Published online Dec 25, 2024. doi: 10.5527/wjn.v13.i4.98880
Revised: September 25, 2024
Accepted: October 20, 2024
Published online: December 25, 2024
Processing time: 122 Days and 0.3 Hours
Globally, diabetic nephropathy (DN) is the primary cause of chronic kidney disease. Currently, renal function is monitored indirectly using measures of serum creatinine, estimated glomerular filtration rate (eGFR), and proteinuria. Novel urinary biomarkers utilized in the early stages of DN have been described; these indicators can be used in the early identification of the disease, which is important for initiating treatment to halt or impediment the advance of diabetic nephro
To estimate neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), and periostin (POSTN) levels as novel urinary biomarkers in DN.
In this hospital based cross-sectional study, a total of 160 patients of both genders aged 18 years or more; 40 healthy participants and 120 patients with diabetes mellitus (DM) were included. Patients with DM were divided into normoalbuminuria (n = 40), microalbuminuria (n = 40), and macroalbuminuria (n = 40) groups as per urine albumin creatinine ratio (uACR). Blood urea, serum creatinine, uACR were measured. Urine NGAL, KIM-1, and POSTN were measured by enzyme linked immunosorbent assay. The eGFR was calculated and compared with urinary markers.
NGAL, KIM-1, and POSTN levels increased significantly in normo, micro, and macroalbuminuria with the highest in the macroalbuminuria group. Albumin creatinine ratio (ACR) showed a positive correlation with NGAL, KIM-1, and POSTN levels. The eGFR showed a weak negative correlation with ACR, NGAL, KIM-1, and POSTN. NGAL was significantly lower in stage 1 compared to stage 2, 3, and 4 kidney disease. KIM-1 was significantly decreased in stage 1 compared to stage 4 kidney disease. POSTN was significantly decreased in stage 1 compared to stage 3 and 4 kidney disease. The receiver operator curve analysis of ACR, NGAL, KIM-1, and POSTN showed good sensitivity of 80%, 75.8%, 63.3%, and 80 % respectively with a cut-off of 12.5 mg/g, 4.5 μg/L, 1.5 ng/mL, and 37.5 ng/mL.
Urinary NGAL and POSTN are independent markers of DN.
Core Tip: Diabetic nephropathy (DN) is a leading cause of chronic kidney disease. Novel urinary biomarkers are useful for detecting DN at an early stage, facilitating timely intervention. Among these, Neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, and periostin have shown promise as early detection markers for DN.
- Citation: Varatharajan S, Jain V, Pyati AK, Neeradi C, Reddy KS, Pallavali JR, Pandiyaraj IP, Gaur A. Neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, and periostin: Novel urinary biomarkers in diabetic nephropathy. World J Nephrol 2024; 13(4): 98880
- URL: https://www.wjgnet.com/2220-6124/full/v13/i4/98880.htm
- DOI: https://dx.doi.org/10.5527/wjn.v13.i4.98880
Diabetes mellitus (DM) has become an epidemic. In modern times, type 2 DM (T2DM) is a potential condition for everyone, owing to the increasing prevalence of obesity and sedentary lifestyle. The incidence of T2DM is expected to rise by 20% in developed nations and by approximately 50%–70% in developing countries between 2000 and 2030[1]. Because of its many consequences, DM is linked with a soaring hazard of morbidity and early fatality. The primary cause of end-stage renal disease (ESRD) and chronic kidney disease (CKD), particularly in advanced nations, is diabetic nephropathy (DN). Renal replacement therapy is estimated to be necessary for 40% of people who have renal failure[2]. Owing to the rising incidence of DN, prompt DN detection is essential to provide appropriate care and avoid ESRD.
A major side effect of DM that arises from modifications in kidney structure and function is DN. Early detection of diagnostic indicators of diabetic kidney disease (DKD) is crucial as medications can halt disease progression and reduce renal function loss. Currently, measurements of serum creatinine, glomerular filtration rate (GFR), and proteinuria are used to monitor renal function indirectly. Although alterations in GFR and creatinine occur in the initial stages of CKD, proteinuria tends to occur in the later phases of the illness. Hence, the albumin excretion rate or estimated GFR (eGFR), which mostly indicates glomerular damage, forms the primary basis for DN screening[3].
A major factor in the pathophysiology of several kidney illnesses is tubulointerstitial injury. The prognosis of kidney disease is highly correlated with the extent of tubulointerstitial injury[4]. Therefore, in patients with DM, the degree of chronic kidney damage may be inferred from renal tubular markers of renal injury. Studies have reported that emerging tubular markers provide early indications of acute kidney damage, including urinary liver fatty acid-binding protein (uLFABP), neutrophil gelatinase-associated lipocalin (NGAL), N-acetyl-β-D-glucosaminidase, and kidney injury molecule-1 (KIM-1)[5-7]. The proximal tubule cells of the kidney express the membrane protein KIM-1 on their apical membrane. KIM-1 may be associated with the advancement or deterioration of DN[8,9]. Moreover, the kidneys do not often contain the cell adhesion molecule periostin (POSTN). On the contrary, it is expressed in the kidney and excreted in the urine in tubulointerstitial lesions. Therefore, POSTN in the urine can act as a marker of renal injury. Since POSTN is high in patients with DM before significant albuminuria develops, it can be used as a diagnostic for diabetic kidney impairment[10].
This study aimed to examine how these novel biomarkers, especially NGAL, KIM-1, and POSTN, might be used to diagnose DN early on.
This hospital-based cross-sectional study with a comparator was carried out at the Department of General Medicine, Tertiary Care Medical College, Southern India, from March 2022 to March 2024. A total of 160 participants, comprising 120 patients with DM and 40 healthy individuals, were included in this study. The sample size was calculated using the Open-Epi software and Fleiss with the continuity correction method for the matched case–control group. The prevalence of DN among the diabetic population was 40% (24). The sample size was calculated to be 22 for each group. However, 40 participants were included in each group to strengthen the study outcome. The sample size was achieved by consecutive sampling. The study comprised both age- and sex-matched healthy comparison population and patients with T2DM who were ≥ 18 years of age. Individuals with systemic arterial hypertension, thyroid disease, nephrotic syndrome, urinary tract infection, known CKD, or chronic liver diseases well as those taking steroids, nephrotoxic drugs, angiotensin receptor blockers, or angiotensin-converting enzyme inhibitors were excluded.
Participants were recruited from the Department of General Medicine, and comparators were recruited from the healthy population. After acquiring informed written consent, the following factors were assessed at baseline during the initial hospital visit: (1) Name; (2) Age; (3) Sex; (4) Religion; (5) Union status; (6) Level of education; (7) Work status; and (8) Soc
The participants were categorized into four groups: (1) Group 1: Comparative healthy subjects (n = 40); (2) Group 2: Patients with DM and normoalbuminuria (n = 40); (3) Group 3: Patients with DM and microalbuminuria (n = 40); and (4) Group 4: Patients with DM and macroalbuminuria (n = 40).
Categorical variables were expressed as frequencies and percentages, and the χ2 or Fischer’s exact test was used to compare them. Utilizing one-way analysis of variance with post-hoc analysis, continuous data were compared and displayed as mean with standard deviation. To find the cut off value for the diagnosis of DN, the receiver-operating characteristic curve (ROC) analysis was employed. P value of less than 0.05 was deemed significant.
This study comprised 160 individuals in total. The study population was comparable concerning age, gender, BMI and alcohol consumption. The duration of DM differed significantly among the groups with the maximum duration in the macroalbuminiuria group. Patients with DM had greater systolic and diastolic blood pressure, particularly in the macroalbuminuria group (Table 1).
| Parameter | Control (n = 40) | Normoalbuminuria (n = 40) | Microalbuminuria (n = 40) | Macroalbuminuria (n = 40) | P value |
| Mean age in years (SD) | 52.83 (7.65) | 49.63 (8.76) | 51.05 (6.54) | 53.73 (10.71) | 0.144 |
| Male | 25 (62.5) | 23 (57.5) | 28 (70) | 26 (65) | 0.704 |
| Smoking habit | 4 (10) | 4 (10) | 4 (10) | 5 (12.5) | 0.978 |
| Alcohol consumption | 7 (17.5) | 8 (20) | 9 (22.5) | 7 (17.5) | 0.932 |
| Mean duration of diabetes mellitus in years (SD) | - | 2.23 (2.41)4,5 | 5.98 (7.08)6 | 8.55 (6.76) | < 0.001 |
| Mean body mass index (SD) (kg/m2) | 24.43 (3.4) | 24.8 (3.7) | 24 (4.29) | 23.8 (3.58) | 0.656 |
| Mean systolic blood pressure (SD) | 118.8 (10.83)1,2,3 | 124.88 (8.7)5 | 128.78 (10.11) | 132.08 (13.8) | < 0.001 |
| Mean diastolic blood pressure (SD) | 78.75 (7.21)2,3 | 81.3 (6.91) | 84.63 (7.52) | 85.18 (10.06) | 0.001 |
Contrary to the microalbuminuria group, the urea level was considerably higher in the macroalbuminuria group. When compared to the control group, there was a significant graded increase in creatinine, FBS, and HbA1c in those with normoalbuminuria to macroalbuminuria. The PPBS also exhibited a similar trend, with the normoalbuminuria group showing significantly elevated values than the control group and lesser values than the macroalbuminuria group. eGFR was significantly decreased in a graded manner, with the lowest rate in the macroalbuminuria group. Urine ACR was significantly elevated in a graded manner, with the highest value in the macroalbuminuria group. The remaining groups had significantly greater levels of NGAL, KIM1, and POSTN than the controls, with macroalbuminuria being the highest. NGAL, KIM1, and POSTN levels were significantly decreased in normoalbuminuria compared with microalbuminuria and macroalbuminuria (Table 2).
| Parameter | Control (n = 40) | Normoalbuminuria (n = 40) | Microalbuminuria (n = 40) | Macroalbuminuria (n = 40) | P value |
| Urea | 24.75 ± 6.78 | 24.23 ± 6.56 | 22.58 ± 10.196 | 28.63 ± 13.52 | 0.042 |
| Creatinine | 0.75 ± 0.431,2,3 | 0.85 ± 0.36 | 0.83 ± 0.45 | 1.18 ± 0.5 | < 0.001 |
| Fasting blood sugar | 92.75 ± 8.321,2,3 | 173.88 ± 83.01 | 199.7 ± 56.15 | 202.78 ± 102.01 | < 0.001 |
| Postprandial blood sugar | 138.55 ± 15.021,2,3 | 268.85 ± 117.934,5 | 337.48 ± 97.98 | 326.9 ± 118.48 | < 0.001 |
| Glycated hemoglobin | 5.03 ± 0.151,2,3 | 8.53 ± 2.07 | 9.5 ± 1.66 | 9.35 ± 2.52 | < 0.001 |
| Estimated glomerular filtration rate | 117.08 ± 30.01 | 111.18 ± 27.35 | 110.88 ± 39.96 | 77 ± 27.48 | < 0.001 |
| Urine albumin creatinine ratio | 5.35 ± 6.491,2,3 | 11.13 ± 6.634,5 | 150.58 ± 81.46 | 1612.55 ± 2064.23 | < 0.001 |
| Neutrophil gelatinase-associated lipocalin (µg/L) | 3.75 ± 2.431,2,3 | 5.28 ± 2.594,5 | 6.85 ± 2.816 | 9.15 ± 2.02 | < 0.001 |
| Kidney injury molecule-1 (ng/mL) | 0.7 ± 0.641,2,3 | 1.33 ± 0.574,5 | 1.85 ± 1.166 | 2.45 ± 0.9 | < 0.001 |
| Periostin (ng/mL) | 29.25 ± 13.151,2,3 | 41.8 ± 24.174,5 | 53.65 ± 14.576 | 64.6 ± 9.1 | < 0.001 |
The duration of DM showed a weak positive correlation with ACR. Creatinine, FBS, PPBS, and HbA1c exhibited significantly positive correlations with ACR, NGAL, and KIM1. ACR was positively correlated with NGAL, KIM1, and POSTN. In contrast, eGFR demonstrated a weak negative correlation with ACR, NGAL, KIM1, and POSTN (Table 3).
| Parameter | ACR | Neutrophil gelatinase-associated lipocalin | Kidney injury molecule-1 | Periostin |
| Duration of diabetes mellitus | R = 0.457, P ≤ 0.001b | - | - | - |
| Creatinine | R = 0.194, P = 0.014a | R = 0.218, P = 0.006b | R = 0.270, P = 0.001b | R = 0.226, P = 0.004b |
| Fasting blood sugar | R = 0.182, P = 0.021a | R = 0.352, P ≤ 0.001b | R = 0.192, P = 0.015a | R = 0.376, P ≤ 0.001b |
| Postprandial blood sugar | R = 0.248, P = 0.002b | R = 0.383, P ≤ 0.001b | R = 0.231, P = 0.003b | R = 0.449, P ≤ 0.001b |
| Glycated hemoglobin | R = 0.225, P = 0.004b | R = 0.425, P ≤ 0.001b | R = 0.295, P ≤ 0.001b | R = 0.406, P ≤ 0.001b |
| ACR | - | R = 0.339, P ≤ 0.001b | R = 0.219, P = 0.005b | R = 0.302, P ≤ 0.001b |
| Estimated glomerular filtration rate | R = -0.292, P ≤ 0.001b | R = -0.325, P ≤ 0.001b | R = -0.299, P ≤ 0.001b | R = -0.275, P ≤ 0.001b |
When the patients were categorized into different stages of renal failure according to the eGFR values, significantly lower ACR and NGAL were observed in stage 1 than in stages 2, 3, and 4. KIM1 was significantly decreased in stage 1 compared with stage 4. Moreover, POSTN was significantly reduced in stage 1 compared with stages 3 and 4 (Table 4).
| Parameter | Stage 1 (n = 76) | Stage 2 (n = 30) | Stage 3 (n = 11) | Stage 4 (n = 3) | P value |
| Albumin creatinine ratio | 240.17 ± 633.921,2,3 | 1078.1 ± 2231.04 | 1344.09 ± 1420.74 | 1860 ± 1893.2 | 0.002 |
| Neutrophil gelatinase-associated lipocalin (µg/L) | 6.36 ± 2.791,2,3 | 8.27 ± 3.15 | 8.27 ± 1.79 | 9.67 ± 2.3 | 0.003 |
| Kidney injury molecule-1 (ng/mL) | 1.67 ± 1.023 | 2.1 ± 0.84 | 2.45 ± 1.12 | 2.67 ± 0.57 | 0.017 |
| Periostin (ng/mL) | 51.04 ± 20.012,3 | 52.63 ± 19.11 | 66.82 ± 9.98 | 69.67 ± 4.5 | 0.034 |
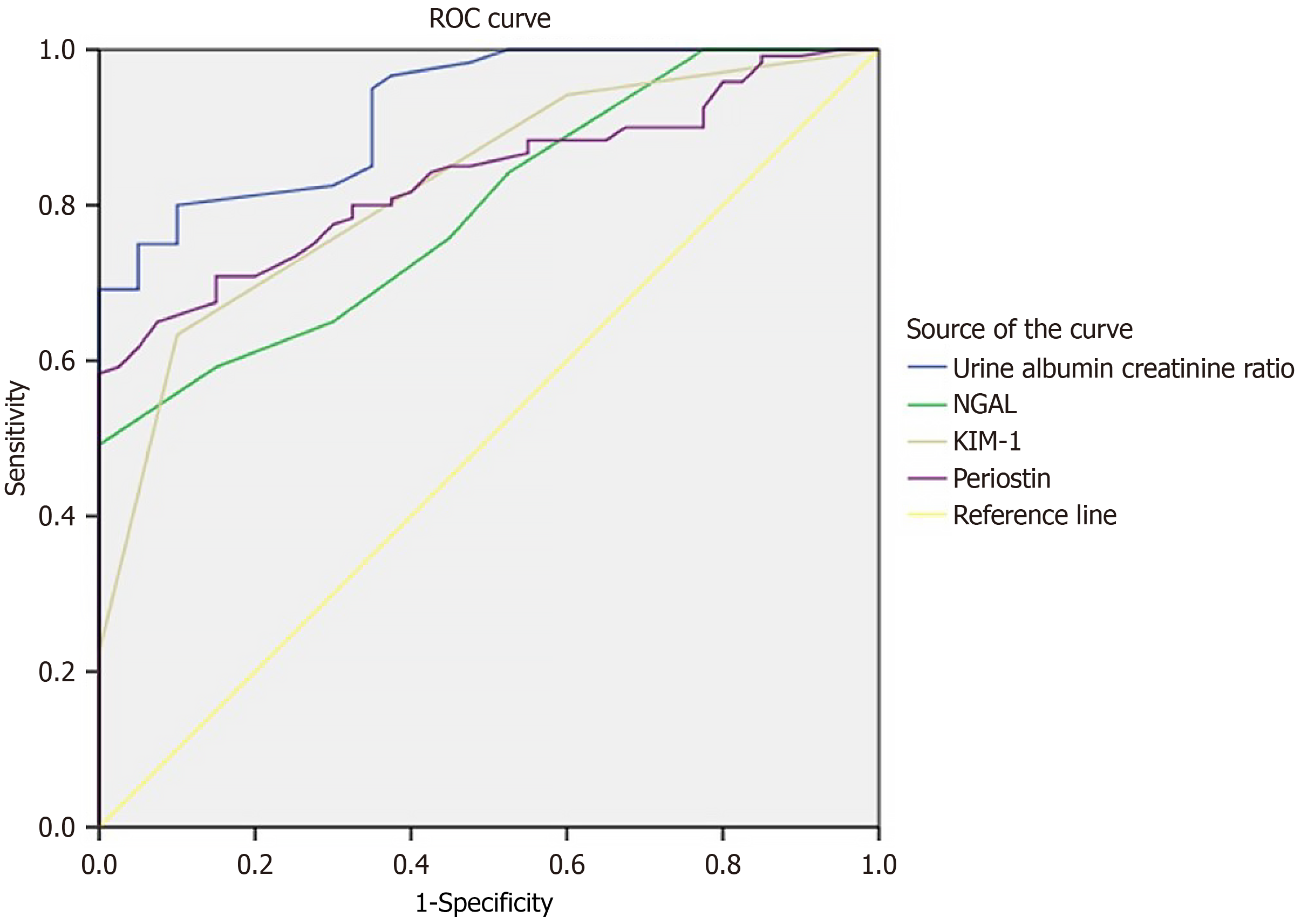
The ROC curve analysis of ACR, NGAL, KIM1, and POSTN showed good sensitivities of 80%, 75.8%, 63.3%, and 80%, respectively, with cut-off values of 12.5 mg/g, 4.5 μg/L, 1.5 ng/mL, and 37.5 ng/mL in predicting DN among the study participants (Table 5, Figure 1).
| Parameter | Area under curve (95%CI) | Sensitivity | Specificity | Cut of value | P value |
| Albumin creatinine ratio | 0.924 (0.882-0.963) | 80 | 90 | 12.5 | < 0.001 |
| Neutrophil gelatinase-associated lipocalin | 0.795 (0.725-0.866) | 75.8 | 55 | 4.5 | < 0.001 |
| Kidney injury molecule-1 | 0.825 (0.757-0.893) | 63.3 | 90 | 1.5 | < 0.001 |
| Periostin | 0.833 (0.773-0.894) | 80 | 67.5 | 37.5 | < 0.001 |
This study showed that when compared to healthy controls, people with DM had significantly higher urine levels of NGAL, KIM-1, and POSTN. Moreover, in patients with DM, these biomarker levels exhibited a notable incremental trend, with the highest levels observed in the macroalbuminuria group. In addition, urinary levels of NGAL, KIM-1, and POSTN were strongly correlated with urinary ACR, FBS, PPBS, and HbA1c and inversely correlated with eGFR. The duration of DM exhibited a weak positive correlation with urinary ACR but was not associated with urinary levels of NGAL, KIM-1, and POSTN.
According to eGFR, study participants were staged from stage 1 to stage 4. Urinary levels of NGAL, KIM-1, and POSTN and urinary ACR were progressively increased significantly from stage 1 to stage 4. The sensitivity and specificity of urinary NGAL, KIM-1, and POSTN in predicting DN among the study participants were 75.8% and 55%with an area under the curve (AUC) of 0.795 (95%CI: 0.725–0.866); 63.3% and 90% with an AUC of 0.825 (95%CI: 0.757–0.893); and 80% and 67.5% with an AUC of 0.833 (95%CI: 0.773–0.894), respectively.
The lipocalin super family’s NGAL, a 25-kDa protein, was first discovered in active neutrophils. However, kidney tubular cells can also produce it in response to tubular damage. Using biomarkers like NGAL, uLFABP, and KIM1, numerous researches have shown the predictive utility of tubular damage markers in anticipating the development and progression of albuminuria[12-14]. Kim et al[15] expressed scepticism on the idea that NGAL serves as an early indicator of DN because, in their trial, significant differences were not observed in NGAL levels among control, normoalbuminuria, and microalbuminuria groups. However, the current study noted elevated NGAL concentrations in urine of patients with DM and normoalbuminuria compared with healthy controls. This finding could be the result of NGAL’s defensive mechanism in reaction to hemodynamic and metabolic stress. These results agree with those of other researchers who have shown that urinary NGAL levels are elevated in people with DM even prior to the microalbuminuria. Hence, NGAL may function as an early biomarker for normoalbuminuric DN[16-20].
In our study, the sensitivity and specificity of urinary NGAL in predicting DN among the study participants were 75.8% and 55%, respectively, with an AUC of 0.795 (95%CI: 0.725–0.866),which is comparable to previous studies. Tang et al[18] assessed the value of urinary NGAL as a preliminary marker of DKD by reviewing 14 observational studies. The findings indicated that urinary NGAL could effectively differentiate patients with DKD from control subjects. Specifically, cross-sectional studies demonstrated a pooled sensitivity and specificity of 82% and 81%, respectively. In contrast, cohort studies reported a higher pooled sensitivity of 96% and a pooled specificity of 89%[18]. Żyłka et al[20] found that the sensitivity of NGAL was 80% and that the specificity was 61%. Quang et al[16] identified that the sensitivity and specificity of NGAL were 60% and 70%, respectively.
The participants were categorized according to their eGFR values, which showed a prominent increase in urinary NGAL levels as the eGFR stage progressed. Additionally, an inverse correlation was noted between urinary NGAL levels and eGFR. These results imply that urinary NGAL may be used as a predictor of eGFR decline in patients with DN. However, Chou et al[21] failed to identify a negative connection between urinary NGAL and eGFR in their study.
Acute kidney damage causes an increase in KIM-1 expression, which is expressed on the proximal tubular membrane cells. Numerous studies have observed that KIM-1 is a reliable indicator of prognosis and a sensitive and specific measure of kidney damage[22]. In our study, patients with DM exhibited significantly higher KIM-1 than healthy subjects. Furthermore, compared with patients having microalbuminuria, those with macroalbuminuria had higher KIM-1 Levels. These results are consistent with the study carried out by Quang et al[16] and El-Ashmawy et al[23]. In their comprehensive review and meta-analysis of 14 studies, Kapoula et al[24] reported that urinary KIM-1 (uKIM-1) can help evaluate early diabetic renal damage. According to their study correlation, urinary ACR (urine albumin creatinine ratio) and uKIM-1 exhibited a statistically significant positive link. Our study also produced a similar finding. This result lends credence to the theory that in DKD, tubular involvement precedes glomerular involvement.
The sensitivity and specificity of uKIM-1 in predicting DM among the study participants were 63.3% and 90%, respectively, with an AUC of 0.825 (95%CI: 0.757–0.893). Żyłka et al[20] documented that the sensitivity and specificity of KIM-1 were 79% and 51%, respectively. Quang et al[16] observed that the sensitivity and specificity of KIM-1 were 62% and 73%, respectively. Kapoula et al[24] stated that the specificity of uKIM-1 for early DN diagnosis was 0.83 (95%CI: 0.69–0.92) and that the predicted overall sensitivity was 0.68 (95%CI: 0.35–0.89). Furthermore, uKIM-1 was inversely correlated with eGFR in our study, a result in line with an earlier investigation by Kapoula et al[24].
The matricellular protein POSTN is involved in wound healing and tissue remodeling. Its expression in healthy kidneys is minimal, but it is highly expressed in various kidney disorders, especially those involving progressive renal fibrosis. POSTN in the urine can indicate the loss of renal tubular cells, as evidenced by an earlier study showing that the protein was highly expressed in tubulointerstitial regions during renal injury[10]. In our study, patients with normoalbuminuria exhibited significantly higher urinary POSTN levels than healthy controls. Additionally, these values increased progressively as the stage of albuminuria worsened, including microalbuminuria and macroalbuminuria. These outcomes are similar to those of a prior work conducted by Satirapoj et al[10]. The sensitivity and specificity of urinary POSTN in predicting DN among the study participants were 80% and 67.5%, respectively, with an AUC of 0.833(95%CI: 0.773–0.894), which is comparable to the investigation by Satirapoj et al[10]. Additionally, POSTN levels were inversely correlated with eGFR levels in the current study.
This study has several potential limitations. Its cross-sectional design restricted the ability to track the study participants over time to observe the progression of kidney injury. Furthermore, the results may not be as broadly applicable as they may be because it was a one-center experiment with a small sample size. Therefore, to fully explore the significance of urine NGAL, KIM-1, and POSTN in identifying and tracking the development of kidney injury, future research needs to concentrate on bigger populations.
The results of this investigation show that urinary NGAL, KIM-1, and POSTN are potential indicators of kidney damage in patients with T2DM during the initial phases of DN. Additionally, higher levels of these biomarkers are indicative of the severity of kidney injury.
| 1. | Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9344] [Cited by in RCA: 8960] [Article Influence: 426.7] [Reference Citation Analysis (1)] |
| 2. | Hoogeveen EK. The Epidemiology of Diabetic Kidney Disease. Kidney Dial. 2022;2:433-442. [DOI] [Full Text] |
| 3. | Campion CG, Sanchez-Ferras O, Batchu SN. Potential Role of Serum and Urinary Biomarkers in Diagnosis and Prognosis of Diabetic Nephropathy. Can J Kidney Health Dis. 2017;4:2054358117705371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 4. | Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J Am Soc Nephrol. 2015;26:1765-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 550] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 5. | McMahon BA, Murray PT. Urinary liver fatty acid-binding protein: another novel biomarker of acute kidney injury. Kidney Int. 2010;77:657-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Shao X, Tian L, Xu W, Zhang Z, Wang C, Qi C, Ni Z, Mou S. Diagnostic value of urinary kidney injury molecule 1 for acute kidney injury: a meta-analysis. PLoS One. 2014;9:e84131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 543] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 8. | Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 496] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 9. | Nielsen SE, Andersen S, Zdunek D, Hess G, Parving HH, Rossing P. Tubular markers do not predict the decline in glomerular filtration rate in type 1 diabetic patients with overt nephropathy. Kidney Int. 2011;79:1113-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Satirapoj B, Tassanasorn S, Charoenpitakchai M, Supasyndh O. Periostin as a tissue and urinary biomarker of renal injury in type 2 diabetes mellitus. PLoS One. 2015;10:e0124055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5:1003-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 474] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 12. | Megallaa MHZ, Abdel Salam MS, Saad NLM, Ahmed Altair MNAH, Amin NG. NGAL (Neutrophil Gelatinase-Associated Lipocalin) as an early biomarker of nephropathy in patients with type 2 diabetes. Alex J Med. 2023;59:52-58. [DOI] [Full Text] |
| 13. | Nielsen SE, Schjoedt KJ, Astrup AS, Tarnow L, Lajer M, Hansen PR, Parving HH, Rossing P. Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Kidney Injury Molecule 1 (KIM1) in patients with diabetic nephropathy: a cross-sectional study and the effects of lisinopril. Diabet Med. 2010;27:1144-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Nielsen SE, Sugaya T, Hovind P, Baba T, Parving HH, Rossing P. Urinary liver-type fatty acid-binding protein predicts progression to nephropathy in type 1 diabetic patients. Diabetes Care. 2010;33:1320-1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Kim SS, Song SH, Kim IJ, Yang JY, Lee JG, Kwak IS, Kim YK. Clinical implication of urinary tubular markers in the early stage of nephropathy with type 2 diabetic patients. Diabetes Res Clin Pract. 2012;97:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Quang TH, Nguyet MP, Thao DP, Thi MH, Phuong Thi Dam L, Thi HH, Van AP, Luong TC, Tuyet MNT, Duy QD, Nhu BD, Duc TN. Evaluation of Urinary Neutrophil Gelatinase Associated Lipocalin and Kidney Injury Molecule-1 as Diagnostic Markers for Early Nephropathy in Patients with Type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes. 2020;13:2199-2207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Wu J, Shao X, Lu K, Zhou J, Ren M, Xie X, Liu J, Xu Y, Ding Y, Shen X, Zhu C. Urinary RBP and NGAL Levels are Associated with Nephropathy in Patients with Type 2 Diabetes. Cell Physiol Biochem. 2017;42:594-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Tang XY, Zhou JB, Luo FQ, Han YP, Zhao W, Diao ZL, Li M, Qi L, Yang JK. Urine NGAL as an early biomarker for diabetic kidney disease: accumulated evidence from observational studies. Ren Fail. 2019;41:446-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Kaul A, Behera MR, Rai MK, Mishra P, Bhaduaria DS, Yadav S, Agarwal V, Karoli R, Prasad N, Gupta A, Sharma RK. Neutrophil Gelatinase-associated Lipocalin: As a Predictor of Early Diabetic Nephropathy in Type 2 Diabetes Mellitus. Indian J Nephrol. 2018;28:53-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Żyłka A, Dumnicka P, Kuśnierz-Cabala B, Gala-Błądzińska A, Ceranowicz P, Kucharz J, Ząbek-Adamska A, Maziarz B, Drożdż R, Kuźniewski M. Markers of Glomerular and Tubular Damage in the Early Stage of Kidney Disease in Type 2 Diabetic Patients. Mediators Inflamm. 2018;2018:7659243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Chou KM, Lee CC, Chen CH, Sun CY. Clinical value of NGAL, L-FABP and albuminuria in predicting GFR decline in type 2 diabetes mellitus patients. PLoS One. 2013;8:e54863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Vaidya VS, Waikar SS, Ferguson MA, Collings FB, Sunderland K, Gioules C, Bradwin G, Matsouaka R, Betensky RA, Curhan GC, Bonventre JV. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1:200-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 259] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 23. | El-ashmawy NE, El-zamarany EA, Khedr NF, Abd El-fattah AI, Eltoukhy SA. Kidney injury molecule-1 (Kim-1): an early biomarker for nephropathy in type II diabetic patients. Int J Diabetes Dev Ctries. 2015;35:431-438. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Kapoula GV, Kontou PI, Bagos PG. Diagnostic Performance of Biomarkers Urinary KIM-1 and YKL-40 for Early Diabetic Nephropathy, in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diagnostics (Basel). 2020;10:909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |