Published online Dec 25, 2024. doi: 10.5527/wjn.v13.i4.98969
Revised: September 9, 2024
Accepted: September 19, 2024
Published online: December 25, 2024
Processing time: 119 Days and 23.7 Hours
Primary immunoglobulin (Ig)-associated mesangiocapillary glomerulonephritis (Ig-MCGN) is an immune complex glomerulonephritis of unknown etiology. It is a common cause of chronic kidney disease in developing countries. There is limited data available on renal and patient outcomes of this disease from deve
To determine the short-term renal and patient outcomes of adults with a tissue-confirmed diagnosis of primary Ig-MCGN at a single center in Pakistan.
A retrospective cohort study of adult patients was conducted on biopsy-proven Ig-MCGN cases diagnosed between 1998 and 2019 at the Sindh Institute of Urology and Transplantation, Karachi, Pakistan. Secondary causes were excluded. The primary endpoint was renal survival without end-stage kidney disease (ESKD) or mortality. The secondary endpoint was the rate of remission during the 2-year follow-up period. Survival curves were made with the use of Kaplan-Meier estimates.
A total of 163 patients were included in the study and their mean follow-up duration was 29.45 months ± 21.28 months. Among baseline characteristics, young age, lower estimated glomerular filtration rate, requirement of kidney replace
The outcomes of primary Ig-MCGN are guarded in Pakistan and require further prospective studies to improve our understanding of this relatively common disease so that more personalized treatment approaches can be de
Core Tip: Primary immunoglobulin (Ig)-associated mesangiocapillary glomerulonephritis (Ig-MCGN) is an immune complex glomerulonephritis of unknown etiology. It is a common cause of chronic kidney disease in developing countries. There is limited data available on renal and patient outcomes from developing countries. In this study, the outcomes of primary Ig-MCGN were guarded and require further prospective studies to improve the understanding of this relatively common disease so that more personalized treatment approaches can be developed.
- Citation: Elahi T, Ahmed S, Mubarak M. Short-term renal and patient outcomes of primary immunoglobulin-associated mesangiocapillary glomerulonephritis: Insights from a developing country. World J Nephrol 2024; 13(4): 98969
- URL: https://www.wjgnet.com/2220-6124/full/v13/i4/98969.htm
- DOI: https://dx.doi.org/10.5527/wjn.v13.i4.98969
Immunoglobulin (Ig)-associated mesangiocapillary glomerulonephritis (Ig-MCGN) (also known as membranoproliferative glomerulonephritis) is a pattern of immune complex-mediated kidney injury due to the deposition of immune complexes containing Igs within the glomeruli[1,2]. The diagnosis of Ig-MCGN is made by histology and immunofluorescence (IF). It is characterized by mesangial hypercellularity, endocapillary proliferation, and duplication of the glo
Mesangiocapillary glomerulonephritis (MCGN) is classified into three categories based on IF results: (1) An immune complex-mediated category with both C3 and IgG present; (2) A complement-only category; and (3) A pauci-immune group[3]. Primary Ig-MCGN falls under the immune complex-mediated group with both C3 and IgG deposits and has no clear secondary cause.
The exact etiology of Ig-MCGN is unknown. It is characterized by activation of the classical complement pathway via antigen-antibody complexes with significant deposition of Igs and complement components[4]. The most common sources of persistent antigenemia with consequent immune complex deposition in glomeruli in adults include various infections such as hepatitis B virus (HBV) and hepatitis C virus (HCV), post-streptococcal glomerulonephritis, and bacterial endocarditis, autoimmune disorders such as systemic lupus erythematosus and cryoglobulinemia, or mo
The underlying etiology of disease is highly heterogenous and is an important cause of nephrotic syndrome in adolescents and young adults. Its prevalence is decreasing in developed countries, likely because of early diagnosis and treatment of infections[8]. Data on renal and patient outcomes of patients with primary Ig-MCGN are limited, particularly from developing countries. It accounts for approximately 7.16% of causes of nephritic syndrome in children and 4.3% in adults[9]. The true incidence is not known in developing countries due to the lack of renal disease registries.
There are many therapeutic challenges to treating this disease because of its heterogeneity and lack of standardized disease management. There are no prospective randomized controlled trials available to guide treatment. Current guidelines recommend a trial of immunosuppression in those who present with renal dysfunction and significant proteinuria[10].
The long-term renal prognosis in primary Ig-MCGN remains unclear. Despite treatment, poor outcomes have been reported in the literature. Approximately 60% of cases progress to end-stage kidney disease (ESKD) within 10 years regardless of treatment[11].
The aim of this study was to determine the clinicopathological characteristics and short-term outcomes of adults with primary Ig-MCGN, irrespective of treatment.
The study was approved by the Institutional Review Board of Sindh Institute of Urology and Transplantation (SIUT), Karachi, Pakistan (No. SIUT-ERC-2020/A-230). The research was conducted in accordance with the ethical principles of the Declaration of Helsinki.
A retrospective observational study was conducted on the clinical records of all adults (> 18 years of age), who were investigated and treated at the Adult Nephrology Section of SIUT, Karachi, Pakistan, with a histopathological diagnosis of MCGN (types I, II, and III) between January 1998 to December 2019 and were on regular follow-up at the Adult Nephrology Clinic for at least two years following renal biopsy. The biopsies were recategorized based on IF results. The diagnosis of primary Ig-MCGN was clinically established after excluding all possible secondary causes. These included: (1) Presence of any chronic infections (e.g., infective endocarditis, and vascular shunt) and systemic diseases; (2) Positive viral serology for HBV and HCV; (3) Positive family history; (4) History of autoimmune diseases, presence of antinuclear antibodies, anti-double stranded (ds)DNA, and rheumatoid factor; (5) Presence of anti-neutrophil cytoplasmic antibody; (6) Lymphoproliferative disorders; and (7) Cryoglobulinemia. As these patients were not acutely ill at presentation or showed evidence of any chronic infections, tests for malaria, leprosy, and other possible infections associated with MCGN were not performed. Because our study participants with MCGN were young, serum or urine protein electrophoresis and serum-free light chains were performed only when there was high clinical suspicion of monoclonal gammopathy. Cases with a biopsy diagnosis of C3GN were excluded. Additionally, all patients with HUS, monoclonal Ig deposition diseases with kappa and lambda staining, and those who had been previously treated with corticosteroids or other immunosuppressive drugs were excluded. Patients with incomplete clinical records or erratic follow-ups were also excluded from this study.
Archived records of all adults with Ig-MCGN were scrutinized for their clinical, laboratory, serological, and histopathological variables at presentation and during follow-up visits to the nephrology clinic. Clinical details included age, sex, indication for biopsy, hypertension, given therapeutic regimens, the requirement for kidney replacement therapy (KRT), and follow-up information. Laboratory parameters including serum creatinine and albumin levels on arrival and follow-ups, and serum complement levels (C3 and C4) were recorded. Urine analysis report, urine protein-to-creatinine ratio (PCR) on arrival and thereafter, and 24-hour urinary protein levels, if available, were recorded. Every effort was taken to collect all available data elements. Cases where main data elements were missing were excluded. The estimated glomerular filtration rate (eGFR) was calculated with the chronic kidney disease epidemiology collaboration creatinine equation[12].
The pathological evaluation included the number of all glomeruli, the number (and proportion) of globally sclerosed glomeruli, the number (and proportion) of glomeruli with true crescents, and their subdivision into fibrous/fibrocellular/cellular crescents, endocapillary hypercellularity, mesangial hypercellularity (diffuse or focal), capillary wall double contours, and the degree of interstitial fibrosis and tubular atrophy (IFTA). IFTA was semi-quantitatively scored as none (0%-5%), mild (6%-25%), moderate (26% to 50%), or severe (> 50%). IF positivity for IgG, IgA, IgM, C3, C1q, kappa, and lambda, their pattern, and the amount of staining was graded as follows: (1) No staining as 0; (2) Mild staining as 1+; (3) Moderate staining as 2+; and (4) Strong staining as 3+, graded on a scale of 0-3+.
All LM-based diagnosed cases of primary MCGN were reclassified by using the IF-based criteria as Ig-MCGN or C3G by two renal pathologists (one with > 20 and the other with 10 years of experience) first independently and then combined in cases of discrepant results. Both pathologists were blinded to patient outcomes. Ig-MCGN was defined as the deposition of IgG ≥ 1+ along with C3 and/or C1q or non-dominant IgA with C3 and/or C1q.
As there are no guidelines for defining remission in Ig-MCGN, we used the following criteria for proliferative lupus nephritis and categorized patients into three groups: (1) Complete remission (CR); (2) Partial remission (PR); and (3) No remission (NR)[13]. CR was defined as urinary dipstick negative or trace for both proteins and blood, serum albumin more than 3.5 g/dL, and eGFR more than 90 mL/minute/1.73 m2. PR was defined as microscopic hematuria or ≥ 1 proteinuria, serum albumin less than 3.5 g/dL, and eGFR less than 90 and more than 60 mL/minute/1.73 m2. NR was defined as persistent proteinuria of > 3 g/day or progression or worsening of renal impairment.
Renal survival was defined as the time from renal biopsy to the first of any of the following events: (1) Starting dialysis; and (2) Receiving a kidney transplant or an eGFR falling to < 15 mL/minute/1.73 m2 at any point during follow-up and then not returning to > 15 mL/minute/1.73 m2 at subsequent time points.
Relapse was defined as a positive dipstick after at least one previous negative test or increased proteinuria (evident on dipstick or increase in PCR) in patients who previously were in PR or CR[9].
The primary endpoint was renal survival defined as survival without ESKD or mortality. The secondary endpoint was the rate of PR or CR during follow-up.
Data analysis was conducted using the Statistical Package for the Social Sciences, version 22.0 (IBM Corp., Armonk, NY, United States). Continuous data were presented as mean ± standard deviation if normally distributed or median with interquartile range (IQR) in case of non-normal distribution. The categorical variables were reported as numbers and percentages, while discrete variables were shown as proportions. The differences between means and proportions of subgroups were analyzed by ANOVA for continuous variables and χ² test for categorical variables. The association between histological class, clinical and laboratory parameters, and patient response to treatment (categorized as complete, partial, or no response at 2 years) was also assessed using ANOVA with Tukey post hoc analysis. Overall survival curves were generated using the Kaplan–Meier method, and differences between survival curves were compared using the log-rank test. A P value less than 0.05 was considered statistically significant.
Between 1998 and 2019, a total of 163 patients with biopsy-proven primary Ig-MCGN were identified after excluding patients with secondary causes, C3G, and inadequate follow-up.
Table 1 presents the demographic, clinical, and serological characteristics of all patients with primary Ig-MCGN. The median age for the study population was 32 years (IQR: 22-42 years), with slight female predominance (51.5% vs 48.4% males). Hypertension was present in 57 (35%) patients. A total of 121 (74.23%) presented with nephrotic-range proteinuria along with microscopic hematuria. The mean eGFR was 53.65 mL/minute/1.73 m2 ± 37.7 mL/minute/1.73 m2. More patients showed C3 complement consumption than C4 at presentation [87 (53.7%) vs 40 (24%)]. Acute kidney injury (AKI) was the predominant indication for biopsy (106, 65%). On presentation, 43 (26.4%) patients required KRT.
| Characteristics | Data among total, n = 163 |
| Age at biopsy in years | 32 (22-42) |
| Sex | |
| Male | 79 (48.4) |
| Female | 84 (51.5) |
| Hypertension | 57 (35) |
| Serum creatinine in mg/dL | 2.7 ± 2.9 |
| Estimated glomerular filtration rate in mL/minute/1.73 m2 | 53.65 ± 37.7 |
| Serum albumin in g/dL | 2.3 ± 0.79 |
| Proteinuria by dipstick | |
| Trace | 5 (3.1) |
| 1+ | 17 (10.7) |
| 2+ | 20 (12.6) |
| 3+ | 76 (46.6) |
| 4+ | 45 (27.6) |
| Microscopic hematuria | |
| Trace | 8 (4.9) |
| 1+ | 17 (10.4) |
| 2+ | 18 (11.04) |
| 3+ | 77 (47.2) |
| 4+ | 43 (26.3) |
| Spot protein-to-creatinine ratio in mg/dL | 3.55 ± 3.19 |
| 24-hour urinary protein in g/day | 4.0 ± 2.61 |
| Serum C3 levels | |
| Low, < 0.8 g/L | 87 (53.7) |
| Normal, > 0.8 g/L | 76 (46.3) |
| Serum C4 levels | |
| Low, < 0.16 g/L | 40 (24) |
| Normal, > 0.16 g/L | 124 (76) |
| Required kidney replacement therapy | 43 (26.4) |
| Indications for renal biopsy | |
| Nephrotic | 27 (16.6) |
| Nephritic | 30 (18.4) |
| Acute kidney injury | 106 (65) |
The renal histopathological features of patients with Ig-MCGN are displayed in Table 2. The main histological pattern observed was diffuse mesangiocapillary proliferation, (77.4%) along with the presence of spikes in 15 (9.4%) patients. Extracellular crescentic proliferation was observed in 57 (35.6%) patients. The mean number of sclerotic glomeruli was 2.05 ± 3.5. A total of 125 (76.68%) had mild or no tubular atrophy. Significant IgG on IF was observed in 88.9% of the patients.
| Characteristics | Data |
| Total glomeruli | 20.36 ± 10.4 |
| Globally sclerosed | 2.05 ± 3.5 |
| Presence of crescents | 57 (35.6) |
| Mesangial proliferation | |
| Focal | 37 (22.6) |
| Diffuse | 126 (77.4) |
| Presence of spikes | 15 (9.4) |
| Arteriolosclerosis | 56 (34.8) |
| Interstitial fibrosis and tubular atrophy | |
| None | 22 (13.49) |
| Mild | 103 (63.1) |
| Moderate | 35 (21.2) |
| Severe | 3 (1.84) |
| Immunofluorescence results | |
| IgA | |
| Negative | 124 (76.1) |
| Trace | 18 (11) |
| 1+ | 19 (11.7) |
| 2+ | 2 (2) |
| 3+ | 0 |
| IgG | |
| Negative | 0 |
| Trace | 1 (0.6) |
| 1+ | 17 (10.4) |
| 2+ | 96 (58.8) |
| 3+ | 49 (30.06) |
| IgM | |
| Negative | 74 (45.4) |
| Trace | 30 (18.4) |
| 1+ | 43 (26.4) |
| 2+ | 11 (6.7) |
| 3+ | 5 (3.1) |
| C3 | |
| Negative | 24 (14.7) |
| Trace | 43 (26.4) |
| 1+ | 70 (42.9) |
| 2+ | 17 (10.4) |
| 3+ | 9 (5.5) |
| C1q | |
| Negative | 91 (55.8) |
| Trace | 17 (10.4) |
| 1+ | 28 (17.1) |
| 2+ | 23 (14.1) |
| 3+ | 4 (2.45) |
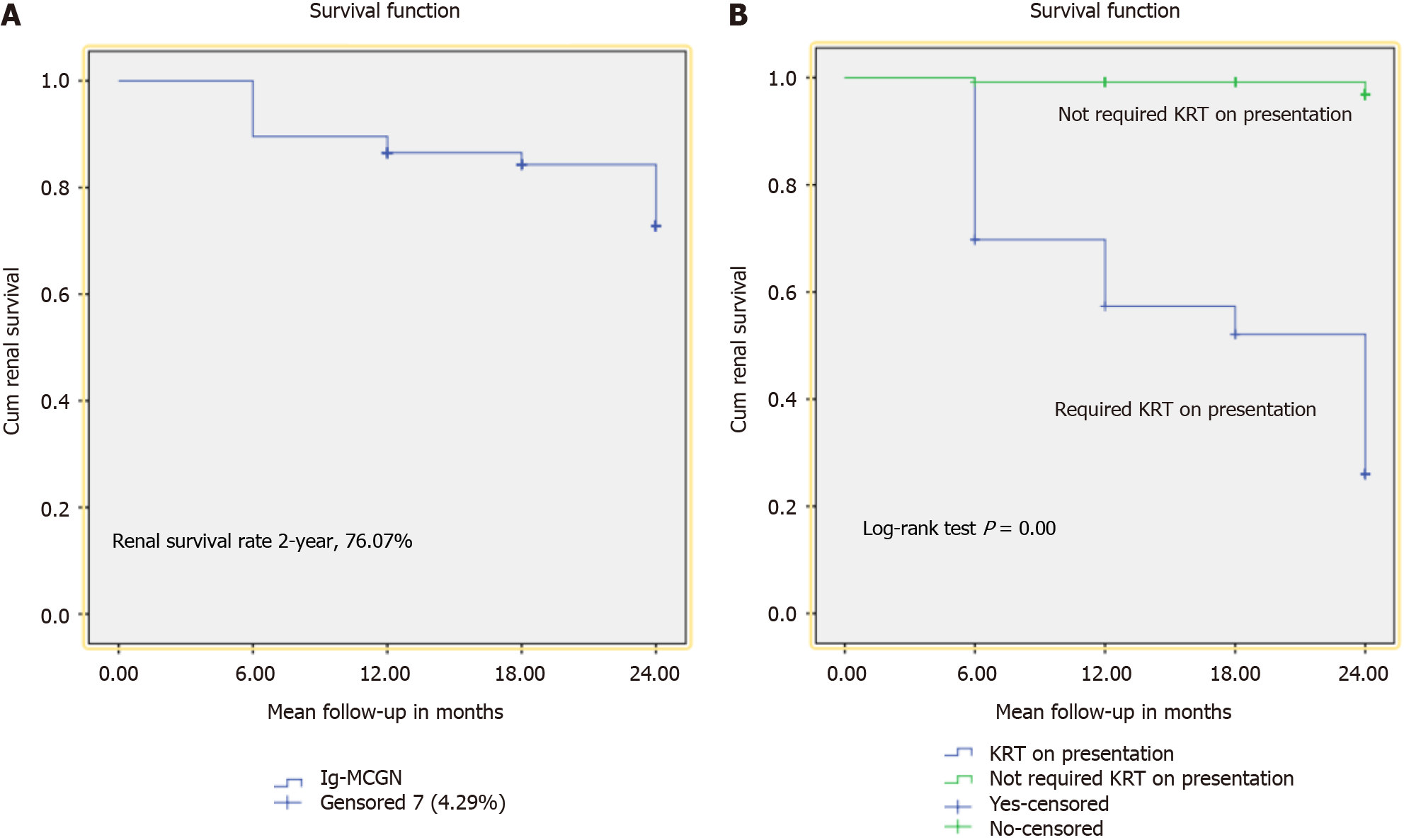
Table 3 illustrates treatment and renal outcomes at the 2-year follow-up in patients with primary Ig-MCGN. Among all, 62 (38.0%) received steroids alone and 21 (12.8%) were treated with combination therapy (steroids and cyclophosphamide). Among those who received steroids only, more cases underwent remission [40 (33.33%) vs 5 (11.62%), P < 0.001], and no one progressed to ESKD with one mortality in patients who did not require KRT on admission. Similarly, in patients who were treated with combination therapy, more cases underwent remission [16 (13.33%) vs 1 (2.32%), P = 0.02], and no one progressed to ESKD with no mortality in patients who did not require KRT on admission. At 2 years, 124 (76.07%) patients were in CR or PR [56 (34.3%) and 68 (41.71%), respectively]. In total, 32 (19.63%) patients progressed to ESKD while 7 (4.29%) patients died. Patients who required KRT on admission achieved CR or PR less frequently than those who did not [3 (6.97%) vs 53 (44.1%), P < 0.001] and [5 (11.6%) vs 63 (52.5%), P < 0.001, respectively]. Similarly, more patients progressed to ESKD [29 (67.41%) vs 3 (2.5%), P < 0.001] with increased mortality [6 (13.9%) vs 1 (0.83%), P < 0.001] in this group. Kaplan–Meier survival analysis was performed by considering the period from treatment initiation to the end of follow-up or death as the time frame. At 2 years, renal survival was 76.07% (Figure 1A) and was significantly better in those who did not require KRT on presentation (log-rank P < 0.001) (Figure 1B).
| Parameters | Overall, n = 163 | KRT required on presentation, n = 43 | KRT not required on presentation, n = 120 | P value |
| Steroids alone | 62 (38.0) | 21 (48.83) | 41 (34.16) | 0.08 |
| CR | 9 (5.52) | 2 (4.65) | 7 (5.83) | 0.42 |
| PR | 36 (22.08) | 3 (6.97) | 33 (27.5) | < 0.001 |
| ESKD | 12 (7.36) | 12 (27.90) | 0 | < 0.001 |
| Mortality | 5 (3.06) | 4 (9.30) | 1 (0.83) | 0.04 |
| Cyclophosphamide + steroids | 21 (12.8) | 5 (11.62) | 16 (13.33) | 0.13 |
| CR | 4 (2.45) | 0 | 4 (3.33) | 0.53 |
| PR | 13 (7.97) | 1 (2.32) | 12 (10) | 0.02 |
| ESKD | 3 (1.84) | 3 (6.97) | 0 | < 0.001 |
| Mortality | 1 (0.61) | 1 (2.32) | 0 | 0.06 |
| Renal outcomes at 2 years | ||||
| CR | 56 (34.3) | 3 (6.97) | 53 (44.1) | < 0.001 |
| PR | 68 (41.71) | 5 (11.6) | 63 (52.5) | < 0.001 |
| ESKD | 32 (19.63) | 29 (67.41) | 3 (2.5) | < 0.001 |
| Mortality | 7 (4.29) | 6 (13.9) | 1 (0.83) | 0.001 |
To compare the clinicopathological parameters at baseline of different patients with the response to treatment (CR/PR/ESKD/mortality), we conducted an ANOVA test. We found that hypertension, degree of proteinuria, and serum co
| Variables | Complete remission, n = 56 | Partial remission, n = 68 | End-stage kidney disease, n = 32 | Mortality, n = 7 | P value |
| Age in years | 32.71 ± 9.84 | 35.71 ± 13.4 | 28.44 ± 11.41 | 29.86 ± 13.50 | 0.03 |
| Hypertension | 24 (42.85) | 21 (30.8) | 10 (31.25) | 2 (28.57) | 0.50 |
| Serum creatinine in mg/dL | 1.94 ± 1.43 | 2.36 ± 2.52 | 4.51 ± 4.49 | 4.69 ± 4.12 | < 0.001 |
| Serum albumin in g/dL | 2.72 ± 0.76 | 2.23 ± 0.76 | 1.78 ± 0.59 | 1.90 ± 0.50 | < 0.001 |
| protein-to-creatinine ratio in g/dL | 3.93 ± 0.879 | 3.73 ± 0.595 | 4.22 ± 0.553 | 3.86 ± 3.78 | 0.114 |
| Estimated glomerular filtration rate in mL/minute/1.73 m2 | 60.33 ± 31.07 | 57.87 ± 41.83 | 38.54 ± 37.14 | 28.20 ± 18.31 | 0.01 |
| Required kidney replacement therapy on admission | 3 (5.35) | 5 (7.35) | 29 (90.62) | 6 (85.71) | < 0.001 |
| Serum C3 levels | |||||
| Low, < 0.8 g/L | 27 (48.21) | 31 (45.5) | 17 (53.12) | 4 (57.14) | 0.84 |
| Normal, > 0.8 g/L | 24 (42.85) | 30 (44.11) | 11 (34.37) | 3 (42.85) | |
| Serum C4 levels | |||||
| Low, < 0.16 g/L | 17 (30.35) | 13 (19.11) | 4 (12.5) | 1 (14.28) | 0.19 |
| Normal, > 0.16 g/L | 33 (58.92) | 49 (72) | 24 (75) | 5 (71.42) | |
| Number of globally sclerosed glomeruli | 0.45 ± 0.80 | 1.75 ± 2.42 | 5.27 ± 5.64 | 3.86 ± 5.36 | < 0.001 |
| Presence of crescents | 10 (17.85) | 25 (36.7) | 17 (53.12) | 5 (71.42) | < 0.001 |
| Mesangial/endocapillary proliferation | |||||
| Focal | 17 (30.35) | 15 (22) | 4 (12.5) | 0 | 0.128 |
| Diffuse | 39 (69.64) | 52 (76.47) | 28 (87.5) | 7 (100) | |
| Presence of spikes | 0 | 12 (17.6) | 3 (9.37) | 0 | 0.006 |
| Interstitial fibrosis and tubular atrophy | |||||
| None | 16 (28.57) | 5 (7.35) | 2 (6.25) | 0 | |
| Mild | 37 (66.07) | 49 (72) | 14 (43.75) | 1 (14.28) | < 0.001 |
| Moderate | 3 (5.35) | 13 (19.11) | 13 (40.62) | 6 (85.71) | |
| Severe | 0 | 0 | 3 (9.37) | 0 |
There is a wide variation in MCGN frequency worldwide, with more cases reported from developing countries over the last two decades. Previous literature has generally reported the overall frequency of idiopathic MCGN rather than isolated primary Ig-MCGN. Additionally, following the reclassification by Sethi et al[2], most studies have focused on the pediatric population, resulting in very limited data available for adults. This retrospective study was conducted to assess the 2-year renal and patient outcomes in adults with primary Ig-MCGN. Our analysis of patients in Pakistan with primary Ig-MCGN reveals several critical clinical, biochemical, and histopathological features that may negatively impact renal outcomes. The 2-year renal survival rate was low in this study. Despite being a single-center study, it is one of the largest studies on this disease compared to previously published studies and is fairly representative of our population.
Primary Ig-MCGN can present at any age. Controversy exists regarding whether the clinical findings at diagnosis can predict long-term renal prognosis. Previous studies have identified older age, hypertension, nephrotic-range proteinuria, and low eGFR as predictors of worse renal outcomes[9]. In the present study, patients were predominantly young, similar to the findings of Chothia et al[8] from South Africa, but in contrast to Nakagawa et al[14] from Japan, who reported a mean age of 62 years in their study population. Additionally, no significant differences have been noted between Asian and Western populations regarding age.
While 35% of our patients were hypertensive at presentation, we did not find a significant correlation between hypertension and outcomes. This contrasts with the findings of Chothia et al[8] and Nakagawa et al[14], who reported a significant adverse association. This discrepancy could be attributed to differences in the prevalence and control of blood pressure (BP). In the study by Chothia et al[8], more than 50% of patients were hypertensive, while Nakagawa et al[14] reported significantly higher systolic and diastolic BP in patients with Ig-MCGN. All patients in our study presented with nephrotic-range proteinuria and microscopic hematuria at the time of biopsy, with AKI being a common indication for biopsy. However, we did not find a significant association between the degree of proteinuria and renal outcomes. This aligns with the findings of Chothia et al[8] and Schmitt et al[11], who reported no differences in the degree of proteinuria between patients who reached the endpoint and those who did not. It appears that poor BP control, rather than the mere presence of hypertension, combined with impaired renal function and consequent protein overload, plays a pathogenic role in disease progression. Therefore, emphasizing BP control in patients with MCGN may be beneficial in improving renal outcomes, as immunosuppression has a limited role in the treatment of these patients, unlike in many other glo
Late presentation of patients is considered an important factor influencing disease outcomes[9]. Many late presenters exhibit markedly elevated serum creatinine (low eGFR) and may show features of uremia or require dialysis at first presentation. The reasons for this may be multifactorial, including lack of awareness, illiteracy or low education levels, poverty, and the absence of edema at disease onset due to subclinical disease. Although we did not evaluate the duration of symptoms in our patients, 26.4% required KRT at presentation. We found that these variables at the time of presen
Besides clinical and laboratory parameters, there is an ongoing debate about whether histopathological findings at presentation can predict progression to ESKD in patients with primary Ig-MCGN. Previous studies have identified the number of sclerosed glomeruli, the presence of crescents, and the degree of interstitial fibrosis as predictors of poor renal outcomes[9]. In our study, we found that the presence of these histological findings at the time of biopsy are poor prognostic factors for renal outcomes, consistent with the study reported by Okpechi et al[15].
Renal survival in primary Ig-MCGN is often reported to be low, with outcomes potentially worse than other glomerular diseases, likely due to the lack of specific therapies for MCGN. Older studies reported 5-year renal survival rates of 51%[11] and 41%[16]. However, these studies often included both adult and pediatric populations and did not exclude secondary causes, limiting their applicability. Existing literature, predominantly from pediatric populations or mixed cohorts, consists mainly of single-center studies with small sample sizes[17-19]. More favorable renal outcomes have been reported in pediatric populations, likely due to the longer time to reach ESKD or mortality outcomes and the shorter duration of published data[20-22]. Nakano et al[19] reported that 31% of patients with primary Ig-MCGN progressed to ESKD over 12 years. In our study, the 2-year renal survival was 76.07%, slightly higher than the 61% reported by Okpechi et al[15] in South Africa but lower than studies from developed countries.
In primary Ig-MCGN, management is still largely untargeted. The 2012 Kidney Disease: Improving Global Outcomes Clinical Practice Guideline for Glomerulonephritis recommends a trial of steroids for patients with nephrotic-range proteinuria and/or abnormal kidney function[10]. Responses to treatment have varied, with good responses[23], poor responses[24], and equivocal responses[19] all reported. This variability may be due to the inclusion of secondary and C3G cases in older studies. Studies showing the effectiveness of cyclophosphamide were conducted before the new classification[25,26]. The present study showed favorable outcomes with steroid treatment alone or in combination with cyclophosphamide in patients not requiring KRT at admission, although only 38% of patients received steroids and none received mycophenolate mofetil. This suggests that immunosuppression alone is not responsible for outcomes and that other factors also play a role. With more targeted therapeutic options on the horizon, the treatment landscape may change profoundly[27]. Recent reports indicate that eculizumab, an anti-C5 monoclonal antibody, ameliorates symptoms in some cases of complement-mediated MCGN, but its efficacy in Ig-MCGN remains an area of active research[28].
We acknowledge that this study has certain limitations. First, it was a retrospective study. Thus, missing information might have slightly influenced the final results. In addition, electron microscopy (EM) findings were not analyzed in this study. Second, its single-center origin limits the generalizability to the entire Pakistani population due to differences in demographics, socio-economic factors, and other variables. Third, we cannot guarantee that secondary causes of Ig-MCGN were completely excluded. Fourth, the relatively short follow-up period (mean of 2 years) limits analysis to short-term outcomes, as ESKD typically develops over years or decades. Fifth, dysproteinemia was not tested in any patient as it was not indicated clinically. Sixth, treatment regimens were not standardized.
Therefore, we continue to have a persistent and renewed concern about the significant gap in our understanding of this disease in Pakistan. The lack of EM findings may have resulted in a lack of critical data elements in this study. However, the entire premise of this study was based on the IF classification of MCGN. We may be missing some yet-to-be-identified sub-clinical chronic antigenemic process, potentially resulting from environmental exposure to drugs (substance abuse) or other toxins, which we have observed to be quite common among these patients. This situation warrants a prospective study of patients with “idiopathic” MCGN, emphasizing a more rigorous evaluation to exclude or identify possible secondary causes, and to ensure completeness of data collection. A multicenter study with a centralized review of the pathology reports and the complete evaluation of renal biopsies including the use of EM will ensure the external validity and generalizability of research findings. For now, however, our primary aim for patients identified with MCGN, where no cause is found, will be to reduce proteinuria and manage BP to target levels.
The outcomes of primary Ig-MCGN in this cohort are poor. Our data suggest that baseline eGFR, the requirement for KRT, and renal histology at presentation have significant predictive value for renal survival. There is a need for pro
| 1. | D'Amico G, Ferrario F. Mesangiocapillary glomerulonephritis. J Am Soc Nephrol. 1992;2:S159-S166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Sethi S, Nester CM, Smith RJ. Membranoproliferative glomerulonephritis and C3 glomerulopathy: resolving the confusion. Kidney Int. 2012;81:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis--a new look at an old entity. N Engl J Med. 2012;366:1119-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 329] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 4. | Iatropoulos P, Noris M, Mele C, Piras R, Valoti E, Bresin E, Curreri M, Mondo E, Zito A, Gamba S, Bettoni S, Murer L, Fremeaux-Bacchi V, Vivarelli M, Emma F, Daina E, Remuzzi G. Complement gene variants determine the risk of immunoglobulin-associated MPGN and C3 glomerulopathy and predict long-term renal outcome. Mol Immunol. 2016;71:131-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 5. | Cook HT, Pickering MC. Clusters Not Classifications: Making Sense of Complement-Mediated Kidney Injury. J Am Soc Nephrol. 2018;29:9-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Fervenza FC, Sethi S, Glassock RJ. Idiopathic membranoproliferative glomerulonephritis: does it exist? Nephrol Dial Transplant. 2012;27:4288-4294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Masani N, Jhaveri KD, Fishbane S. Update on membranoproliferative GN. Clin J Am Soc Nephrol. 2014;9:600-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Chothia MY, Panday AS, Coetzee L, Bates W. Outcomes of immunoglobulin-associated mesangiocapillary glomerulonephritis: A South African experience. Nephrology (Carlton). 2020;25:765-774. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Orth SR, Ritz E. The nephrotic syndrome. N Engl J Med. 1998;338:1202-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 250] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Notice. Kidney International Supplements. 2012;2:139. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 338] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 11. | Schmitt H, Bohle A, Reineke T, Mayer-Eichberger D, Vogl W. Long-term prognosis of membranoproliferative glomerulonephritis type I. Significance of clinical and morphological parameters: an investigation of 220 cases. Nephron. 1990;55:242-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20319] [Cited by in RCA: 20173] [Article Influence: 1260.8] [Reference Citation Analysis (0)] |
| 13. | Renal Disease Subcommittee of the American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria. The American College of Rheumatology response criteria for proliferative and membranous renal disease in systemic lupus erythematosus clinical trials. Arthritis Rheum. 2006;54:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 199] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | Nakagawa N, Mizuno M, Kato S, Maruyama S, Sato H, Nakaya I, Sugiyama H, Fujimoto S, Miura K, Matsumura C, Gotoh Y, Suzuki H, Kuroki A, Yoshino A, Nakatani S, Hiromura K, Yamamoto R, Yokoyama H, Narita I, Isaka Y. Demographic, clinical characteristics and treatment outcomes of immune-complex membranoproliferative glomerulonephritis and C3 glomerulonephritis in Japan: A retrospective analysis of data from the Japan Renal Biopsy Registry. PLoS One. 2021;16:e0257397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Okpechi IG, Dlamini TA, Duffield M, Rayner BL, Moturi G, Swanepoel CR. Outcome of patients with primary immune-complex type mesangiocapillary glomerulonephritis (MCGN) in Cape Town South Africa. PLoS One. 2014;9:e113302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Chan MK, Chan KW, Chan PC, Fang GX, Cheng IK. Adult-onset mesangiocapillary glomerulonephritis: a disease with a poor prognosis. Q J Med. 1989;72:599-607. [PubMed] |
| 17. | Okuda Y, Ishikura K, Hamada R, Harada R, Sakai T, Hamasaki Y, Hataya H, Fukuzawa R, Ogata K, Honda M. Membranoproliferative glomerulonephritis and C3 glomerulonephritis: frequency, clinical features, and outcome in children. Nephrology (Carlton). 2015;20:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Kawasaki Y, Kanno S, Ono A, Suzuki Y, Ohara S, Sato M, Suyama K, Hashimoto K, Hosoya M. Differences in clinical findings, pathology, and outcomes between C3 glomerulonephritis and membranoproliferative glomerulonephritis. Pediatr Nephrol. 2016;31:1091-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Nakano M, Karasawa K, Moriyama T, Uchida K, Nitta K. Characteristics of membranoproliferative glomerulonephritis based on a new classification at a single center. Clin Exp Nephrol. 2019;23:852-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Holle J, Berenberg-Goßler L, Wu K, Beringer O, Kropp F, Müller D, Thumfart J. Outcome of membranoproliferative glomerulonephritis and C3-glomerulopathy in children and adolescents. Pediatr Nephrol. 2018;33:2289-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Kirpalani A, Jawa N, Smoyer WE, Licht C; Midwest Pediatric Nephrology Consortium. Long-Term Outcomes of C3 Glomerulopathy and Immune-Complex Membranoproliferative Glomerulonephritis in Children. Kidney Int Rep. 2020;5:2313-2324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Nakagawa N, Hasebe N, Hattori M, Nagata M, Yokoyama H, Sato H, Sugiyama H, Shimizu A, Isaka Y, Maruyama S, Narita I. Clinical features and pathogenesis of membranoproliferative glomerulonephritis: a nationwide analysis of the Japan renal biopsy registry from 2007 to 2015. Clin Exp Nephrol. 2018;22:797-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Nargund P, Kambham N, Mehta K, Lafayette RA. Clinicopathological features of membranoproliferative glomerulonephritis under a new classification. Clin Nephrol. 2015;84:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Puri P, Walters GD, Fadia MN, Konia M, Gibson KA, Jiang SH. The impact of reclassification of C3 predominant glomerulopathies on diagnostic accuracy, outcome and prognosis in patients with C3 glomerulonephritis. BMC Nephrol. 2020;21:265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Faedda R, Satta A, Tanda F, Pirisi M, Bartoli E. Immunosuppressive treatment of membranoproliferative glomerulonephritis. Nephron. 1994;67:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Braun MC, West CD, Strife CF. Differences between membranoproliferative glomerulonephritis types I and III in long-term response to an alternate-day prednisone regimen. Am J Kidney Dis. 1999;34:1022-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Caliskan Y, Torun ES, Tiryaki TO, Oruc A, Ozluk Y, Akgul SU, Temurhan S, Oztop N, Kilicaslan I, Sever MS. Immunosuppressive Treatment in C3 Glomerulopathy: Is it Really Effective? Am J Nephrol. 2017;46:96-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Bomback AS. Eculizumab in the treatment of membranoproliferative glomerulonephritis. Nephron Clin Pract. 2014;128:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |