Published online Mar 25, 2025. doi: 10.5527/wjn.v14.i1.99044
Revised: December 7, 2024
Accepted: December 27, 2024
Published online: March 25, 2025
Processing time: 192 Days and 10 Hours
Equations for estimation glomerular filtration rate (eGFR) have been associated with poor clinical performance and their clinical accuracy and reliability have been called into question.
To assess the longitudinal changes in measured glomerular filtration rate (mGFR) in patients with autosomal dominant polycystic kidney disease (ADPKD).
Analysis of an ambispective data base conducted on consecutive patients diagnosed with ADPKD. The mGFR was assessed by iohexol clearance; while eGFR was calculated by three different formulas: (1) The chronic kidney disease epidemiology collaboration (CKD-EPI); (2) Modification of diet in renal disease (MDRD); and (3) The 24-hour urine creatinine clearance (CrCl). The primary end-points were the mean change in mGFR between the baseline and final visit, as well as the comparison of the mean change in mGFR with the change estimated by the different formulas.
Thirty-seven patients were included in the study. As compared to baseline, month-6 mGFR was significantly decrease by -4.4 mL/minute ± 10.3 mL/minute (P = 0.0132). However, the CKD-EPI, MDRD, and CrCl formulas underestimated this change by 48.3%, 89.0%, and 45.8% respectively, though none of these differences reached statistical significance (P = 0.3647; P = 0.0505; and P = 0.736, respectively). The discrepancies between measured and estimated glomerular filtration rate values, as evaluated by CKD-EPI (r = 0.29, P = 0.086); MDRD (r = 0.19, P = 0.272); and CrCl (r = 0.09, P = 0.683), were not correlated with baseline mGFR values.
This study indicated that eGFR inaccurately reflects the decline in mGFR and cannot reliably track changes over time. This poses significant challenges for clinical decision-making, particularly in treatment strategies.
Core Tip: This analysis of an ambispective data base aimed to evaluate the longitudinal changes in measured glomerular filtration rate (mGFR) and the estimation glomerular filtration rate (eGFR). Glomerular filtration rate (GFR) in patients with autosomal dominant polycystic kidney disease (ADPKD) and their relationship between baseline eGFR and final mGFR. The three formulas for estimating GFR were notably imprecise and unreliable, especially for tracking changes in GFR in individuals with ADPKD. The change in mGFR was underestimated by 48.3%, 89.0%, and 45.8% by the chronic kidney disease epidemiology collaboration, modification of diet in renal disease, and the 24-hour urine creatinine clearance formulas, respectively, although none of these underestimations were statistically significant. These results could signifi
- Citation: Fernandez JM, Rodriguez-Pérez JC, Lorenzo-Medina MM, Rodriguez-Esparragon F, Quevedo-Reina JC, Hernandez-Socorro CR. Longitudinal assessment of measured and estimated glomerular filtration-rate in autosomal dominant polycystic kidney disease: Real practice experience. World J Nephrol 2025; 14(1): 99044
- URL: https://www.wjgnet.com/2220-6124/full/v14/i1/99044.htm
- DOI: https://dx.doi.org/10.5527/wjn.v14.i1.99044
Autosomal dominant polycystic kidney disease (ADPKD) is a prevalent monogenic disorder affecting individuals worldwide[1,2]. The hallmark of the disease is the development of multiple cysts gradually compressing renal paren
ADPKD involves complex renal cyst formation processes, including cell proliferation, apoptosis, altered cell phe
Following the onset of ESKD, ADPKD progression tends to be constant and can be monitored by glomerular filtration rate (GFR)[7]. However, during the early stages of the disease, due to glomerular hyperfiltration, kidney function remains intact[8,9]. Renal volume, indeed, increases years before GFR starts to decrease, which means that GFR is not an accurate early predictor of ADPKD progression[9,10].
In clinical practice, renal function might be estimated by rutinary and standard techniques creatinine-based formulas[11]. Many different equations that focused on estimating renal function have been described. However, their clinical performance has been questioned[12,13].
In general terms, the mean difference between the estimation GFR (eGFR) and the measured GRF (mGFR) is about 30%, although this difference may be even greater[11,14]. The current evidence evaluating the clinical performance in patients with ADPKD is limited[15-17]. In a previous study published by our group, estimating GFR by formulas did not provide reliable results[14].
The disparities observed between mGFR and eGFR measurements may exert detrimental effects on patient prognosis, potentially leading to treatment delays. Moreover, the consequences of variations in mGFR over time on clinical disease progression remain poorly understood. Consequently, there exists a need to investigate the longitudinal clinical performance of eGFR and its correlation with mGFR throughout follow-up periods, which could yield valuable insights into disease management and patient outcomes.
The intention of this study was to evaluate the changes of mGFR over time in patients with ADPKD. In addition, this study assessed the changes in eGFR (measured by different formulas) over time and the relationship between baseline eGFR and final mGFR.
This was a retrospective analysis of a prospective data base conducted on consecutive patients diagnosed with ADPKD (all of them PKD1) attended in the third-level University Hospital of Gran Canaria Doctor Negrín (HUGCDN) (Las Palmas de Gran Canaria; Spain).
The study protocol received approval from the Ethics Committee of the HUGCDN (Protocol VO 05-2017; Review Board approval, No. 170071; May 2017). This study adhered to the principles outlined in the Good Clinical Practice/International Council for Harmonization Guidelines, the Declaration of Helsinki, and all relevant country-specific regulations governing clinical research, prioritizing the highest level of individual protection.
Prior to participation, written informed consent was obtained from all patients. To ensure anonymity, any potentially identifying information was either encrypted or omitted from the data.
This study included patients, aged > 18 years; diagnosed with ADPKD, clinically stable[18]; and with a measured CKD-EPI > 60 mL/minutes (i.e., absence of acute kidney injury, active infectious diseases, or cardiovascular events within the three months prior to the study enrollment).
Patients with allergy to iodine, active malignant tumor, uremia or impending dialysis, severe psychiatric disorders, or those who were pregnant or nursing were excluded.
mGFR: On the study visit day (baseline), a 5 mL intravenous injection of iohexol solution (Omnipaque 300, GE Healthcare) was administered over 2 minutes. Iohexol levels were assessed using dried blood spot samples, which were subsequently forwarded to the University of La Laguna (Tenerife, Spain) for analysis[19]. Plasma clearance of iohexol was determined following the method described by Krutzén et al[20].
EGFR: Simultaneously to the clearance of iohexol, serum creatinine (enzimatic method) and cystatin-C (inmunoturbidimetric method) were determined to calculate eGFR by different formulas. The chronic kidney disease epidemiology collaboration (CKD-EPI)[21], the modification of diet in renal disease (MDRD)[22], and 24-hour urine creatinine clearance (CrCl) equations were used to calculate eGFR.
The primary end-points were the mean change in mGFR between the baseline and final visit, as well as the comparison of the mean change in mGFR with the change estimated by the different formulas.
The secondary end-points were the comparison of mGFR and eGFR at baseline and at the end of the study and the changes in blood and urine analysis data from baseline to month-6.
To assess the clinical performance of eGFR formulas, in addition to considering the overall study population, we stratified the study sample according to their baseline mGFR (median split). Patients were, therefore, divided in those with a baseline mGFR ≤ 80 mL/minutes and those with a baseline mGFR > 80 mL/minutes.
Statistical analysis was performed using MedCalc® Statistical Software version 22.023 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2024).
The Shapiro-Wilks test was used for assessing quantitative variables normality.
In normally distribute variables repeated measures analysis of variance was used to analyzed the changes in mGFR and eGFR; while if such variables were no normally distribute, the Friedman test was use.
The Bland-Altman method with 95% limits of agreement was used to assess the differences between mGFR (determined as reference method) and the eGFR (estimated by CKD-EPI, MDRD, and CrCl formulas) at baseline and at the last follow-up visit. In addition, this method was also used to determine the differences between the mean changes (basal vs final) among the different techniques.
Linear regression analysis (considering Pearson correlation coefficient) was performed to assess the relationship month-6 changes between measured and estimated GFR values, as well as the relationship between month-6 changes in eGFR and baseline mGFR.
Additionally, the concordance correlation coefficient (CCC)[23] was performed to assess agreement between the mGFR (used as reference method) and the eGFR (by CKD-EPI, MDRD, and CrCl formulas). The CCC contains a measurement of precision Ρ (which measures how far each observation deviates from the best-fit line) and accuracy Cb (that measures how far the best-fit line deviates from the 45° line through the origin)[23]. CCC ranges from 0 to 1 and it should be interpreted as poor (< 0.9); moderate (≥ 0.90 to ≤ 0.95); substantial (> 0.95 to ≤ 0.99); and almost perfect (> 0.99)[24].
A total of 37 subjects, 19 (51.4%) females and 18 (48.6%) males, were included in the study. The mean ± SD age was 40.2 years ± 15.3 years. Twenty-two (59.5%) patients had systemic hypertension (21 of them well controlled with medication). Thirty-one (83.8%) patients had a family history of ADPKD.
The Table 1 shows the main demographic and clinical characteristics of the study sample.
| Variable | n = 37 |
| Age (years) | |
| mean ± SD | 40.2 ± 15.3 |
| Median (IqR) | 37.0 (31.0-50.0) |
| Body mass index (kg/m2) | |
| mean ± SD | 25.4 ± 5.8 |
| Median (IqR) | 24.5 (21.8-27.4) |
| Gender | |
| Female | 19 (51.4) |
| Male | 18 (48.6) |
| Diabetes mellitus | |
| Yes | 0 (0.0) |
| No | 37 (100.0) |
| Dyslipidemia | |
| Yes | 4 (11.1) |
| No | 32 (88.9) |
| HBP | |
| Yes | 22 (59.5) |
| No | 15 (40.5) |
| HBP controlled | |
| Yes | 21 (95.5) |
| No | 1 (4.5) |
| Systolic blood pressure (mmHg) | |
| mean ± SD | 130.9 ± 15.5 |
| Median (IqR) | 131.0 (118.8-140.0) |
| Diastolic blood pressure (mmHg) | |
| mean ± SD | 79.4 ± 9.0 |
| Median (IqR) | 80.0 (72.8-84.3) |
| Cardiovascular events | |
| Yes | 0 (0.0) |
| No | 37 (100.0) |
| Smoker status | |
| Never | 32 (86.5) |
| Former | 2 (5.4) |
| Current | 3 (8.1) |
| Family history of autosomal dominant polycystic kidney disease | |
| Yes | 31 (86.1) |
| No | 5 (13.9) |
An overview of the blood and urine analysis data of the study sample is shown in Supplementary Table 1.
The mean (SD) the mGRF at baseline was 85.6 mL/minute ± 24.6 mL/minute (median: 81.0 mL/minute; interquartile range: 69.4-101.0 mL/minute); with 17 (45.9%) and 20 (54.1%) patients with a baseline mGFR ≤ 80 mL/minute and > 80 mL/minute, respectively.
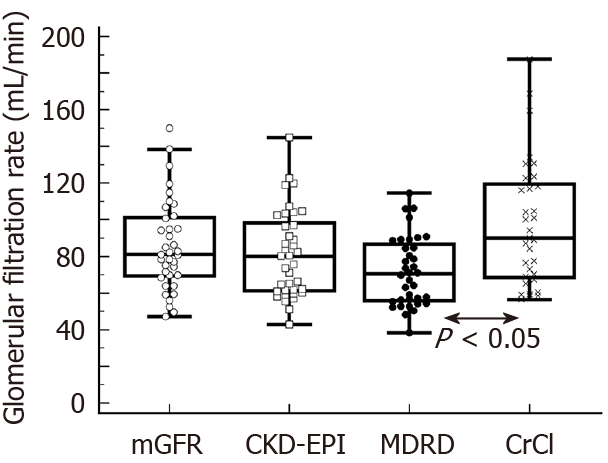
Additionally, the mean (SD) estimated GFR at baseline was 81.3 mL/minute ± 23.7 mL/minute; 71.7 mL/minute ± 18.9 mL/minute; and 97.4 mL/minute ± 34.3 mL/minute according to the CKD-EPI, MDRD, and CrCl formulas, respectively.
The Figure 1 shows the baseline mGRF and eGRF. The CCC showed poor agreement between mGFR and eGFR regardless the formula used or the baseline mGFR (Table 2).
| mGFR (reference), overall study sample (n = 37) | |||
| CCC (95%CI) | Precision | Accuracy | |
| CKD-EPI | 0.576 (0.318-0.754) | 0.585 | 0.983 |
| MDRD | 0.457 (0.223-0.641) | 0.582 | 0.786 |
| CrCl | 0.598 (0.376-0.755) | 0.697 | 0.858 |
| mGFR ≤ 80 (n = 17) | |||
| CCC (95%CI) | Precision | Accuracy | |
| CKD-EPI | 0.315 (-0.038 to 0.599) | 0.425 | 0.741 |
| MDRD | 0.356 (-0.069 to 0.672) | 0.414 | 0.862 |
| CrCl | 0.418 (0.064-0.679) | 0.599 | 0.698 |
| mGFR > 80 (n = 20) | |||
| CCC (95%CI) | Precision | Accuracy | |
| CKD-EPI | 0.429 (0.069-0.690) | 0.515 | 0.833 |
| MDRD | 0.277 (0.033-0.490) | 0.529 | 0.524 |
| CrCl | 0.315 (-0.037 to 0.597) | 0.423 | 0.745 |
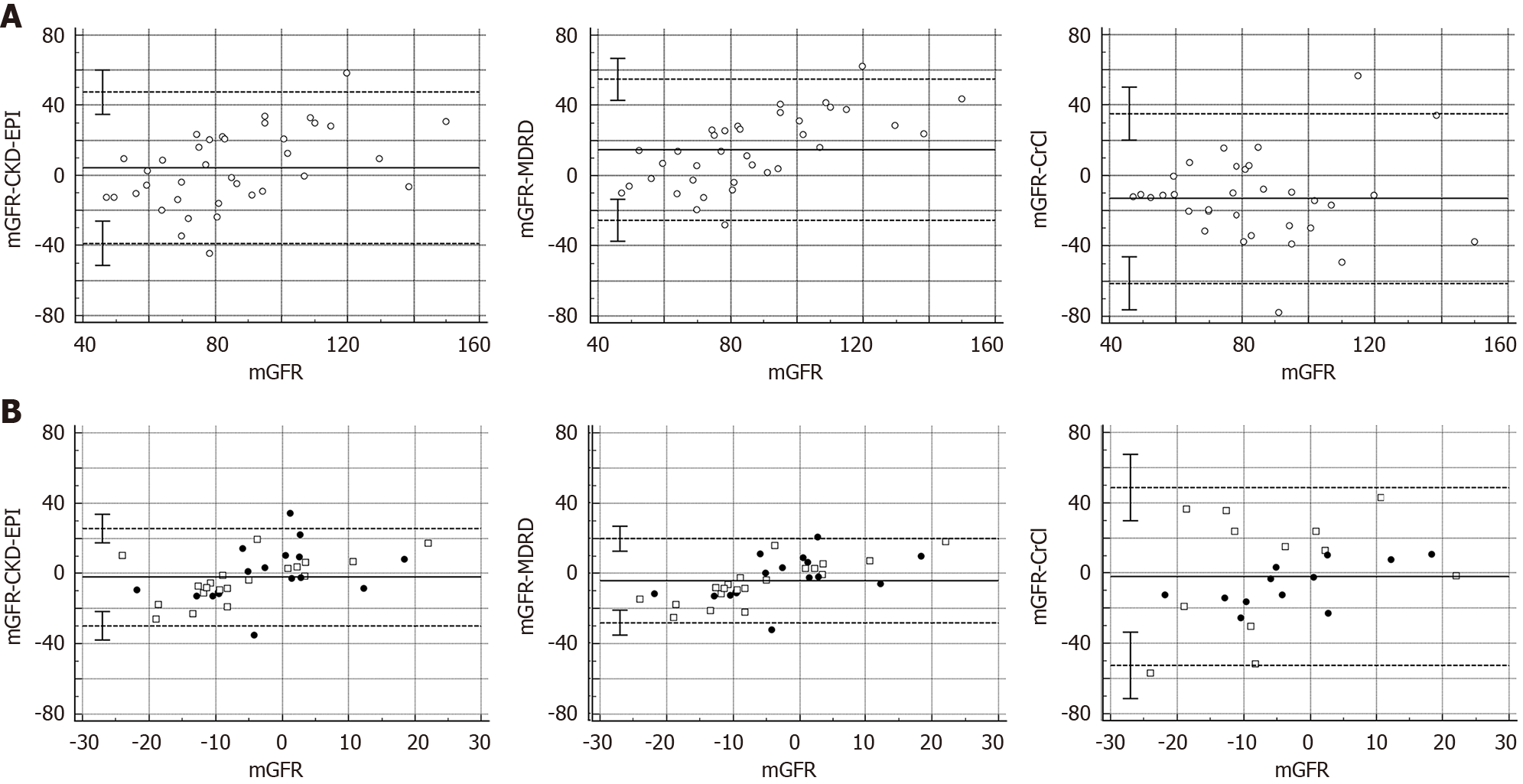
Additionally, the Bland-Almant plots (Figure 2A) show that as compared to mGFR, systematic mean difference (95%CI) were 4.31 mL/minute (95%CI: -3.01 to 11.64); 14.64 mL/minute (95%CI: 7.71-21.57); and -13.07 mL/minute (95%CI: -21.80 to -4.36) for the CKD-EPI; MDRD; and CrCl formulas, respectively.
There was a borderline, but not significant, correlation between serum creatinine levels and mGFR at baseline (slope:
The Table 3 shows an overview of the mGFR and eGFR changes vs baseline in the overall study sample and according to their baseline mGFR (mGFR ≤ 80 mL/minute and > 80 mL/minute).
| Glomerular filtration rate (mL/minute) | ||||
| Overall (n = 37) | mGFR ≤ 80 (n = 17) | mGFR > 80 (n = 20) | ||
| Iohexol | Baseline (mean ± SD) | 85.6 ± 24.6 | 65.6 ± 10.2 | 102.7 ± 19.8 |
| Month-6 (mean ± SD) | 81.2 ± 26.2 | 63.2 ± 16.1 | 96.6 ± 16.3 | |
| Difference | ||||
| mean ± SD | -4.4 ± 10.3 | -2.4 ± 9.5 | -6.1 ± 10.8 | |
| 95%CI | -7.8 to -1.0 | -7.2 to 2.5 | -11.2 to -1.1 | |
| P value | 0.0132a | 0.2842b | 0.0136b | |
| Chronic kidney disease epidemiology collaboration | Baseline (mean ± SD) | 81.3 ± 23.7 | 71.2 ± 20.8 | 89.9 ± 23.0 |
| Month-6 (mean ± SD) | 79.0 ± 23.9 | 69.1 ± 24.2 | 87.5 ± 20.6 | |
| Difference | ||||
| mean ± SD | -2.3 ± 12.7 | -2.2 ± 15.2 | -2.4 ± 10.4 | |
| 95%CI | -6.5 to 2.0 | -10.0 to 5.7 | -7.3 to 2.5 | |
| P value | 0.2824a | 0.4691b | 0.4939b | |
| Modification of diet in renal disease | Baseline (mean ± SD) | 71.7 ± 18.9 | 63.6 ± 17.6 | 78.2 ± 17.7 |
| Month-6 (mean ± SD) | 71.2 ± 19.2 | 63.5 ± 18.6 | 77.4 ± 17.7 | |
| Difference | ||||
| mean ± SD | -0.5 ± 9.3 | -0.1 ± 11.9 | -0.8 ± 6.9 | |
| 95%CI | -3.6 to 2.7 | -6.4 to 6.2 | -4.0 to 2.3 | |
| P value | 0.7560a | 0.7119b | 0.5196b | |
| Creatinine clearancec | Baseline (mean ± SD) | 100.6 ± 34.1 | 74.0 ± 15.4 | 127.3 ± 27.4 |
| Month-6 (mean ± SD) | 98.2 ± 34.0 | 77.6 ± 19.2 | 118.9 ± 33.5 | |
| Difference | ||||
| mean ± SD | -2.4 ± 24.5 | 3.6 ± 10.3 | -8.4 ± 32.7 | |
| 95%CI | -12.7 to 8.0 | -2.9 to 10.1 | -29.2 to 12.4 | |
| P value | 0.7642b | 0.3668b | 0.4697b | |
In the overall study sample, at month 6, mGFR significantly decreased by -4.4 ± 10.3 vs baseline (P = 0.0132). The mGFR change was underestimated by 48.3%, 89.0%, and 45.8% by the CKD-EPI, MDRD, and CrCl formulas, although none of them was statistically significant (P = 0.3647, P = 0.0505, and P = 0.736, respectively).
The Bland-Almant plots (Figure 2B) show that as compared to mGFR change from baseline to month-6, systematic mean difference (95%CI) were -2.12 mL/minute (95%CI: -6.83 to 2.57); -4.11 mL/minute (95%CI: -8.23 to 0.01); and -1.96 mL/minute (-12.85 to 8.93) for the CKD-EPI, MDRD, and CrCl formulas, respectively.
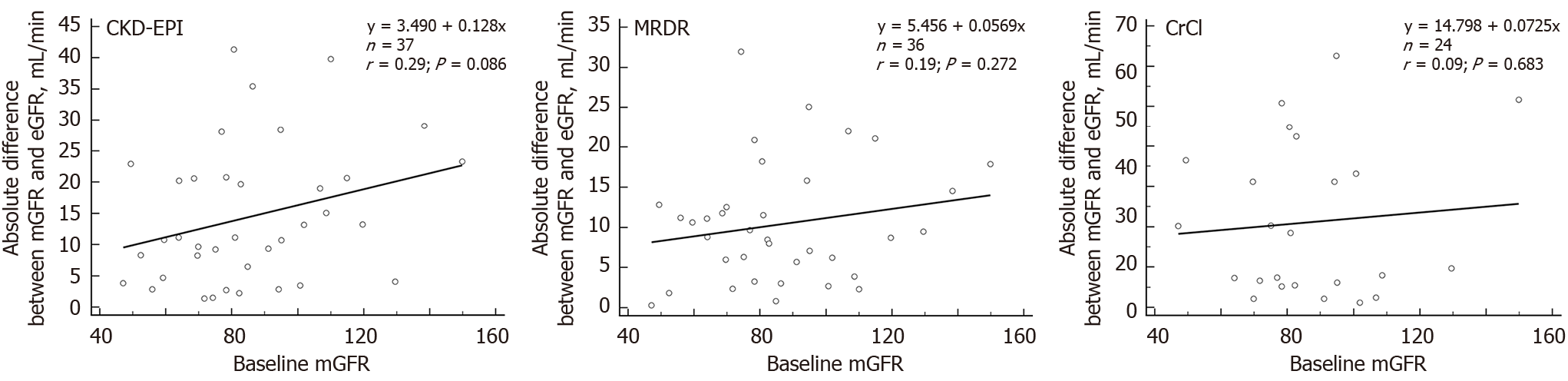
The absolute differences between measured and estimated GFR values assessed by CKD-EPI (r = 0.29, P = 0.086); MDRD (r = 0.19, P = 0.272); and CrCl (r = 0.09, P = 0.683) were no related to baseline mGFR values (Figure 3).
In the overall study sample, no significant correlation was found between mGFR changes and changes estimated either by the CKD-EPI formula (slope: 0.32; 95%CI: -0.9 to 0.73; r = 0.26; P = 0.0126); the MDRD formula (slope: 0.21; 95%CI:
Regarding blood and urine analysis data, except for thrombocytes and cystatin-C (which were significantly increased at month-6, P = 0.0054 and P = 0.0067, respectively), none of the variables showed significant changes over the study follow-up (Supplementary Table 2).
The current study assessed the clinical performance of three formulas to estimate GFR, namely CKD-EPI, MDRD, and CrCl, as compared to measured GFR assessed by the suggested gold standard procedure (i.e., the iohexol plasma clearance technique)[19,20,25] in a cohort of ADPKD patients with 1-2 chronic kidney disease (CKD) stages.
The results of this study indicated that the three formulas for estimating GFR were significantly inaccurate and unreliable, particularly for tracking GFR changes in individuals with ADPKD. Additionally, this inaccuracy was consistent regardless of the level of kidney function, affecting even those with mild-to-moderate renal insufficiency. Indeed, month-6 changes estimated by the three formulas failed to correlate to any appreciable extent with measured changes. Moreover, data were biased by a systematic underestimation of estimated GFR changes that ranged from 45.8% by CrCl formula to 89.0% by MDRD formula. On the other hand, estimated month-6 GFR changes were no associated with baseline mGFR, which indicated a wide and unpredictable deviation of estimated data.
The results of this study confirmed the current evidence indicating that that prediction formulas do not accurately estimate GFR[14-17].
The key finding of the study was that eGFR formulas failed to accurately capture mGFR changes over time in individuals with ADPKD, a group highly susceptible to CKD progression.
The inaccurate estimation of actual GFR values and the unreliable prediction of GFR changes over time by the CKD-EPI, MDRD, and CrCl formulas might be associated with two important clinical issues. The first one is that this lack of precision and reliability prevents these formulas from being used for assessing the impact of experimental treatments on the progressive loss of renal function in patients with ADPKD. The second implication is that these formulas failed to diagnose rapid progressors towards advanced CKD accurately. This failure can negatively affect clinical management by preventing the establishment of appropriate therapeutic strategies[26,27].
Recognizing ADPKD patients who are at elevated risk for rapid progression to requiring kidney replacement therapy has become increasingly important due to the advent of potential novel treatments[26,28]. It is, therefore, essential to establish criteria for rapid progression in patients with ADPKD to facilitate the selection of disease-modifying therapies and the recruitment of participants for clinical trials[28,29].
From a clinical perspective, failing to identify patients with rapid progression will postpone the starting of treatment in patients who could benefit from it[30,31]. Conversely, misdiagnosing patients with slow renal function decline or stable condition as rapid progressors may unnecessarily expose them to adverse effects, such as liver injury, severe polyuria, and hypernatremia, while also increasing the societal costs associated with the disease[30,31].
The findings of the current study corroborate the conclusions reported by Miquel-Rodríguez et al[31], who employed a methodology congruent with our approach. Their study revealed that formulas to estimate GFR, whether based on creatinine and/or cystatin-C, were inadequate in detecting the temporal changes and progression of renal function in patients with ADPKD. They highlighted two principal implications of this discrepancy, namely the failure to correctly identify individuals with rapid disease progression, and the misclassification of patients with stable GFR or moderate disease progression as rapid progressors[31].
Porrini et al[12] reported that a single measurement of serum creatinine or cystatin-C could be associated with a value of mGFR ranging from 30 mL/minute to 90 mL/minute, indicating a variability of 200%. In our study, we found a significant correlation between cystatin-C levels and mGFR, while the correlation between serum creatinine and mGFR was borderline, but not significant. Despite this correlation, we noted comparable variability to that reported by Porrini et al[12] or Rodríguez et al[14].
For instance, serum creatinine values ranging between 0.87-0.88 were linked with mGFR ranging from 68.6 mL/minute to 149.9 mL/minute. Similarly, levels of cystatin-C ranging between 0.84 and 0.85 were associated with mGFR ranging from 78.3 mL/minute to 110.7 mL/minute.
It should be mentioned that, as compared to baseline, cystatin-C levels were significantly increased at month-6 in our sample. Therefore, it might be conjectured that individuals with ADPKD may experience heightened inflammatory activity, leading to elevated cystatin-C levels regardless of GFR, thereby potentially underestimating actual renal function.
The present study has several limitations that warrant consideration when interpreting its findings. The most important one was the relatively small sample size within subgroups, potentially limiting the ability to draw robust comparisons. Nevertheless, the study included a pertinent number of patients, adequate for meaningful stratification. The 6-month follow-up period in our study may be insufficient to fully capture disease-associated complications. Further
The main strength of the study is that it has been carried out under conditions of real clinical practice, which offers a more accurate representation of how these tools work outside of controlled research environments. Another strength was the use of a standardize method for measuring GFR, as it is the iohexol plasma clearance technique.
In summary, the findings of this study clearly indicate that eGFR inadequately and imprecisely reflected the decline in mGFR. Furthermore, eGFR equations exhibited unreliable estimation of actual GFR values and were unable to detect changes in GFR over time. These results might critically impact on clinical decision-making, particularly on treatment strategies and highlighted the need for a more effective and efficient method to assess kidney function and its evolution over time. Finally, it will be necessary to develop future research, ideally prospective and multicenter, to evaluate the clinical performance of these new tools in daily practice.
The authors would like to thank Dr. Porrini E and Dr. Lima SL for their collaboration during the research.
| 1. | Martínez V, Comas J, Arcos E, Díaz JM, Muray S, Cabezuelo J, Ballarín J, Ars E, Torra R. Renal replacement therapy in ADPKD patients: a 25-year survey based on the Catalan registry. BMC Nephrol. 2013;14:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Ponticelli C, Moroni G, Reggiani F. Autosomal Dominant Polycystic Kidney Disease: Is There a Role for Autophagy? Int J Mol Sci. 2023;24:14666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Chebib FT, Torres VE. Autosomal Dominant Polycystic Kidney Disease: Core Curriculum 2016. Am J Kidney Dis. 2016;67:792-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 4. | Cornec-Le Gall E, Audrézet MP, Renaudineau E, Hourmant M, Charasse C, Michez E, Frouget T, Vigneau C, Dantal J, Siohan P, Longuet H, Gatault P, Ecotière L, Bridoux F, Mandart L, Hanrotel-Saliou C, Stanescu C, Depraetre P, Gie S, Massad M, Kersalé A, Séret G, Augusto JF, Saliou P, Maestri S, Chen JM, Harris PC, Férec C, Le Meur Y. PKD2-Related Autosomal Dominant Polycystic Kidney Disease: Prevalence, Clinical Presentation, Mutation Spectrum, and Prognosis. Am J Kidney Dis. 2017;70:476-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Bergmann C, Guay-Woodford LM, Harris PC, Horie S, Peters DJM, Torres VE. Polycystic kidney disease. Nat Rev Dis Primers. 2018;4:50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 497] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 6. | Grantham JJ, Mulamalla S, Swenson-Fields KI. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2011;7:556-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 7. | Rizk D, Chapman AB. Cystic and inherited kidney diseases. Am J Kidney Dis. 2003;42:1305-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Perrone R. Imaging progression in polycystic kidney disease. N Engl J Med. 2006;354:2181-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Helal I, Reed B, McFann K, Yan XD, Fick-Brosnahan GM, Cadnapaphornchai M, Schrier RW. Glomerular hyperfiltration and renal progression in children with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6:2439-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF Jr, Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP; CRISP Investigators. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 559] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 11. | García-Maset R, Bover J, Segura de la Morena J, Goicoechea Diezhandino M, Cebollada Del Hoyo J, Escalada San Martin J, Fácila Rubio L, Gamarra Ortiz J, García-Donaire JA, García-Matarín L, Gràcia Garcia S, Isabel Gutiérrez Pérez M, Hernández Moreno J, Mazón Ramos P, Montañés Bermudez R, Muñoz Torres M, de Pablos-Velasco P, Pérez-Maraver M, Suárez Fernández C, Tranche Iparraguirre S, Luis Górriz J. Information and consensus document for the detection and management of chronic kidney disease. Nefrologia (Engl Ed). 2022;42:233-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Porrini E, Ruggenenti P, Luis-Lima S, Carrara F, Jiménez A, de Vries APJ, Torres A, Gaspari F, Remuzzi G. Estimated GFR: time for a critical appraisal. Nat Rev Nephrol. 2019;15:177-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 207] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 13. | Escamilla-Cabrera B, Luis-Lima S, Gallego-Valcarce E, Sánchez-Dorta NV, Negrín-Mena N, Díaz-Martín L, Cruz-Perera C, Hernández-Valles AM, González-Rinne F, Rodríguez-Gamboa MJ, Estupiñán-Torres S, Miquel-Rodríguez R, Cobo-Caso MÁ, Delgado-Mallén P, Fernández-Suárez G, González-Rinne A, Hernández-Barroso G, González-Delgado A, Torres-Ramírez A, Jiménez-Sosa A, Ortiz A, Gaspari F, Hernández-Marrero D, Porrini EL. The error of estimated GFR in predialysis care. Sci Rep. 2024;14:5219. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Rodríguez RM, Luis-Lima S, Fernandez JM, Gómez MVP, Toledo BG, Cobo M, Delgado-Mallén P, Escamilla B, Marco CO, Estupiñán S, Perera CC, Mena NN, Martín LD, Reyes SP, González IH, González-Rinne F, González-Delgado A, Ferrer-Moure C, Zulueta BL, Torres A, Rodriguez Pérez JC, Gaspari F, Ortiz A, Porrini E. Estimated GFR in autosomal dominant polycystic kidney disease: errors of an unpredictable method. J Nephrol. 2022;35:2109-2118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Orskov B, Borresen ML, Feldt-Rasmussen B, Østergaard O, Laursen I, Strandgaard S. Estimating glomerular filtration rate using the new CKD-EPI equation and other equations in patients with autosomal dominant polycystic kidney disease. Am J Nephrol. 2010;31:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Spithoven EM, Meijer E, Boertien WE, Sinkeler SJ, Tent H, de Jong PE, Navis G, Gansevoort RT. Tubular secretion of creatinine in autosomal dominant polycystic kidney disease: consequences for cross-sectional and longitudinal performance of kidney function estimating equations. Am J Kidney Dis. 2013;62:531-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Ruggenenti P, Gaspari F, Cannata A, Carrara F, Cella C, Ferrari S, Stucchi N, Prandini S, Ene-Iordache B, Diadei O, Perico N, Ondei P, Pisani A, Buongiorno E, Messa P, Dugo M, Remuzzi G; GFR-ADPKD Study Group. Measuring and estimating GFR and treatment effect in ADPKD patients: results and implications of a longitudinal cohort study. PLoS One. 2012;7:e32533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359:1477-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 375] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 19. | Luis-Lima S, Gaspari F, Negrín-Mena N, Carrara F, Díaz-Martín L, Jiménez-Sosa A, González-Rinne F, Torres A, Porrini E. Iohexol plasma clearance simplified by dried blood spot testing. Nephrol Dial Transplant. 2018;33:1597-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Krutzén E, Bäck SE, Nilsson-Ehle I, Nilsson-Ehle P. Plasma clearance of a new contrast agent, iohexol: a method for the assessment of glomerular filtration rate. J Lab Clin Med. 1984;104:955-961. [PubMed] |
| 21. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20319] [Cited by in RCA: 20106] [Article Influence: 1256.6] [Reference Citation Analysis (0)] |
| 22. | Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3805] [Cited by in RCA: 4225] [Article Influence: 222.4] [Reference Citation Analysis (0)] |
| 23. | Lin L, Torbeck LD. Coefficient of accuracy and concordance correlation coefficient: new statistics for methods comparison. PDA J Pharm Sci Technol. 1998;52:55-59. [PubMed] |
| 24. | Mcbride GB. A Proposal for Strength-of-agreement Criteria for Lin's Concordance Correlation Coefficient. 2005. |
| 25. | Gaspari F, Perico N, Ruggenenti P, Mosconi L, Amuchastegui CS, Guerini E, Daina E, Remuzzi G. Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J Am Soc Nephrol. 1995;6:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 276] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 26. | Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, Ouyang J, McQuade RD, Blais JD, Czerwiec FS, Sergeyeva O; REPRISE Trial Investigators. Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease. N Engl J Med. 2017;377:1930-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 417] [Article Influence: 52.1] [Reference Citation Analysis (1)] |
| 27. | Kramers BJ, van Gastel MDA, Boertien WE, Meijer E, Gansevoort RT. Determinants of Urine Volume in ADPKD Patients Using the Vasopressin V2 Receptor Antagonist Tolvaptan. Am J Kidney Dis. 2019;73:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS; TEMPO 3:4 Trial Investigators. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1179] [Cited by in RCA: 1145] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 29. | European Medicines Agency. Summary of medicinal product characteristics Jinarc. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002788/WC500187921.pdf. |
| 30. | Li X, Li W, Li Y, Dong C, Zhu P. The safety and efficacy of tolvaptan in the treatment of patients with autosomal dominant polycystic kidney disease: A systematic review and meta-analysis. Nefrologia (Engl Ed). 2023;43:731-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 31. | Miquel-Rodríguez R, González-Toledo B, Pérez-Gómez MV, Cobo-Caso MÁ, Delgado-Mallén P, Estupiñán S, Cruz-Perera C, Díaz-Martín L, González-Rinne F, González-Delgado A, Torres A, Gaspari F, Hernández-Marrero D, Ortiz A, Porrini E, Luis-Lima S. Measured and Estimated Glomerular Filtration Rate to Evaluate Rapid Progression and Changes over Time in Autosomal Polycystic Kidney Disease: Potential Impact on Therapeutic Decision-Making. Int J Mol Sci. 2024;25:5036. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |