Published online Dec 25, 2024. doi: 10.5527/wjn.v13.i4.98709
Revised: September 29, 2024
Accepted: October 8, 2024
Published online: December 25, 2024
Processing time: 126 Days and 19.5 Hours
Recently, new findings have been clarified concerning both pathogenesis and treatment of IgA nephritis. The four hits theory has been confirmed but several genetic wide association studies have allowed finding several genes connected with the pathogenesis of the disease. All these new genes apply to each of the four hits. Additionally, new discoveries concerning the microbiota and its connection with immune system and IgA generation have allowed finding out the role of the mucosa in IgA nephropathy pathogenesis. The IgA treatment is also changed included the future possibilities. The treatment of the chronic kidney disease, associated with the nephropathy, is mandatory, since the beginning of the disease. The classical immunosuppressive agents have poor effect. The corticosteroids remain an important cornerstone in any phase of the disease. More effect is related to the treatment of B cells and plasma cells. In particular, in very recent studies have been documented the efficacy of anti B cell-activating factor and anti A proliferation-inducing ligand agents. Most of these studies are to date in phase II/III. Finally, new agents targeting complement are arising. These agents also are still in randomized trials and act principally in hit 4 where the immunocomplexes in the mesangium activate the different pathways of the complement cascade.
Core Tip: Genetic wide association studies (GWAS) and studies on microbiota allowed finding new aspects in pathogenesis of IgA nephropathy. GWAS allowed finding new genes related to the different IgA nephropathy phases. Microbiota allowed finding new roles exerted by microbes in mucosa immune system and in IgA pathogenesis. Treatment of chronic kidney disease must be always associated to the immune treatment. In the latter fundamental role is exerted by steroids. Recent studies found the relevance of anti B cell-activating factor and anti A proliferation-inducing ligand agents even if the majority of these studies are still in phases II/III. Similarly in premarketing studies are the anti-complement agents.
- Citation: Salvadori M, Rosso G. What is new in the pathogenesis and treatment of IgA glomerulonephritis. World J Nephrol 2024; 13(4): 98709
- URL: https://www.wjgnet.com/2220-6124/full/v13/i4/98709.htm
- DOI: https://dx.doi.org/10.5527/wjn.v13.i4.98709
The aim of this study has been to check what is new in the pathogenesis and treatment of the IgA glomerulonephritis, one of the most common nephropathies in the world. Indeed, genetic wide association studies (GWAS) have allowed to confirm the old hypothesis of the four hits pathogenesis, but added several genes involved in the pathogenesis. Similarly, new findings have been found in the fields of microbiota, as important causes of the disease. The treatment also has been reviewed. The necessity to add to the immune therapy, the treatment of the chronic kidney disease (CKD) has been confirmed. Concerning the immunological therapy, in addition to the corticosteroids, new drugs anti B cell-activating factor (BAFF) and A proliferation-inducing ligand (APRIL) and anti-complement cascade are in trials. Some of them are already in phase III and ready to enter the marketing.
IgA glomerulonephritis is the most common form of primary glomerulonephritis worldwide. Up to 50% of patients with IgA nephropathy (IgAN) develop end-stage kidney disease (ESKD) and require dialysis, and 10% of patients on dialysis have IgAN. Sixty percent of IgAN patients with ESKD receive one or more kidney transplants. Overall, 200000 people in the United States have IgAN, and 200000 people in Europe have IgAN. Most likely, in Asia, this glomerulonephritis is more common, 130000 people in Japan have IgAN, and 800000 people in China have IgAN.
IgAN is characterized by high heterogeneity and high-unmet medical needs. It is characterized by dominant IgA glomerular deposits, highly variable clinical presentation[1] and different histological features such as interesting glomeruli, tubules, interstitial and vessels. In addition, the clinical course may be highly variable[1]. The mechanisms responsible for the presentation and development of IgAN are incompletely understood, and the development of highly targeted therapies for this disease has been limited[2].
Under normal conditions, the hinge region is unique to IgA1. Indeed, it consists of repeating sequences of proline, serine and threonine, and it carries multiple O-linked carbohydrate side chains. Each O-glycan chain is based on a core N-acetyl galactosamine unit that is O-linked to serine or threonine. Moreover, abnormalities in O-glycosylation represent hit 2 of the development of IgA nephritis.
The interactions of different pathogenic elements are required to produce IgAN. The fraction of total serum IgA that has a propensity for mesangial deposition is small, and the fraction capable of initiating glomerulonephritis is still small[3]. The response of the mesangium, particularly the mesangial cells, to the deposited IgA is critical for the development of IgAN. Failure of mesangial cell clearance mechanisms could contribute to mesangial IgA accumulation, whereas mesangial cell activation with the release of proinflammatory cytokines and extracellular matrix components drives glomerulonephritis and glomerulosclerosis. Without an appropriate genetic predisposition to develop IgAN, IgA deposi
A multi-hit mechanism has been proposed to clarify the pathways involved in the pathogenesis of IgAN.
Accordingly, in hit 1, galactose-deficient IgA1 is produced by a subpopulation of IgA1-secreting cells. IgA1 production may be affected by the IgAN-associated locus on chromosome 22q12.2[4]. Hit 2 includes the formation of antiglycan antibodies with specific characteristics of the variable region of the heavy chain that recognize galactose-deficient IgA1. In hit 3, immune complexes are formed from autoantigens (galactose-deficient IgA1) and O-glycan-specific antibodies. Hits 2 and 3 may be regulated by the three major histocompatibility complex loci on chromosome 6p21 associated with the risk of IgAN[4]. In hit 4, there is the deposition of pathogenic immune complexes in the mesangium, activation of mesangial cells and induction of glomerular injury. Hits 3 and 4 may be affected by the genotype at the complement factor H locus on chromosome 1q32, which regulates the alternative complement cascade. One pathway involves the formation of immune complexes in the circulation and their subsequent mesangial deposition[5-8]. In an alternative pathway, some aberrantly glycosylated IgA1 molecules are in the mesangium as lanthanide deposits and are later bound by newly generated antiglycan antibodies to form immune complexes in situ, which activate mesangial cells[9] (Table 1).
| Hit | Pathogenetic process | Putative environmental factors involved | Putative genetic factors involved | Potential clinical biomarkers | Potential novel therapeutic approaches |
| 1 | Hereditary increase in circulating galactose-deficient IgA1 | Potential role of mucosal exposure to infectious of dietary antigens | Strong evidence for high heritability of serum galactose-deficient IgA1 level. Potential role of chromosome 22q12.2 | Serum galactose-deficient IgA1 level (HAA based ELISA) | Suppression of synthesis of galactose-deficient IgA1. Enzymatic boost of galactose transfer to IgA1 hinge region O-glycans |
| 2 | Circulating antibody directed against galactose-deficient IgA1 | Potential role of mucosal exposure to infectious or dietary antigens | Potential role of three MHC-II loci in antigen presentation and humoral response to galactose-deficient IgA1 O-glycans | Serum anti-glycan antibodies (dot-blot assay) | Alteration of processing and presentation of galactose-deficient IgA1 O-glycopeptides. Specific B-cell depletion therapy |
| 3 | Formation of pathogenic IgA1-containing immune complexes | Unknown | Unknown | Circulating and/or urinary immune complexes | Competitive blockade of immune complex formation by non-cross-linking anti-glycan antibodies or specific glycopeptides |
| 4 | Mesangial deposition of IgA1-containing immune complexes, cell activation and initiation of glomerular injury | Unknown | Protective effect of common deletion in CFHR1 and CFHR3 | Circulating and/or urinary complement degradation products, or novel markers of glomerular injury | Suppression of the alternative complement pathway. Targeted CFHR1/3 depletion. Blocking mesangial cell signaling induced by nephritogenic IgA1-containing immune complexes |
According to the data shown, there are different pathogenic processes according to the hit considered, different putative environmental factors, different putative genetic factors, different potential clinical biomarkers and, therefore, different potential novel therapeutic approaches[10].
The importance of genetics in determining IgAN and high serum galactose deficient (Gd)-IgA1 levels has been documented by several GWAS, as documented by the review of Xu et al[11] and as shown in Table 2[11-19]. One of the most important cited studies is the one conducted by Kiryluk et al[20], in 2023. This study was conducted on a large population (10146 kidney biopsy-diagnosed IgAN patients and 28751 matched controls across 17 international cohorts). Additionally, the study defined 30 independent risk loci in IgAN.
| Ref. | Published date | Ancestry | GWAS population | Genome-wide significant loci (candidate gene) |
| Susceptibility to IgA nephropathy | ||||
| Feehally et al[12] | 2010 | European ancestry | 244 cases and 4980 healthy controls | 6p21 (HLA) |
| Gharavi et al[4] | 2011 | Chinese and European ancestry | 3144 cases and 2822 healthy controls | 6p21 (HL-DQB1/DRB1; PSMB9/TAPI; HLA-DPA1/DPB2), 1q32 (CFHR3/R1), 22q12 (HORMAD2) |
| Yu et al[13] | 2011 | Chinese ancestry | 1434 cases and 4270 healthy controls | 6p21 (HLA), 8p23 (DEFAs), 17p13 (TNFSF13), 22q12 (MTMR3) |
| Kiryluk et al[14] | 2014 | European and East Asian ancestry | 7658 cases and 12954 healthy controls | 6p21 (HLA-DQ-HLA-DR; TAP1-PSMB8; HLA-DP), 1p13 (VAV3), 1q32 (CFHR3-CFHR1 deletion), 8p23 (DEFAs), 9q34 (CARD9), 16p11 (ITGAM-ITGAX), 17p13 (TNFSF13), 22q12 (HORMAD2) |
| Li et al[15] | 2015 | Chinese ancestry | 1434 cases and 10661 healthy controls | 6p21 (HLA), 3q27 (ST6GAL1), 8p23 (DEFA), 8q22 (ODF1-KLF10), 11p11 (ACCS), 16p11 (ITGAX-ITGAM, 22q12 (HORMAD2) |
| Jeong et al[16] | 2019 | Korean ancestry | 188 cases and 455 healthy controls | 10p15 (ANKRD16) |
| Li et al[17] | 2020 | Chinese and European ancestry | 2628 cases and 11563 healthy controls | 6p21 (HLA), 1q23 (FCRL3), 1p36 (PAD14), 6p25 (DUSP22/IRF4), 8p23 (DEFA), 16p11 (ITGAX-ITGAM), 17p13 (TNPSF12-TNPSF13), 22q12 (MTMR3/HORMAD2) |
| Zhou et al[18] | 2021 | Chinese ancestry | 601 cases and 4076 healthy controls | 6p21 (GABBR1), suggestive genes (TGFB1, CCR6, STAT3, CFB) |
| Li et al[19] | 2023 | Chinese ancestry | 2378 cases and 15642 healthy controls | 6p21 (HLA), 6p21.1 (VEGFA), 16q22.2 (PKD1 L3), 17p13 (TNFSF13) |
| Kiryluk et al[20] | 2023 | European and East Asian ancestry | 10146 cases and 28751 healthy controls | 6p21 (HLA), 8 known non-HLA loci (CFH, FCRL3, IRF4/DUSP22, DEFA ¼, CARD9, ITGAM/ITGAX, TNFSF 13/12, MTMR3/HORMAD2/LIF/OSM), 16 new non HLA loci (TNFSF4/18, CD28, REL, PF4V1, LY86, LYN, ANXA13, TNFSF8/15, ZMIZ1, REEP3, OVOL1/RELA, ETS1, IGH, IRF8, TnFRSF13B, and FCAR) CCR6 (only in the East Asian cohorts) |
| Serum Gd-IgA1 levels | ||||
| Gale et al[21] | 2017 | European and Chinese ancestry | 513 subsets | 7p21 (C1GALT1) |
| Kiryluk et al[22] | 2017 | European and East Asian ancestry | 2633 subsets | 7p21 (C1GALT1), Xq24 (C1GALT1C1) |
| Wang et al[23] | 2021 | Chinese ancestry | 1127 patients with IgAN | 7p22 (C1GALT1), 9q22 (GALNT12) |
Overall, the review of Xu et al[11] supported the multi-hit hypothesis. Indeed, genes responsible for the dysregulation of mucosal innate immunity (DEFA, CARD 9, TNFSF 13), genes responsible for immune cell proliferation and abnormal IgA production (LYN, FCRL3, CD28; TNFGF12/13), genes responsible for the production of galactose-deficient IgA1 (CIGALT1, GALNT12) and, at the circulatory level, genes responsible for the formation of autoantibodies against galactose-deficient IgA1 (HLA-DRA) have been identified. Finally, at the kidney level, genes responsible for the activation of the complement system and cytokine production have been identified (ITGAM-ITGAX).
Genetics alone are important in promoting the development of IgA glomerulonephritis, but the abnormal handling of the microbiota and the inflammatory phenotype are similarly important factors[20].
Alterations in the gut microbiome are considered novel factors involved in the pathogenesis of IgAN. Dysbiosis ultimately results in changes in microbial functions, including changes in biochemical processes and fermentation. Loss of immune equilibrium could increase the susceptible of the host to intestinal infections. Intestinal infections prime the class switch of B cells, leading to the overproduction of Gd-IgA1. Excess Gd-IgA1 acts as an autoantigen and is bound to autoantibodies such as IgG or IgA. These immune complexes are deposited in the glomerular mesangium, where they are involved in processes such as mesangial proliferation. A series of downstream pathways, including the complement system, are activated, leading to inflammation and glomerular injury[21-28]
Mucosal infections are implicated in the pathogenesis of IgAN through three hypotheses. According to one hypothesis, specific pathogens are believed to be involved in the initiation and progression of IgAN. Several pathogens can be directly detected in renal tissue. Several other pathogens, such as Helicobacter pylori and poliovirus, affect the production of IgA1 and the hypogalactosylation process[29,30]. According to another hypothesis, the occurrence of tonsillitis is believed to be related to IgAN. Clinically there is a close relationship between upper respiratory tract infections and hematuria. Morphologic analyses have shown that tonsils are important producing sites of Gd-IgA1. Additional evidence includes microbiome analysis of tonsillar crypts in IgAN, genetic association analysis (HORMAD2) and the effectiveness of population-based tonsillectomy[31]. Thus, chronic and persistent tonsillitis might be involved in disease pathogenesis. According to a third hypothesis, intestinal infections caused by alterations in the gut microbiome have been recognized as critical inducers of the pathogenesis of IgAN[32]. Indeed, persistent antigenic stimulation causes aberrant mucosal immune responses, affecting the class switch of B-cell and the overproduction of IgA. Additionally, the gut microbiota post-translational modifies IgA1 in autoimmune glomerulonephritis[33].
A recent study from He et al[34] analyzed the potential roles of the oral microbiota in the pathogenesis of IgAN. In this study, saliva samples from 31 patients affected by IgAN and 30 controls were collected and analyzed via 16S rRNA gene sequencing. Microbial diversity was reduced in IgAN patients and microbes, such as Capnocytophaga and Haemophilus were associated with increased levels of proteinuria. In addition, in IgAN, metabolic pathways such as glycosphingolipid synthesis and N-glycan biosynthesis were enriched. The authors conclude that alterations in the oral microbiota are associated with IgAN. In another study, Currie et al[35] characterized the tonsil microbiota of patients affected by IgAN. These authors reported an elevated presence of the genus Neisseria. In vitro the authors found that in mice affected by IgAN, with the enzyme-linked immune spot assay, the kidneys of these mice contained anti Neisseria-specific IgA secreting cells.
For many years, the relevance of the microbiota in IgAN has been recognized[36]. In 2011, the first description of the gut microbiota in animal models of IgAN was published. By the beginning of 2020, disturbance of the intestinal microflora was recognized to be important for the severity of IgAN, as was the potential of the gut microbiota as a specific biomarker in the pathogenesis of IgAN. More recently, microbial profiles have been shown to be useful for distinguishing IgAN patients, and the gut microbiota in newly diagnosed IgAN patients with CKD stages 1-2 is different from that in healthy controls.
In a recent study, Zhu et al[37] reported that in IgAN, the gut microbiome regulates the production of hypoglycosylated IgA1 via the TLR4 signaling pathway. Indeed, the overproduction of Gd-IgA1 correlated with gut dysbiosis, and TLR4 and B-cell stimulators and the TLR4 inhibitor; TAK242 inhibited the overproduction of Gd-IgA1 and B-cell stimulators in peripheral blood mononuclear cells from IgAN patients and controls. In another recent study from Cai et al[38], systematic microbiome dysbiosis was associated with IgAN. The study enrolled 505 participants and performed 16S rRNA gene sequencing on samples from the mouth, pharynx, gut and urine. The microbial abundance in the samples was used to predict IgAN, and an accuracy of 0.879 was obtained.
In addition to genetics and the microbiome, other factors intervene in the genesis of IgAN.
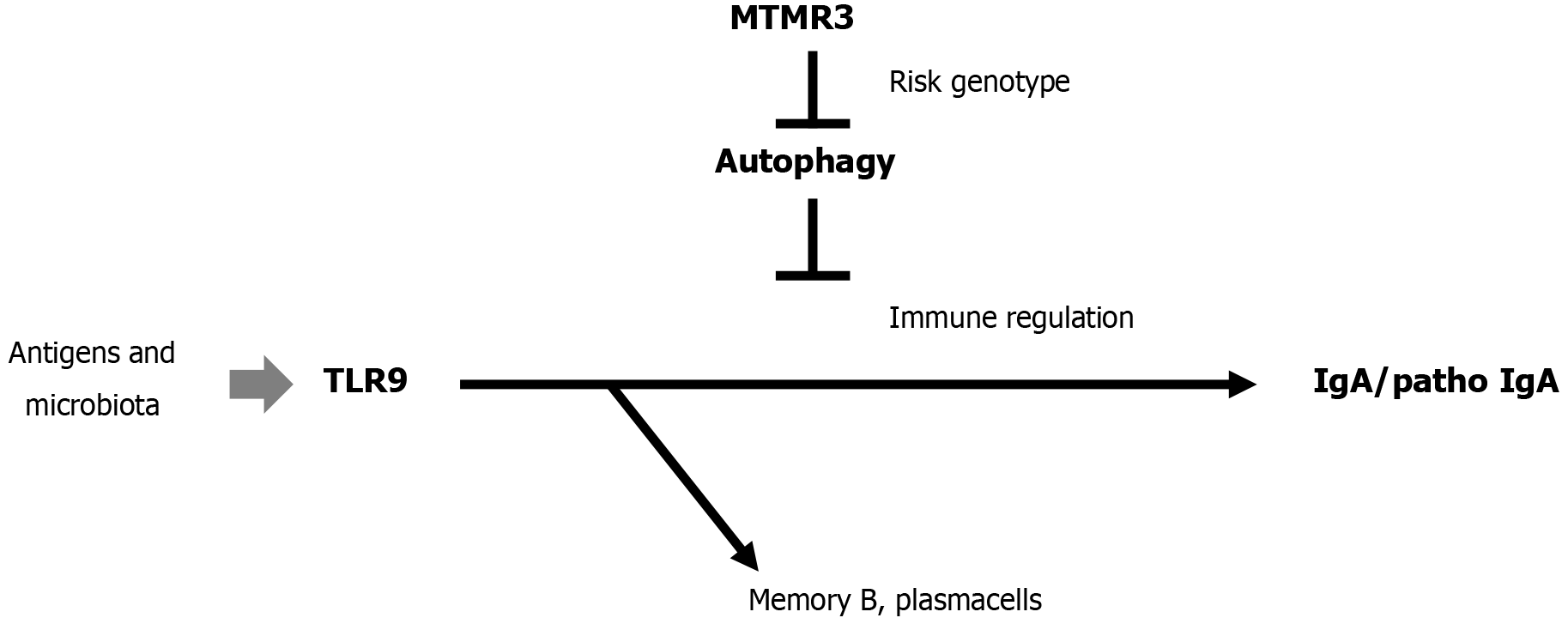
Toll-like receptor 9 and 7 stimulation induces aberrant expression of a proliferation-inducing ligand by tonsillar germinal center B cells in IgAN[39], and (Myotubularin-related protein 3 gene) MTMR risk alleles increase Toll-like receptor 9-induced IgA immunity in IgAN[40]. Therefore, targeting IgA-regulating genes has therapeutic importance as shown in Figure 1.
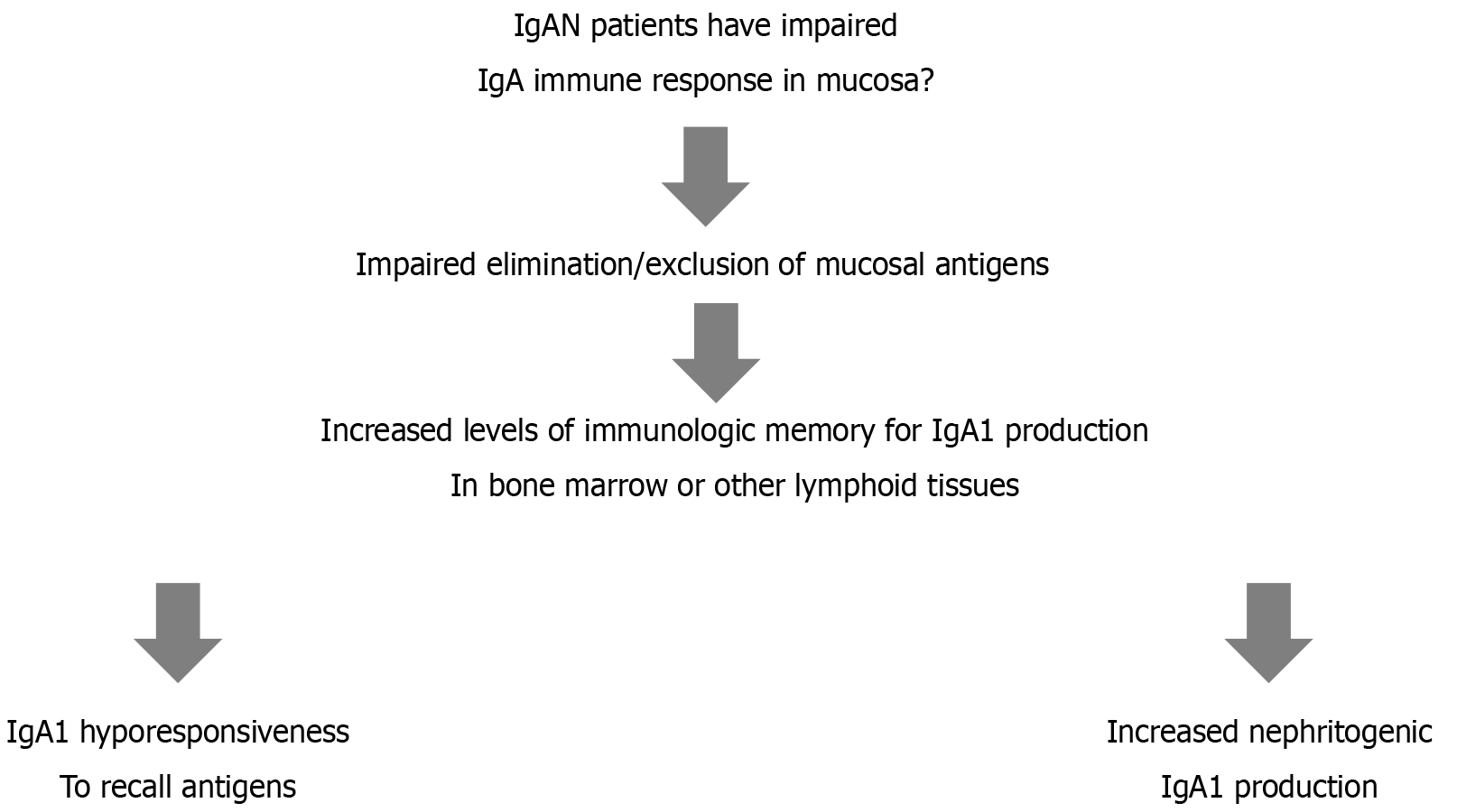
Another important factor is the existence of a mucosa bone marrow axis in patients with IgAN[41]. As shown in Figure 2, IgAN patients have an impaired IgA immune response in the mucosa that causes impaired elimination/exclusion of mucosal antigens. Therefore, there is an increased level of immunologic memory for IgA1 production in bone marrow or other lymphoid tissues. This ultimately causes IgAN1 hyporesponsiveness to recall antigens and increased production of nephritogenic IgA1.
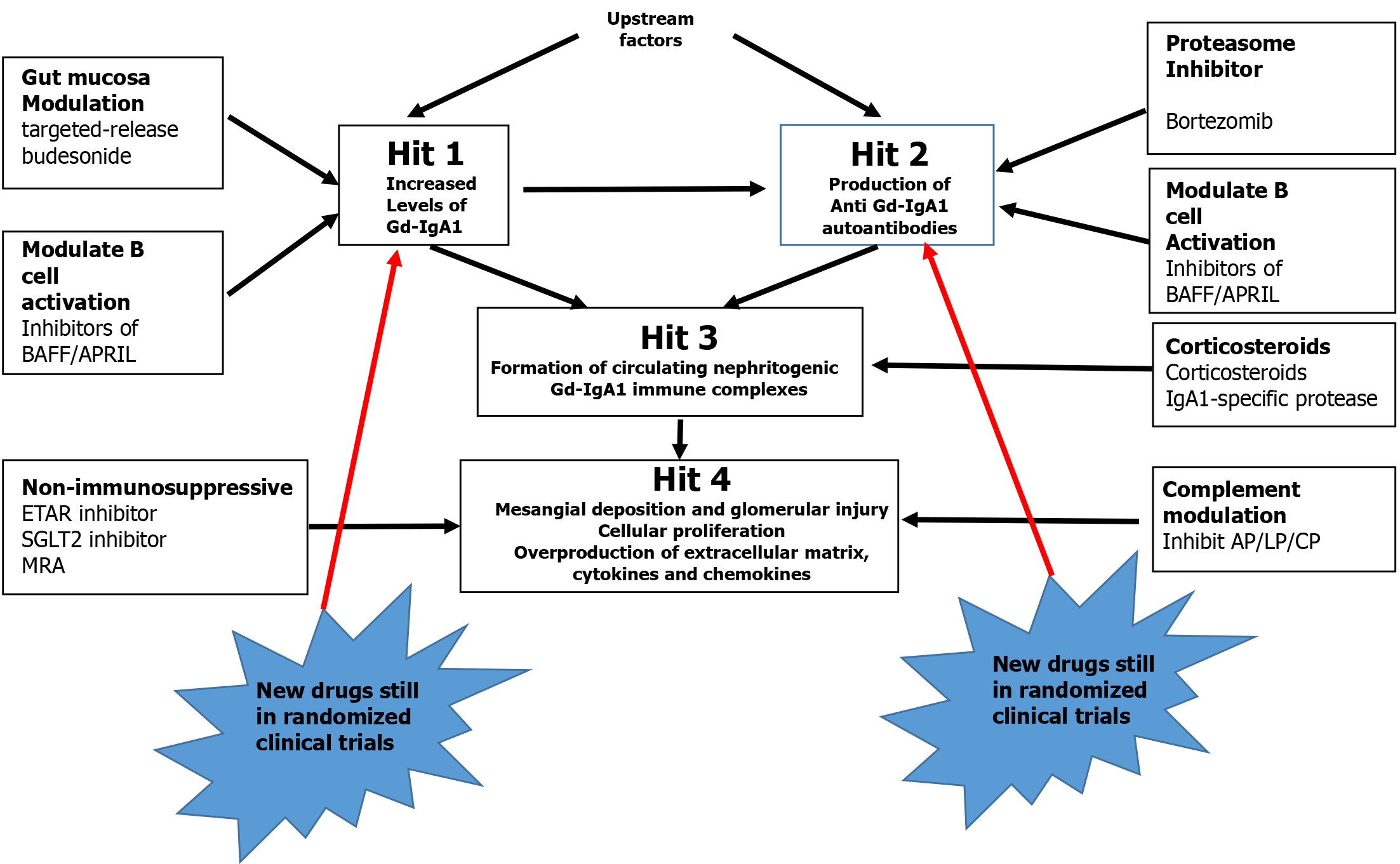
Figure 3 shows the potential therapeutic targets for IgAN based on the findings of GWASs[42]. Basing on what is described, it can be stated that: IgAN is a heterogeneous disease with complex underlying pathophysiology, and current options are variable and often not sufficient. The evolving understanding of the pathogenic pathways of IgAN has led to the discovery and development of new treatments for more targeted therapies. Identifying better biomarkers and establishing evaluative systems for new treatments may help in identify more precise targeted treatments for patients with IgAN.
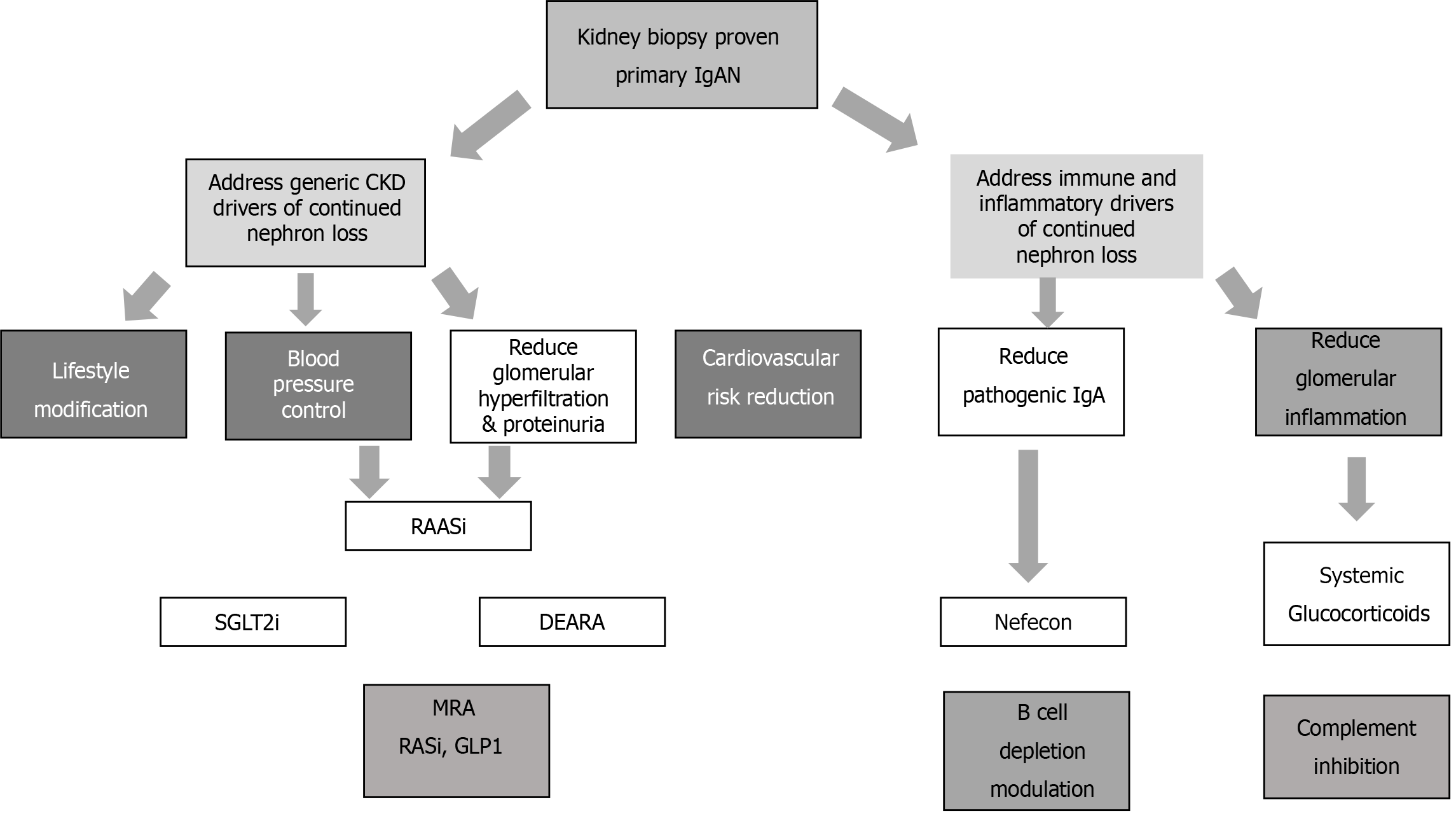
Figure 3 shows the different treatments according to the four hit theories confirmed by GWAS. From the clinical point of view is better to distinguish the different treatments according Figure 4. One the one hand, we have to address treatment against immune inflammation; on the other hand, we have to address different stages of CKD. In all patients, these treatments should be addressed simultaneously.
Like CKD of different origins, IgAN is essential to prevent or control CKD if it is present.
This can be addressed in different ways, as shown in Figure 4. First, optimal control of blood pressure and proteinuria is essential. These factors delay CKD progression in patients with any type of renal disease (including IgAN)[43].The optimal control of blood pressure includes several aspects of lifestyle modification, such as salt restriction, dietary modification, weight reduction, smoking cessation and physical activity. Medications may include angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers (ARBs)[44-47].
Sodium-glucose cotransporter inhibitors (SGLT2is) inhibit glucose reabsorption from the proximal tubule and cause glycosuria and natriuresis. Glycosuria is essential in controlling prediabetes and diabetes, whereas natriuresis contributes to a reduction in plasma volume and in preload. Additionally, intraglomerular pressure and glomerular hyperfiltration are reduced with a nephroprotective action[48]. Two studies[49,50] documented a protective effect in CKD patients. A different molecule still belonging to SGLT2 (empaglifozin) has been used in the EMPA-KIDNEY trial. Among 6609 CKD patients, 817 patients with IgAN were included in the trial. Overall, empaglifozin was associated with a reduction in kidney disease progression[51].
In prediabetic patients with IgAN, the efficacy of glucagon-like peptide-1 (GLP-1) receptor agonists has been proven. In a meta-analysis by Salamah et al[52], its efficacy was proven in 2903 patients with prediabetes and CKD. Unfortunately, how many patients are affected by IgAN has not been clarified, but owing to the good results of the study, GLP-1 may be added to the drugs used in CKD patients.
In the HAKA multicenter retrospective study on patients affected by advanced CKD[53], the efficacy of other drugs, such as renin-angiotensin system inhibitors and mineralocorticoid receptor antagonists, was proven to be affected principally by heart complications when given in association with SGLT2. Sparsentan is a molecule that targets dual endothelin angiotensin receptor antagonist. In addition to the vascular effect, it ameliorates glomerular hypercellularity and inflammatory-gene networks. In a recent mouse model, its efficacy in IgAN was documented[54]. The study PROTECT is designed to determine the long-term antiproteinuric and nephroprotective potential of treatment with sparsentan compared head-to-head with active control irbesartan, an ARB, in patients with IgAN. There is a strong scientific rationale for the dual antagonism of endothelin and angiotensin receptors in IgAN, which is supported by extensive preclinical data. The PROTECT study interim analysis clearly revealed the superiority of sparsentan in reducing proteinuria compared with the active control irbesartan.
Immunosuppressive treatment for IgAN should principally be considered for patients at high risk of evolution because of the clinical, pathogenetic and evolution of renal function. Several immunosuppressive treatments (cyclophosphamide, azathioprine, calcineurin inhibitors) have not been shown to be effective in treating IgAN[55]. Uniform data concerning the use of mycophenolate mofetil are not available[56-58]. In these patients, corticosteroids in combination with supportive care are recommended.
Among the different glucocorticoids, the target-release formulation of budesonide should be considered. Budesonide is a drug that is released in the distal ileum and acts on IgA1 released from Peyer patches. Budesonide acts on IgA1 produced by the mucosa, which is the main source of undergalactosylated IgA1.
Budesonide has been studied in an randomized controlled trial (RCT) phase 3 trial (NefIgArd)[59]. A total of 199 patients were enrolled. At 9 months, a reduction in proteinuria and a reduction in the epidermal growth factor receptor (EGFR) were observed. These results were confirmed by other controlled and uncontrolled studies[60-62]. Overall, the principal studies with corticosteroids are reported in Table 3.
| Ref. | Medication | Initial dose | Taper | Total exposure |
| Lv et al[63] | Methylprednisolone | 0.4 mg/kg orally once daily | Reduce daily dose by 4 mg every month | 6 months |
| Lv et al[64] | Methylprednisolone | 0.6 to 0.8 mg/kg orally once daily | Reduce daily dose by 8 mg every month | 6 months |
| Manno et al[65] | Prednisone | 1 mg/kg orally per day | Reduce daily dose by 0.2 mg/kg every month | 6 months |
| Lv et al[66] | Prednisone | 0.8 to 1 mg/kg orally per day | Reduce daily dose by 5 to 10 mg every 2 weeks | 6 months |
| Pozzi et al[67] | Methylprednisolone | Methylprednisolone 1 g IV for 3 days, followed by prednisolone | None | 6 months |
| Rauen et al[68] | Prednisolone/prednisone | Prednisolone or prednisone 05 mg/kg orally ever other day | None | 6 months |
| Fellström et al[59] | TRF- budesonide | 16 mg orally daily | Reduce dose to 8 mg once daily for 2 weeks | 9 months |
In the largest RCT on glucocosteroids in IgA nephritis (TESTING)[64], 503 patients were enrolled. Proteinuria greater than 1 g /day was present in all patients, and oral methylprednisolone was given at dosage of 0.6/0.8 mg/kg/day. A reduction in proteinuria and a reduction in the loss of EGFR were observed, but there was an excess of infection. The study was repeated by the same authors with a reduced corticosteroid dose (0.4 mg/kg/daily)[63], obtaining similar results with a lower rate of infection. Another study by Manno et al[65] and Lv et al[66] reported good results with the use of prednisone in combination with ACE inhibitors. Pozzi et al[67] obtained similarly good results with a different strategy. The patients were given 1 g of IV methylprednisolone for 3 days at the beginning of months 1, 3 and 5, followed by 0.5 mg of prednisone orally every other day. Finally, Rauen et al[68] obtained very good results in the STOP-IgAN trial[68], who administered prednisolone or prednisone at the dose of 0.5 mg/kg orally every other day in addition to com
As B cells are responsible for producing Gd-IgA1, targeting B cells is considered an optimal treatment to improve renal outcomes in patients with IgAN. Rituximab, a chimeric monoclonal agent that targets CD20, was used in a controlled trial to control IgAN[69]. A study of 34 patients documented a lack of efficacy. Since then, several other drugs have been used to control IgAN. The majority are still in clinical development and are shown in Table 4.
| Agent | Phase | Registration number | Mechanism of action |
| Inhibition of endothelin A receptor and angiotensin II subtype 1 receptor inhibitor | |||
| Sparsentan | III | NCT03762850 | Endothelin A receptor and angiotensin II subtype 1 receptor inhibitor |
| Atrasentan | III | NCT04573478 | Endothelin A receptor antagonist |
| Plasma cell depletion | |||
| Felzartamab | II | NCT05065970 | Monoclonal IgG1 antibody targeting CD38 |
| Bortezomib | NA | NCT05383547 | Proteasome inhibitor that depletes plasma cells |
| Inhibition of BAFF/APRIL signaling | |||
| Blisibimod | II/III | NCT02062684 | Monoclonal antibody against both soluble and membrane BAFF |
| Sibeprenlimab | NCT05248646 | Monoclonal IgG2k antibody targeting APRIL | |
| BION-1301 | I/II | NCT03945318 | Monoclonal IgG4 antibody targeting APRIL |
| Atacicept | IIb | NCT04716231 | BAFF/APRIL dual inhibitor |
| Telitacicept | II | NCT04905212 | BAFF/APRIL dual inhibitor |
| Povetacicept | I | NCT05034484 | BAFF/APRIL dual inhibitor |
| Zigakibart (BION-1301) | III | NCT05852938 | Monoclonal IgG4 antibody targeting APRIL |
| Inhibition of immune complex-activated complement activity | |||
| Avacopan | II | NCT02384317 | Anti-C5ab receptor antagonist |
| Ravulizumab | II | NCT04564339 | Long-acting C5-blocking antibody |
| Cemdisiran | II | NCT03841448 | Small interfering RNA-targeting C5 |
| APL-2 | II | NCT03453619 | Cyclic peptide inhibitor of C3 and C3b |
| Iptacopan | III | NCT04578834 | Small-molecule inhibitor of complement factor B |
| IONIS-B-LRx | II | NCT04014335 | Antisense inhibitor of complement factor B messenger ribonucleic acid |
| Narsoplimab | III | NCT03608033 | Human monoclonal antibody against MASP-2 |
We have previously described the beneficial effect of Sparsentan, which is among the no- immunosuppressive drugs, on IgAN[70]. Atresantan is a selective endothelin A receptor antagonist used in IgAN patients in the ALIGN trial[71] for the treatment of IgAN with deterioration of renal function. Felzartamab is a monoclonal IgG1 antibody that targets CD38 on plasma cells[72]. It is used in a phase II trial (NCT05065970) with decreasing renal function. Bortezomib, a proteasome inhibitor, depletes plasma cells and, in addition to multiple myeloma has been used for to reduce proteinuria in a pilot trial[73]. TNF ligand superfamily member 13 B (BAFF and APRIL) are important triggers of autoimmunity and are involved in several types of glomerulonephritis, included IgAN. These data indicate that alterations in the BAFF/APRIL system affect the capacity of the innate immune system to regulate B-cell activation. BAFF and type I interferons function together in different forms of glomerulonephritis pathogenesis in both Toll-like receptor-dependent and Toll-like receptor-independent manners.
BAFF and APRIL are type II transmembrane proteins located on myeloid cells, lymphocytes and epithelial cells. BAFF can be processed into a soluble cytokine after cleavage at a furin protease site. APRIL is soluble and has been cleaved intracellularly. Both BAFF and APRIL are responsible for B-cell maturation, proliferation and survival after binding to their receptors. BAFF receptors include B-cell maturation antigen (BCMA) and BAFF receptor. The APRIL receptors are BCMA and transmembrane activator and cyclophilin ligand interactor (TACI). Another receptor for APRIL is heparan sulfate proteoglycan, which is responsible for the homing of plasma cells to the bone marrow[74].
Both APRIL[75] and BAFF[76] are extremely elevated in IgAN patients with respect to controls. Most likely, according to Schrezenmeier et al[77], APRIL is more active than BAFF in IgAN. According to the already cited GWAS of Kiryluk et al[20], the gene TNFSF13 encodes APRIL as a susceptibility gene. According to the study of Zhai et al[75], there is a correlation between GdIgA1 and APRIL According to a study from Han et al[78], the serum and mucosal levels of APRIL correlate with the severity and prognosis of IgAN. APRIL, rather than BAFF, is involved in IgAN recurrence after transplantation. Indeed, a study from Martín-Penagos et al[79] reported that this increase in the expression of proliferation-inducing ligands precedes the recurrence of IgAN in kidney transplant recipients. Kim et al[80], have reported a pathogenic role of APRIL in murine IgAN. Therefore, Myette et al[81] reported that an antibody targeting APRIL is a safe and effective treatment for murine IgAN.
Recently, several drugs have been used to control BAFF and APRIL in human patients affected by IgAN. All these studies are to date in different phases of RCTs. Blisibimob is a monoclonal antibody against both soluble and membrane BAFF. In a phase II/III study (BRIGHT-SC study) (NCT020 62684)[82], Blisibimob reduced proteinuria in IgAN patients. Sibenprelimab is a monoclonal IgG2k antibody that targets APRIL. In a phase II trial conducted by Mathur et al[83] (NCT05248646), the 24-hour urinary protein-to-creatinine ratio decreased significantly, and the decrease was dose-dependent. Zigakibart (BION-1301) is a monoclonal IgG4 antibody that targets APRIL In a phase I/II, a multicenter trial investigating the safety, tolerability, pharmacokinetics and pharmacodynamics of BION-1301[84] (NCT03945318) was conducted in 40 healthy volunteers and adults with IgAN.
Recently, the same drug was the subject of a phase III RCT (NCT05852938) (BEYOND study)[85]. This study will document and confirm the beneficial effects on proteinuria, the EGFR and key safety measures in IgAN patients at risk of progressive loss of kidney function. Atacicept is a BAFF/APRIL dual inhibitor in a phase III study (ORIGIN 3) (NCT04716231)[86]. It is a soluble TACI immunoglobulin fusion protein. This study aimed to asses to check the reduction in Gd-IgA1 antibody levels and proteinuria in IgAN patients. Telitacicept, another BAFF/APRIL inhibitor (NCT049505212)[87], is in a phase II trial and, given to 44 patients affected by IgAN, is associated with a dose related reduction in IgA and proteinuria and a reduction in the EGFR. Povetacicept (ALPN-303) is an enhanced dual APRIL/BAFF antagonist in a phase I/II trial (NCT05732402). In a study conducted by Evans et al[88], multiple dose levels were evaluated in patients with IgAN, membranous nephropathy, lupus nephritis and ANCA vasculitis. Povatacicept has been shown to be effective in improving the clinical outcomes of patients with autoantibody-related autoimmune diseases.
Inhibitors of immune-complex-activated complement activity constitute the last group of agents. These agents operate on the fourth hit after immune complex deposition in the mesangium and consequent complement activation. Both the lectin pathway and alternative pathway are activated, and glomerular inflammation is generated by interleukin 6 and tumor growth factor beta 1[89]. Glomerular C3 deposition leads to a poor clinical outcome[90]. Several phase II/III studies to date are ongoing, targeting different phases of complement activation. Avacopan is an anti-C5ab receptor antagonist that has been proven to improve the protein-creatinine ratio[91]. Ravalizumab is a long-acting C5-blocking antibody. Its efficacy is under investigation by the SANCTUARY study, NCT04564339[92]. Cemdisaran is a small interfering RNA that targets C5. To date, this agent is in a phase 2, randomized, double-blind, placebo-controlled trial in adult patients with IgAN (NCT03841448)[93].
APL-2 is a cyclic peptide inhibitor of C3 and C3b. To date, a phase 2 study (NCT03453619)[94] was performed to evaluate the safety and biological activity in patients with IgAN, lupus nephritis membranous nephropathy and C3 glomerulopathy.
Similarly, Iptacopan is a small-molecule inhibitor of complement factor B. This agent reduces proteinuria in IgAN patients and will be further evaluated in a phase III APPLAUSE-IgAN trial (NCT04578834)[95]. IONIS-B-LRx is an antisense inhibitor of complement factor B messenger ribonucleic acid. In phase II, its effect on plasma factor B levels and serum AH50, and CH50 activity in patients with IgAN is being evaluated (NCT04014335)[96]. Narsoplimab is a human monoclonal antibody against mannan-associated lectin-binding serine protease-2 that inhibits the lectin pathway. In a phase II trial, Narsoplimab reduced proteinuria[97]. The safety and efficacy of the agent is currently being evaluated in the phase III ARTEMIS-IGAN trial (NCT03608033)[98].
In conclusion, the vast majority of these agents are still in different phases of randomized clinical trials. Obviously, we have selected the ongoing, enrolling and more promising trials. B-cell modulation with anti-APRIL or with the combination of anti-BLYS/BAFF and APRIL appears promising. Early signals suggest tolerable administration and limited adverse effects, with no significant concerns for infection to date. These agents seem to have potent, stable effects on the EGFR while maintaining a reduction in the Gd-IgA level, proteinuria and likely hematuria. We are waiting to see if phase III can identify differences between agents and classes.
Since a long time[99] the main limitation in evaluating the drug efficacy in IgAN has been the few controlled randomized studies. This fact is true also to date, also because of the new agents on trial. A revision of the main limitations and gaps of the drug used has been recently made by Caster et al[100]. According this study tonsillectomy may be used, but is limited by the geographic genes. The use of SGLT2 inhibitors is still awaiting the results of controlled studies, in particular the analysis of the empagliflozin study. Main problem with the use of the targeted release budesonide are that long-term outcomes are still pending and the high cost of the treatment. Studies on the use of corticosteroids are the STOP IgAN and the TESTING trial. The first has the limitation that the ongoing study did not document differences in the kidney outcomes between the two groups on study. The second documented a high incidence of adverse effects. The principal agent on study against B-Cell, is the anti-April. Concerning this agent we are still awaiting the phase 3 results, checking in particular the infections rate and the infusion reaction. Similarly, infections and infuse reactions concern the use of anti plasma-cell agents. Recently, several agents inhibiting complement have been proposed in the treatment of IgAN. The main knowledge gap is the evaluation of the relative contribution to the disease of the lectin and the alternative pathways and the importance of determining the dominant pathway driving IgAN progression[101]. In addition, all these studies are still in phase 2 or 3.
Following the still valid four hit hypotheses, new recent studies allowed finding new aspects in the pathogenesis and in the treatment of IgAN. Several GWAS studies found genes responsible for the dysregulation of mucosal immunity, gene responsible for the immune cell proliferation, genes responsible for the production of galactose-deficient IgA1 and genes responsible for the activation of complement. For the treatment is essential to associate the classic CKD treatment to the more specific immune treatment. Standard immunosuppressive therapy has poor utility, except for the corticosteroids treatment. Targeting B cells and plasma cells seems to be essential. Among these agents, those targeting BAFF and APRIL have the higher utility. These agents are still in phase II or III, but their results seem to be important in several nephropathies, included the IgAN. Other new agents, still in RCTs are the anti-complement agents, which acts principally in the fourth hit stage.
| 1. | Tan M, Li W, Zou G, Zhang C, Fang J. Clinicopathological features and outcomes of IgA nephropathy with hematuria and/or minimal proteinuria. Kidney Blood Press Res. 2015;40:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Al-Lawati AI, Reich HN. Is there a role for immunosuppression in immunoglobulin A nephropathy? Nephrol Dial Transplant. 2017;32:i30-i36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Barratt J, Feehally J, Smith AC. Pathogenesis of IgA nephropathy. Semin Nephrol. 2004;24:197-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Gharavi AG, Kiryluk K, Choi M, Li Y, Hou P, Xie J, Sanna-Cherchi S, Men CJ, Julian BA, Wyatt RJ, Novak J, He JC, Wang H, Lv J, Zhu L, Wang W, Wang Z, Yasuno K, Gunel M, Mane S, Umlauf S, Tikhonova I, Beerman I, Savoldi S, Magistroni R, Ghiggeri GM, Bodria M, Lugani F, Ravani P, Ponticelli C, Allegri L, Boscutti G, Frasca G, Amore A, Peruzzi L, Coppo R, Izzi C, Viola BF, Prati E, Salvadori M, Mignani R, Gesualdo L, Bertinetto F, Mesiano P, Amoroso A, Scolari F, Chen N, Zhang H, Lifton RP. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet. 2011;43:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 495] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 5. | Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest. 2009;119:1668-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 236] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 6. | Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 373] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 7. | Julian BA, Novak J. IgA nephropathy: an update. Curr Opin Nephrol Hypertens. 2004;13:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Mestecky J, Tomana M, Moldoveanu Z, Julian BA, Suzuki H, Matousovic K, Renfrow MB, Novak L, Wyatt RJ, Novak J. Role of aberrant glycosylation of IgA1 molecules in the pathogenesis of IgA nephropathy. Kidney Blood Press Res. 2008;31:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Glassock RJ. The pathogenesis of IgA nephropathy. Curr Opin Nephrol Hypertens. 2011;20:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 597] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 11. | Xu LL, Zhou XJ, Zhang H. An Update on the Genetics of IgA Nephropathy. J Clin Med. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 12. | Feehally J, Farrall M, Boland A, Gale DP, Gut I, Heath S, Kumar A, Peden JF, Maxwell PH, Morris DL, Padmanabhan S, Vyse TJ, Zawadzka A, Rees AJ, Lathrop M, Ratcliffe PJ. HLA has strongest association with IgA nephropathy in genome-wide analysis. J Am Soc Nephrol. 2010;21:1791-1797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 210] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 13. | Yu XQ, Li M, Zhang H, Low HQ, Wei X, Wang JQ, Sun LD, Sim KS, Li Y, Foo JN, Wang W, Li ZJ, Yin XY, Tang XQ, Fan L, Chen J, Li RS, Wan JX, Liu ZS, Lou TQ, Zhu L, Huang XJ, Zhang XJ, Liu ZH, Liu JJ. A genome-wide association study in Han Chinese identifies multiple susceptibility loci for IgA nephropathy. Nat Genet. 2011;44:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 14. | Kiryluk K, Li Y, Scolari F, Sanna-Cherchi S, Choi M, Verbitsky M, Fasel D, Lata S, Prakash S, Shapiro S, Fischman C, Snyder HJ, Appel G, Izzi C, Viola BF, Dallera N, Del Vecchio L, Barlassina C, Salvi E, Bertinetto FE, Amoroso A, Savoldi S, Rocchietti M, Amore A, Peruzzi L, Coppo R, Salvadori M, Ravani P, Magistroni R, Ghiggeri GM, Caridi G, Bodria M, Lugani F, Allegri L, Delsante M, Maiorana M, Magnano A, Frasca G, Boer E, Boscutti G, Ponticelli C, Mignani R, Marcantoni C, Di Landro D, Santoro D, Pani A, Polci R, Feriozzi S, Chicca S, Galliani M, Gigante M, Gesualdo L, Zamboli P, Battaglia GG, Garozzo M, Maixnerová D, Tesar V, Eitner F, Rauen T, Floege J, Kovacs T, Nagy J, Mucha K, Pączek L, Zaniew M, Mizerska-Wasiak M, Roszkowska-Blaim M, Pawlaczyk K, Gale D, Barratt J, Thibaudin L, Berthoux F, Canaud G, Boland A, Metzger M, Panzer U, Suzuki H, Goto S, Narita I, Caliskan Y, Xie J, Hou P, Chen N, Zhang H, Wyatt RJ, Novak J, Julian BA, Feehally J, Stengel B, Cusi D, Lifton RP, Gharavi AG. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 511] [Cited by in RCA: 472] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 15. | Li M, Foo JN, Wang JQ, Low HQ, Tang XQ, Toh KY, Yin PR, Khor CC, Goh YF, Irwan ID, Xu RC, Andiappan AK, Bei JX, Rotzschke O, Chen MH, Cheng CY, Sun LD, Jiang GR, Wong TY, Lin HL, Aung T, Liao YH, Saw SM, Ye K, Ebstein RP, Chen QK, Shi W, Chew SH, Chen J, Zhang FR, Li SP, Xu G, Shyong Tai E, Wang L, Chen N, Zhang XJ, Zeng YX, Zhang H, Liu ZH, Yu XQ, Liu JJ. Identification of new susceptibility loci for IgA nephropathy in Han Chinese. Nat Commun. 2015;6:7270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Jeong KH, Kim JS, Lee YH, Kim YG, Moon JY, Kim SK, Kang SW, Kim TH, Lee SH, Kim YH; Representing the KNOW-CKD Study Group. Genome-wide association study identifies new susceptible loci of IgA nephropathy in Koreans. BMC Med Genomics. 2019;12:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Li M, Wang L, Shi DC, Foo JN, Zhong Z, Khor CC, Lanzani C, Citterio L, Salvi E, Yin PR, Bei JX, Wang L, Liao YH, Chen J, Chen QK, Xu G, Jiang GR, Wan JX, Chen MH, Chen N, Zhang H, Zeng YX, Liu ZH, Liu JJ, Yu XQ. Genome-Wide Meta-Analysis Identifies Three Novel Susceptibility Loci and Reveals Ethnic Heterogeneity of Genetic Susceptibility for IgA Nephropathy. J Am Soc Nephrol. 2020;31:2949-2963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 18. | Zhou XJ, Tsoi LC, Hu Y, Patrick MT, He K, Berthier CC, Li Y, Wang YN, Qi YY, Zhang YM, Gan T, Li Y, Hou P, Liu LJ, Shi SF, Lv JC, Xu HJ, Zhang H. Exome Chip Analyses and Genetic Risk for IgA Nephropathy among Han Chinese. Clin J Am Soc Nephrol. 2021;16:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Li M, Wang YN, Wang L, Meah WY, Shi DC, Heng KK, Wang L, Khor CC, Bei JX, Cheng CY, Aung T, Liao YH, Chen QK, Gu JR, Kong YZ, Lee J, Chong SA, Subramaniam M, Foo JN, Cai FT, Jiang GR, Xu G, Wan JX, Chen MH, Yin PR, Dong XQ, Feng SZ, Tang XQ, Zhong Z, Tan EK, Chen N, Zhang H, Liu ZH, Tai ES, Liu JJ, Yu XQ. Genome-Wide Association Analysis of Protein-Coding Variants in IgA Nephropathy. J Am Soc Nephrol. 2023;34:1900-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Kiryluk K, Sanchez-Rodriguez E, Zhou XJ, Zanoni F, Liu L, Mladkova N, Khan A, Marasa M, Zhang JY, Balderes O, Sanna-Cherchi S, Bomback AS, Canetta PA, Appel GB, Radhakrishnan J, Trimarchi H, Sprangers B, Cattran DC, Reich H, Pei Y, Ravani P, Galesic K, Maixnerova D, Tesar V, Stengel B, Metzger M, Canaud G, Maillard N, Berthoux F, Berthelot L, Pillebout E, Monteiro R, Nelson R, Wyatt RJ, Smoyer W, Mahan J, Samhar AA, Hidalgo G, Quiroga A, Weng P, Sreedharan R, Selewski D, Davis K, Kallash M, Vasylyeva TL, Rheault M, Chishti A, Ranch D, Wenderfer SE, Samsonov D, Claes DJ, Akchurin O, Goumenos D, Stangou M, Nagy J, Kovacs T, Fiaccadori E, Amoroso A, Barlassina C, Cusi D, Del Vecchio L, Battaglia GG, Bodria M, Boer E, Bono L, Boscutti G, Caridi G, Lugani F, Ghiggeri G, Coppo R, Peruzzi L, Esposito V, Esposito C, Feriozzi S, Polci R, Frasca G, Galliani M, Garozzo M, Mitrotti A, Gesualdo L, Granata S, Zaza G, Londrino F, Magistroni R, Pisani I, Magnano A, Marcantoni C, Messa P, Mignani R, Pani A, Ponticelli C, Roccatello D, Salvadori M, Salvi E, Santoro D, Gembillo G, Savoldi S, Spotti D, Zamboli P, Izzi C, Alberici F, Delbarba E, Florczak M, Krata N, Mucha K, Pączek L, Niemczyk S, Moszczuk B, Pańczyk-Tomaszewska M, Mizerska-Wasiak M, Perkowska-Ptasińska A, Bączkowska T, Durlik M, Pawlaczyk K, Sikora P, Zaniew M, Kaminska D, Krajewska M, Kuzmiuk-Glembin I, Heleniak Z, Bullo-Piontecka B, Liberek T, Dębska-Slizien A, Hryszko T, Materna-Kiryluk A, Miklaszewska M, Szczepańska M, Dyga K, Machura E, Siniewicz-Luzeńczyk K, Pawlak-Bratkowska M, Tkaczyk M, Runowski D, Kwella N, Drożdż D, Habura I, Kronenberg F, Prikhodina L, van Heel D, Fontaine B, Cotsapas C, Wijmenga C, Franke A, Annese V, Gregersen PK, Parameswaran S, Weirauch M, Kottyan L, Harley JB, Suzuki H, Narita I, Goto S, Lee H, Kim DK, Kim YS, Park JH, Cho B, Choi M, Van Wijk A, Huerta A, Ars E, Ballarin J, Lundberg S, Vogt B, Mani LY, Caliskan Y, Barratt J, Abeygunaratne T, Kalra PA, Gale DP, Panzer U, Rauen T, Floege J, Schlosser P, Ekici AB, Eckardt KU, Chen N, Xie J, Lifton RP, Loos RJF, Kenny EE, Ionita-Laza I, Köttgen A, Julian BA, Novak J, Scolari F, Zhang H, Gharavi AG. Genome-wide association analyses define pathogenic signaling pathways and prioritize drug targets for IgA nephropathy. Nat Genet. 2023;55:1091-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 21. | Gale DP, Molyneux K, Wimbury D, Higgins P, Levine AP, Caplin B, Ferlin A, Yin P, Nelson CP, Stanescu H, Samani NJ, Kleta R, Yu X, Barratt J. Galactosylation of IgA1 Is Associated with Common Variation in C1GALT1. J Am Soc Nephrol. 2017;28:2158-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 22. | Kiryluk K, Li Y, Moldoveanu Z, Suzuki H, Reily C, Hou P, Xie J, Mladkova N, Prakash S, Fischman C, Shapiro S, LeDesma RA, Bradbury D, Ionita-Laza I, Eitner F, Rauen T, Maillard N, Berthoux F, Floege J, Chen N, Zhang H, Scolari F, Wyatt RJ, Julian BA, Gharavi AG, Novak J. GWAS for serum galactose-deficient IgA1 implicates critical genes of the O-glycosylation pathway. PLoS Genet. 2017;13:e1006609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 23. | Wang YN, Zhou XJ, Chen P, Yu GZ, Zhang X, Hou P, Liu LJ, Shi SF, Lv JC, Zhang H. Interaction between GALNT12 and C1GALT1 Associates with Galactose-Deficient IgA1 and IgA Nephropathy. J Am Soc Nephrol. 2021;32:545-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Berthoux F, Suzuki H, Thibaudin L, Yanagawa H, Maillard N, Mariat C, Tomino Y, Julian BA, Novak J. Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol. 2012;23:1579-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 25. | Chen P, Yu G, Zhang X, Xie X, Wang J, Shi S, Liu L, Lv J, Zhang H. Plasma Galactose-Deficient IgA1 and C3 and CKD Progression in IgA Nephropathy. Clin J Am Soc Nephrol. 2019;14:1458-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 26. | Zhao N, Hou P, Lv J, Moldoveanu Z, Li Y, Kiryluk K, Gharavi AG, Novak J, Zhang H. The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int. 2012;82:790-796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 27. | Zhao YF, Zhu L, Liu LJ, Shi SF, Lv JC, Zhang H. Pathogenic role of glycan-specific IgG antibodies in IgA nephropathy. BMC Nephrol. 2017;18:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Yeo SC, Cheung CK, Barratt J. New insights into the pathogenesis of IgA nephropathy. Pediatr Nephrol. 2018;33:763-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 29. | Liu XZ, Zhang YM, Jia NY, Zhang H. Helicobacter pylori infection is associated with elevated galactose-deficient IgA1 in IgA nephropathy. Ren Fail. 2020;42:539-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Soylu A, Berktaş S, Sarioğlu S, Erbil G, Yilmaz O, Demir BK, Tufan Y, Yeşilirmak D, Türkmen M, Kavukçu S. Saccharomyces boulardii prevents oral-poliovirus vaccine-induced IgA nephropathy in mice. Pediatr Nephrol. 2008;23:1287-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Miura N, Imai H, Kikuchi S, Hayashi S, Endoh M, Kawamura T, Tomino Y, Moriwaki K, Kiyomoto H, Kohagura K, Nakazawa E, Kusano E, Mochizuki T, Nomura S, Sasaki T, Kashihara N, Soma J, Tomo T, Nakabayashi I, Yoshida M, Watanabe T. Tonsillectomy and steroid pulse (TSP) therapy for patients with IgA nephropathy: a nationwide survey of TSP therapy in Japan and an analysis of the predictive factors for resistance to TSP therapy. Clin Exp Nephrol. 2009;13:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Rollino C, Vischini G, Coppo R. IgA nephropathy and infections. J Nephrol. 2016;29:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 33. | Gleeson PJ, Benech N, Chemouny J, Metallinou E, Berthelot L, da Silva J, Bex-Coudrat J, Boedec E, Canesi F, Bounaix C, Morelle W, Moya-Nilges M, Kenny J, O'Mahony L, Saveanu L, Arnulf B, Sannier A, Daugas E, Vrtovsnik F, Lepage P, Sokol H, Monteiro RC. The gut microbiota posttranslationally modifies IgA1 in autoimmune glomerulonephritis. Sci Transl Med. 2024;16:eadl6149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 34. | He JW, Zhou XJ, Hou P, Wang YN, Gan T, Li Y, Liu Y, Liu LJ, Shi SF, Zhu L, Lv JC, Zhang H. Potential Roles of Oral Microbiota in the Pathogenesis of Immunoglobin A Nephropathy. Front Cell Infect Microbiol. 2021;11:652837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Currie EG, Coburn B, Porfilio EA, Lam P, Rojas OL, Novak J, Yang S, Chowdhury RB, Ward LA, Wang PW, Khaleghi K, An J, Crome SQ, Hladunewich MA, Barbour SJ, Cattran DC, Parekh RS, Licht C, John R, Kaul R, Croitoru K, Gray-Owen SD, Guttman DS, Gommerman JL, Reich HN. Immunoglobulin A nephropathy is characterized by anticommensal humoral immune responses. JCI Insight. 2022;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 36. | Wang J, Wang X, Cai X, Pan D. Global trends and hotspots in IgA nephropathy: a bibliometric analysis and knowledge map visualization from 2012 to 2023. Int Urol Nephrol. 2023;55:3197-3207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 37. | Zhu Y, He H, Sun W, Wu J, Xiao Y, Peng Y, Hu P, Jin M, Liu P, Zhang D, Xie T, Huang L, He W, Wei M, Wang L, Xu X, Tang Y. IgA nephropathy: gut microbiome regulates the production of hypoglycosilated IgA1 via the TLR4 signaling pathway. Nephrol Dial Transplant. 2024;39:1624-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 38. | Cai F, Zhou C, Jiao N, Liang X, Ye Z, Chen W, Yang Q, Peng H, Tang Y, Niu C, Zhao G, Wang Z, Zhang G, Yu X. Systematic Microbiome Dysbiosis Is Associated with IgA Nephropathy. Microbiol Spectr. 2023;11:e0520222. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Muto M, Manfroi B, Suzuki H, Joh K, Nagai M, Wakai S, Righini C, Maiguma M, Izui S, Tomino Y, Huard B, Suzuki Y. Toll-Like Receptor 9 Stimulation Induces Aberrant Expression of a Proliferation-Inducing Ligand by Tonsillar Germinal Center B Cells in IgA Nephropathy. J Am Soc Nephrol. 2017;28:1227-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 40. | Wang YN, Gan T, Qu S, Xu LL, Hu Y, Liu LJ, Shi SF, Lv JC, Tsoi LC, Patrick MT, He K, Berthier CC, Xu HJ, Zhou XJ, Zhang H. MTMR3 risk alleles enhance Toll Like Receptor 9-induced IgA immunity in IgA nephropathy. Kidney Int. 2023;104:562-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Suzuki Y, Tomino Y. The mucosa-bone-marrow axis in IgA nephropathy. Contrib Nephrol. 2007;157:70-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Lai KN, Tang SC, Schena FP, Novak J, Tomino Y, Fogo AB, Glassock RJ. IgA nephropathy. Nat Rev Dis Primers. 2016;2:16001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 375] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 43. | Xie X, Liu Y, Perkovic V, Li X, Ninomiya T, Hou W, Zhao N, Liu L, Lv J, Zhang H, Wang H. Renin-Angiotensin System Inhibitors and Kidney and Cardiovascular Outcomes in Patients With CKD: A Bayesian Network Meta-analysis of Randomized Clinical Trials. Am J Kidney Dis. 2016;67:728-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 305] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 44. | Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, Cook HT, Fervenza FC, Gibson KL, Glassock RJ, Jayne DRW, Jha V, Liew A, Liu ZH, Mejía-Vilet JM, Nester CM, Radhakrishnan J, Rave EM, Reich HN, Ronco P, Sanders JF, Sethi S, Suzuki Y, Tang SCW, Tesar V, Vivarelli M, Wetzels JFM, Lytvyn L, Craig JC, Tunnicliffe DJ, Howell M, Tonelli MA, Cheung M, Earley A, Floege J. Executive summary of the KDIGO 2021 Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100:753-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 464] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 45. | Praga M, Gutiérrez E, González E, Morales E, Hernández E. Treatment of IgA nephropathy with ACE inhibitors: a randomized and controlled trial. J Am Soc Nephrol. 2003;14:1578-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 223] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 46. | Barbour SJ, Canney M, Coppo R, Zhang H, Liu ZH, Suzuki Y, Matsuzaki K, Katafuchi R, Induruwage D, Er L, Reich HN, Feehally J, Barratt J, Cattran DC; International IgA Nephropathy Network. Improving treatment decisions using personalized risk assessment from the International IgA Nephropathy Prediction Tool. Kidney Int. 2020;98:1009-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 47. | Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, Scolari F, Schena FP, Remuzzi G. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999;354:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 590] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 48. | Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation. 2016;134:752-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 966] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 49. | Wheeler DC, Toto RD, Stefánsson BV, Jongs N, Chertow GM, Greene T, Hou FF, McMurray JJV, Pecoits-Filho R, Correa-Rotter R, Rossing P, Sjöström CD, Umanath K, Langkilde AM, Heerspink HJL; DAPA-CKD Trial Committees and Investigators. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021;100:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 251] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 50. | Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383:1436-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1586] [Cited by in RCA: 3142] [Article Influence: 628.4] [Reference Citation Analysis (1)] |
| 51. | Herrington WG, Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson JR, Preiss D, Judge P, Mayne KJ, Ng SYA, Sammons E, Zhu D, Hill M, Stevens W, Wallendszus K, Brenner S, Cheung AK, Liu ZH, Li J, Hooi LS, Liu W, Kadowaki T, Nangaku M, Levin A, Cherney D, Maggioni AP, Pontremoli R, Deo R, Goto S, Rossello X, Tuttle KR, Steubl D, Petrini M, Massey D, Eilbracht J, Brueckmann M, Landray MJ, Baigent C, Haynes R; The EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388:117-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1116] [Cited by in RCA: 1237] [Article Influence: 618.5] [Reference Citation Analysis (2)] |
| 52. | Salamah HM, Marey A, Abugdida M, Abualkhair KA, Elshenawy S, Elhassan WAF, Naguib MM, Malnev D, Durrani J, Bailey R, Tsyunchyk A, Ibrahim L, Zavgorodneva Z, Sherazi A. Efficacy and safety of glucagon-like peptide-1 receptor agonists on prediabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetol Metab Syndr. 2024;16:129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 53. | Quiroga B, Ortiz A, Núñez S, Kislikova M, González Sanchidrián S, Broseta JJ, Albines ZS, Escamilla Cabrera B, Rivero Viera Y, Rodriguez Santarelli D, Salanova Villanueva L, Lopez Rodriguez F, Cancho Castellano B, Ibáñez Cerezon M, Gutierrez Rivas CP, Aresté N, Campos Gutiérrez B, Ródenas Gálvez A, Glucksmann Pizá MC, Balda Manzanos S, Soldevila A, Rodríguez Gayo L, Moral Berrio E, Ortega Diaz M, Beltrán Catalán S, Puente García A, Ángel Rojas M, Sosa Barrios RH, Santana Zapatero H, Rangel Hidalgo G, Martinez Canet AM, Díez J; Cardiorenal Medicine Group (CaReSEN) of the Spanish Society of Nephrology. Treatment of Chronic Heart Failure in Advanced Chronic Kidney Disease: The HAKA Multicenter Retrospective Real-World Study. Cardiorenal Med. 2024;14:202-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 54. | Reily C, Moldoveanu Z, Pramparo T, Hall S, Huang ZQ, Rice T, Novak L, Komers R, Jenkinson CP, Novak J. Sparsentan ameliorates glomerular hypercellularity and inflammatory-gene networks induced by IgA1-IgG immune complexes in a mouse model of IgA nephropathy. Am J Physiol Renal Physiol. 2024;326:F862-F875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 55. | Natale P, Palmer SC, Ruospo M, Saglimbene VM, Craig JC, Vecchio M, Samuels JA, Molony DA, Schena FP, Strippoli GF. Immunosuppressive agents for treating IgA nephropathy. Cochrane Database Syst Rev. 2020;3:CD003965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 56. | Maes BD, Oyen R, Claes K, Evenepoel P, Kuypers D, Vanwalleghem J, Van Damme B, Vanrenterghem YF. Mycophenolate mofetil in IgA nephropathy: results of a 3-year prospective placebo-controlled randomized study. Kidney Int. 2004;65:1842-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 170] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 57. | Frisch G, Lin J, Rosenstock J, Markowitz G, D'Agati V, Radhakrishnan J, Preddie D, Crew J, Valeri A, Appel G. Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: a double-blind randomized controlled trial. Nephrol Dial Transplant. 2005;20:2139-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 58. | Hou FF, Xie D, Wang J, Xu X, Yang X, Ai J, Nie S, Liang M, Wang G, Jia N; MAIN Trial Investigators. Effectiveness of Mycophenolate Mofetil Among Patients With Progressive IgA Nephropathy: A Randomized Clinical Trial. JAMA Netw Open. 2023;6:e2254054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 59. | Fellström BC, Barratt J, Cook H, Coppo R, Feehally J, de Fijter JW, Floege J, Hetzel G, Jardine AG, Locatelli F, Maes BD, Mercer A, Ortiz F, Praga M, Sørensen SS, Tesar V, Del Vecchio L; NEFIGAN Trial Investigators. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phase 2b trial. Lancet. 2017;389:2117-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 269] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 60. | Barratt J, Lafayette R, Kristensen J, Stone A, Cattran D, Floege J, Tesar V, Trimarchi H, Zhang H, Eren N, Paliege A, Rovin BH; NefIgArd Trial Investigators. Results from part A of the multi-center, double-blind, randomized, placebo-controlled NefIgArd trial, which evaluated targeted-release formulation of budesonide for the treatment of primary immunoglobulin A nephropathy. Kidney Int. 2023;103:391-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 131] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 61. | Lopez-Martinez M, Torres I, Bermejo S, Moreso F, Garcia-Carro C, Vergara A, Ramos N, Perello M, Gabaldon A, Azancot MA, Bolufer M, Toapanta N, Bestard O, Agraz-Pamplona I, Soler MJ. Enteric Budesonide in Transplant and Native IgA Nephropathy: Real-World Clinical Practice. Transpl Int. 2022;35:10693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 62. | Ismail G, Obrişcă B, Jurubiţă R, Andronesi A, Sorohan B, Vornicu A, Sinescu I, Hârza M. Budesonide versus systemic corticosteroids in IgA Nephropathy: A retrospective, propensity-matched comparison. Medicine (Baltimore). 2020;99:e21000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 63. | Lv J, Zhang H, Wong MG, Jardine MJ, Hladunewich M, Jha V, Monaghan H, Zhao M, Barbour S, Reich H, Cattran D, Glassock R, Levin A, Wheeler D, Woodward M, Billot L, Chan TM, Liu ZH, Johnson DW, Cass A, Feehally J, Floege J, Remuzzi G, Wu Y, Agarwal R, Wang HY, Perkovic V; TESTING Study Group. Effect of Oral Methylprednisolone on Clinical Outcomes in Patients With IgA Nephropathy: The TESTING Randomized Clinical Trial. JAMA. 2017;318:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 372] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 64. | Lv J, Wong MG, Hladunewich MA, Jha V, Hooi LS, Monaghan H, Zhao M, Barbour S, Jardine MJ, Reich HN, Cattran D, Glassock R, Levin A, Wheeler DC, Woodward M, Billot L, Stepien S, Rogers K, Chan TM, Liu ZH, Johnson DW, Cass A, Feehally J, Floege J, Remuzzi G, Wu Y, Agarwal R, Zhang H, Perkovic V; TESTING Study Group. Effect of Oral Methylprednisolone on Decline in Kidney Function or Kidney Failure in Patients With IgA Nephropathy: The TESTING Randomized Clinical Trial. JAMA. 2022;327:1888-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 183] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 65. | Manno C, Torres DD, Rossini M, Pesce F, Schena FP. Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant. 2009;24:3694-3701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 66. | Lv J, Zhang H, Chen Y, Li G, Jiang L, Singh AK, Wang H. Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: a randomized controlled trial. Am J Kidney Dis. 2009;53:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 165] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 67. | Pozzi C, Bolasco PG, Fogazzi GB, Andrulli S, Altieri P, Ponticelli C, Locatelli F. Corticosteroids in IgA nephropathy: a randomised controlled trial. Lancet. 1999;353:883-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 350] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 68. | Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, Panzer U, Peters H, Benck U, Mertens PR, Kuhlmann U, Witzke O, Gross O, Vielhauer V, Mann JF, Hilgers RD, Floege J; STOP-IgAN Investigators. Intensive Supportive Care plus Immunosuppression in IgA Nephropathy. N Engl J Med. 2015;373:2225-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 499] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 69. | Lafayette RA, Canetta PA, Rovin BH, Appel GB, Novak J, Nath KA, Sethi S, Tumlin JA, Mehta K, Hogan M, Erickson S, Julian BA, Leung N, Enders FT, Brown R, Knoppova B, Hall S, Fervenza FC. A Randomized, Controlled Trial of Rituximab in IgA Nephropathy with Proteinuria and Renal Dysfunction. J Am Soc Nephrol. 2017;28:1306-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 191] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 70. | Hunter-Dickson M, Wong MG. The role of endothelin receptor antagonists in IgA nephropathy. Nephrology (Carlton). 2024;29 Suppl 2:30-33. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 71. | Turner CG, Hayat MJ, Otis JS, Quyyumi AA, Wong BJ. The effect of endothelin a receptor inhibition and biological sex on cutaneous microvascular function in non-Hispanic Black and White young adults. Physiol Rep. 2024;12:e16149. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 72. | Maixnerova D, Tesar V. Emerging role of monoclonal antibodies in the treatment of IgA nephropathy. Expert Opin Biol Ther. 2023;23:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 73. | Hartono C, Chung M, Perlman AS, Chevalier JM, Serur D, Seshan SV, Muthukumar T. Bortezomib for Reduction of Proteinuria in IgA Nephropathy. Kidney Int Rep. 2018;3:861-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 74. | Vincent FB, Morand EF, Schneider P, Mackay F. The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol. 2014;10:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 315] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 75. | Zhai YL, Zhu L, Shi SF, Liu LJ, Lv JC, Zhang H. Increased APRIL Expression Induces IgA1 Aberrant Glycosylation in IgA Nephropathy. Medicine (Baltimore). 2016;95:e3099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 76. | Xin G, Shi W, Xu LX, Su Y, Yan LJ, Li KS. Serum BAFF is elevated in patients with IgA nephropathy and associated with clinical and histopathological features. J Nephrol. 2013;26:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 77. | Schrezenmeier E, Jayne D, Dörner T. Targeting B Cells and Plasma Cells in Glomerular Diseases: Translational Perspectives. J Am Soc Nephrol. 2018;29:741-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 78. | Han SS, Yang SH, Choi M, Kim HR, Kim K, Lee S, Moon KC, Kim JY, Lee H, Lee JP, Jung JY, Kim S, Joo KW, Lim CS, Kang SW, Kim YS, Kim DK. The Role of TNF Superfamily Member 13 in the Progression of IgA Nephropathy. J Am Soc Nephrol. 2016;27:3430-3439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 79. | Martín-Penagos L, Benito-Hernández A, San Segundo D, Sango C, Azueta A, Gómez-Román J, Fernández-Fresnedo G, López-Hoyos M, Ruiz JC, Rodrigo E. A proliferation-inducing ligand increase precedes IgA nephropathy recurrence in kidney transplant recipients. Clin Transplant. 2019;33:e13502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 80. | Kim YG, Alvarez M, Suzuki H, Hirose S, Izui S, Tomino Y, Huard B, Suzuki Y. Pathogenic Role of a Proliferation-Inducing Ligand (APRIL) in Murine IgA Nephropathy. PLoS One. 2015;10:e0137044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 81. | Myette JR, Kano T, Suzuki H, Sloan SE, Szretter KJ, Ramakrishnan B, Adari H, Deotale KD, Engler F, Shriver Z, Wollacott AM, Suzuki Y, Pereira BJG. A Proliferation Inducing Ligand (APRIL) targeted antibody is a safe and effective treatment of murine IgA nephropathy. Kidney Int. 2019;96:104-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 82. | Williams CEC, Lamond M, Marro J, Chetwynd AJ, Oni L. A narrative review of potential drug treatments for nephritis in children with IgA vasculitis (HSP). Clin Rheumatol. 2023;42:3189-3200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 83. | Mathur M, Barratt J, Chacko B, Chan TM, Kooienga L, Oh KH, Sahay M, Suzuki Y, Wong MG, Yarbrough J, Xia J, Pereira BJG; ENVISION Trial Investigators Group. A Phase 2 Trial of Sibeprenlimab in Patients with IgA Nephropathy. N Engl J Med. 2024;390:20-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 88] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 84. | Yeo SC, Barratt J. The contribution of a proliferation-inducing ligand (APRIL) and other TNF superfamily members in pathogenesis and progression of IgA nephropathy. Clin Kidney J. 2023;16:ii9-ii18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 85. | Trimarchi H, Barrat J, Radhakrishnan J, Khawaja Z, Cambell K, Perkovic V. WCN-1837 BEYOND: a phase 3, randomized, double-blind, placebocontrolled trial of Zigakibart in adults with IgA nephropathy. Kidney Int Rep. 2024;9:S177. |
| 86. | Lafayette R, Barbour S, Israni R, Wei X, Eren N, Floege J, Jha V, Kim SG, Maes B, Phoon RKS, Singh H, Tesař V, Lin CJF, Barratt J. A phase 2b, randomized, double-blind, placebo-controlled, clinical trial of atacicept for treatment of IgA nephropathy. Kidney Int. 2024;105:1306-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 41] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 87. | Lv J, Liu L, Hao C, Li G, Fu P, Xing G, Zheng H, Chen N, Wang C, Luo P, Xie D, Zuo L, Li R, Mao Y, Dong S, Zhang P, Zheng H, Wang Y, Qin W, Wang W, Li L, Jiao W, Fang J, Zhang H. Randomized Phase 2 Trial of Telitacicept in Patients With IgA Nephropathy With Persistent Proteinuria. Kidney Int Rep. 2023;8:499-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 85] [Reference Citation Analysis (0)] |
| 88. | Evans LS, Lewis KE, DeMonte D, Bhandari JG, Garrett LB, Kuijper JL, Ardourel D, Wolfson MF, Debrot S, Mudri S, Kleist K, Griffin LL, Hebb L, Sanderson RJ, Wang N, Seaberg M, Chunyk AG, Yang J, Hong Y, Maria Z, Messenheimer DJ, Holland PM, Peng SL, Rixon MW, Dillon SR. Povetacicept, an Enhanced Dual APRIL/BAFF Antagonist That Modulates B Lymphocytes and Pathogenic Autoantibodies for the Treatment of Lupus and Other B Cell-Related Autoimmune Diseases. Arthritis Rheumatol. 2023;75:1187-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 89. | Maillard N, Wyatt RJ, Julian BA, Kiryluk K, Gharavi A, Fremeaux-Bacchi V, Novak J. Current Understanding of the Role of Complement in IgA Nephropathy. J Am Soc Nephrol. 2015;26:1503-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 247] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 90. | Xie M, Zhu Y, Wang X, Ren J, Guo H, Huang B, Wang S, Wang P, Liu Y, Liu Y, Zhang J. Predictive prognostic value of glomerular C3 deposition in IgA nephropathy. J Nephrol. 2023;36:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 91. | Bruchfeld A, Magin H, Nachman P, Parikh S, Lafayette R, Potarca A, Miao S, Bekker P. C5a receptor inhibitor avacopan in immunoglobulin A nephropathy-an open-label pilot study. Clin Kidney J. 2022;15:922-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 92. | Bartoli G, Dello Strologo A, Grandaliano G, Pesce F. Updates on C3 Glomerulopathy in Kidney Transplantation: Pathogenesis and Treatment Options. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 93. | Rizk DV, Maillard N, Julian BA, Knoppova B, Green TJ, Novak J, Wyatt RJ. The Emerging Role of Complement Proteins as a Target for Therapy of IgA Nephropathy. Front Immunol. 2019;10:504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 94. | Fu DJ, Bagga P, Naik G, Glinton S, Faes L, Liefers B, Lima R, Wignall G, Keane PA, Ioannidou E, Ribeiro Reis AP, McKeown A, Scheibler L, Patel PJ, Moghul I, Pontikos N, Balaskas K. Pegcetacoplan Treatment and Consensus Features of Geographic Atrophy Over 24 Months. JAMA Ophthalmol. 2024;142:548-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 95. | Rizk DV, Rovin BH, Zhang H, Kashihara N, Maes B, Trimarchi H, Perkovic V, Meier M, Kollins D, Papachristofi O, Charney A, Barratt J. Targeting the Alternative Complement Pathway With Iptacopan to Treat IgA Nephropathy: Design and Rationale of the APPLAUSE-IgAN Study. Kidney Int Rep. 2023;8:968-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 96. | Caravaca-Fontán F, Gutiérrez E, Sevillano ÁM, Praga M. Targeting complement in IgA nephropathy. Clin Kidney J. 2023;16:ii28-ii39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 97. | Lafayette RA, Rovin BH, Reich HN, Tumlin JA, Floege J, Barratt J. Safety, Tolerability and Efficacy of Narsoplimab, a Novel MASP-2 Inhibitor for the Treatment of IgA Nephropathy. Kidney Int Rep. 2020;5:2032-2041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 98. | Selvaskandan H, Kay Cheung C, Dormer J, Wimbury D, Martinez M, Xu G, Barratt J. Inhibition of the Lectin Pathway of the Complement System as a Novel Approach in the Management of IgA Vasculitis-Associated Nephritis. Nephron. 2020;144:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 99. | Laville M, Alamartine E. Treatment options for IgA nephropathy in adults: a proposal for evidence-based strategy. Nephrol Dial Transplant. 2004;19:1947-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 100. | Caster DJ, Lafayette RA. The Treatment of Primary IgA Nephropathy: Change, Change, Change. Am J Kidney Dis. 2024;83:229-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 101. | Tesař V, Radhakrishnan J, Charu V, Barratt J. Challenges in IgA Nephropathy Management: An Era of Complement Inhibition. Kidney Int Rep. 2023;8:1730-1740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |