10 years of their disease course[13,14]. Therefore, early prediction of long-term renal outcomes is crucial.
Discrepant renal outcomes have been reported across various Asian studies, potentially due to differences in sample size, time between symptom onset and treatment initiation, histological classifications (classes III, IV, and V lesions), remission criteria, treatment regimens, follow-up durations, relapses, and flares[15-17]. Better survival rates have been linked to timely referrals to nephrologists, heightened awareness, the success of new induction regimens, and overall advancements in medical care. As a result, the management objectives for PLN can be divided into short-term goals (preventing flares) and long-term aims (preserving renal function)[18-20]. The initiation of induction therapy with cyclophosphamide (CYC) has improved patient survival, with a 5-year survival rate of 82% for class IV LN[21].
Although extensive research on LN treatment outcomes and survival has been conducted in developed countries, the issue remains underexplored in developing nations. Despite the higher prevalence of LN in Asian countries compared to Europe, data from South Asian countries, particularly Pakistan, is scarce. A study by Rabbani et al[22] reported the frequency of renal involvement among Pakistani patients with SLE and those who progressed to ESKD in 2009[23]. Since then, no comprehensive data on renal outcomes, whether short- or long-term, has been published from Pakistan. Further research is needed to understand the factors related to therapy non-response and the survival outcomes of patients with LN, particularly those with PLN.
Hence, the current study aimed to address these gaps in knowledge by determining the clinicopathological characteristics and long-term outcomes in patients with PLN.
The present study was approved by the institutional review board of the Sindh Institute of Urology and Transplantation (SIUT) (SIUT-ERC-2020/A-227; Karachi, Pakistan). The research was conducted in accordance with the ethical principles of the Declaration of Helsinki.
A retrospective review was conducted on the medical records of all adults over 18 years old who were referred to the renal division of SIUT in Karachi, Pakistan, with a serological and histopathological diagnosis of PLN from January 1998 to December 2019. These patients were followed up at the renal clinic for at least 5 years after their renal biopsy.
The medical records of all adult patients diagnosed with PLN were examined for various clinical, biochemical, serological, and histopathological parameters at initial presentation and during subsequent follow-ups at the renal clinic. Clinical information reviewed included age at SLE diagnosis and renal biopsy, sex, history of constitutional symptoms, oliguria, duration of symptoms prior to admission, a comprehensive review of extra-renal manifestations, and physical examination findings such as hypertension, edema, rash, and other pre-biopsy signs. Additionally, the records detailed the treatment regimens administered, the necessity for kidney replacement therapy (KRT), and follow-up data spanning a minimum of 5 years.
Laboratory parameters such as serum creatinine and albumin levels were recorded both at presentation and during follow-up visits. Serum complement levels (complement component 3 [C3] and C4) were noted and categorized as low or normal. Additionally, detailed urine reports, urine protein creatinine ratio (PCR) upon arrival and subsequently, and 24-hour urinary protein levels (if available) were documented. The estimated glomerular filtration rate (eGFR) was calculated using the CKD epidemiology collaboration creatinine equation[24].
The histological assessment included evaluating the total number of glomeruli, the number of sclerosed glomeruli, and the number and proportion of glomeruli with crescents. These crescents were further classified into cellular, fibrocellular, and fibrous types. Other parameters included mesangial hypercellularity (either diffuse or focal), endocapillary hypercellularity, capillary wall double contours, and the extent of interstitial fibrosis and tubular atrophy, which were semi-quantitatively graded as none (0%), mild (6%-25%), moderate (26%-50%), or severe (> 50%). Additionally, immunofluorescence (IF) was used to detect the presence of immunoglobulin A (IgA), IgG, IgM, C3, C1q, κ, and λ, with their staining patterns and intensity rated on a scale from 0 to 3+ (0: No staining; 1+: Mild; 2+: Moderate; and 3+: Severe).
Patients with PLN (classes III and IV, with or without class V) underwent induction therapy with intravenous (IV) methylprednisolone at a dose of 0.5-1 g/day for 3 days, followed by oral prednisolone according to the department’s protocol. Prednisolone was initially administered at 1 mg/kg/day for 4 to 8 weeks and then gradually tapered by 10 mg every 2 weeks to reach a maintenance dose of 5 mg/day to 10 mg/day within 4 to 6 months. All patients also received IV CYC according to the National Institutes of Health regimen[25], which consisted of monthly pulses of 500-1000 mg/m² of body surface area for 6 months. This was followed by maintenance therapy with prednisolone (5-10 mg/day) and either azathioprine (AZA) at 2 mg/kg or mycophenolate mofetil (MMF) at 1-2 g/day, as determined by the treating clinicians. Notably, MMF was not used as induction therapy in this study. Patients with mixed PLN were treated with a similar regimen. Hydroxychloroquine at a dose of 200–400 mg was administered to all patients unless contraindicated or restricted by financial constraints.
PLN was defined as classes III and IV LN, while classes II and V were designated as non-proliferative LN.
Complete remission (CR) was characterized by normal urinalysis results (dipstick negative or trace for both proteins and blood), serum albumin levels above 3.5 g/dL, and an eGFR greater than 90 mL/minute/1.73 m².
Partial remission (PR) was identified by abnormal urinalysis findings, such as microscopic hematuria or ≥ 1 proteinuria, serum albumin levels below 3.5 g/dL, and an eGFR ranging from 60 mL/minute/1.73 m² to 90 mL/minute/1.73 m².
No remission was defined as persistent proteinuria of more than 3 g/day or progressive or worsening renal function.
Renal survival was measured as the duration from renal biopsy to the occurrence of any of these events: (1) Initiation of dialysis; (2) Receiving a kidney transplant; and (3) eGFR dropping below 15 mL/minute/1.73 m² at any point during follow-up and not returning above 15 mL/minute/1.73 m² in subsequent checks.
Renal relapse was defined as the reappearance of a positive dipstick (after previously being negative) or an increase in proteinuria (evident on dipstick or an increase in PCR) in patients who had achieved PR or CR.
The main objective was to measure renal survival, which was characterized by the absence of ESKD or death. The secondary objective focused on evaluating the rate of PR or CR during the follow-up period.
The data analysis was conducted using the Statistical Package for the Social Sciences, version 22.0 (IBM Corp, Armonk, NY, United States). Continuous data are presented as the mean ± SD or median with interquartile range. Categorical data are displayed as numbers and percentages, while discrete data are shown as proportions. Differences in means and proportions were assessed using analysis of variance (ANOVA) for continuous variables and the χ² test for categorical variables. Overall survival curves were generated using the Kaplan–Meier method, and differences between these curves were evaluated using a log-rank test. P < 0.05 was considered statistically significant.
From 1998 to 2019, a total of 207 patients were diagnosed with biopsy-confirmed PLN. Among these, 103 patients (49.8%) exhibited ISN/RPS histology class IV, while 43 patients (20.8%) were classified as class III. The remaining 30% of patients had mixed PLN with class V.
Table 1 provides an overview of the demographic, clinical, and serological features of patients diagnosed with PLN. The average age at SLE onset was 24.12 years ± 8.89 years, whereas the average age at LN onset was 26.63 years ± 8.61 years. The interval between the onset of SLE and LN averaged 12.21 months ± 14.58 months. Females were dominant comprising 184 (88.9%) patients, while there were 23 (11.1%) male patients. Hypertension was present in 126 (60.9%) patients. Extra-renal manifestations included constitutional symptoms (98.6%), rash (78.3%), polyarthalgia/arthritis (97.6%), lung involvement (24.2%), and central nervous system–associated symptoms (18.4%). A total of 144 (69.56%) patients exhibited nephrotic range proteinuria in conjunction with microscopic hematuria. The average eGFR was 75.21 mL/minute/1.73 m² ± 42.59 mL/minute/1.73 m². On presentation, 25 (12.1%) patients were oliguric, of whom 19 (9.2%) patients required KRT. A total of 138 (66.7%) patients showed C4 complement consumption; 133 (64.3%) patients were on immunosuppression prior to renal biopsy; and 144 (69.56%) patients received maintenance treatment with AZA, while the remaining 63 (30.4%) patients were treated with MMF.
| n = 207 | |
| Age at systemic lupus erythematosus diagnosis (years), mean ± SD | 24.12 ± 8.89 |
| Age at renal biopsy (years), mean ± SD | 26.63 ± 8.61 |
| Sex | |
| Male | 23 (11.1) |
| Female | 184 (88.9) |
| Weight (kg), mean ± SD | 51.47 ± 11.97 |
| Duration between onset of symptoms and biopsy (months), mean ± SD | 12.21 ± 14.58 |
| Hypertension | 126 (60.9) |
| Oliguria at presentation | 25 (12.1) |
| Macroscopic hematuria | 3 (1.4) |
| Edema | 44 (64.7) |
| Constitutional symptoms | 204 (98.6) |
| Sinus/ENT | 3 (1.4) |
| Skin rash/purpura | 162 (78.3) |
| Lung involvement | 50 (24.2) |
| Arthritis/polyarthalgia | 202 (97.6) |
| Neurological | 38 (18.4) |
| Renal biopsy International Society of Nephrology/Renal Pathology Society classification | |
| III | 43 (20.8) |
| IV | 103 (49.8) |
| III/IV | 6 (2.9) |
| III/V | 5 (2.4) |
| IV/V | 50 (24.2) |
| Antiphospholipid syndrome positive | 20 (9.7) |
| Extractable nuclear antigen positive | 20 (9.7) |
| Proteinuria (dipstick) | |
| 1+ | 9 (4.3) |
| 2+ | 50 (24.2) |
| 3+ | 119 (57.5) |
| 4+ | 25 (12.1) |
| Microscopic hematuria | |
| Trace | 38 (18.4) |
| 1+ | 31 (15) |
| 2+ | 44 (21.3) |
| 3+ | 66 (31.9) |
| 4+ | 14 (6.8) |
| Serum creatinine (mg/dL), mean ± SD | 1.67 ± 1.79 |
| Estimated glomerular filtration rate (mL/minute/1.73 m²), mean ± SD | 75.21 ± 42.59 |
| Serum albumin (g/dL), mean ± SD | 2.30 ± 0.62 |
| Spot protein creatinine ratio (g/dL) or 24 hours urinary protein (g/day), mean ± SD | 3.54 ± 3.13 |
| Serum C3 levels | |
| Low (< 0.8) | 171 (82.6) |
| Normal (> 0.8) | 36 (17.4) |
| Serum C4 levels | |
| Low (< 0.16) | 138 (66.7) |
| Normal (> 0.16) | 69 (33.3) |
| Kidney replacement therapy on admission | 19 (9.2) |
| Immunosuppression before biopsy | 133 (64.3) |
| Maintenance treatment | |
| Azathioprine | 144 (69.56) |
| Mycophenolate mofetil | 63 (30.43) |
| Hydroxychloroquine | 131 (63.28) |
Table 2 shows the renal histopathological features of patients diagnosed with PLN. The predominant histological pattern identified was diffuse proliferative glomerulonephritis (76.32%), either occurring alone or in conjunction with class V. Extracellular crescentic proliferation was detected in 74 patients, accounting for 35.7% of the cohort. The average proportion of sclerotic glomeruli was 1.31 ± 2.60, which corresponded to 6.69%. A total of 154 (74.39%) biopsies had mild or no tubular atrophy. The complete full house pattern on IF was observed in 80% of the patients.
| n = 207 | |
| Total glomeruli ± SD | 19.43 ± 7.39 |
| Globally sclerosed ± SD | 1.31 ± 2.60 |
| Presence of crescents | 74 (35.7) |
| Mesangial/endocapillary proliferation | |
| Focal | 49 (23.67) |
| Diffuse | 158 (76.32) |
| Interstitial fibrosis/tubular atrophy | |
| None | 43 (20.7) |
| Mild | 111 (53.62) |
| Moderate | 38 (18.35) |
| Severe | 3 (1.44) |
| IgA | 133 (64.3) |
| IgG | 164 (79.2) |
| IgM | 160 (77.3) |
| C3 | 180 (87) |
| C1q | 160 (77.3) |
Table 3 illustrates renal functional parameters during the 5 years of follow-up. After 6 months of induction therapy, 186 (89.8%) patients had achieved CR and PR (64 [30.9%] and 122 [58.9%], respectively). Seven (3.4%) were dialysis-dependent, and fourteen (6.8%) patients died. Patients who did not require KRT at the time of admission had a higher rate of CR and PR, with 62 patients (32.97%) achieving CR compared to 2 patients (10.52%) (P = 0.04), and 113 patients (60.1%) achieving PR compared to 9 patients (47.36%) (P = 0.28). Moreover, fewer patients in this group progressed to ESKD, with 4 patients (2.12%) compared to 3 patients (15.7%) (P = 0.002). Additionally, mortality was lower in this group, with 9 patients (4.78%) dead in this group compared to 5 patients (26.3%) in the group requiring KRT (P < 0.001).
| Overall (n = 207) | Required KRT on presentation (n = 19) | Not required KRT on presentation (n = 188) | P value | |
| Renal outcomes after induction with pulse cyclophosphamide at 6 months | ||||
| CR | 64 (30.9) | 2 (10.52) | 62 (32.97) | 0.04 |
| PR | 122 (58.9) | 9 (47.36) | 113 (60.1) | 0.28 |
| ESKD | 7 (3.4) | 3 (15.7) | 4 (2.12) | 0.002 |
| Mortality | 14 (6.8) | 5 (26.3) | 9 (4.78) | 0.000 |
| Final outcomes at 5 years | ||||
| CR | 94 (45.4) | 3 (15.7) | 91 (48.40) | 0.005 |
| PR | 47 (22.7) | 2 (10.52) | 45 (23.9) | 0.170 |
| ESKD | 38 (18.35) | 8 (42.10) | 30 (15.95) | 0.010 |
| Mortality | 28 (13.52) | 6 (31.57) | 22 (11.7) | 0.016 |
| Renal relapses during 5-year follow-up | 34 (16.4) | 0 | 34 (18.0) | 0.043 |
| Azathioprine | 30 (88.2) | 0 | 30 (88.2) | |
| Mycophenolate mofetil | 4 (11.76) | 4 (11.76) | ||
| Re-biopsy | 23 (11.1) | 1 (5.2) | 22 (11.70) | 0.67 |
At the 5-year mark, a total of 141 patients (68.11%) achieved either CR or PR, with 94 patients (45.4%) in CR and 47 patients (22.7%) in PR. During this period, 38 patients (18.4%) progressed to ESKD, and 28 patients (13.52%) passed away. Among those requiring KRT at admission, fewer attained CR and PR, with 3 patients (15.7%) reaching CR compared to 91 patients (48.40%) (P = 0.005) and 2 patients (10.52%) achieving PR compared to 45 patients (23.9%) (P = 0.17). Additionally, a higher proportion of these patients progressed to ESKD, with 8 patients (42.10%) compared to 30 patients (15.95%) (P = 0.002), and mortality was higher, with 6 patients (31.57%) compared to 22 patients (11.7%) (P = 0.016).
During the 5-year follow-up period, 34 patients (16.4%) experienced renal relapses, all of whom did not require KRT at presentation. Of these patients, 23 (11.1%) underwent re-biopsy. The relapse rate was higher among patients treated with AZA, with 30 patients (88.2%), compared to those treated with MMF, with 4 patients (11.76%).
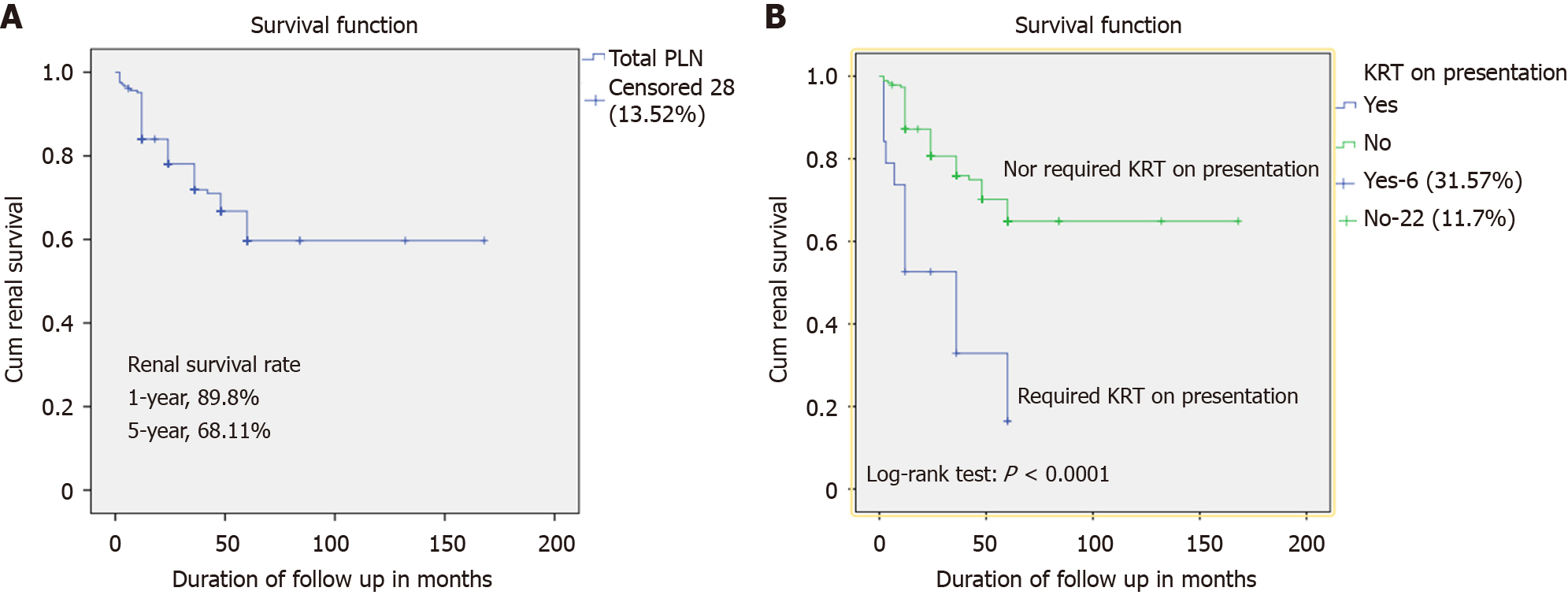
Kaplan-Meier survival analysis was performed, considering the period from treatment initiation to the end of follow-up or death as the timeframe. Renal survival was 89.8% at 6 months and 68.11% at 5 years (Figure 1A). The survival rate was significantly better in patients who did not require KRT at presentation (P < 0.001) (Figure 1B).
To evaluate the mean baseline values of parameters in different patient categories such as CR, PR, ESKD, and mortality, an ANOVA test was performed to compare their responses to treatment. It was found that age, duration between onset of symptoms and biopsy, and degree of proteinuria had no significant correlation with the outcomes. The outcomes showed a strong correlation with the presence of hypertension, baseline eGFR, the need for KRT at the time of admission, and renal histology. Patients who achieved CR had a lower incidence of hypertension, with 40 patients (42.5%) affected, and demonstrated higher eGFR values, averaging 90.27 mL/minute/1.73 m² ± 30.08 mL/minute/1.73 m², than those patients with PR (71.62 mL/kg/m² ± 40.29 mL/kg/m2), ESKD (56.31 mL/kg/m² ± 44.96 mL/kg/m2), and died (60.25 mL/kg/m² ± 41.68 mL/kg/m2) (P < 0.001). Similarly, fewer patients required KRT on admission in patients with CR (3 [3.19%]) and PR (2 [4.25%]) than those who progressed to ESKD (8 [21.05%]) and died (6 [21.42%]) (P = 0.001). Patients with fewer globally sclerosed glomeruli, fewer crescents (30 [31.9%]), focal proliferation, and mild to no tubular atrophy (87.6%) observed through light microscopy, experienced higher rates of CR and PR (P < 0.001). These findings are detailed in Table 4.
| Variable | Complete remission (n = 94) | Partial remission | End-stage kidney disease (n = 38) | Mortality (n = 28) | P value |
| Age (years), mean ± SD | 26.21 ± 45 7.54 | 27.89 ± 11.05 | 25.87 ± 7.98 | 26.14 ± 8.37 | 0.699 |
| Duration between onset of symptoms and biopsy (months), mean ± SD | 10.98 ± 13.94 | 11.79 ± 10.85 | 16.63 ± 21.44 | 11.07 ± 9.40 | 0.225 |
| Hypertension | 40 (42.5) | 31 (65.95) | 13 (34.21) | 24 (85.71) | < 0.0001 |
| Serum creatinine (mg/dL), mean ± SD | 1.14 ± 0.82 | 1.49 ± 1.01 | 2.54 ± 2.50 | 2.59 ± 2.99 | < 0.0001 |
| Serum albumin on admission (g/dL), mean ± SD | 2.42 ± 0.70 | 2.32 ± 0.55 | 2.05 ± 0.44 | 2.16 ± 0.50 | 0.014 |
| Protein creatinine ratio (g/dL), mean ± SD | 3.27 ± 2.82 | 4.17 ± 3.94 | 3.05 ± 2.26 | 4.16 ± 3.71 | 0.45 |
| Estimated glomerular filtration rate (mL/minute/1.73 m²) mean ± SD | 90.27 ± 30.08 | 71.62 ± 40.29 | 56.31 ± 44.96 | 60.25 ± 41.68 | < 0.0001 |
| Required kidney replacement therapy on admission | 3 (3.19) | 2 (4.25) | 8 (21.05) | 6 (21.42) | 0.001 |
| Mesangial/endocapillary proliferation | |||||
| Focal | 33 (35.10) | 11 (23.40) | 4 (10.52) | 1 (3.571) | 0.001 |
| Diffuse | 61 (64.89) | 36 (76.59) | 34 (89.47) | 27 (96.42) | |
| Number of globally sclerosed glomeruli, mean ± SD | 0.55 ± 1.2 | 1.44 ± 2.22 | 2.62 ± 4.05 | 1.80 ± 3.21 | < 0.0001 |
| Presence of crescents | 30 (31.9) | 18 (38.29) | 15 (39.47) | 11 (39.28) | 0.010 |
| Interstitial fibrosis/tubular atrophy | < 0.0001 | ||||
| None | 36 (38.29) | 4 (8.51) | 2 (5.26) | 2 (7.14) | |
| Mild | 49 (52.12) | 30 (63.8) | 22 (57.89) | 10 (35.7) | |
| Moderate | 9 (9.57) | 13 (27.65) | 14 (36.8) | 12 (42.85) | |
| Severe | 0 | 0 | 0 | 4 (14.2) |
LN is a highly heterogeneous disease with variable treatment responses. Despite advancements in current treatments, many patients with PLN still progress to ESKD. This study provides a comprehensive analysis of treatment outcomes, survival status, and associated factors for PLN patients in the South-Asian context, specifically from Pakistan. Despite being a single-center study, it is one of the largest studies to thoroughly assess various factors impacting PLN treatment outcomes and survival rates, utilizing a relatively large sample size.
In this study, baseline clinical and laboratory variables showed no correlation between outcomes and age, symptom duration, or 24-hour urinary protein, aligning with findings by Aliyi et al[26]. While proteinuria reduction serves as a marker for renal outcomes, our study identified significant predictors of outcomes, including reduced baseline eGFR, presence of hypertension, need for KRT at presentation, underlying renal histology (ISN/RPS class IV compared to class III), increased globally sclerosed glomeruli, presence of crescents, and moderate to severe IF/TA. These results are consistent with Dhir et al[27] from India.
In the current study, we observed that the overall remission at the end of induction therapy was 89.8%: (1) CR in 30.9 % of patients; and (2) PR in 58.9% of patients. This finding aligns with a study conducted by Chan et al[15] in China, where nearly 90% of patients achieved remission following induction therapy with prednisolone combined with either MMF or CYC. In contrast, the remission rate was relatively higher compared to studies conducted by Prasad et al[16] in India, and Rasheed et al[17] in Iraq, in which 70% and 53% of patients achieved remission at 6 months, respectively. Moreover, the current finding was relatively lower than a study done by Aliyi et al[26] in Ethiopia and George et al[28] in India, which reported 92.5% and 94.1% of remission at the end of induction therapy, respectively. The considerable variation in remission rates can be attributed to several factors, including differences in sample size, patient age, baseline renal function, chosen treatment regimens (MMF with prednisolone vs CYC alone or AZA alone), remission criteria, racial disparities among the studied population, and variations in disease histopathology. At 5 years of follow-up, 68.11% of patients with PLN in our study achieved remission. Out of these, 45.4% of them had a CR, while 22.7% had a PR. This finding is comparable with a study reported by Prasad et al[16] from India in which nearly 69% of patients achieved remission at the last follow-up. The overall remission rate found in this study was lower compared to a previous study conducted in Ethiopia[26], which reported an overall remission (CR plus PR) rate of approximately 86.5%. This disparity is largely attributed to variations in the selection of treatment regimens for induction therapy (CYC with prednisolone or MMF with prednisolone), genetics, socioeconomic status, histological classes (PLN vs all classes), and type of regimen selected for maintenance therapy.
Our research identified a notable disparity in renal relapse rates between maintenance treatments, with AZA having a rate of 88.2% and MMF having a rate of 11.76%. These findings are in contrast to the outcomes observed in the MAINTAIN trial[29], which showed no difference in renal relapse rate between these two maintenance regimens. The MAINTAIN trial has a similar design to our study, in which all the patients received induction with IV CYC only, but only for 3 months with a low dose. This incongruity might be ascribed to the variation in the dose and the duration of pulse CYC.
There are limited data on the rate of ESKD due to the shorter follow-up duration and some studies only covering the induction period. However, one of the major strengths of this study is that it offers valuable insights into ESKD progression over a 5-year follow-up period, with 18.4% of patients progressing to ESKD, higher than the 15% reported by Prasad et al[16] from India at 10 years. The higher rate may be attributed to differences in renal dysfunction severity, KRT requirements at presentation, and maintenance therapy regimens as more patients on AZA progressed to ESKD.
We observed a mortality rate of 6.8% at 6 months, increasing to 13.5% at 5 years, higher than previously reported studies[29,30]. Prasad et al[16] reported a similar mortality rate (13%) from India but at 10 years. There are multiple factors responsible for this. First, we studied only PLN in which the majority were in histological class IV. Second, 9.2% of patients required KRT on arrival because of late presentation. Thirdly, due to the risk of increased opportunistic infections during the intensive phase, there is a possibility of developing pneumonitis, urinary tract infections, and bloodstream infections with sepsis.
In the current study, renal survival at first-year follow-up was 86.4%. This finding is in keeping with two previous studies[15,26]. But renal survival at 5 years was 68.11%, much lower than that reported by Prasad et al[16] and Dhir et al[27] for Indian patients (89% and 79%, respectively). A possible explanation for the apparent discrepancy is that induction with a combination of IV CYC and corticosteroids produced similar renal survival at 6 months as induction with MMF and corticosteroids reported in published literature[15,26]. It is a maintenance therapy, whether AZA or MMF, which affects the renal outcomes thereafter, as 70% of our patients were on AZA because of financial constraints.
Without any prospective controlled trials, the management of PLN presents considerable difficulties. Ongoing investigations into new drugs for LN hold promise for more personalized treatments, as improved assessment of disease activity and outcomes, along with an expanded armamentarium, become available[31].
One of the key strengths of this study is that it encompasses one of the largest adult cohorts of biopsy-confirmed PLN from a developing South Asian country. The data are very scarce, and no data on the outcomes of PLN from Pakistan have been published. Additionally, our patients have been monitored for more than 5 years, with individual evaluations of treatment outcomes. Moreover, our study provides data on ESKD and mortality rates at the 5-year mark, which are often absent in many previously published PLN studies. However, this study had several limitations as well. First, being a retrospective study, the absence of certain data may have impacted the final analysis. In addition, the retrospective study design may not fully control all the potential confounding factors. Second, since this study was based on data from a single center, it may not fully represent the entire population of the country, which limits the generalizability of the results. Third, the high cost and inconsistent supply of immunosuppressive medications led to most patients being transitioned from MMF to AZA, especially in the early phase of the study. This affected the universality of the results and hindered the accurate evaluation of the different drugs' efficacy.
Our findings indicate that baseline eGFR, the necessity for KRT, and the presence of diffuse proliferative disease at presentation are strong predictors of renal survival. Short-term renal outcomes were good with a combination of IV CYC and corticosteroids-based induction therapy, but 5-year renal outcomes were worse with AZA-based maintenance therapy than with MMF with more ESKD and mortality.
We gratefully acknowledge Mr. Iqbal Mujtaba, the Department of Clinical Research, Dr. Safdar and his team, and the Department of Electronic Health Record System for making this task easier.
| 1. | Hoi A, Igel T, Mok CC, Arnaud L. Systemic lupus erythematosus. Lancet. 2024;403:2326-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 89] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 2. | Burgos PI, McGwin G Jr, Pons-Estel GJ, Reveille JD, Alarcón GS, Vilá LM. US patients of Hispanic and African ancestry develop lupus nephritis early in the disease course: data from LUMINA, a multiethnic US cohort (LUMINA LXXIV). Ann Rheum Dis. 2011;70:393-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Pons-Estel GJ, Catoggio LJ, Cardiel MH, Bonfa E, Caeiro F, Sato E, Massardo L, Molina-Restrepo JF, Toledano MG, Barile-Fabris LA, Amigo MC, Acevedo-Vásquez EM, Abadi I, Wojdyla D, Alarcón-Riquelme ME, Alarcón GS, Pons-Estel BA; GLADEL. Lupus in Latin-American patients: lessons from the GLADEL cohort. Lupus. 2015;24:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Wang YF, Zhang Y, Lin Z, Zhang H, Wang TY, Cao Y, Morris DL, Sheng Y, Yin X, Zhong SL, Gu X, Lei Y, He J, Wu Q, Shen JJ, Yang J, Lam TH, Lin JH, Mai ZM, Guo M, Tang Y, Chen Y, Song Q, Ban B, Mok CC, Cui Y, Lu L, Shen N, Sham PC, Lau CS, Smith DK, Vyse TJ, Zhang X, Lau YL, Yang W. Identification of 38 novel loci for systemic lupus erythematosus and genetic heterogeneity between ancestral groups. Nat Commun. 2021;12:772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 159] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 5. | Mok CC, Hamijoyo L, Kasitanon N, Chen Y, Chen S, Yamaoka K, Oku K, Li MT, Zamora L, Bae SC, Navarra S, Morand EF, Tanaka Y. The Asia-Pacific League of Associations for Rheumatology consensus statements on the management of systemic lupus erythematosus. Lancet Rheumatol. 2021;3:e517-e531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M; International Society of Nephrology Working Group on the Classification of Lupus Nephritis; Renal Pathology Society Working Group on the Classification of Lupus Nephritis. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 976] [Cited by in RCA: 1086] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 7. | Fanouriakis A, Kostopoulou M, Andersen J, Aringer M, Arnaud L, Bae SC, Boletis J, Bruce IN, Cervera R, Doria A, Dörner T, Furie RA, Gladman DD, Houssiau FA, Inês LS, Jayne D, Kouloumas M, Kovács L, Mok CC, Morand EF, Moroni G, Mosca M, Mucke J, Mukhtyar CB, Nagy G, Navarra S, Parodis I, Pego-Reigosa JM, Petri M, Pons-Estel BA, Schneider M, Smolen JS, Svenungsson E, Tanaka Y, Tektonidou MG, Teng YO, Tincani A, Vital EM, van Vollenhoven RF, Wincup C, Bertsias G, Boumpas DT. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann Rheum Dis. 2024;83:15-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 365] [Article Influence: 365.0] [Reference Citation Analysis (0)] |
| 8. | Floege J, Barbour SJ, Cattran DC, Hogan JJ, Nachman PH, Tang SCW, Wetzels JFM, Cheung M, Wheeler DC, Winkelmayer WC, Rovin BH; Conference Participants. Management and treatment of glomerular diseases (part 1): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;95:268-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 178] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 9. | Moroni G, Quaglini S, Gallelli B, Banfi G, Messa P, Ponticelli C. The long-term outcome of 93 patients with proliferative lupus nephritis. Nephrol Dial Transplant. 2007;22:2531-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Moroni G, Depetri F, Ponticelli C. Lupus nephritis: When and how often to biopsy and what does it mean? J Autoimmun. 2016;74:27-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Yap DY, Tang CS, Ma MK, Lam MF, Chan TM. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant. 2012;27:3248-3254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | Saxena R, Mahajan T, Mohan C. Lupus nephritis: current update. Arthritis Res Ther. 2011;13:240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Hanly JG, O'Keeffe AG, Su L, Urowitz MB, Romero-Diaz J, Gordon C, Bae SC, Bernatsky S, Clarke AE, Wallace DJ, Merrill JT, Isenberg DA, Rahman A, Ginzler EM, Fortin P, Gladman DD, Sanchez-Guerrero J, Petri M, Bruce IN, Dooley MA, Ramsey-Goldman R, Aranow C, Alarcón GS, Fessler BJ, Steinsson K, Nived O, Sturfelt GK, Manzi S, Khamashta MA, van Vollenhoven RF, Zoma AA, Ramos-Casals M, Ruiz-Irastorza G, Lim SS, Stoll T, Inanc M, Kalunian KC, Kamen DL, Maddison P, Peschken CA, Jacobsen S, Askanase A, Theriault C, Thompson K, Farewell V. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford). 2016;55:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 418] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 14. | Zhang B, Xie S, Su Z, Song S, Xu H, Chen G, Cao W, Yin S, Gao Q, Wang H. Heme oxygenase-1 induction attenuates imiquimod-induced psoriasiform inflammation by negative regulation of Stat3 signaling. Sci Rep. 2016;6:21132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Chan TM, Tse KC, Tang CS, Mok MY, Li FK; Hong Kong Nephrology Study Group. Long-term study of mycophenolate mofetil as continuous induction and maintenance treatment for diffuse proliferative lupus nephritis. J Am Soc Nephrol. 2005;16:1076-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 322] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 16. | Prasad N, Kurian J, Agarwal V, Bhadauria D, Behera M, Yacha M, Kushwaha R, Agrawal V, Jain M, Gupta A. Long-term outcomes of lupus nephritis treated with regimens based on cyclophosphamide and mycophenolate mofetil. Lupus. 2020;29:845-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Rasheed IJ, Mousa BA, Makki HMA. Short-term outcome of proliferative lupus nephritis: a single center study. IJMPS. 2017;7:19-30. |
| 18. | Hanaoka H, Iida H, Kiyokawa T, Takakuwa Y, Kawahata K. Early achievement of deep remission predicts low incidence of renal flare in lupus nephritis class III or IV. Arthritis Res Ther. 2018;20:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Koo HS, Kim S, Chin HJ. Remission of proteinuria indicates good prognosis in patients with diffuse proliferative lupus nephritis. Lupus. 2016;25:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Dall'Era M, Cisternas MG, Smilek DE, Straub L, Houssiau FA, Cervera R, Rovin BH, Mackay M. Predictors of long-term renal outcome in lupus nephritis trials: lessons learned from the Euro-Lupus Nephritis cohort. Arthritis Rheumatol. 2015;67:1305-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 218] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 21. | Radhakrishnan J, Kunis CL, D'Agati V, Appel GB. Cyclosporine treatment of lupus membranous nephropathy. Clin Nephrol. 1994;42:147-154. [PubMed] |
| 22. | Rabbani MA, Tahir MH, Siddiqui BK, Ahmad B, Shamim A, Shah SM, Ahmad A. Renal involvement in systemic lupus erythematosus in Pakistan. J Pak Med Assoc. 2005;55:328-332. [PubMed] |
| 23. | Rabbani MA, Habib HB, Islam M, Ahmad B, Majid S, Saeed W, Shah SM, Ahmad A. Survival analysis and prognostic indicators of systemic lupus erythematosus in Pakistani patients. Lupus. 2009;18:848-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20319] [Cited by in RCA: 20173] [Article Influence: 1260.8] [Reference Citation Analysis (0)] |
| 25. | Austin HA 3rd, Klippel JH, Balow JE, le Riche NG, Steinberg AD, Plotz PH, Decker JL. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med. 1986;314:614-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 787] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 26. | Aliyi O, Worku B, Hassen M, Muhammed OS. Treatment outcome and survival status among adult patients treated for lupus nephritis in selected tertiary hospitals of Ethiopia. Sci Rep. 2024;14:5603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Dhir V, Aggarwal A, Lawrence A, Agarwal V, Misra R. Long-term outcome of lupus nephritis in Asian Indians. Arthritis Care Res (Hoboken). 2012;64:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | George J, Sankaramangalam KP, Sinha A, Hari P, Dinda AK, Bagga A. Lupus Nephritis in Indian Children: Flares and Refractory Illness. Indian Pediatr. 2018;55:478-481. [PubMed] |
| 29. | Houssiau FA, D'Cruz D, Sangle S, Remy P, Vasconcelos C, Petrovic R, Fiehn C, de Ramon Garrido E, Gilboe IM, Tektonidou M, Blockmans D, Ravelingien I, le Guern V, Depresseux G, Guillevin L, Cervera R; MAINTAIN Nephritis Trial Group. Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial. Ann Rheum Dis. 2010;69:2083-2089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 342] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 30. | Dooley MA, Jayne D, Ginzler EM, Isenberg D, Olsen NJ, Wofsy D, Eitner F, Appel GB, Contreras G, Lisk L, Solomons N; ALMS Group. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med. 2011;365:1886-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 421] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 31. | Furie RA, Aroca G, Cascino MD, Garg JP, Rovin BH, Alvarez A, Fragoso-Loyo H, Zuta-Santillan E, Schindler T, Brunetta P, Looney CM, Hassan I, Malvar A. B-cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2022;81:100-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 250] [Article Influence: 83.3] [Reference Citation Analysis (0)] |