Published online Mar 25, 2025. doi: 10.5527/wjn.v14.i1.101515
Revised: October 21, 2024
Accepted: December 3, 2024
Published online: March 25, 2025
Processing time: 124 Days and 20.7 Hours
Chronic kidney disease (CKD), which represents a significant global health concern, is characterized by a gradual decline in kidney function, leading to complications such as electrolyte imbalance, cardiovascular disease, and immune dysfunction. Standard CKD management includes dietary modifications, ketoanalogues supplementation, blood pressure and blood glucose control, hydration maintenance, and treatment of the underlying causes. Emerging evidence has indicated a significant role of the gut microbiota in CKD, and that dysbiosis of the gut microbiota contributes to the progression of CKD towards end-stage renal disease. Probiotics and prebiotics have recently garnered attention owing to their potential to enhance gastrointestinal health and well-being by restoring the balance of the gut microbiota. Specific probiotic strains, including Lactobacillus and Bifidobacterium, promote beneficial bacterial growth, suppress harmful bacteria, and exert anti-inflammatory, antihypertensive, and antidiabetic effects. The combination of Streptococcus thermophilus, Lactobacillus acidophilus, Bifidobacterium longum, and Bacillus coagulans has demonstrated potential as a therapeutic formulation for CKD management in various studies, highlighting its promise in treating CKD; however, supporting evidence remains limited, making it crucial to conduct further investigations to determine the specific effects of different probiotic formulations on outcomes in patients with CKD.
Core Tip: Chronic kidney disease (CKD) is a major global health challenge marked by a gradual decline in kidney function and associated complications. Recent studies have suggested that dysbiosis of the gut microbiota plays a significant role in CKD progression to end-stage renal disease. Probiotics and prebiotics, particularly strains such as Lactobacillus and Bifidobacterium and combinations including Streptococcus thermophilus and Bacillus coagulans, show promise in managing CKD by restoring the gut microbiota balance. Although these findings are promising, further research is essential to comprehensively understand the impact of specific probiotic formulations on CKD outcomes and their potential as therapeutic interventions.
- Citation: Chafekar D. Optimizing chronic kidney disease management: The potential of a multi-strain probiotic formulation. World J Nephrol 2025; 14(1): 101515
- URL: https://www.wjgnet.com/2220-6124/full/v14/i1/101515.htm
- DOI: https://dx.doi.org/10.5527/wjn.v14.i1.101515
Chronic kidney disease (CKD) is a global health concern that affects millions of people worldwide, and is characterized by a gradual loss of kidney function over time. As CKD progresses, it leads to a range of complications, including electrolyte imbalance, cardiovascular disease, and compromised immune function[1]. Globally, approximately 850 million individuals have kidney disorders, representing a pressing public health challenge. CKD is particularly impactful, with a worldwide prevalence of 13.4%[2]. Asia bears a substantial burden, with an estimated CKD prevalence of 434.3 million, including 65.6 million individuals in the advanced stages. However, CKD prevalence across the Asia-Pacific region exhibits notable variability, ranging from 4.7% to 17.4%, with China and India experiencing the highest disease prevalence[3]. This heightened prevalence in Asia, accounting for approximately 34% of the total population, is attributed to a high incidence of risk factors such as diabetes, hypertension, obesity, and cardiovascular disease[4]. From 1990 to 2019, most Asian countries have witnessed a staggering increase of more than 100% in CKD incidence, deaths, prevalent cases, and disability-adjusted life years[5]. The repercussions of CKD extend beyond health, impacting hospitalization rates and productivity, and imposing a considerable economic burden on patients, payers, and healthcare infrastructure[6,7].
The prevention and management of CKD involves a multifaceted approach encompassing both primary and secondary preventive measures[8]. Primary prevention strategies aim to mitigate risk factors associated with CKD, such as diabetes, hypertension, and obesity. Public health campaigns promoting lifestyle modifications, such as a balanced diet, regular physical activity, controlling alcohol consumption, and controlling blood pressure (BP), also play a pivotal role in preventing the onset of CKD[4,9]. Secondary treatment strategies for CKD involve early detection through the regular monitoring of BP, blood sugar level, serum creatinine level, epidermal growth factor receptor, and proteinuria. Treatment includes lifestyle modification, control of sugars, lowering lipids with statins, control of BP, proteinuria with the use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs), and nephroprotective strategies with sodium-glucose transport protein 2 inhibitors[10]. The standard of care for CKD treatment involves a conservative approach that focuses on slowing the disease progression, managing symptoms, and preventing complications[11]. The components of the standard conservative management of CKD have been outlined in detail below.
Dietary interventions, including electrolyte supplementation and protein restriction, are recommended to alleviate the burden on the kidneys, as they play an important role in the etiology and progression of CKD[11]. CKD impairs fluid, electrolyte, and pH balance, as well as toxin and waste excretion and vitamin D metabolism, commonly resulting in complications such as hyperkalemia, hyperphosphatemia, chronic metabolic acidosis, bone deterioration, and edema. Meticulous monitoring and dietary management of protein, phosphorus, potassium, sodium, and calcium levels can help to mitigate these symptoms[12-14]. Early dietary restriction and a very low-protein diet in the standard treatment of CKD can slow disease progression, reduce uremic toxins, manage metabolic acidosis, control BP, improve nutritional status, decrease proteinuria, and regulate phosphorus and potassium levels, thereby potentially reducing the need for medication, and improving overall patient outcomes[15].
Ketoanalogue supplementation offers an additional therapeutic approach to slow CKD progression[16]. Common examples of these supplements include alpha-ketoisocaproic acid, alpha-ketoisovaleric acid, and alpha-keto-beta-methylvaleric acid, among others[17]. All of these are precursors to corresponding amino acids (AAs); in the body, these precursors undergo a transamination reaction, in which an amino group is transferred from an AA donor to the ketoacid, resulting in the formation of a new AA. This process allows the body to synthesize essential AAs, without increasing the nitrogen load, which is particularly advantageous for patients with CKD, as it helps to maintain the protein balance while reducing the burden on the kidneys[18,19]. The updated Kidney Disease Outcomes Quality Initiative nutrition guidelines indicate that a very low-protein diet providing 0.3-0.4 g of dietary protein per kilogram per day, supplemented with ketoanalogues of essential AAs, may be considered as an effective dietary regimen to mitigate the risk of end-stage kidney disease (ESKD)[16].
Pharmacological interventions, such as ACE inhibitors and ARBs, are frequently prescribed to manage BP and reduce proteinuria, thereby slowing CKD progression[20,21]. Both ARBs and ACE inhibitors help to lower BP by inhibiting the renin-angiotensin-aldosterone system, which reduces the production of angiotensin II, a potent vasoconstrictor. This leads to vasodilation, decreased BP, and reduced renal strain. By managing BP effectively, ARBs and ACE inhibitors help delay progression to ESKD and improve overall renal outcomes in patients with CKD[22].
Effective glycemic control is crucial for delaying the onset of diabetes-related complications, including CKD, as elevated blood glucose levels can exacerbate kidney damage by leading to glomerular hyperfiltration and contributing to the development of diabetic nephropathy[23,24]. Commonly recommended medications include metformin (500-2000 mg/day), depending on renal function; sodium-glucose transport protein 2 inhibitors, such as dapagliflozin (10 mg/day) or empagliflozin (10-25 mg/day); glucagon like peptide-1 receptor agonists, such as liraglutide (0.6-1.8 mg/day) or dulaglutide (0.75-1.5 mg/week); dipeptidyl peptidase 4 inhibitors, such as sitagliptin (25-100 mg/day) or linagliptin (5 mg/day); and insulin in the more advanced stages of CKD[25].
Maintaining proper hydration in patients with CKD is crucial for preserving kidney function and overall health as it helps to ensure that the kidneys can effectively filter waste products and toxins from the blood, thereby preventing their accumulation[26]. In addition, it aids in regulating BP, which is vital in CKD management, as a high BP can further damage the kidneys. Hydration therapy comprises oral fluids (water), intravenous fluids, or electrolyte solutions (sodium, potassium, and bicarbonate)[27].
Nephrotoxic drugs can exacerbate kidney impairment by causing direct cellular toxicity, reducing renal blood flow, or obstructing renal tubules[28,29]. Common examples include nonsteroidal anti-inflammatory drugs, such as ibuprofen and naproxen, certain antibiotics such as aminoglycosides (e.g., gentamicin), and chemotherapeutic agents such as cisplatin, etc[30]. Avoiding these drugs or using them with caution and under strict medical supervision can help to mitigate risks and preserve kidney function in patients with CKD.
The primary etiologies of CKD include diabetes mellitus, hypertension, glomerulonephritis, polycystic kidney disease, and recurrent urinary tract infections[31]. Addressing the underlying causes of CKD is vital for slowing disease progression, preserving kidney function, and improving patient outcomes and quality of life (QOL)[32].
Probiotics, defined as live microorganisms that confer health benefits when administered in adequate quantities, have gained considerable attention for their potential to promote gastrointestinal health and overall well-being[33]. Growing evidence has suggested that the gut microbiota plays a crucial role in CKD by interacting with key organ systems such as the brain, kidney, and immune system, and that its dysbiosis is linked to various chronic diseases such as CKD. There is growing recognition of the potential role of modifying the gut-kidney axis through the use of prebiotics and probiotics as a therapeutic strategy to delay the progression of CKD to end-stage renal disease. By intervening earlier in the disease course by providing conservative therapies, including the strategic use of prebiotics and probiotics, it is possible reduce the need for more invasive and expensive treatments. One of the primary mechanism through which probiotics exhibit their therapeutic potential in CKD is the modulation of the gut microbiota, as CKD is associated with an imbalance in the gut microbial community, leading to the production and absorption of uremic toxins[34]. Probiotics, particularly Lactobacillus and Bifidobacterium, restore this balance by promoting the growth of beneficial bacteria, and inhibiting their proliferation of harmful ones. This modulation contributes to a reduction in the production of uremic toxins, subsequently alleviating the burden on compromised kidneys[34]. Inflammation has further been characterized as a pathophysiological hallmark of CKD, and contributes to its progression to various cardiovascular complications such as atherosclerosis, etc. Probiotics have been observed to promote the production of anti-inflammatory cytokines [e.g., interleukin (IL)-10] and inhibit pro-inflammatory pathways (e.g., Tumor necrosis factor α, IL-5, IL-6, and endotoxins), thereby attenuating the chronic inflammatory state associated with CKD[34,35].
Another significant benefit of probiotics in CKD management is their ability to regulate BP. Hypertension is both a cause and a consequence of CKD, which exacerbates the progression of renal damage[36]. Certain probiotic strains, including Lactobacillus and Bifidobacterium, have demonstrated antihypertensive effects by acting on the renin-angiotensin-aldosterone system pathway and inhibiting ACE, thereby offering a nonpharmacological approach to BP control, and potentially complementing traditional antihypertensive strategies[36]. In CKD, maintaining an optimal nutritional status is crucial but challenging due to dietary restrictions and malabsorption issues. Probiotics, particularly those with prebiotic properties, can improve nutrient absorption by promoting the growth of beneficial bacteria involved in the fermentation of non-digestible fibers. This fermentation process yields short-chain fatty acids (SCFAs), which not only support the health of the colonic epithelium, but also contribute to overall nutritional well-being[37].
The use of probiotics in the treatment of CKD is evolving, and accumulating evidence has suggested their potential benefits in addressing various aspects of the disease. However, it is crucial to acknowledge the need for further research to establish the optimal strains, dosages, and duration of probiotic supplementation for CKD[34]. Individual variations in the gut microbiota composition, CKD stage, and etiology warrant tailored approaches to maximize their efficacy. Herein, we conducted a systematic literature review to evaluate the role of probiotics in CKD management. Relevant studies were identified through a comprehensive search of relevant databases, including PubMed, ScienceDirect, and Cochrane, with results restricted to English-language articles published in the past 10 years. Keywords such as “probiotics”, “CKD”, “gut microbiota”, and “dosage” were used to select high-quality studies based on relevance and documented outcomes related to probiotic interventions in CKD. Data synthesis emphasized strain specificity, clinical efficacy, and patient-reported outcomes, particularly in studies involving Lactobacillus, Bifidobacterium, and Bacillus species. A qualitative analysis of the selected studies provided insights into the potential benefits of probiotic supplementation in patients with CKD, including its effects on gut microbiota modulation, reduction of uremic toxins, and improved clinical outcomes. This review explores the scientific rationale and emerging evidence supporting the use of prebiotics and probiotics in CKD management, thereby highlighting their potential to preserve kidney function and improve patient outcomes along with scientific data supporting their beneficial role in CKD.
The gut-kidney axis is an intricate network connecting the gastrointestinal tract (GIT) and kidneys, which is fundamental for maintaining physiological homeostasis. This bidirectional communication system involves a dynamic interplay between the patient’s gut microbiota, intestinal barrier, and renal function[38]. The gut microbiota is a diverse community of microorganisms residing in the GIT, comprising bacteria, viruses, fungi, and other microbes, which functions as the core of the gut-kidney axis. It plays a pivotal role in various processes, such as the metabolism of nutrients, including indigestible polysaccharides, lipids, vitamins, and AAs, and regulation of the immune system and intestinal function[39,40]. In the gut, these microorganisms ferment undigested carbohydrates to produce SCFAs[41]. These serve as an energy source for colonic epithelial cells and affect renal function by modulating BP and electrolyte balance[42]. Furthermore, the gut microbiota contributes to the synthesis of certain vitamins, and participates in the metabolism of dietary compounds that may influence kidney health[43]. The integrity of the intestinal barrier is another critical component of the gut-kidney axis. The intestinal barrier, formed by a single layer of epithelial cells, acts as a selective gatekeeper to regulate the passage of substances between the gut and bloodstream[44]. This prevents the translocation of harmful microorganisms and their byproducts, ensuring that only essential nutrients are absorbed. Disruption of this barrier, often referred to as “leaky gut”, has been associated with various renal disorders[45]. The inflammatory responses triggered by the translocation of bacteria or their products may further contribute to kidney injury and dysfunction. The bidirectional transport of substances between the gut and kidneys is a dynamic aspect of this axis. The kidneys play a central role in maintaining fluid and electrolyte balance and in excreting waste products and excess fluids to regulate BP. Simultaneously, the gut absorbs nutrients, electrolytes, and water, thereby maintaining systemic homeostasis[46]. The reabsorption of electrolytes such as sodium is a coordinated process involving both the gut and kidneys. The delicate balance maintained by this bidirectional transport ensures proper functioning of both systems. Understanding the normal physiology of the gut-kidney axis has significant implications for human health. Research has suggested that dysregulation of this axis is associated with various conditions, including CKD, renal fibrosis, and autoimmune kidney disorders[47]. Consequently, there is a growing interest in therapeutic interventions targeting the gut-kidney axis to manage or prevent kidney diseases. Investigating the nuances of this axis provides insights into the normal physiological processes governing gut and kidney health and offers potential avenues for developing novel therapeutic strategies for renal disorders.
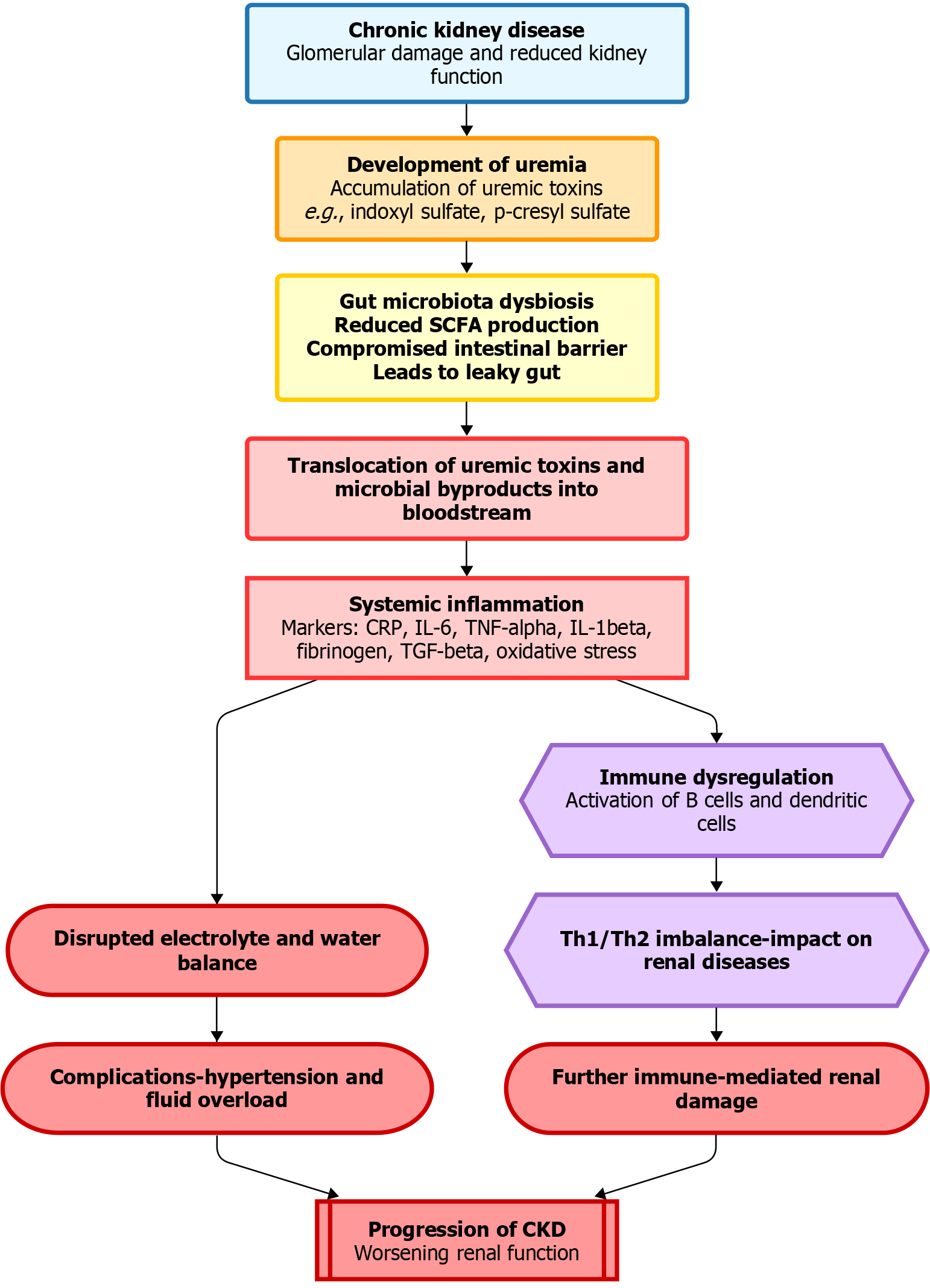
In conditions such as CKD, the gut-kidney axis undergoes substantial physiological changes in the context of uremia, a condition characterized by the retention of uremic toxins due to impaired renal function. In the presence of uremia, this bidirectional communication system, involving the interplay between the GIT and kidneys, disrupts the gut microbiota composition, intestinal barrier integrity, and substance transport. Uremia in CKD is closely linked to alterations in gut microbiota (Figure 1)[34]. Disruption in the balance of microbial species in the GIT can lead to dysbiosis. Uremic toxins, such as indoxyl sulfate (IS) and p-cresyl sulfate, are byproducts of microbial metabolism that accumulate during impaired renal excretion. These toxins have been implicated in the pathogenesis of renal dysfunction and systemic inflammation, thereby highlighting the profound impact of uremia on the gut microbiota[48].
The fermentation of undigested carbohydrates by the gut microbiota results in the production of SCFAs, which play a crucial role in maintaining gut health and influencing various physiological processes, including BP regulation and electrolyte balance. However, in uremia, alterations in gut microbiota composition may disrupt SCFA production, potentially contributing to disturbances in renal homeostasis[49]. The integrity of the intestinal barrier, a key component of the gut-kidney axis, is also compromised under uremic conditions[50]. Increased gut permeability, often colloquially referred to as “leaky gut”, allows the translocation of uremic toxins and microbial byproducts into the bloodstream, thus triggering inflammatory responses. Disruption of the intestinal barrier can exacerbate systemic inflammation and contribute to CKD progression[51]. Uremia also affects the bidirectional transport of substances between the gut and kidneys. Impaired renal function leads to disturbances in the electrolyte and water balance, influencing fluid homeostasis maintained by the gut-kidney axis. This imbalance contributes to complications associated with CKD, including hypertension and fluid overload[52].
The gut-kidney axis is intricately linked to immune regulation, and alterations in this axis underlie the immune dysregulation observed under uremic conditions. The gut-associated lymphoid tissue, a significant component of the immune system in the GIT, communicates with the renal immune system. Changes in the gut microbiota composition and increased gut permeability may affect immune-mediated renal disease in the context of CKD and uremia[53]. Understanding the changes in the gut-kidney axis linked to uremia will have important implications for therapeutic interventions. Modulation of the gut microbiota, restoration of intestinal barrier integrity, and optimization of substance transport are emerging strategies for mitigating the impact of uremia on CKD progression and associated complications[54].
Emerging research has explored the potential benefits of pre- and probiotics in the management of CKD, offering new avenues for therapeutic interventions.
Modulation of the gut microbiota: Probiotic microorganisms predominantly belong to the genera Lactobacillus and Bifidobacterium, and can positively influence the composition of the gut microbiota. Previous studies have demonstrated that probiotic supplementation can restore this balance by increasing the abundance of beneficial strains. In contrast, prebiotics are non-digestible fibers that serve as food sources for beneficial gut bacteria. They promote the growth of bacteria, thereby enhancing their activity and overall diversity[55-57].
Uremic toxin reduction: Dysbiosis in CKD leads to an altered gut environment that promotes the production of uremic toxins, such as IS and p-cresol sulfate. These toxins enter the bloodstream and contribute to systemic inflammation and kidney damage. Probiotics have been shown to reduce the production of uremic toxins by modulating the gut microbiota. Specific bacterial strains can enzymatically degrade uremic toxins, rendering them less harmful, or promoting their excretion. This detoxifying effect can alleviate the burden on the compromised kidneys and slow CKD progression[58].
Immunomodulation and inflammation: Probiotics have been studied for their immunomodulatory effects, with evidence indicating their ability to downregulate proinflammatory pathways. Probiotics may mitigate the inflammatory response in CKD by enhancing the production of anti-inflammatory cytokines, and inhibiting the release of proinflammatory mediators. Additionally, prebiotics contribute to this anti-inflammatory effect by fostering the growth of bacteria that produce SCFAs, which function as metabolites with anti-inflammatory properties that help maintain gut barrier integrity and modulate immune responses[59].
BP regulation: Hypertension is a common complication of CKD, and a major risk factor for its progression. The gut microbiota has been implicated in BP regulation through the production of bioactive compounds, such as SCFA’s and certain peptides. Probiotics have been studied for their potential antihypertensive effects. By modulating the gut microbiota and producing bioactive peptides that inhibit ACE, probiotics could contribute to BP control in patients with CKD[36].
Nutritional support: Nutritional management is crucial in CKD to prevent malnutrition and support overall health. However, dietary restrictions in CKD, such as limited protein and phosphorus intake, can pose challenges in achieving adequate nutrition. Prebiotics, as non-digestible fibers, serve as a source of energy for beneficial bacteria in the gut. Moreover, the fermentation of prebiotics produces metabolites, such as SCFAs, which serve as an energy source for the colonic epithelium. This could be particularly beneficial in patients with CKD, in which maintaining gastrointestinal health is crucial for nutrient absorption and overall well-being[60].
Several strains of probiotics can be used to treat CKD, including Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus casei, Bifidobacterium longum, Streptococcus thermophilus, etc. In the form of capsules, tablets, powders, sachets, syrups, etc. Typically in dosage ranges of 2 × 109 to 20 × 109 colony-forming units[35,61]. Prebiotics such as inulin and fructooligosaccharides are commonly utilized in CKD to promote the growth of beneficial bacteria that can reduce the production of uremic toxins. These prebiotics also help to improve bowel regularity, and may contribute to better management of metabolic acidosis in patients[62]. In addition, various symbiotic formulations are used to treat CKD, including probiotics and prebiotics, wherein probiotics restore the balance of good/bad bacteria, whereas prebiotics support its growth by serving as a food source[63].
Although randomized controlled trials have reported positive outcomes with probiotics in non-dialysis CKD stages 3-5, studies involving populations undergoing dialysis have yielded mixed results[64]. The probiotics-supplemented low-protein diet in CKD trial (registration number NCT04204005, conducted from March 13, 2017 to December 31, 2020) demonstrated the effective control and modulation of microbiota-derived proatherogenic toxins in patients with CKD[65]. In the CKD-Renal Epidemiology and Information Network, clinical trial (NCT03381950, conducted from November 10, 2010 to October 11, 2019), yogurt and probiotic consumption were associated with a reduced risk of inflammation in patients with CKD[66]. The 2014 INCMNSZ clinical trial (conducted at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico) included a randomized controlled study examining changes in blood urea levels, showing a reduction of > 10% in serum urea concentrations[67]. In the observational study of kibow biotics in chronic kidney failure patients on dialysis (NCT01450709, conducted from April 2011 to November 2012), biochemical parameters such as blood urea nitrogen (BUN), serum creatinine, and uric acid revealed statistically significant differences in BUN levels between the placebo and probiotic treatment groups[68]. The NATURE 3.1 - new approach for the reduction of renal uremic toxins clinical trial (NCT03815786, conducted from January 24, 2019 to February 15, 2022) demonstrated the effectiveness of the symbiotic NATUREN G® in reducing serum-free, mild intestinal permeability, abdominal pain, and constipation syndromes[69].
A recent study by Zhong et al[70] explored the impact of washed microbiota transplantation (WMT), a modified fecal microbiota transplantation method, on patients with renal dysfunction, a condition characterized by altered gut microbiota composition. CKD often involves microbiota changes, and interventions targeting this microbial imbalance have shown promise CKD treatment. A comparative analysis revealed a significant difference in the microbial community structure between individuals with renal dysfunction and healthy controls. Notably, patients undergoing WMT experienced significant improvements in renal parameters, including serum creatinine levels and estimated glomerular filtration rate. The adverse events associated with WMT were minimal (2.91%). Post-WMT, patients exhibited an increased Shannon index and abundance of probiotic bacteria in their gut microbiota, aligning more closely with healthy profiles. Urine analysis revealed high levels of toxic metabolites, indicating enhanced excretion. Overall, this study concluded that WMT is a safe and effective method for enhancing renal function by modulating the gut microbiota, and promoting the elimination of toxic metabolites, underscoring the potential of gut microbiota interventions in CKD management[70]. The results of other animal and human studies are presented in Table 1[67-88].
| Investigator name | Probiotics used | Study characteristics | Results obtained |
| Clinical studies in chronic kidney disease patients | |||
| Natarajan et al[68] | Streptococcus thermophilus, Lactobacillus acidophilus, and Bifidobacterium longum | A randomized, double-blind, placebo-controlled crossover study in 22 subjects | Reductions in levels of C-reactive protein (-8.61 mg/L) and total indoxyl glucuronide (-0.11 mg%) were observed and it was concluded that the probiotic formulation was safe to administer to ESRD patients on haemodialysis |
| Group A: Treatment group | |||
| Group B: Control group | |||
| Hida et al[71] | Bifidobacterium infantis, Lactobacillus acidophilus, and Enterococcus faecalis | Single-centre, randomized, controlled, observational trial | Lebenin led to reduction in the enterobactericiea populations along with p-cresol and indole in haemodialysis-refractory uremic patients |
| Group A (n = 12): Control | |||
| Group B (n = 20): Treatment | |||
| Takayama et al[72] | Bifidobacterium longum strain, Lactic acid bacillus | Single-centre, non-randomized, placebo-controlled trial in 22 patients for a duration of 5 weeks | It was observed following the study period that the pre-hemodialysis serum levels of IS significantly decreased in Bifidobacterium-treated patients (before: 4.9 +/- 1.7 mg/dL, after 5 weeks: 3.5 +/- 1.3 mg/dL). However, they did not decrease in the Lac B-treated patients (before: 4.8 +/- 1.4 mg/dL, after 5 weeks: 5.2 +/- 2.0 mg/dL). It was concluded that the administration of Bifidobacterium species to haemodialysis patients was effective in reducing the serum levels of IS by correcting the intestinal microflora |
| Nakabayashi et al[73] | Symbiotic: Lactobacillus casei strain, Shirota and Bifidobacterium breve strain, Yakult + pre- biotic (galacto-oligosaccharides) three times a day for 2 weeks | Single-centre, observational trial in 9 haemodialysis patients for a duration of 4 weeks | It was observed that the synbiotic treatment resulted in normalization of bowel habits and a decrease of serum p-cresol levels in haemodialysis patients |
| Simenhoff et al[74] | Oral Lactobacillus acidophilus | Single-centre, observational trial in 8 haemodialysis patients | Lactobacillus acidophilus was observed to improve small bowel pathobiology by modifying metabolic actions of small bowel bacterial overgrowth, reducing in vivo generation of toxins and carcinogens and promoting nutrition with no adverse side effects |
| Rossi et al[75] | Synbiotic: Lactobacillus, Bifidobacteria and Streptococcus genera + prebiotic (inulin, fructooligosaccarides, and galactooligosaccarides) | Randomized, double-blind, placebo-controlled, crossover trial in 37 patients of stage 4-5 CKD for a duration of 6 weeks | Synbiotic therapy in CKD patients did not significantly reduce serum IS levels (-2 μmol/L) but did decrease serum PCS levels (-14 μmol/L) and favourably modified the stool microbiome |
| Wang et al[76] | Bifobacterium bifidum, Bifidobacterium catenulatum, Bifidobacterium longum, and Lactobacillus plantarum | A randomised, double-blind, placebo-controlled trial in 39 peritoneal dialysis patients (21 in the probiotics group and 18 in the placebo group) for a duration of 12 months | The levels of serum TNF-α, IL-5, IL-6, and endotoxin were significantly decreased after 6 months of probiotic treatment, while levels of serum IL-10 were significantly increased. On the other hand, there were no significant changes in levels of serum cytokines and endotoxin in the placebo group after 6 months. The residual renal function was preserved in patients receiving probiotics |
| Viramontes-Hörner et al[77] | Synbiotic: Lactobacillus acidophilus and Bifidobacterium lactis + prebiotic (inulin) | A randomized, placebo-controlled, double-blinded, clinical trial in 42 haemodialysis patients for a duration of 2 months | The intervention group showed a significant reduction in the prevalence of monthly of vomit, heartburn, and stomach ache, as well as a significant decrease in GIS severity compared with control group after 2 months of the study. Also, no symbiotic-related adverse side effects were shown in these patients. Clinical studies with longer follow-up and sample size are required to confirm these results |
| Group A (n = 22): Intervention group nutritional counselling + symbiotic gel | |||
| Group B (n = 20): Control group nutritional counselling + placebo | |||
| Ranganathan et al[78] | Lactobacillus acidophilus, Bifidobacterium longum, and Streptococcus thermophilus, for a total of 1.5 × 1010 CFU | A 6-month prospective, randomized, double-blind, placebo-controlled crossover trial in 46 outpatients of CKD stages 3 and 4; group A: Placebo; group B: Probiotic | It was observed following the treatment period that the oral ingestion of probiotics (90 billion CFUs/day) was well tolerated and safe. BUN levels decreased in 29 patients (63%) creatinine levels in 20 patients (43%), and uric acid levels in 15 patients (33%). Almost all subjects experienced a substantial overall improvement in QOL (86%) |
| After 3 months crossover; group A: Probiotic; group B: Placebo | |||
| Miranda Alatriste et al[67] | Lactobacillus casei Shirota | A simple randomized, controlled clinical trial in 30 patients of stage 3-4 CKD for a duration of 8 weeks | Upon evaluation, it was observed that the patients treated with 16 × 109 CFU showed a > 10% decrease in the blood urea concentrations and it was significant as compared to the baseline measurement |
| Group A: 8 × 109 CFU Lactobacillus casei Shirota | |||
| Group B: 16 × 109 Lactobacillus casei Shirota CFU | |||
| Pavan[79] | Synbiotic: Prebiotic + probiotic | A 12-month prospective, randomized, controlled, open-label, observational trial in 24; patients of CKD stage 3-4 | It was observed that prebiotic and probiotic supplementation was able to delay the declining glomerular filtration rate than receiving LPD alone (-11.6 ± 8.6 vs |
| Group A (n = 12): LPD + prebiotic + probiotic supplementation | |||
| Group B (n = 12): LPD only (protein 0.8 g/kg body weight/day) | |||
| Cruz-Mora et al[80] | Synbiotic: Lactobacillus acidophilus and Bifidobacterium lactis + prebiotic (inulin) + 1.5 g of omega-3 fatty acids (eicosapentaenoic and docosahexaenoic acid) and vitamins (complex B, folic acid, ascorbic acid, and vitamin E) | Single-centre, double-blind, placebo-controlled trial in 18 patients of ESRD diagnosis with renal replacement therapy (haemodialysis) | It was observed at the end of the study duration that the probiotic supplementation led to an increase in the Bifidobacterial counts in fecal samples and improved GIS (test group start 12 and end 9) compared with control group (start 11 and end 11) |
| Group A: Nutritional counselling + symbiotic gel (Lactobacillus acidophilus and Bifidobacterium bifidum 2 × 1012 CFU) | |||
| Group B: Nutritional counselling + placebo | |||
| Guida et al[81] | Synbiotic: Lactobacillus acidophilus, Bifidobacterium bifidum, Lactobacillus casei, Lactobacillus rhamnosus, and Bifidobacterium longum + prebiotic (inulin, fructooligosaccharides, maltodextrin, corn starch, magnesium stearate, silicon dioxide) | A double-blind randomized placebo controlled clinical trial in 30 patients of stage 3-4 CKD | It was observed that the probiotic formulation lowered total plasma p-cresol concentrations however, it did not ameliorate GIS in non-dialyzed CKD patients but the investigators concluded that synbiotics deserved attention as possible tools to delay CKD progression towards ESRD |
| Group A: Placebo (n = 12) | |||
| Group B: Synbiotic (n = 18) | |||
| Ranganathan et al[82] | Streptococcus thermophilus, Lactobacillus acidophilus and Bifidobacterium longum | A 6-month, prospective, randomized, controlled, double blinded, crossover, placebo-controlled pilot scale trial in 16 patients of stages 3-4 CKD | Probiotic supplementation was associated greater mean reduction of BUN concentration (-2.93 mmol/L) as compared to the placebo (4.52 mmol/L). No GI or infectious complications were noted in any subject and their QOL was improved |
| Taki et al[83] | Bifidobacterium longum | Single-centre, non-randomized-placebo controlled trial in 27 heamodialysis patients for a duration of 12 weeks | It was observed that the oral administration of Bifidobacterium longum in a gastroresistant capsule significantly decreased the serum levels of homocysteine in HD patients (before: 39.2 ± 3.6 µmol/L; 12th week: 34.8 ± 2.8 µmol/L). The most effective dose of Bifidobacterium longum for decreasing the serum levels of homocysteine (reduction rate: 13.3%) was found to be 6.0 × 109 CFU/day |
| Ando et al[84] | Bifidobacterium longum | Single-centre, observational trial in 27 patients of CKD patients all stages for a duration of 6 months | A significant retardation of the progression of renal failure was observed in patients and there was no adverse effect observed in any case |
| Studies in experimental chronic kidney disease | |||
| Andrade-Oliveira et al[85] | Bifidobacterium adolescentis or Bifidobacterium longum | Evaluation of the role of SCFA in an acute kidney injury mice model bilateral kidney ischemia reperfusion injury 2 weeks | Treatment with SCFAs, especially acetate, reduced kidney damage after kidney ischemia and reperfusion injury. Also, low levels of activated neutrophils and macrophages, infiltrating macrophages and activated dendritic cells (CD11c+CD40+) were observed in acetate-treated mice |
| Ranganathan et al[86] | Sporosarcina pasteurii | 18 Sprague-Dawley uremic rats (5/6 nephrectomy) of 16 weeks | It was observed that feeding with 109 CFU of Sporosarcina pasteurii prolonged the lifespan of uremic rats by at least 22.3% thus increasing the average survival time after surgery from 115.8 ± 16.02 days to 148.5 ± 5.7 days. Moreover, it attenuated the increase in BUN levels in the treatment group. Whereas, in the placebo group, average BUN levels increased by 120 ± 5.8% from baseline to time of death, indicating progression of azotemia in nephrectomized animals |
| Group A: Control group | |||
| Group B: Placebo group | |||
| Group C: Probiotic group | |||
| Prakash et al[87] | Polymeric membrane artificial cells (semipermeable microcapsules) containing genetically engineered live Escherichia coli DH5 cells | An observational study to determine the effect of semipermeable microcapsules containing genetically engineered live Escherichia coli DH5 cells in male Wistar uremic rats (5/6 nephrectomy) | In the treatment group, it was observed that the bacteria were able to reduce urea levels from 52.08 ± 2.06 to 10.58 ± 0.85 mg/dL by day 7. This suggested that the genetically engineered cells normalized the plasma urea level in uremic rats with induced kidney failure |
| Group A: Normal rats receiving empty microcapsules | |||
| Group B: Uremic rats receiving empty microcapsules | |||
| Group C: Uremic rats receiving semipermeable microcapsules containing genetically engineered live Escherichia coli DH5 cells | |||
| Ranganathan et al[88] | Various combinations of probiotics | A prospective, blinded, placebo-controlled pilot-study to evaluate the effect of probiotic combination in 5/6th nephrectomized Sprague-Dawley rats with chronic renal failure | Following the 16 weeks of treatment, regimens C and D significantly prolonged the life span of uremic rats, in addition to showing a reduction in BUN levels, concluding that supplementation of probiotic formulation to uremic rats slowed the progression of azotemia, thus leading to prolonged life span of uremic rats |
| Group A: Casein based diet + Control | |||
| Group B: Placebo (casein-based diet without probiotics) | |||
| Group C: Bacillus pasteurii | |||
| Group D: Sporolac (R) | |||
| Group E: Kibow cocktail | |||
| Group F: Chr. Hansen cocktail | |||
| Group G: Econom | |||
The selection of specific probiotic strains is crucial for formulating effective products, and a combination of multiple strains can offer synergistic benefits. This study explored the rationale and key differentials of a mixed-strain probiotic comprising Streptococcus thermophilus, Lactobacillus acidophilus, Bifidobacterium longum, and Bacillus coagulans. The various factors that must be considered when selecting a specific probiotic strain are outlined below.
The inclusion of multiple strains in a probiotic formulation is based on the synergistic effects of several strains. Streptococcus thermophilus is known for its ability to produce lactase, which aids in lactose digestion. Similarly, Lactobacillus acidophilus contributes to lactic acid production and creates an acidic environment that inhibits the growth of harmful bacteria. Furthermore, Bifidobacterium longum is associated with the metabolism of complex carbohydrates, and promotes the production of SCFAs with anti-inflammatory properties. In addition, Bacillus coagulans, a spore-forming bacterium, offers stability and survival advantages under the harsh conditions of the GIT[33]. A combination of two or more of these strains may offer several benefits as they may exert a synergistic action.
Streptococcus thermophilus and Lactobacillus acidophilus work together to support the breakdown of lactose and maintain an acidic environment, which can be particularly beneficial for individuals with lactose intolerance. In addition, Bifidobacterium longum, which resides predominantly in the colon, contributes to the fermentation of non-digestible fibers, producing SCFAs that nourish the colonic epithelium and help maintain gut barrier function. Bacillus coagulans, a spore-forming bacterium, survives in an acidic stomach environment and reaches the intestines in a viable form to exert its probiotic effects[89].
The gut is a key player in the immune system, and the gut microbiota play a crucial role in modulating the immune responses. Streptococcus thermophilus and Lactobacillus acidophilus have demonstrated immunomodulatory properties, including stimulation of immunoglobulin production and enhancement of macrophage activity. Bifidobacterium longum indirectly contributes to immune homeostasis by promoting balanced gut microbiota. Bacillus coagulans, in its spore form, can stimulate the production of anti-inflammatory cytokines, and contribute to a regulated immune response[90].
The ability of probiotics to inhibit the growth of pathogenic bacteria is a critical aspect of their function. The mixed-strain formulation leveraged the inherent antimicrobial properties of each strain to create a hostile environment for harmful bacteria. Streptococcus thermophilus and Lactobacillus acidophilus produce lactic acid, which lowers the pH of the gut, and creates an unfavorable environment for pathogen growth. Bifidobacterium longum contributes to the inhibition of pathogenic bacteria by producing SCFAs. Bacillus coagulans, with its spore-forming nature, can also effectively compete with and suppress the growth of harmful microorganisms[91].
The survivability of probiotic strains under the harsh conditions of the GIT is a critical factor in their effectiveness. Bacillus coagulans, a spore-forming bacterium, exhibits enhanced stability and survival rates compared to non-spore-forming strains. The spore-forming nature of Bacillus coagulans further allows it to withstand the acidic environment of the stomach, ensuring that a higher number of viable cells reach the intestines where they can exert their probiotic effects. This resilience enhances the overall stability and shelf life of mixed-strain formulations, providing a product that delivers promising benefits to the consumer[92].
The efficacy of many probiotic formulations has been supported by clinical evidence; for example, the mixed-strain combination of Streptococcus thermophilus, Lactobacillus acidophilus, Bifidobacterium longum, and Bacillus coagulans has been the subject of many studies that have demonstrated the individual and collective benefits of these strains in promoting gastrointestinal health, modulating immune responses, and inhibiting the growth of pathogenic bacteria. Synergistic interactions among strains have been shown to enhance their overall efficacy compared to single-strain formulations. In CKD management, probiotics show potential benefits, although variability in outcomes has been identified, owing to differences in strains, study designs, sample sizes, and patient characteristics. Trials varied in sample sizes (8-46 participants) and duration (5 weeks to 12 months), influencing outcomes such as uremic toxin levels, inflammatory markers, and QOL. Strain-specific effects, such as those observed in Lactobacillus casei and Bifidobacterium longum, further highlight the need for standardized large-scale trials to establish efficacy and optimize treatment protocols for patients with CKD[93].
Bacillus coagulans stands out among the strains in this mixed formulation because of its spore-forming nature. This characteristic provides several advantages, including enhanced survivability, stability, and ease of formulation. This spore-forming capability allows Bacillus coagulans to remain dormant until it reaches the intestine, where conditions are more favorable for germination and probiotic activity. This feature is particularly advantageous for ensuring that a higher number of viable cells reach the target site. Additionally, spore-forming probiotics commonly exhibit greater resistance to environmental factors, such as heat and humidity, making them more robust during storage and distribution[94].
Probiotic supplementation is most effective when aligned with consumer habits and preferences. A mixed-strain formulation encapsulating the benefits of Streptococcus thermophilus, Lactobacillus acidophilus, Bifidobacterium longum, and Bacillus coagulans provides a comprehensive approach to support gastrointestinal health. The rationale behind the mixed-strain probiotic formulation of Streptococcus thermophilus, Lactobacillus acidophilus, Bifidobacterium longum, and Bacillus coagulans involves the synergy of their mechanisms of action, which target different aspects of gastrointestinal health, immune modulation, and pathogen inhibition. Consumers benefit from the convenience of obtaining multiple strains from a single product, which simplifies their supplementation routines. This can enhance compliance, as individuals are more likely to adhere to a regimen that is easy to follow. The diverse range of benefits offered by each strain in the formulation further addresses various aspects of health, thus appealing to a broader audience seeking holistic well-being[95].
As research in the field of probiotics continues to evolve, future perspectives may involve a deeper understanding of the interactions among these strains and their specific effects on different populations and conditions. Technological advances may also contribute to the development of tailored probiotic formulations based on individualized microbiome assessments. In conclusion, the mixed-strain probiotic formulation represents a comprehensive and synergistic approach to promote health, and its differential advantages make it a promising candidate for those seeking a well-rounded probiotic supplement. However, further research and clinical studies are required to elucidate the specific benefits and applications of this formulation in diverse populations and health contexts. Large-scale, well-designed clinical trials will be essential to establish the efficacy and safety of these interventions in diverse CKD populations[96].
Probiotics show promise in managing CKD; however, significant limitations regarding strain specificity and dosage remain. Existing studies have used different probiotic strains and lacked standardized dosages, complicating efficacy assessments[34,35]. Lactobacillus and Bifidobacterium strains also offer potential benefits, such as modulating the gut microbiota and reducing uremic toxins; however, their effectiveness depends on the CKD stage and individual factors[70]. Dosage inconsistencies further complicate clinical outcomes, highlighting the need for rigorous trials to determine strain-specific actions, optimal dosages, and long-term safety[36].
In conclusion, the ongoing research on the complex interactions between gut microbial health and renal function highlights the potential of probiotics as a valuable adjunctive therapy for the comprehensive care of patients with CKD. Several studies have demonstrated that probiotics can effectively reduce levels of urea, BUN, ammonia, plasma p-cresol, IS, and p-cresyl sulfate in patients with CKD. Although evidence is currently limited, the probiotic combination of Streptococcus thermophilus, Lactobacillus acidophilus, Bifidobacterium longum, and Bacillus coagulans appears to be a promising formulation. However, the variability in dosages and protocols used in prior studies necessitates large-scale studies to assess the efficacy of probiotics, particularly in patients with stages 3 CKD and 4 CKD, to determine whether early intervention can offer significant benefits and enhance patient QOL. In addition, the emerging role of enzybiotics presents a novel opportunity for targeted metabolic interventions that can further reduce toxins and improve renal function, thus complementing the effects of probiotics and prebiotics. Currently, a combination of Streptococcus thermophilus, Lactobacillus acidophilus, Bifidobacterium longum, and Bacillus coagulans is the most rational and promising formulation for CKD management. However, further investigation of the specific impact of various probiotic formulations on improving outcomes in patients are essential.
| 1. | Romagnani P, Remuzzi G, Glassock R, Levin A, Jager KJ, Tonelli M, Massy Z, Wanner C, Anders HJ. Chronic kidney disease. Nat Rev Dis Primers. 2017;3:17088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 627] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 2. | Hill NR, Fatoba ST, Oke JL, Hirst JA, O'Callaghan CA, Lasserson DS, Hobbs FD. Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0158765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2648] [Cited by in RCA: 2328] [Article Influence: 258.7] [Reference Citation Analysis (0)] |
| 3. | Liyanage T, Toyama T, Hockham C, Ninomiya T, Perkovic V, Woodward M, Fukagawa M, Matsushita K, Praditpornsilpa K, Hooi LS, Iseki K, Lin MY, Stirnadel-Farrant HA, Jha V, Jun M. Prevalence of chronic kidney disease in Asia: a systematic review and analysis. BMJ Glob Health. 2022;7:e007525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 145] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 4. | Li PK, Garcia-Garcia G, Lui SF, Andreoli S, Fung WW, Hradsky A, Kumaraswami L, Liakopoulos V, Rakhimova Z, Saadi G, Strani L, Ulasi I, Kalantar-Zadeh K. Kidney Health for Everyone Everywhere: From Prevention to Detection and Equitable Access to Care. Can J Kidney Health Dis. 2020;7:2054358120910569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Aashima, Nanda M, Sharma R, Jani C. The burden of chronic kidney disease in Asia, 1990-2019: Examination of estimates from global burden of disease 2019 study. Nephrology (Carlton). 2022;27:610-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 6. | Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1265] [Cited by in RCA: 1532] [Article Influence: 127.7] [Reference Citation Analysis (0)] |
| 7. | Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7995] [Cited by in RCA: 8518] [Article Influence: 405.6] [Reference Citation Analysis (0)] |
| 8. | World Health Organization. Global status report on noncommunicable diseases 2014. [cited 17 September 2024]. Available from: https://iris.who.int/bitstream/handle/10665/148114/9789241564854_eng.pdf. |
| 9. | Kelly JT, Su G, Zhang L, Qin X, Marshall S, González-Ortiz A, Clase CM, Campbell KL, Xu H, Carrero JJ. Modifiable Lifestyle Factors for Primary Prevention of CKD: A Systematic Review and Meta-Analysis. J Am Soc Nephrol. 2021;32:239-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 169] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 10. | Li PK, Garcia-Garcia G, Lui SF, Andreoli S, Fung WW, Hradsky A, Kumaraswami L, Liakopoulos V, Rakhimova Z, Saadi G, Strani L, Ulasi I, Kalantar-Zadeh K; World Kidney Day 2020 Steering Committee. Kidney health for everyone everywhere - from prevention to detection and equitable access to care. Braz J Med Biol Res. 2020;53:e9614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 11. | National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60:850-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 829] [Cited by in RCA: 917] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 12. | Abiemo EE, Alonso A, Nettleton JA, Steffen LM, Bertoni AG, Jain A, Lutsey PL. Relationships of the Mediterranean dietary pattern with insulin resistance and diabetes incidence in the Multi-Ethnic Study of Atherosclerosis (MESA). Br J Nutr. 2013;109:1490-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Nakayama M, Okuda S, Tamaki K, Fujishima M. Short- or long-term effects of a low-protein diet on fibronectin and transforming growth factor-beta synthesis in Adriamycin-induced nephropathy. J Lab Clin Med. 1996;127:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Wang AY, Mallamaci F, Zoccali C. What is central to renal nutrition: protein or sodium intake? Clin Kidney J. 2023;16:1824-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Rhee CM, Wang AY, Biruete A, Kistler B, Kovesdy CP, Zarantonello D, Ko GJ, Piccoli GB, Garibotto G, Brunori G, Sumida K, Lambert K, Moore LW, Han SH, Narasaki Y, Kalantar-Zadeh K. Nutritional and Dietary Management of Chronic Kidney Disease Under Conservative and Preservative Kidney Care Without Dialysis. J Ren Nutr. 2023;33:S56-S66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Ariyanopparut S, Metta K, Avihingsanon Y, Eiam-Ong S, Kittiskulnam P. The role of a low protein diet supplemented with ketoanalogues on kidney progression in pre-dialysis chronic kidney disease patients. Sci Rep. 2023;13:15459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 17. | Fuchs M, Engel J, Campos M, Matejec R, Henrich M, Harbach H, Wolff M, Weismüller K, Menges T, Heidt MC, Welters ID, Krüll M, Hempelmann G, Mühling J. Intracellular alpha-keto acid quantification by fluorescence-HPLC. Amino Acids. 2009;36:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Yen CL, Fan PC, Chen JJ, Kuo G, Hsiao CC, Chen CY, Tu YR, Hsu HH, Chen YC, Chang CH. Ketoanalogues Supplemental Low Protein Diet Safely Decreases Short-Term Risk of Dialysis among CKD Stage 4 Patients. Nutrients. 2022;14:4020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 19. | Koppe L, Cassani de Oliveira M, Fouque D. Ketoacid Analogues Supplementation in Chronic Kidney Disease and Future Perspectives. Nutrients. 2019;11:2071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS; AIPRD Study Group. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 790] [Cited by in RCA: 705] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 21. | Pugh D, Gallacher PJ, Dhaun N. Management of Hypertension in Chronic Kidney Disease. Drugs. 2019;79:365-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 211] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 22. | Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 448] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 23. | MacIsaac RJ, Jerums G, Ekinci EI. Effects of glycaemic management on diabetic kidney disease. World J Diabetes. 2017;8:172-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Hahr AJ, Molitch ME. Management of diabetes mellitus in patients with chronic kidney disease. Clin Diabetes Endocrinol. 2015;1:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 25. | de Boer IH, Khunti K, Sadusky T, Tuttle KR, Neumiller JJ, Rhee CM, Rosas SE, Rossing P, Bakris G. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2022;102:974-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 138] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 26. | Bouby N, Clark WF, Roussel R, Taveau C, Wang CJ. Hydration and kidney health. Obes Facts. 2014;7 Suppl 2:19-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V. Chronic kidney disease. Lancet. 2021;398:786-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 752] [Article Influence: 188.0] [Reference Citation Analysis (1)] |
| 28. | Kim SY, Moon A. Drug-induced nephrotoxicity and its biomarkers. Biomol Ther (Seoul). 2012;20:268-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 29. | Kwiatkowska E, Domański L, Dziedziejko V, Kajdy A, Stefańska K, Kwiatkowski S. The Mechanism of Drug Nephrotoxicity and the Methods for Preventing Kidney Damage. Int J Mol Sci. 2021;22:6109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 30. | Džidić-Krivić A, Sher EK, Kusturica J, Farhat EK, Nawaz A, Sher F. Unveiling drug induced nephrotoxicity using novel biomarkers and cutting-edge preventive strategies. Chem Biol Interact. 2024;388:110838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 31. | Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet. 2017;389:1238-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1654] [Cited by in RCA: 2417] [Article Influence: 302.1] [Reference Citation Analysis (0)] |
| 32. | Neale EP, Rosario VD, Probst Y, Beck E, Tran TB, Lambert K. Lifestyle Interventions, Kidney Disease Progression, and Quality of Life: A Systematic Review and Meta-analysis. Kidney Med. 2023;5:100643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 33. | Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4055] [Cited by in RCA: 5535] [Article Influence: 503.2] [Reference Citation Analysis (2)] |
| 34. | Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 822] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 35. | Fagundes RAB, Soder TF, Grokoski KC, Benetti F, Mendes RH. Probiotics in the treatment of chronic kidney disease: a systematic review. J Bras Nefrol. 2018;40:278-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 36. | Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 364] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 37. | Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2256] [Cited by in RCA: 3178] [Article Influence: 397.3] [Reference Citation Analysis (0)] |
| 38. | Liu X, Wang X, Zhang P, Fang Y, Liu Y, Ding Y, Zhang W. Intestinal homeostasis in the gut-lung-kidney axis: a prospective therapeutic target in immune-related chronic kidney diseases. Front Immunol. 2023;14:1266792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Fujisaka S, Watanabe Y, Tobe K. The gut microbiome: a core regulator of metabolism. J Endocrinol. 2023;256:e220111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 65] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 40. | Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016;14:e1002533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2328] [Cited by in RCA: 2914] [Article Influence: 323.8] [Reference Citation Analysis (0)] |
| 41. | den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2408] [Cited by in RCA: 3269] [Article Influence: 272.4] [Reference Citation Analysis (3)] |
| 42. | Natarajan N, Pluznick JL. From microbe to man: the role of microbial short chain fatty acid metabolites in host cell biology. Am J Physiol Cell Physiol. 2014;307:C979-C985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 43. | D'Argenio G, Mazzacca G. Short-chain fatty acid in the human colon. Relation to inflammatory bowel diseases and colon cancer. Adv Exp Med Biol. 1999;472:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 2705] [Article Influence: 169.1] [Reference Citation Analysis (0)] |
| 45. | Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 216] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 46. | Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, Jurutka PW. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013;92:77-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 508] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 47. | Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25:657-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 529] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 48. | Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jörres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W; European Uremic Toxin Work Group (EUTox). Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1178] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 49. | Evenepoel P, Meijers BK, Bammens BR, Verbeke K. Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl. 2009;S12-S19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 323] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 50. | Vaziri ND, Yuan J, Norris K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am J Nephrol. 2013;37:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 279] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 51. | Lau WL, Kalantar-Zadeh K, Vaziri ND. The Gut as a Source of Inflammation in Chronic Kidney Disease. Nephron. 2015;130:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 52. | Vaziri ND, Zhao YY, Pahl MV. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol Dial Transplant. 2016;31:737-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 273] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 53. | Evenepoel P, Poesen R, Meijers B. The gut-kidney axis. Pediatr Nephrol. 2017;32:2005-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 209] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 54. | Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target. Am J Kidney Dis. 2016;67:483-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 275] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 55. | Fijan S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health. 2014;11:4745-4767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 583] [Cited by in RCA: 569] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 56. | Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. 2013;6:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 647] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 57. | Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, Berenjian A, Ghasemi Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods. 2019;8:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 776] [Article Influence: 129.3] [Reference Citation Analysis (0)] |
| 58. | Wu IW, Hsu KH, Lee CC, Sun CY, Hsu HJ, Tsai CJ, Tzen CY, Wang YC, Lin CY, Wu MS. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant. 2011;26:938-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 372] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 59. | Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2130] [Cited by in RCA: 2403] [Article Influence: 200.3] [Reference Citation Analysis (0)] |
| 60. | Lehto M, Groop PH. The Gut-Kidney Axis: Putative Interconnections Between Gastrointestinal and Renal Disorders. Front Endocrinol (Lausanne). 2018;9:553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 61. | Zirker L. Probiotic Use in Chronic Kidney Disease Patients. J Renal Nutr. 2014;24:e47-e49. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Vacca M, Celano G, Lenucci MS, Fontana S, Forgia FM, Minervini F, Scarano A, Santino A, Dalfino G, Gesualdo L, De Angelis M. In Vitro Selection of Probiotics, Prebiotics, and Antioxidants to Develop an Innovative Synbiotic (NatuREN G) and Testing Its Effect in Reducing Uremic Toxins in Fecal Batches from CKD Patients. Microorganisms. 2021;9:1316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 63. | Pei M, Wei L, Hu S, Yang B, Si J, Yang H, Zhai J. Probiotics, prebiotics and synbiotics for chronic kidney disease: protocol for a systematic review and meta-analysis. BMJ Open. 2018;8:e020863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Tian N, Li L, Ng JK, Li PK. The Potential Benefits and Controversies of Probiotics Use in Patients at Different Stages of Chronic Kidney Disease. Nutrients. 2022;14:4044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 65. | De Mauri A, Carrera D, Bagnati M, Rolla R, Vidali M, Chiarinotti D, Pane M, Amoruso A, Del Piano M. Probiotics-Supplemented Low-Protein Diet for Microbiota Modulation in Patients with Advanced Chronic Kidney Disease (ProLowCKD): Results from a Placebo-Controlled Randomized Trial. Nutrients. 2022;14:1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 66. | Wagner S, Merkling T, Metzger M, Koppe L, Laville M, Boutron-Ruault MC, Frimat L, Combe C, Massy ZA, Stengel B, Fouque D. Probiotic Intake and Inflammation in Patients With Chronic Kidney Disease: An Analysis of the CKD-REIN Cohort. Front Nutr. 2022;9:772596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 67. | Miranda Alatriste PV, Urbina Arronte R, Gómez Espinosa CO, Espinosa Cuevas Mde L. Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutr Hosp. 2014;29:582-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 68. | Natarajan R, Pechenyak B, Vyas U, Ranganathan P, Weinberg A, Liang P, Mallappallil MC, Norin AJ, Friedman EA, Saggi SJ. Randomized controlled trial of strain-specific probiotic formulation (Renadyl) in dialysis patients. Biomed Res Int. 2014;2014:568571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 69. | Cosola C, Rocchetti MT, di Bari I, Acquaviva PM, Maranzano V, Corciulo S, Di Ciaula A, Di Palo DM, La Forgia FM, Fontana S, De Angelis M, Portincasa P, Gesualdo L. An Innovative Synbiotic Formulation Decreases Free Serum Indoxyl Sulfate, Small Intestine Permeability and Ameliorates Gastrointestinal Symptoms in a Randomized Pilot Trial in Stage IIIb-IV CKD Patients. Toxins (Basel). 2021;13:334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 70. | Zhong HJ, Xie X, Chen WJ, Zhuang YP, Hu X, Cai YL, Zeng HL, Xiao C, Li Y, Ding Y, Xue L, Chen M, Zhang J, Wu Q, He XX. Washed microbiota transplantation improves renal function in patients with renal dysfunction: a retrospective cohort study. J Transl Med. 2023;21:740. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 71. | Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron. 1996;74:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 213] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 72. | Takayama F, Taki K, Niwa T. Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am J Kidney Dis. 2003;41:S142-S145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 73. | Nakabayashi I, Nakamura M, Kawakami K, Ohta T, Kato I, Uchida K, Yoshida M. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: a preliminary study. Nephrol Dial Transplant. 2011;26:1094-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 74. | Simenhoff ML, Dunn SR, Zollner GP, Fitzpatrick ME, Emery SM, Sandine WE, Ayres JW. Biomodulation of the toxic and nutritional effects of small bowel bacterial overgrowth in end-stage kidney disease using freeze-dried Lactobacillus acidophilus. Miner Electrolyte Metab. 1996;22:92-96. [PubMed] |
| 75. | Rossi M, Johnson DW, Morrison M, Pascoe EM, Coombes JS, Forbes JM, Szeto CC, McWhinney BC, Ungerer JP, Campbell KL. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial. Clin J Am Soc Nephrol. 2016;11:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 274] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 76. | Wang IK, Wu YY, Yang YF, Ting IW, Lin CC, Yen TH, Chen JH, Wang CH, Huang CC, Lin HC. The effect of probiotics on serum levels of cytokine and endotoxin in peritoneal dialysis patients: a randomised, double-blind, placebo-controlled trial. Benef Microbes. 2015;6:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 77. | Viramontes-Hörner D, Márquez-Sandoval F, Martín-del-Campo F, Vizmanos-Lamotte B, Sandoval-Rodríguez A, Armendáriz-Borunda J, García-Bejarano H, Renoirte-López K, García-García G. Effect of a symbiotic gel (Lactobacillus acidophilus + Bifidobacterium lactis + inulin) on presence and severity of gastrointestinal symptoms in hemodialysis patients. J Ren Nutr. 2015;25:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 78. | Ranganathan N, Ranganathan P, Friedman EA, Joseph A, Delano B, Goldfarb DS, Tam P, Rao AV, Anteyi E, Musso CG. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv Ther. 2010;27:634-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 79. | Pavan M. Influence of prebiotic and probiotic supplementation on the progression of chronic kidney disease. Minerva Urol Nefrol. 2016;68:222-226. [PubMed] |
| 80. | Cruz-Mora J, Martínez-Hernández NE, Martín del Campo-López F, Viramontes-Hörner D, Vizmanos-Lamotte B, Muñoz-Valle JF, García-García G, Parra-Rojas I, Castro-Alarcón N. Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. J Ren Nutr. 2014;24:330-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 81. | Guida B, Germanò R, Trio R, Russo D, Memoli B, Grumetto L, Barbato F, Cataldi M. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: a randomized clinical trial. Nutr Metab Cardiovasc Dis. 2014;24:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 82. | Ranganathan N, Friedman EA, Tam P, Rao V, Ranganathan P, Dheer R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: a 6-month pilot scale trial in Canada. Curr Med Res Opin. 2009;25:1919-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 83. | Taki K, Takayama F, Niwa T. Beneficial effects of Bifidobacteria in a gastroresistant seamless capsule on hyperhomocysteinemia in hemodialysis patients. J Ren Nutr. 2005;15:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 84. | Ando Y, Miyata Y, Tanba K, Saito O, Muto S, Kurosu M, Homma S, Kusano E, Asano Y. [Effect of oral intake of an enteric capsule preparation containing Bifidobacterium longum on the progression of chronic renal failure]. Nihon Jinzo Gakkai Shi. 2003;45:759-764. [PubMed] |
| 85. | Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJ, de Almeida DC, Bassi EJ, Moraes-Vieira PM, Hiyane MI, Rodas AC, Peron JP, Aguiar CF, Reis MA, Ribeiro WR, Valduga CJ, Curi R, Vinolo MA, Ferreira CM, Câmara NO. Gut Bacteria Products Prevent AKI Induced by Ischemia-Reperfusion. J Am Soc Nephrol. 2015;26:1877-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 380] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 86. | Ranganathan N, Patel BG, Ranganathan P, Marczely J, Dheer R, Pechenyak B, Dunn SR, Verstraete W, Decroos K, Mehta R, Friedman EA. In vitro and in vivo assessment of intraintestinal bacteriotherapy in chronic kidney disease. ASAIO J. 2006;52:70-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 87. | Prakash S, Chang TM. Microencapsulated genetically engineered live E. coli DH5 cells administered orally to maintain normal plasma urea level in uremic rats. Nat Med. 1996;2:883-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 139] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 88. | Ranganathan N, Patel B, Ranganathan P, Marczely J, Dheer R, Chordia T, Dunn SR, Friedman EA. Probiotic amelioration of azotemia in 5/6th nephrectomized Sprague-Dawley rats. ScientificWorldJournal. 2005;5:652-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 89. | Plaza-Díaz J, Ruiz-Ojeda FJ, Vilchez-Padial LM, Gil A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients. 2017;9:555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 274] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 90. | Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25:716-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 833] [Article Influence: 138.8] [Reference Citation Analysis (0)] |
| 91. | Marco ML, Heeney D, Binda S, Cifelli CJ, Cotter PD, Foligné B, Gänzle M, Kort R, Pasin G, Pihlanto A, Smid EJ, Hutkins R. Health benefits of fermented foods: microbiota and beyond. Curr Opin Biotechnol. 2017;44:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 761] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 92. | Sanders ME, Merenstein DJ, Ouwehand AC, Reid G, Salminen S, Cabana MD, Paraskevakos G, Leyer G. Probiotic use in at-risk populations. J Am Pharm Assoc (2003). 2016;56:680-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 93. | Kwoji ID, Aiyegoro OA, Okpeku M, Adeleke MA. Multi-Strain Probiotics: Synergy among Isolates Enhances Biological Activities. Biology (Basel). 2021;10:322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 94. | Nataraj BH, Ali SA, Behare PV, Yadav H. Postbiotics-parabiotics: the new horizons in microbial biotherapy and functional foods. Microb Cell Fact. 2020;19:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 320] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 95. | Parvez S, Malik KA, Ah Kang S, Kim HY. Probiotics and their fermented food products are beneficial for health. J Appl Microbiol. 2006;100:1171-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 750] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 96. | Seifert S, Bub A, Franz CM, Watzl B. Probiotic Lactobacillus casei Shirota supplementation does not modulate immunity in healthy men with reduced natural killer cell activity. J Nutr. 2011;141:978-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |