Published online Jun 25, 2025. doi: 10.5527/wjn.v14.i2.101419
Revised: December 27, 2024
Accepted: January 14, 2025
Published online: June 25, 2025
Processing time: 208 Days and 0.1 Hours
Private insurance coverage is associated with higher rates of living donor kidney transplantation (LDKT) but whether this is attributable to confounding is not known.
To study the association between increased access to private health insurance and LDKT.
Retrospective cohort study using United States transplant registry data. We identified incident candidates aged 22-29 years who were waitlisted for a kidney-only transplant from 2005-2014, excluding prior transplant recipients and those with missing data. We calculated the hazard of LDKT after waitlisting for those with private insurance vs other insurance pre-Affordable Care Act (ACA) vs post-ACA, using death and delisting as competing events, for candidates affected by the policy change (age 22-25 years) vs those who were not (age 26-29 years).
A total of 13817 candidates were included, of whom 46% were age 22-25 years and 54% were age 26-29 years. Among candidates aged 22-25 years at listing, those listed post-ACA were more likely to have private insurance compared to those listed pre-ACA (42% vs 35%), but there was no difference in private insurance coverage between eras among candidates aged 26-29 years at listing. In adjusted competing risk regression, privately insured patients age 22-25 years were less likely to receive a LDKT post-ACA compared to pre-ACA [hazard ratio (HR) = 0.88, 95%CI: 0.78-1.00], as were those aged 22-25 years old with other insurance types (HR = 0.80, 95%CI: 0.69-0.92). These associations were not seen among candidates age 26-29 years.
Candidates age 22-25 years were likelier to have private insurance post-ACA, without an increased rate in LDKT. Demonstrations of associations between insurance and LDKT are likely attributable to residual confounding.
Core Tip: In this retrospective cohort study using United States transplant registry data from 2005-2014, we found that although kidney transplant candidates age 22-25 years were more likely to have private insurance following the Affordable Care Act policy change expanding eligibility to remain on parental insurance, this shift in payer mix was not associated with higher rates of living donor kidney transplantation. These data suggest that insurance type itself is not a direct determinant of access to living donor kidney transplant; rather the association of private insurance with higher transplantation rates in prior observational studies is likely a result of unmeasured demographic confounding.
- Citation: Perry K, Yu M, Adler JT, Maclay LM, Cron DC, Mohan S, Husain SA. Association between private insurance and living donor kidney transplant: Affordable Care Act as a natural experiment. World J Nephrol 2025; 14(2): 101419
- URL: https://www.wjgnet.com/2220-6124/full/v14/i2/101419.htm
- DOI: https://dx.doi.org/10.5527/wjn.v14.i2.101419
Living donor kidney transplant is the optimal long-term treatment for patients with end-stage kidney disease (ESKD) given that it is a cost-effective solution[1,2] that has better long-term quality of life[3-6] and survival[7-10] outcomes over dialysis or deceased donor transplantation. Despite these benefits, only a minority of patients with ESKD in the United States ever receive a living donor transplant[11,12]. Several modifiable and unmodifiable socioeconomic and demo
Following the implementation of the Affordable Care Act (ACA) in 2009, individuals who would normally have lost access to their parents’ private insurance at age 22 years were permitted to remain on their parents’ private plans until they reach age of 26 years. This change created a natural experiment enabling the study of the impact of private insurance coverage on living donor kidney transplantation (LDKT). Overall, the national volume of LDKT involving recipients of all ages remained similar before and after the policy change. Understanding changes in payer mix and the associated changes in preemptive waitlisting rates for patients with ESKD in the age group affected vs unaffected by this policy change vs others may elucidate the expected impact of increased access to private insurance and inform ESKD coverage policy initiatives. We hypothesized that, compared to young transplant candidates age 26-29 years not impacted by the policy change, those age 22-25 years at listing would have a greater proportion of private insurance coverage at the time of listing following ACA implantation but that rates of LDKT would be similar before and after the policy.
We conducted a retrospective cohort study using United States transplant registry data from the Organ Procurement and Transplantation Network (OPTN) Standard Transplant Analysis and Research data set. This study was approved by the institutional review board of Columbia University Medical Center. The analysis was done using deidentified data from a national registry of waitlisted patients, therefore informed consent was not required.
We identified all incident candidates aged 22 years to 29 years who were waitlisted for a kidney-only transplant from 2005 to 2014 (Supplementary Figure 1). This age range was selected to provide one group of candidates with expanded access to parental private insurance resulting from the ACA policy change (age 22-25 years), as well as a control group similar in age that did not have access to parental insurance before or after the policy change (age 26-29 years). This time period was used to reflect the time period following the ACA policy change to the rollout of the Kidney Allocation System, which was implemented in December 2014 and a period of equal duration before the policy change. We excluded patients who had received any prior solid organ transplant (n = 1793), or who had missing body mass index (BMI) and height or weight data (n = 182).
We classified the final cohort of candidates into four analytic groups based on age at listing and era: (1) 22-25 years olds waitlisted in 2005-2009 (pre-ACA); (2) 2010-2014 (post-ACA); (3) 26-29 years olds waitlisted in 2005-2009 (pre-ACA); and (4) 2010-2014 (post-ACA). Follow up time was truncated six years after waitlisting date.
Candidate characteristics at the time of waitlisting were compared between the two eras within age groups using Pearson χ² tests for categorical variables, and Kruskal-Wallis tests for continuous variables. Column percentages are given for categorical characteristics. Medians with interquartile ranges are presented for continuous characteristics. Candidate characteristics were then also compared between patients with private insurance vs other insurance types among patients aged 22-25 years old in each era.
We used competing risks regression to assess the likelihood of LDKT after waitlisting in the presence of competing risks by calculating the cumulative incidence function for the probability of LDKT and treating deceased donor kidney transplantation, death, and waitlist removal as competing events. We first used unadjusted competing risk regression to determine the subhazard of LDKT by era, separately for each age group (i.e. 22-25 years and 26-29 years). We next computed adjusted regression models including age at listing, sex, race, BMI at listing, diabetes status, educational attainment, employment status, cause of kidney disease, preemptive listing status, and insurance type. We then repeated these competing risk regression models after further stratifying each age group by insurance type (private vs all others).
In order to determine if the observed effect was due primarily to an increase of patients on Medicaid, whose expansion was also part of the ACA, we conducted a sensitivity analysis repeating the same competing risk analysis, unadjusted and adjusted, limited to candidates residing in the twenty-four states that did not expand Medicaid prior to January 1, 2015 (Alabama, Alaska, Florida, Georgia, Idaho, Indiana, Kansas, Louisiana, Maine, Mississippi, Missouri, Montana, Nebraska, North Carolina, Oklahoma, Pennsylvania, South Carolina, South Dakota, Tennessee, Texas, Utah, Virginia, Wisconsin, and Wyoming).
A total of 13817 kidney transplant candidates with age 22-29 years who were added to the waitlist from 2005-2014 were included in the analysis, of whom 6308 (46%) were age 22-25 years and 7509 (54%) were age 26-29 years (Supplementary Figure 1) at listing. Both age groups were similar, with White race and male sex being the largest categories in both (Table 1). When comparing candidate characteristics in each age group before ACA implementation (2005-2009) and after ACA implementation (2010-2014), sex, race, employment status, and pre-listing dialysis use were similar between eras for both groups.
| 22-25 years old | 26-29 years old | |||||||
| All | 2005-2009 (pre-ACA) | 2010-2014 (post-ACA) | P value | All | 2005-2009 (pre-ACA) | 2010-2014 (post-ACA) | P value | |
| Age at listing (year) (median, 25%th- | 24 (23, 25) | 24 (23, 25) | 24 (23, 25) | 0.0062 | 28 (27, 29) | 28 (27, 29) | 28 (27, 29) | 0.1773 |
| Race/ethnicity | ||||||||
| White | 2543 (38.84) | 1250 (38.83) | 1293 (38.85) | 0.205 | 2993 (36.96) | 1516 (37.08) | 1477 (36.843 | 0.367 |
| Black | 1871 (28.58) | 949 (29.48) | 922 (27.70) | 2559 (31.60) | 1316 (32.18) | 1243 (31.00) | ||
| Hispanic | 1606 (24.53) | 779 (24.20) | 827 (24.85) | 1785 (22.04) | 892 (21.81) | 893 (22.27) | ||
| Other | 527 (8.05) | 241 (7.49) | 286 (8.59) | 762 (9.41) | 365 (8.93) | 397 (9.90) | ||
| Gender | ||||||||
| Male | 3692 (56.39) | 1803 (56.01) | 1889 (56.76) | 0.558 | 4376 (54.03) | 2182 (53.36) | 2194 (54.71) | 0.219 |
| Female | 2855 (43.61) | 1415 (43.99) | 1439 (43.24) | 3723 (45.97) | 1907 (46.64) | 1816 (45.29) | ||
| Body mass index (kg/m2) | 24.33 (21.27, 29.15) | 24.41 (21.33, 29.03) | 24.23 (21.16, 29.23) | 0.431 | 25.59 (22.15, 30.29) | 25.46 (22.13, 30.00) | 25.72 (22.19, 30.78) | 0.0088 |
| Diabetes status | ||||||||
| No diabetes | 6120 (93.48) | 2994 (93.01) | 3126 (93.93) | 6789 (83.39) | 3410 (83.39) | 3379 (84.26) | ||
| Diabetes | 427 (6.52) | 225 (6.99) | 202 (6.07) | 0.132 | 1310 (16.17) | 679 (16.61) | 631 (15.74) | 0.288 |
| Educational attainment | ||||||||
| Less than high school | 249 (3.80) | 136 (4.22) | 113 (3.40) | < 0.001 | 330 (4.07) | 171 (4.18) | 159 (3.97) | < 0.001 |
| High school graduate or general equivalency diploma | 3136 (47.89) | 1572 (48.84) | 1564 (47.00) | 3549 (43.71) | 1784 (43.63) | 1756 (43.79) | ||
| Some college | 2060 (31.46) | 901 (27.99) | 1159 (34.83) | 2234 (27.58) | 1049 (25.65) | 1185 (29.55) | ||
| College graduate or higher | 771 (11.78) | 342 (10.62) | 429 (12.89) | 1498 (18.50) | 689 (16.85) | 809 (20.17) | ||
| Missing or unknown | 331 (5.06) | 269 (8.33) | 63 (1.89) | 497 (6.14) | 396 (9.68) | 101 (2.52) | ||
| Employment status | ||||||||
| Not employed | 4200 (64.15) | 1998 (62.07) | 2202 (66.17) | < 0.001 | 4776 (58.97) | 2345 (57.35) | 2431 (60.62) | < 0.001 |
| Employed | 2004 (30.61) | 980 (30.44) | 1024 (30.77) | 2891 (35.70) | 1419 (34.70) | 1472 (36.71) | ||
| Missing or unknown | 343 (5.24) | 241 (7.49) | 102 (3.06) | 432 (5.33) | 325 (7.95) | 107 (2.67) | ||
| Primary cause of renal failure | ||||||||
| Cystic kidney disease | 137 (2.09) | 55 (1.71) | 82 (2.46) | 0.019 | 197 (2.43) | 82 (2.01) | 115 (2.87) | 0.001 |
| Diabetes mellitus | 298 (4.55) | 164 (5.09) | 134 (4.03) | 1110 (13.71) | 565 (13.82) | 545 (13.59) | ||
| Hypertension | 1017 (15.53) | 525 (16.31) | 492 (14.78) | 1461 (18.04) | 775 (18.95) | 686 (17.11) | ||
| Glomerulonephritis | 2837 (43.33) | 1375 (42.72) | 1462 (43.93) | 3231 (39.89) | 1567 (38.32) | 1664 (41.50) | ||
| Other/unknown | 2258 (34.49) | 1100 (34.17) | 1158 (34.80) | 2100 (25.93) | 1100 (26.90) | 1000 (24.94) | ||
| Pre-emptively waitlisted | ||||||||
| No | 5155 (28.74) | 2588 (80.40) | 2567 (77.13) | 0.001 | 6170 (76.18) | 3190 (78.01) | 2980 (74.31) | < 0.001 |
| Yes | 1392 (21.26) | 631 (19.60) | 761 (22.87) | 1929 (23.82) | 899 (21.99) | 1030 (25.69) | ||
| Primary payer at waitlist registration | ||||||||
| Private | 2537 (38.75) | 1140 (35.41) | 1397 (41.98) | < 0.001 | 3156 (38.97) | 1602 (39.18) | 1554 (38.75) | 0.090 |
| Medicare | 2634 (40.23) | 1371 (42.59) | 1263 (37.95) | 3397 (41.94) | 1736 (42.46) | 1661 (41.42) | ||
| Medicaid | 1184 (18.08) | 599 (18.61) | 585 (17.58) | 1322 (16.32) | 629 (15.38) | 693 (17.28) | ||
| All others | 192 (2.93) | 109 (3.39) | 83 (2.49) | 224 (2.77) | 122 (2.98) | 102 (2.54) | ||
| Waitlist outcomes | ||||||||
| Deceased donor transplant | 2416 (36.90) | 1191 (37.00) | 1225 (36.81) | < 0.001 | 2988 (36.89) | 1563 (38.22) | 1425 (35.54) | < 0.001 |
| Living donor transplant | 1858 (28.38) | 933 (28.98) | 925 (27.79) | 2036 (25.14) | 1014 (24.80) | 1022 (25.49) | ||
| Died on waitlist | 427 (6.52) | 251 (7.80) | 176 (5.29) | 591 (7.30) | 357 (8.73) | 234 (5.84) | ||
| Removed from waitlist for reason other than transplant or death | 1648 (25.17) | 804 (24.98) | 844 (25.36) | 2258 (27.88) | 1114 (27.24) | 1144 (28.53) | ||
| Still on waitlist | 198 (3.02) | 40 (1.24) | 158 (4.75) | 226 (2.79) | 41 (1.00) | 185 (4.61) | ||
Among candidates aged 22-25 years at listing, those listed between 2010-2014 (post-ACA) were significantly more likely to have private insurance compared to those listed from 2005-2009 (pre-ACA) (42% vs 35%) (Table 1). However, there was no significant difference in private insurance coverage between eras observed in the 26-29 years age group not impacted by the ACA policy change (39% in both eras).
When examining candidates age 22-25 years at listing, the demographics of those who had private insurance were similar among those listed 2005-2009 vs 2010-2014 (Supplementary Table 1). Privately insured candidates were most commonly of White race and male sex. Privately insured candidates also most commonly had a high school diploma or general equivalency diploma, glomerulonephritis as primary disease, and were not pre-emptively waitlisted before initiating dialysis. Although there was an observed increase in the proportion of patients who were listed as not employed (2005-2009: 39%, 2010-2014: 46%), this was paired with a similar decrease in employment status listed as “missing/unknown”.
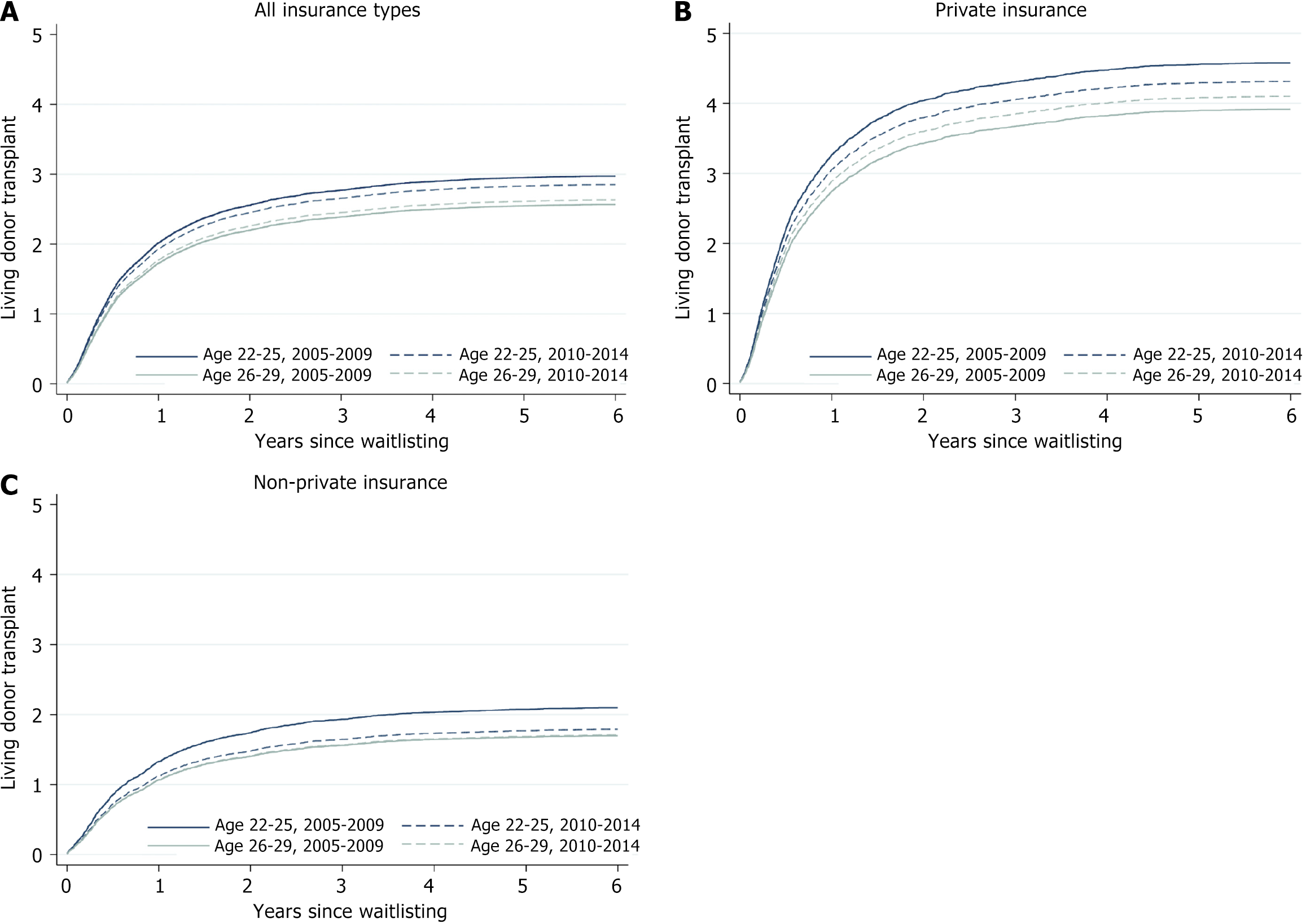
Despite the difference in payor mix between eras for the age 22-25 years group, there was no significant difference in the observed proportion receiving a living donor transplant by end of follow up (Table 1). Similarly, there was no difference between eras in the proportion of candidates aged 26-29 years at listing who received a living donor transplant. Among all groups, the cumulative incidence of living donor kidney transplant was highest for candidates aged 22-25 years pre-ACA, followed by 22-25 years post-ACA, then 26-29 years post-ACA, and finally 26-29 years pre-ACA (Figure 1A). This order was similar when limiting the analysis only to candidates with private insurance or only candidates with other insurance types (Figure 1B and C).
In adjusted competing risk regression, privately insured patients ages 22-25 years were less likely to receive a living donor kidney transplant post-ACA compared to pre-ACA [hazard ratio (HR) = 0.88, 95% CI: 0.78-1.00, P = 0.04], as were patients aged 22-25 years old with other types of insurance (HR = 0.80, 95% CI: 0.69-0.92, P = 0.002) (Table 2). These associations were not seen among candidates aged 26-29 years at listing (Table 2).
| Unadjusted | Adjusted model1 | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age 22-25 years (Group Impacted By Policy Change) | ||||||
| Post-ACA vs pre-ACA, whole cohort | 0.95 | 0.87-1.04 | 0.26 | 0.85 | 0.77-0.93 | 0.001 |
| Post-ACA vs pre-ACA, privately insured | 0.92 | 0.81-1.03 | 0.15 | 0.88 | 0.78-1.00 | 0.04 |
| Post-ACA vs pre-ACA, no private insurance | 0.84 | 0.73-0.97 | 0.02 | 0.80 | 0.69-0.92 | 0.002 |
| Age 26-30 years (Control Group) | ||||||
| Post-ACA vs pre-ACA, whole cohort | 1.03 | 0.94-1.13 | 0.48 | 1.00 | 0.91-1.09 | 0.98 |
| Post-ACA vs pre-ACA, privately insured | 1.07 | 0.95-1.19 | 0.28 | 1.01 | 0.90-1.14 | 0.82 |
| Post-ACA vs pre-ACA, no private insurance | 1.01 | 0.88-1.16 | 0.92 | 0.98 | 0.85-1.13 | 0.77 |
Results were similar in a sensitivity analysis restricted to candidates listed in states without Medicaid expansion (Supplementary Table 2).
In this retrospective cohort study using United States transplant registry data from 2005-2014, we found that although kidney transplant candidates age 22-25 years were more likely to have private insurance following the ACA policy change expanding eligibility to remain on parental insurance, this shift in payer mix was not associated with a higher rate of LDKT. Rather, both privately insured candidates and candidates with other forms of insurance in this age group appear to be less likely to receive a living donor kidney transplant after the ACA, an effect that was not observed in the older cohort not affected by the ACA policy change. These data suggest that, in this young adult population, insurance type itself is not a direct determinant of access to living donor kidney transplant; rather the association of private insurance with higher transplantation rates in prior observational studies is likely a result of unmeasured demographic confounding—i.e. characteristics of the privately insured population.
The association of private insurance coverage and outcomes for patients with ESKD has been of increased interest following the Marietta Memorial Hospital Employee Health Benefit Plan v. DaVita Inc. decision by the Supreme Court in June 2022[16]. As a result of that decision, it is possible that in coming years, it will be more difficult for patients with ESKD to maintain their private insurance, making it essential to understand the potential implications of this on access to transplantation–and in particular living donor transplantation. However, given the many dissimilarities between individuals with access to different types of insurance, it is difficult to say whether insurance type itself affects patient outcomes instead of an unmeasured confounding factor. By using a natural experiment design with a built in intervention and control group, with the implementation of the ACA as the intervention, we can better study whether or not gaining access to private insurance itself leads to increased rates of living donor transplantation.
Previous literature suggested that patients with private insurance are more likely to be evaluated, waitlisted, receive any transplant, and receive a living donor transplant compared to patients with public insurance (i.e. Medicare or Medicaid), so it would follow that expansion of private insurance in any group would improve health outcomes in that group[13-15]. Our findings to the contrary suggest that interventions aimed at expanding access to specific types of insurance are unlikely to impact living donor transplant rates. They also emphasize the need for better capture of individual-level socioeconomic status data in national transplant registries in order to better understand and address disparities in access to transplantation. More importantly, however, they suggest that expansion of public coverage to medically vulnerable populations is not likely to provide inferior outcomes compared to private insurance coverage.
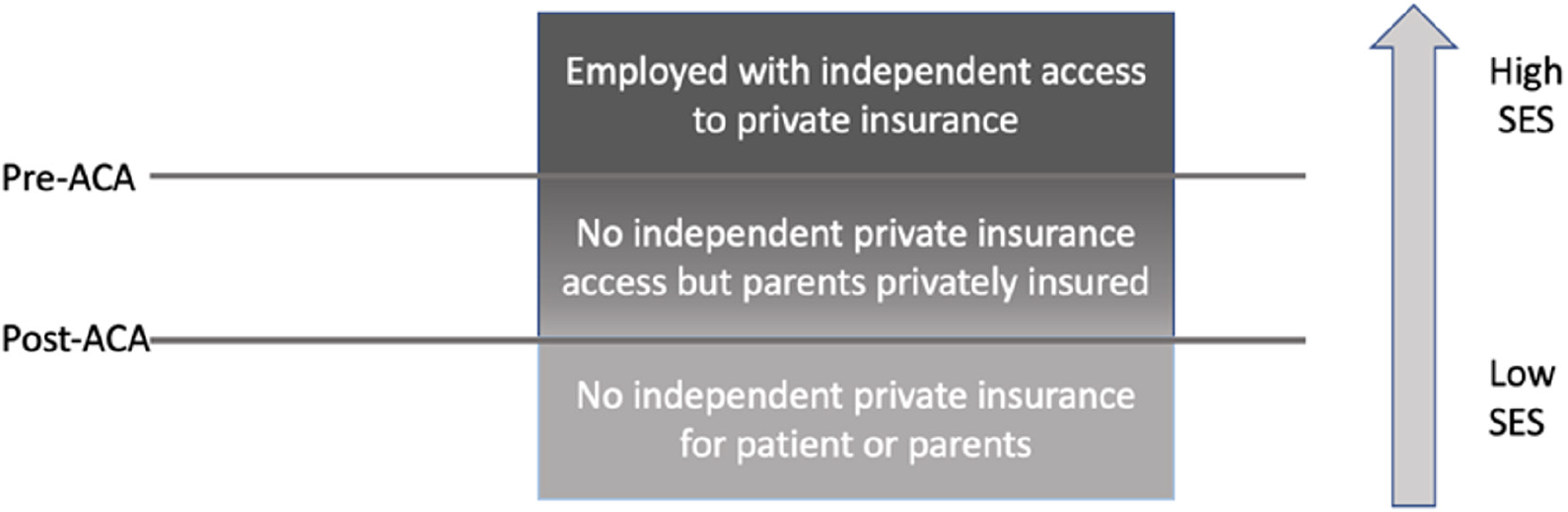
Paradoxically, we found that even though private insurance was associated with a higher rate of living donor transplantation than other types of insurance, despite a post-ACA shift towards more private insurance coverage among candidates age 22-25 years, these candidates were less likely to receive a living donor transplant post-ACA, an effect that was also observed when analyzing only candidates with private insurance or only those with other forms of insurance. We hypothesize that understanding insurance type as capturing one dimension of a multi-dimensional concept of socioeconomic status helps reconcile these findings (Figure 2). Candidates can be conceptually divided into three groups with decreased socioeconomic advantage: (1) An employed group with independent access to private insurance; (2) A group with no independent private insurance access but parents who are privately insured; and (3) A group with no independent private insurance for either the patients or the parents. The second group would have been part of the “non-privately insured group” prior to the ACA and then part of the “privately insured group” after the ACA. As a result, the socioeconomic status of both privately insured candidates and other candidates age 22-25 years decreased post-ACA, as the most advantaged candidates among those who previously would have been non-privately insured were instead privately insured.
Strengths of our study included the use of the ACA as a “natural experiment” to assess the association between insurance and health outcomes for patients with ESKD in the absence of an ability to conduct a clinical trial. Limitations include the inability to account for other key individual-level socioeconomic characteristics that are not included in the OPTN registry, including income data. Further, in the absence of randomized trial data assigning candidates to different insurance types, the lack of an association between insurance type and living donor transplantation that we observed is itself possibly subject to residual confounding. However, we believe that other investigators may consider using a similar natural experiment design around the ACA’s implementation to study the association between payer type and outcomes in other health domains.
In conclusion, we found that although kidney transplant candidates age 22-25 years were more likely to have private insurance post-ACA compared to pre-ACA, those listed in the post-ACA period were less likely to receive a living donor transplant. This result suggests that insurance type itself is not independently associated with living donor transplant rates, but instead that prior demonstrations of associations between insurance and living donor transplantation were likely attributable to residual confounding. Further research is needed to elucidate how to develop insurance expansion strategies that optimize transplant rates.
| 1. | Axelrod DA, Schnitzler MA, Xiao H, Irish W, Tuttle-Newhall E, Chang SH, Kasiske BL, Alhamad T, Lentine KL. An economic assessment of contemporary kidney transplant practice. Am J Transplant. 2018;18:1168-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 259] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 2. | de Groot IB, Veen JI, van der Boog PJ, van Dijk S, Stiggelbout AM, Marang-van de Mheen PJ; PARTNER-study group. Difference in quality of life, fatigue and societal participation between living and deceased donor kidney transplant recipients. Clin Transplant. 2013;27:E415-E423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Gross CR, Limwattananon C, Matthees B, Zehrer JL, Savik K. Impact of transplantation on quality of life in patients with diabetes and renal dysfunction. Transplantation. 2000;70:1736-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Kugler C, Gottlieb J, Warnecke G, Schwarz A, Weissenborn K, Barg-Hock H, Bara C, Einhorn I, Haverich A, Haller H. Health-related quality of life after solid organ transplantation: a prospective, multiorgan cohort study. Transplantation. 2013;96:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med. 2001;344:726-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 354] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 6. | Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation. 2002;74:1377-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 557] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 7. | Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, Ojo AO, Port FK. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294:2726-2733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 556] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 8. | Pinson CW, Feurer ID, Payne JL, Wise PE, Shockley S, Speroff T. Health-related quality of life after different types of solid organ transplantation. Ann Surg. 2000;232:597-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3684] [Cited by in RCA: 3857] [Article Influence: 148.3] [Reference Citation Analysis (1)] |
| 11. | Johansen KL, Zhang R, Huang Y, Patzer RE, Kutner NG. Association of race and insurance type with delayed assessment for kidney transplantation among patients initiating dialysis in the United States. Clin J Am Soc Nephrol. 2012;7:1490-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Schold JD, Gregg JA, Harman JS, Hall AG, Patton PR, Meier-Kriesche HU. Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol. 2011;6:1760-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 13. | Keith D, Ashby VB, Port FK, Leichtman AB. Insurance type and minority status associated with large disparities in prelisting dialysis among candidates for kidney transplantation. Clin J Am Soc Nephrol. 2008;3:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | King KL, Husain SA, Jin Z, Brennan C, Mohan S. Trends in Disparities in Preemptive Kidney Transplantation in the United States. Clin J Am Soc Nephrol. 2019;14:1500-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 15. | Harhay MN, McKenna RM, Boyle SM, Ranganna K, Mizrahi LL, Guy S, Malat GE, Xiao G, Reich DJ, Harhay MO. Association between Medicaid Expansion under the Affordable Care Act and Preemptive Listings for Kidney Transplantation. Clin J Am Soc Nephrol. 2018;13:1069-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Maliha G, Glickman JD, McCoy MS. Ensuring Equitable Access to Dialysis: The Medicare Secondary Payer Act in Marietta Memorial Hospital Employee Health Benefit Plan v. DaVita, Inc. J Am Soc Nephrol. 2022;33:1814-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |