Published online Dec 25, 2023. doi: 10.5527/wjn.v12.i5.182
Peer-review started: May 23, 2023
First decision: August 16, 2023
Revised: September 1, 2023
Accepted: September 25, 2023
Article in press: September 25, 2023
Published online: December 25, 2023
Processing time: 213 Days and 4.4 Hours
Gliflozins or Sodium glucose cotransporter 2 inhibitors (SGLT2i) are relatively novel antidiabetic medications that have recently been shown to represent favorable effects on patients’ cardiorenal outcomes. However, there is shortage of data on potential disparities in this therapeutic effect across different patient subpopulations.
To investigate differential effects of SGLT2i on the cardiorenal outcomes of heart failure patients across left ventricular ejection fraction (LVEF) levels.
Literature was searched systematically for the large randomized double-blind controlled trials with long enough follow up periods reporting cardiovascular and renal outcomes in their patients regarding heart failure status and LVEF levels. Data were then meta-analyzed after stratification of the pooled data across the LVEF strata and New York Heart Associations (NYHA) classifications for heart failure using Stata software version 17.0.
The literature search returned 13 Large clinical trials and 13 post hoc analysis reports. Meta-analysis of the effects of gliflozins on the primary composite outcome showed no significant difference in efficacy across the heart failure subtypes, but higher efficacy were detected in patient groups at lower NYHA classifications (I2 = 46%, P = 0.02). Meta-analyses across the LVEF stratums revealed that a baseline LVEF lower than 30% was associated with enhanced improvement in the primary composite outcome compared to patients with higher LVEF levels at the borderline statistical significance (HR: 0.70, 95%CI: 0.60 to 0.79 vs 0.81, 95%CI: 0.75 to 0.87; respectively, P = 0.06). Composite renal outcome was improved significantly higher in patients with no heart failure than in heart failure patients with preserved ejection fraction (HFpEF) (HR: 0.60, 95%CI: 0.49 to 0.72 vs 0.94, 95%CI: 0.74 to 1.13; P = 0.04). Acute renal injury occurred significantly less frequently in heart failure patients with reduced ejection fraction who received gliflozins than in HFpEF (HR: 0.67, 95%CI: 51 to 0.82 vs 0.94, 95%CI: 0.82 to 1.06; P = 0.01). Volume depletion was consistently increased in response to SGLT2i in all the subgroups.
Heart failure patients with lower LVEF and lower NYHA sub-classifications were found to be generally more likely to benefit from therapy with gliflozins. Further research are required to identify patient subgroups representing the highest benefits or adverse events in response to SGLT2i.
Core Tip: Compared to placebo, treatment with Sodium glucose cotransporter 2 inhibitors improve cardiorenal outcomes in a broad range of disorders with significant heterogeneity in the subgroup of patients who are likely to benefit most from the treatment across their heart failure subtypes, New York Heart Associations classifications and ejection fraction levels. There are also adverse events associated with these drugs that deserve further research.
- Citation: Taheri S. Heterogeneity in cardiorenal protection by Sodium glucose cotransporter 2 inhibitors in heart failure across the ejection fraction strata: Systematic review and meta-analysis. World J Nephrol 2023; 12(5): 182-200
- URL: https://www.wjgnet.com/2220-6124/full/v12/i5/182.htm
- DOI: https://dx.doi.org/10.5527/wjn.v12.i5.182
Anti-hyperglycemic medications have been shown to improve cardiovascular outcomes and renal health in a range of disorders; yet in specific patient subpopulations there is a possibility that their side effects outweigh the protection they offer. For the same reason, large and expensive clinical trials have been conducted to investigate their impact on health entities, and protective roles have been reported for a number of these drugs that went beyond their antihyperglycemic effects[1,2].
Gliflozins or Sodium glucose cotransporter 2 inhibitors (SGLT2i) are relatively novel antidiabetic medications that lower blood levels of glucose through increasing its urinary excretion and therefore they also induce weight loss[1]. Recently a number of large clinical trials have shown significant cardiorenal protection by these drugs in a spectrum of diseases including patients with type 2 diabetes mellitus (T2DM), heart failure and chronic kidney diseases. However, the patient populations were inconsistent in these trials in several aspects, and there is a need for further research regarding the potential factors that might contribute in this effect. In fact, a number of systematic reviews have already been published covering a broad spectrum of cardiac, renal and metabolic factors, including meta-analyses showing significant improvements in the composite outcomes of cardiovascular death or hospitalizations in heart failure patients with either preserved (HFpEF) or reduced ejection fraction (HFrEF)[3-6]. The purpose of this systematic review and meta-analysis is to examine potential effects of SGLT2i therapy on the composite or specific cardiac or renal outcomes in heart failure patients across baseline left ventricular ejection fraction (LVEF) levels.
Supplementary Figure 1 summarizes the search strategy of the current systematic review. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist was followed in this study (Supplementary Figure 2). A systematic search of the literature was performed using Cochrane Library, Reference Citation Analysis, nejm.org, and EuropePMC search engines to April 15, 2023. Pubmed/MEDLINE could not be reached due to internet filtering. Further search of the literature was performed using Google Scholar to find the post hoc analyses and substudies from the included large randomized controlled trials, regarding the subjects of interest for this systematic review (Figure 1).
In order to minimize potential publication biases, the inclusion criteria assigned eligibility only to the reports of double-blind and placebo-controlled trials if they were large (defined as at least 1000 subjects in the SLGT2i arm and at least half as many patients in the placebo arm) with long enough follow up time (at least 6 mo), assessing SGLT2i, and reported any of the efficacy or safety outcomes of interest in this review, as specified. Finally 27 studies (13 trials and 14 post hoc analyses) were found and reviewed[7-33].
The evaluated outcomes in this systematic review and meta-analysis included the primary composite outcome as defined by each study and irrespective of the disparities between them, cardiovascular death, the composite outcome of cardiovascular death or hospitalization (or an urgent visit) for heart failure, composite renal outcome (serious renal events defined by different studies and irrespective of potential differences between trials) and death from any cause.
Specific renal outcomes: As mentioned above, the composite renal outcomes were inconsistently defined by different studies and included a heterogeneous combinations of the following indicators: Doubling of serum creatinine measures, substantial decrease in estimated glomerular filtration rate (i.e. ≥ 40% decrease; falling below 60 to less than 15 mL/min/1.73 m2 in different studies), end-stage kidney disease; renal replacement therapy initiation (i.e. dialysis or renal transplantation), and renal death. Wherever there were reports from more than one combination of renal outcomes, the one with the larger spectrum was used as the composite renal outcome for inclusion into the meta-analysis. Other renal outcomes that were evaluated in this study included renal disease progression/worsening renal function, acute kidney injury/acute renal failure, volume depletion, and diabetic ketoacidosis.
Heart failure subtypes: Data for primary outcomes of interests were extracted and meta-analysis were conducted across specific stratification strategies. The patients’ heart failure status and the type of heart failure (i.e. HFpEF), HFrEF and mid-range/mildly-reduced ejection fraction (HFmrEF) were also extracted. The definition of HFpEF has varied across different trials, with HFpEF defined as EF > 40% in the EMPEROR-Preserved[16] and DELIVER[18], and as EF ≥ 50% in the SCORED[13], CANVAS[12], EMPA-REG OUTCOME[21], and SOLOIST-WHF[17] trials. Likewise, HFrEF was inconsistently defined as EF < 40% in the SCORED[13] and SOLOIST-WHF[17], as ≤ 40% in the EMPEROR-Reduced[15] and DAPA-HF[14], as EF < 45% in DECLARE-TIMI 58[33], as EF ≤ 45% in the VERTIS-CV[24], and as EF < 50% in the CANVAS[22] and EMPA-REG OUTCOME[21] trials. Heart failure with mildly reduced (mid-range) EF was consistently defined as EF between 40%-49%. Only in Supplementary Figure 3, LVEF rates between 35% and 55% were also considered HFmrEF. Finally, heart failure not-otherwise-specified (nos) as patients diagnosed with heart failure (presence of signs and symptoms of HF, elevated levels of natriuretic peptides in the plasma and evidence of structural heart disease - left ventricular hypertrophy or left atrial remodeling - or the presence of diastolic dysfunction) with no further stratifications. Patients who had baseline LVEF ranged within the definitions but without the documented diagnosis of heart failure were excluded from the respective subgroups.
LVEF stratums: Meta-analyses of the outcomes were repeated after stratification of the LVEF rates by LVEF stratums (i.e. documented heart failure patients with LVEF measures above or below the cutoff values of 30%, 40%, 45%, 50%, and 60%). However, since the outcome analyses across all the predefined LVEF cutoff points were not exactly performed by all the reviewed studies, an alternative approach was employed wherever there were reports that fell in ranges totally within the study subgroups defined across the cutoff points of this study; e.g. in meta-analysis of outcomes across LVEF of 40%, if a trial had only provided data of LVEF over 50% or below 30%, the data were included as LVEF over 40% or ≤ 40%, respectively (since LVEF values ≥ 50% falls totally within the range of > 40% and LVEF < 30% falls fully within the range of ≤ 40%). But data of patients with LVEF < 50% was not included into meta-analysis of patients with LVEF < 40%, since it doesn’t totally fall within the specified range. Moreover, if data was available for two LVEF ranges for any particular study, both falling within the meta-analysis ranges, the one that was closest to the cutoff and therefore encompassed the largest possible patient population was chosen for inclusion (e.g. if LVEF > 45% and > 50% were available for a trial, in meta-analysis of outcomes across LVEF 40%, data of LVEF > 45% was included in the reports of LVEF > 40%).
Hazard ratio (HR) and 95% confidence interval (CI) were pooled using a random-effects DerSimonian and Laird model. Inverse of the variance was used to assign weights to each study. Heterogeneity among studies was assessed using the Higgins I2 value. Meta-regression analysis was conducted using mixed-effects modelling to evaluate factors potentially explaining any observed heterogeneity for the study outcomes (i.e. composite study outcome, cardiovascular death and/or heart failure hospitalizations and composite or specific renal outcomes). Meta-regression models using demographic or disease-specific baseline data (i.e. age, gender, ethnicity, glycated hemoglobin, past medical history, etc.) inputs were not possible due the lack of the baseline data discriminately reported across the study groups (i.e. heart failure subtypes, LVEF cutoff levels and NYHA). The only factor that could be included into meta-regression without controversy was the type of gliflozins employed. Some other factors were also used for this purpose (including mean study follow-up time, T2DM and chronic kidney disease (CKD) as inclusion criterions to the study) which might sound controversial since the follow up times could be inconsistent in patient subgroups, as were T2DM and CKD status in studies not having them as inclusion criterions. Even though, no observed heterogeneity in any of the meta-analyses could be explained by the gliflozin type, with no significant effect returned by meta-regression analysis. The same observation was made for meta-regression analysis of the more controversial factors mentioned above.
No special dosage preferences were made for trials in which more than one SGLT2i dosage had been sought and the pooled effects were used for analyses wherever applicable and otherwise, data from the higher SGLT2i dosage was considered. Subgroup analysis was conducted to assess for variability of therapeutic effects across the LVEF stratums, heart failure subtypes and NYHA subclass populations. Study quality was assessed using version 2 of the Cochrane risk-of-bias tool. 2-tailed P values with statistical significance specified at 0.05 were used in all analyses. Stata version 17 (Stata Corp.) and Microsoft Excel 2013 (Microsoft Corp.) were used for analyses.
The literature search returned 13 large clinical trials evaluating impact of SGLT2i on the outcome of patients[7-19], and their characteristics are summarized in Table 1. Fourteen more studies reporting post hoc analysis of the reviewed trials were also found and reviewed[20-33]. Five trials were on heart failure patients, in seven trials only diabetic patients included and four trials were conducted specifically on patients with chronic kidney diseases. Patients’ data and outcome reports were extracted regarding their heart failure status and included in the meta-analyses.
| Ref. | Ref-post-hoc | Trial | Year | Follow (yr) | N | Participants | Diabetes proportion % | Heart failure proportion (%) | SGLT2i | Primary outcome |
| [7] | 21 | EMPA-REG OUTCOME | 2015 | 3.1 | 7020 | T2DM with established CVD; eGFR ≥ 30 mL/min/1.73 m2 | 7020 (100) | 706 (10) | Empagliflozin | CV death+non-fatal MI+non-fatal stroke |
| [8] | 22 | CANVAS/CANVAS-R | 2017 | 2.4 | 10142 | T2DM with CVD or multiple RFs for CVD; eGFR ≥ 30 mL/min/1.73 m2 | 10142 (100) | 1461 (14) | Canagliflozin | CV death+non-fatal MI+non-fatal stroke |
| [9] | 23 | DECLARE–TIMI58 | 2019 | 4.2 | 17160 | T2DM with CVD or multiple RFs for CVD | 17160 (100) | 1724 (10) | Dapagliflozin | CV death+MI+ischemic stroke |
| [10] | 24 | VERTIS-CV | 2020 | 3.5 | 8246 | T2DM with established CVD; eGFR ≥ 30 mL/min/1.73 m2 | 8246 (100) | 1958 (24) | Ertugliflozin | CV death+non-fatal MI+non-fatal stroke |
| [11] | 25 | CREDENCE | 2019 | 2.6 | 4401 | T2DM with CVD+albuminuria (uACR 300–5000); eGFR 30–90 mL/min/1.73 m2 | 4401 (100) | 652 (15) | Canagliflozin | ESKD, doubling of serum creatinine/death from renal/CV cause |
| [12] | 26, 27 | DAPA-CKD | 2020 | 2.4 | 4304 | CVD + albuminuria +/- T2DM (eGFR 25-75 mL/min/1.73 m2) | 2906 (68) | 468 (11) | Dapagliflozin | ESKD, sustained ≥ 50% eGFR decline, death from renal or CV cause |
| [13] | - | SCORED | 2020 | 1.3 | 10584 | T2DM with CVD & RFs for CVD; (GFR) of 30 to 60 mL/min/1.73 m2 | 10 584 (100) | 3283 (31) | Sotagliflozin | CV death and hospitalizations and urgent visits for HF |
| [14] | 28, 29 | DAPA-HF | 2019 | 1.5 | 4744 | HF (EF ≤ 40% & NYHA class II–IV) +/- T2DM; eGFR ≥ 30 mL/min/1.73 m2 | 2139 (45) | 4744 (100) | Dapagliflozin | Worsening HF and CV death |
| [15] | 31 | EMPEROR-Reduced | 2020 | 1.3 | 3730 | HF (EF ≤ 40% & NYHA class II–IV) +/- T2DM | 1856 (50) | 3730 (100) | Empagliflozin | Composite of HF hospitalization and CV death |
| [16] | EMPEROR-Preserved | 2021 | 26.2 months | 5988 | HF (EF > 40% & NYHA class II–IV) +/- T2DM; eGFR ≥ 20 mL/min/1.73 m2 | 2938 (49) | 5988 (100) | Empagliflozin | Composite of cardiovascular death or hospitalization for HF | |
| [17] | 32 | SOLOIST-WHF | 2020 | 0.75 | 1222 | T2DM & recent hospitalization for HF; eGFR ≥ 30 mL/min/1.73 m2 | 1222 (100) | 1222 (100) | Sotagliflozin | CV death and hospitalizations and urgent visits for HF |
| [18] | 30 | DELIVER | 2022 | 2.3 | 6263 | HF (EF > 40% & NYHA class II–IV) +/- T2DM | 3150 (50) | 6263 (100) | Dapagliflozin | Hospitalization for HF or an urgent visit for HF or CV death |
| [19] | EMPA-KIDNEY | 2023 | 2.0 | 6609 | CKD [eGFR > 20 & < 45 OR 45 < eGFR < 90 mL/min/1.73 m2 & (proteinuria)] | 3040 (46) | 658 (10) | Empagliflozin | eGFR to < 10 OR decrease in eGFR of ≥ 40% OR renal death |
Meta-analysis of the effects of gliflozins on the primary composite outcomes (cardiorenal events as defined by each study) showed that compared to placebo, SGLT2i significantly decreased the event rates (HR: 0.78, 95%CI: 0.73 to 0.83, I2 = 53.7%), with no significant difference in efficacy across the heart failure status or subtypes (P = 0.49, Figure 2A). Likewise, when cardiovascular death and/or urgent visits/hospitalization for heart failure was used as the outcome, gliflozins were superior to placebo with no heterogeneity between the subgroups (HR: 0.76, 95%CI: 0.72 to 0.79, P = 0.68, I2 = 0%, Figure 2B). Compared to placebo, SGLT2i therapy was again found to be significantly associated with lower cardiovascular death (HR: 0.84, 95%CI: 0.78 to 0.90, I2 = 19.9%) and all-cause mortality (HR: 0.86, 95%CI: 0.81 to 0.91, I2 = 32.1%), with no significant difference between the subgroups [P = 0.98 (Supplementary Figure 3) and P = 0.21 (Figure 3), respectively]. However, a trend toward higher effectiveness was observed for patients with HFrEF vs HFpEF; though it failed to reach the statistical significance just at the borderline level; P = 0.07 (Supplementary Figure 4).
Although no significant difference was detected in efficacy measures between the heart failure subtypes in any of the above-mentioned meta-analyses, interestingly SGLT2i seem to offer significant benefits in survival outcome (i.e. cardiovascular death or all-cause mortality) only to HFrEF or (to a lesser degree) HFmrEF patients, and the respective outcome effects did not reach significance level for HFpEF (HR: 0.89, 95%CI: 0.75 to 1.02; HR: 0.96, 95%CI: 0.88 to 1.05; respectively, Supplementary Figure 3 and Figure 3).
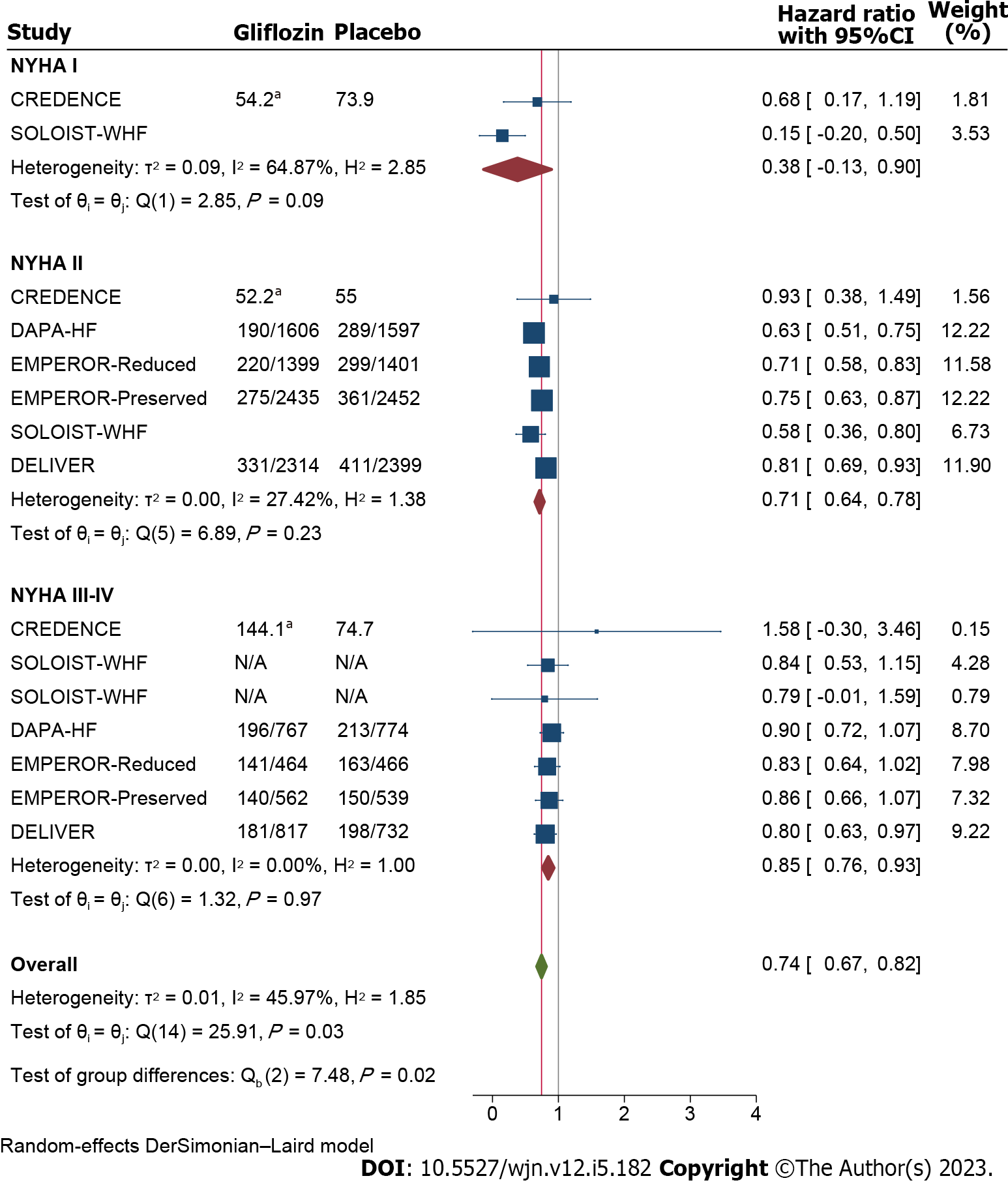
Meta-analyses of the primary composite outcomes across NYHA classes revealed significant improvement in the outcome rates [HR: 0.74(0.67-0.82)], although as is illustrated in Figure 4, this favorable effect was not consistent across all the NYHA subclasses and those at lower classes significantly better responded to SGLT2i (I2 = 46%, P = 0.02; Figure 4).
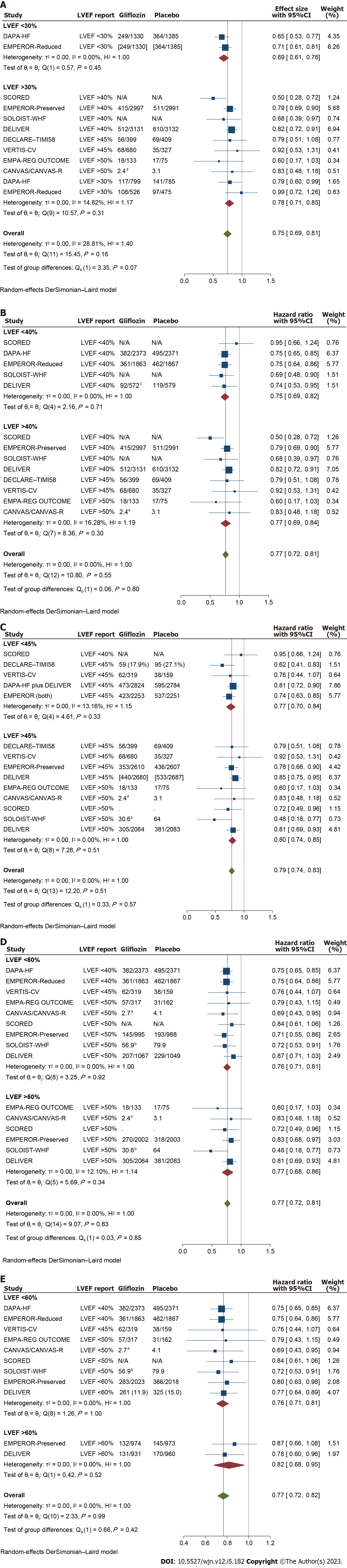
Meta-analyses were repeated across the LVEF stratums, irrespective of the authors’ definitions of the heart failure subtypes. The primary composite outcomes across all the LVEF cutoff levels showed significant efficacy for gliflozins compared to placebo, with no significant difference between the subgroups. Notably, patients with a baseline LVEF of 30% or less represented enhanced improvement in the primary composite outcome compared to patients with LVEF over 30%, but at the borderline statistical significance (HR: 0.70, 95%CI: 0.60 to 0.79 vs 0.81, 95%CI: 0.75 to 0.87; respectively, P = 0.06; Supplementary Figure 4).
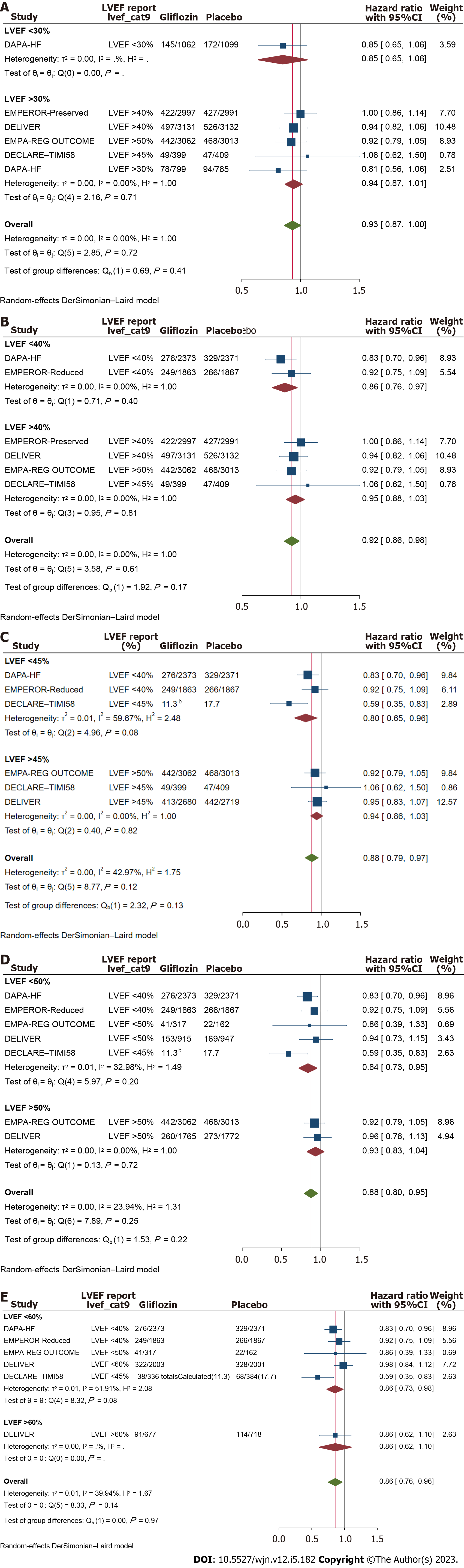
Similar to the results of primary outcome analyses, meta-analysis of the composite outcome of ‘cardiovascular death or hospitalizations (or urgent visits) due to heart failure’ exhibited significant improvement in response to treatment with SGLT2i at all the LVEF levels though again, compared to patients with LVEF above 30%, the subgroup of patients with the baseline LVEF of 30% or less showed a stronger response to gliflozins at borderline significance (HR: 0.69, 95%CI: 0.61 to 0.76 vs 0.78, 95%CI: 0.71 to 0.85; P = 0.07). Further analyzes at higher cutoff values showed no significant difference for the respective outcome (P > 0.4 for all; Figure 5). All-cause mortality also showed significant benefit across LVEF stratums with the relatively best effect size in patients with LVEF ≤ 40% (versus LVEF > 40%) but no statistical significance was reached; Figure 6.
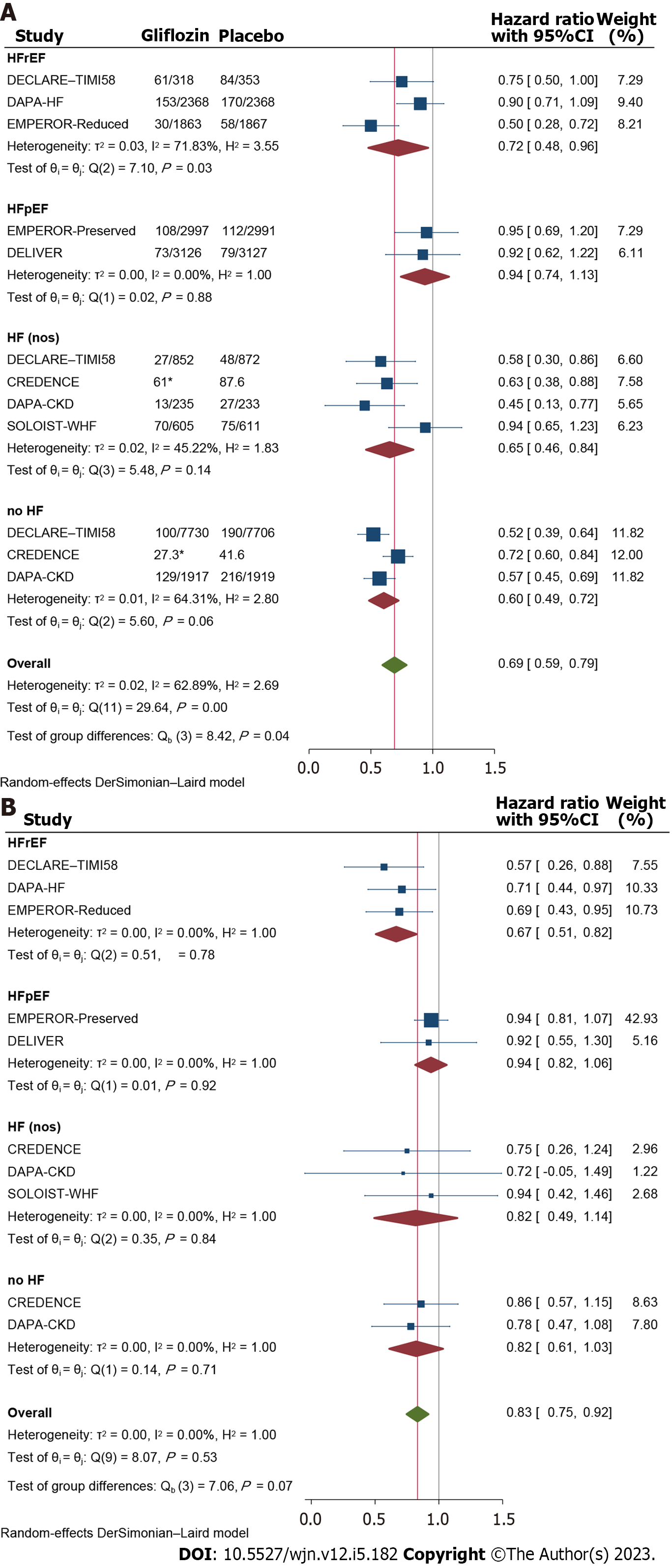
Composite renal events: Composite renal events was an unspecific terminology that comprised a diverse spectrum of unfavorable renal events (described in methods) As could be perceived from Figure 6A, SGLT2i significantly improved composite renal events as compared to the placebo-treated group (HR: 0.69, 95%CI: 0.59 to 0.79), but significant difference across the meta-analysis patient groups was observed with HFpEF and no-heart failure patients representing the lowest and the highest response rates, respectively (HR: 0.94, 95%CI: 0.74 to 1.13 and 0.60, 95%CI: 0.49 to 0.72, respectively); P = 0.04, Figure 7.
Acute kidney injury (or acute renal failure) was also shown to occure significantly less frequently in patients receiving SGLT2i compared to placebo (HR: 0.83, 95%CI: 0.75 to 0.92; Figure 7B); however this effect was not consistent across the heart failure groups and HFrEF and HFpEF patients respectively represented the highest and the lowest response rates with significant difference between, after excluding other subgroups from the meta-analysis (HR: 0.67, 95%CI: 51 to 0.82 vs 0.94, 95%CI: 0.82 to 1.06; P = 0.01, Supplementary Figure 5).
Renal disease progression or worsening renal function: Gliflozins significantly reduced renal disease progression in the meta-analysis (HR: 0.63, 95%CI: 0.55 to 0.71). But unlike the composite renal event, no significant difference was found regarding the heart failure status or across subtypes (P = 0.52; Supplementary Figure 6).
Volume depletion: As is evident from Supplementary Figure 7, SGLT2i therapy was associated with significantly higher rates of volume depletion in the pooled data meta-analysis with no significant difference across the study subgroups (HR: 1.14, 95%CI: 1.02 to 1.26; P = 0.33).
Diabetic ketoacidosis: As is summarized in Supplementary Table 1, diabetic ketoacidosis was a rare observation in both the SGLT2i and placebo groups, and therefore meta-analyses were not possible. The distribution of the outcomes between the two groups reveals no heterogeneity.
In this meta-analysis of 13 large clinical trials, data of 45918 patients were screened and significant but inequivalent protective effects for SGLT2i were found across the patients’ LVEF strata, regarding a spectrum of cardiovascular and renal outcomes. Compared to HFpEF patients, HFrEF exhibited more dramatic response to gliflozins in a good number of the predefined outcomes. This finding is in contrast to a previous study in which authors found equivalent efficacy in heart failure patients across a full spectrum of LVEF[30]. One reason for this disparity could be related to the number of studies and patients entered into the analysis, with the current study encompassing substantially larger population (including data from the mentioned study). As well, in the current study the analyses were performed across different cutoff points compared to the analyses across the spectrum of LVEF, which leaves only a limited number of subjects for each subgroup. Moreover the spectrum of specific outcomes investigated in the current study was relatively broader.
Previous review articles have explored several predicting factors on response to SGLT2i. In a comprehensive review, Baigent et al[20] analyzed the impact of diabetes mellitus on the cardiorenal protective effects of SGLT2i treatment and found no disparity regarding diabetes status. In another review study, Zelniker et al[2] reported that the cardiovascular benefits of gliflozins in diabetic population seem to be largely confined to patients with established atherosclerotic cardiovascular disease. Bhatia et al[34] provided evidence for SGLT2i protective effects in a broader range of cardiac, renal and metabolic derangements, and in another very recent post hoc analysis from DELIVER trial, Peikert et al[35] reported substantial improvements in a large range of symptoms, functionality indices, and quality of life in HFmrEF/HFpEF patients in response to SGLT2i. The current systematic review provides further data on the variability of response to gliflozins in heart failure patients regarding their LVEF levels and NYHA classifications, which could have significant clinical implications for the practitioners.
It is noteworthy that all the clinical trials reviewed in this study have compared the outcome of patients receiving gliflozins vs placebo. Although this verifies favorable effects for the drug, it doesn’t provide robust evidence that this protective effect outweighs the advantages that are expectable from conventional medications prescribed in this patients; therefore it is still an open question as to whether or not gliflozins’ protection outweighs the conventional medications or is there some sort of synergistic relationship between them. But this was out of the scope of the current systematic review, and future studies are required to issue this questions.
Cardiac outcomes: Gliflozins significantly improved the primary composite outcome of cardiovascular death and hospitalizations in patients with or without heart failure and across all the subgroups. However this effect seemed to be skewed in favor of HFrEF compared to HFpEF (the number of patients needed to be treated to save one additional patient from the primary composite outcome in the HFpEF was twice as large as the HFrEF in CANVAS/CANVAS-R trial[22] and 2.9 times for EMPEROR-Reduced vs either EMPEROR-Preserved or DELIVER[16,18]; this result was not reproduced in EMPA-REG OUTCOME trial[21]). Reanalyses of the patients’ composite outcomes as described above (i.e. cardiovascular death or associated hospitalizations) were based on arbitrary definitions of heart failure subgroups by LVEF levels, inconsistently made by the authors in the different trials; therefore in order to have more precise conclusions, definitive cutoff points across LVEF were set and sought for the evaluation of the outcome, and it has been revealed that for a number of major outcomes, the benefit from SGLT2i therapy reaches significant difference in favor of the patients with lower LVEF, at the cutoff point of 30% (Supplementary Figure 4 and Figure 5). Interestingly, repeating the meta-analysis across NYHA classifications showed significantly enhanced therapeutic effects for patients at lower vs higher NYHA subclasses. These findings broaden our understanding on the subgroups of the heart failure patients who are likely to benefit most from the SGLT2i.
Death outcomes: Meta-analysis of the impact of SGLT2i on cardiovascular death and all-cause mortality also exhibited benefit with relative but none-significant difference between the subgroups (Figures 3 and 6 and Supplementary
Renal specific outcom: Renal outcomes are of special interest in patients with either heart failure or diabetes mellitus and a main focus of attention in most of the reviewed trials. Although previous systematic reviews have shown the benefits of SGLT2i on renal events[20], potential variability in the magnitude of this protection across LVEF rates could have clinical implcations. Interestingly, results of the meta-analysis of composite renal outcomes were consistent with the respective analyses on the cardiovascular outcomes, with the HFrEF patients responding relatively but not significantly better to the treatment than HFpEF, though with an unexpected finding of detecting the most pronounced renal protective effects in patients without heart failure (Figure 7A). This offers that gliflozins’ renoprotective effects are unlikely to be associated with their heart failure modifying effects and deserves further investigations.
In the meta-analyses of more specific renal outcomes, acute kidney injury was reduced by 32% in patients with HFrEF compared to only 6% in HFpEF, a difference that was statistically significant (Figure 7B). On the other hand, not every specific renal outcome benefited by SGLT2i, and volume depletion had been shown to be significantly exacerbated by 14% compared to patients receiving placebo. This finding warns of the possible risks to patients receiving gliflozins and emphasisthe need for close monitoring of patients for signs of volume depletion.
Limitations and strengths: There are strengths and limitations associated with this study that warrants further discussion. Different patient populations (exclusive inclusion of patients with diabetes mellitus, chronic kidney disease or heart failure, or variations in the proportions of these patients in different studies), large variations in the follow up times, and inconsistencies in the outcome definitions and reports between the reviewed trials are a number of limitations that could undermine the findings of this study. The principle strength of the current systematic review is providing a stratified outcome analysis across the LVEF stratums of patients with heart failure, and introducing the patient subgroups that are most or least likely to benefit from treatment with gliflozins. Identifying the patient populations that don’t benefit the treatment gives a message to the scientific community that further research and developments are needed.
In conclusion, compared to placebo, SGLT2i have shown significant therapeutic effects in patients with or without heart failure regarding cardiovascular and renal outcomes. These effects are generally more pronounced in HFrEF patients at the lowest LVEF levels compared to HFpEF, with no survival advantage for the latter group. Patients with lower NYHA classifications were also found to respond more vigorously to the study drugs. Further well-designed studies are needed to determine other potential factors with significant roles in response to gliflozins.
Gliflozins have been shown effective to improve outcomes in patients with heart failure.
Finding the indications for the prescription of gliflozins would help to concentrate research on subgroups that need further research and novel therapeutic approach.
To find the subpopulations of heart failure patients that benefit most from Sodium glucose cotransporter 2 (SGLT2) inhibitors based on their left ventricular ejection fraction levels.
A systematic review and meta-analysis of data of patients receiving gliflozin thepay in large and robust randomized double-blind placebo trials was conducted. Meta-analyses were conducted after stratification of the patients based on their left ventricular ejection fraction (LVEF) levels.
Gliflozins were generally superior to placebo in improving composite outcome of patients with heart failure across LVEF levels. This therapeutic effects were more pronounced in patients with reduced LVEF and low New York Heart Associations classes. No survival benefit was detected for patients with preserved ejection fraction disease.
Gliflozins are effective in improving the outcome in patients with heart failure.
Further research would be needed to examine the magnitude of gliflozins' efficacy as well as its cost-effectiveness compared to the other therapeutic options in this patient population.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wu QN, China S-Editor: Liu JH L-Editor: A P-Editor: Zhao S
| 1. | Braunwald E. Gliflozins in the Management of Cardiovascular Disease. N Engl J Med. 2022;386:2024-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 153] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 2. | Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 1903] [Article Influence: 317.2] [Reference Citation Analysis (0)] |
| 3. | Gager GM, Gelbenegger G, Jilma B, von Lewinski D, Sourij H, Eyileten C, Filipiak K, Postula M, Siller-Matula JM. Cardiovascular Outcome in Patients Treated With SGLT2 Inhibitors for Heart Failure: A Meta-Analysis. Front Cardiovasc Med. 2021;8:691907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Herrington WG, Savarese G, Haynes R, Marx N, Mellbin L, Lund LH, Dendale P, Seferovic P, Rosano G, Staplin N, Baigent C, Cosentino F. Cardiac, renal, and metabolic effects of sodium-glucose co-transporter 2 inhibitors: a position paper from the European Society of Cardiology ad-hoc task force on sodium-glucose co-transporter 2 inhibitors. Eur J Heart Fail. 2021;23:1260-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Tsampasian V, Elghazaly H, Chattopadhyay R, Ali O, Corballis N, Chousou PA, Clark A, Garg P, Vassiliou VS. Sodium glucose co-transporter 2 inhibitors in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022;29:e227-e229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Banerjee M, Pal R, Nair K, Mukhopadhyay S. SGLT2 inhibitors and cardiovascular outcomes in heart failure with mildly reduced and preserved ejection fraction: A systematic review and meta-analysis. Indian Heart J. 2023;75:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 7. | Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375:323-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2303] [Cited by in RCA: 2520] [Article Influence: 280.0] [Reference Citation Analysis (0)] |
| 8. | Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4498] [Cited by in RCA: 5357] [Article Influence: 669.6] [Reference Citation Analysis (0)] |
| 9. | Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4652] [Cited by in RCA: 4207] [Article Influence: 701.2] [Reference Citation Analysis (0)] |
| 10. | Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, Shih WJ, Gantz I, Terra SG, Cherney DZI, McGuire DK; VERTIS CV Investigators. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N Engl J Med. 2020;383:1425-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 1001] [Article Influence: 200.2] [Reference Citation Analysis (0)] |
| 11. | Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:2295-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2826] [Cited by in RCA: 3930] [Article Influence: 655.0] [Reference Citation Analysis (0)] |
| 12. | Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383:1436-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1586] [Cited by in RCA: 3068] [Article Influence: 613.6] [Reference Citation Analysis (1)] |
| 13. | Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Inzucchi SE, Kosiborod MN, Cherney DZI, Dwyer JP, Scirica BM, Bailey CJ, Díaz R, Ray KK, Udell JA, Lopes RD, Lapuerta P, Steg PG; SCORED Investigators. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N Engl J Med. 2021;384:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 778] [Article Influence: 194.5] [Reference Citation Analysis (0)] |
| 14. | McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 4378] [Article Influence: 729.7] [Reference Citation Analysis (0)] |
| 15. | Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383:1413-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1875] [Cited by in RCA: 3123] [Article Influence: 624.6] [Reference Citation Analysis (0)] |
| 16. | Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M; EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385:1451-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3009] [Cited by in RCA: 2715] [Article Influence: 678.8] [Reference Citation Analysis (0)] |
| 17. | Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B; SOLOIST-WHF Trial Investigators. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med. 2021;384:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 1255] [Article Influence: 313.8] [Reference Citation Analysis (0)] |
| 18. | Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, Jhund PS, Belohlavek J, Chiang CE, Borleffs CJW, Comin-Colet J, Dobreanu D, Drozdz J, Fang JC, Alcocer-Gamba MA, Al Habeeb W, Han Y, Cabrera Honorio JW, Janssens SP, Katova T, Kitakaze M, Merkely B, O'Meara E, Saraiva JFK, Tereshchenko SN, Thierer J, Vaduganathan M, Vardeny O, Verma S, Pham VN, Wilderäng U, Zaozerska N, Bachus E, Lindholm D, Petersson M, Langkilde AM; DELIVER Trial Committees and Investigators. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med. 2022;387:1089-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 1397] [Article Influence: 465.7] [Reference Citation Analysis (0)] |
| 19. | The EMPA-KIDNEY Collaborative Group; Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson JR, Preiss D, Judge P, Mayne KJ, Ng SYA, Sammons E, Zhu D, Hill M, Stevens W, Wallendszus K, Brenner S, Cheung AK, Liu ZH, Li J, Hooi LS, Liu W, Kadowaki T, Nangaku M, Levin A, Cherney D, Maggioni AP, Pontremoli R, Deo R, Goto S, Rossello X, Tuttle KR, Steubl D, Petrini M, Massey D, Eilbracht J, Brueckmann M, Landray MJ, Baigent C, Haynes R. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388:117-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1116] [Cited by in RCA: 1182] [Article Influence: 591.0] [Reference Citation Analysis (2)] |
| 20. | Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists' Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400:1788-1801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 488] [Article Influence: 162.7] [Reference Citation Analysis (0)] |
| 21. | Savarese G, Uijl A, Lund LH, Anker SD, Asselbergs F, Fitchett D, Inzucchi SE, Koudstaal S, Ofstad AP, Schrage B, Vedin O, Wanner C, Zannad F, Zwiener I, Butler J. Empagliflozin in Heart Failure With Predicted Preserved Versus Reduced Ejection Fraction: Data From the EMPA-REG OUTCOME Trial. J Card Fail. 2021;27:888-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Figtree GA, Rådholm K, Barrett TD, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Matthews DR, Shaw W, Neal B. Effects of Canagliflozin on Heart Failure Outcomes Associated With Preserved and Reduced Ejection Fraction in Type 2 Diabetes Mellitus. Circulation. 2019;139:2591-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 23. | Verma S, McMurray JJV. The Serendipitous Story of SGLT2 Inhibitors in Heart Failure. Circulation. 2019;139:2537-2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Cosentino F, Cannon CP, Cherney DZI, Masiukiewicz U, Pratley R, Dagogo-Jack S, Frederich R, Charbonnel B, Mancuso J, Shih WJ, Terra SG, Cater NB, Gantz I, McGuire DK; VERTIS CV Investigators. Efficacy of Ertugliflozin on Heart Failure-Related Events in Patients With Type 2 Diabetes Mellitus and Established Atherosclerotic Cardiovascular Disease: Results of the VERTIS CV Trial. Circulation. 2020;142:2205-2215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 182] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 25. | Neuen BL, Oshima M, Perkovic V, Agarwal R, Arnott C, Bakris G, Cannon CP, Charytan DM, Edwards R, Górriz JL, Jardine MJ, Levin A, Neal B, De Nicola L, Pollock C, Rosenthal N, Wheeler DC, Mahaffey KW, Heerspink HJL. Effects of canagliflozin on serum potassium in people with diabetes and chronic kidney disease: the CREDENCE trial. Eur Heart J. 2021;42:4891-4901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 26. | McMurray JJV, Wheeler DC, Stefánsson BV, Jongs N, Postmus D, Correa-Rotter R, Chertow GM, Hou FF, Rossing P, Sjöström CD, Solomon SD, Toto RD, Langkilde AM, Heerspink HJL; DAPA-CKD Trial Committees and Investigators. Effects of Dapagliflozin in Patients With Kidney Disease, With and Without Heart Failure. JACC Heart Fail. 2021;9:807-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 27. | Heerspink HJL, Sjöström CD, Jongs N, Chertow GM, Kosiborod M, Hou FF, McMurray JJV, Rossing P, Correa-Rotter R, Kurlyandskaya R, Stefansson BV, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Effects of dapagliflozin on mortality in patients with chronic kidney disease: a pre-specified analysis from the DAPA-CKD randomized controlled trial. Eur Heart J. 2021;42:1216-1227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 28. | Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett J, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Vinh PN, Schou M, Tereshchenko S, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD, Johanson P, Greasley PJ, Boulton D, Bengtsson O, Jhund PS, McMurray JJV. Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients With Heart Failure With and Without Diabetes. JAMA. 2020;323:1353-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 356] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 29. | Desai AS, Jhund PS, Claggett BL, Vaduganathan M, Miao ZM, Kondo T, Barkoudah E, Brahimi A, Connolly E, Finn P, Lang NN, Mc Causland FR, McGrath M, Petrie MC, McMurray JJV, Solomon SD. Effect of Dapagliflozin on Cause-Specific Mortality in Patients With Heart Failure Across the Spectrum of Ejection Fraction: A Participant-Level Pooled Analysis of DAPA-HF and DELIVER. JAMA Cardiol. 2022;7:1227-1234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 30. | Jhund PS, Kondo T, Butt JH, Docherty KF, Claggett BL, Desai AS, Vaduganathan M, Gasparyan SB, Bengtsson O, Lindholm D, Petersson M, Langkilde AM, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Køber L, Lam CSP, Martinez FA, Sabatine MS, Shah SJ, Solomon SD, McMurray JJV. Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat Med. 2022;28:1956-1964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 156] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 31. | Butler J, Packer M, Filippatos G, Ferreira JP, Zeller C, Schnee J, Brueckmann M, Pocock SJ, Zannad F, Anker SD. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur Heart J. 2022;43:416-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 184] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 32. | Verma S, Anker SD, Butler J, Bhatt DL. Early initiation of SGLT2 inhibitors is important, irrespective of ejection fraction: SOLOIST‐WHF in perspective. ESC Heart Failure. 2020;7:3261-3267. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Bonaca MP, Ruff CT, Desai AS, Goto S, Johansson PA, Gause-Nilsson I, Johanson P, Langkilde AM, Raz I, Sabatine MS, Wiviott SD. Effect of Dapagliflozin on Heart Failure and Mortality in Type 2 Diabetes Mellitus. Circulation. 2019;139:2528-2536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 396] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 34. | Bhatia K, Jain V, Gupta K, Bansal A, Fox A, Qamar A, Damman K, Vaduganathan M. Prevention of heart failure events with sodium-glucose co-transporter 2 inhibitors across a spectrum of cardio-renal-metabolic risk. Eur J Heart Fail. 2021;23:1002-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Peikert A, Chandra A, Kosiborod MN, Claggett BL, Desai AS, Jhund PS, Lam CSP, Inzucchi SE, Martinez FA, de Boer RA, Hernandez AF, Shah SJ, Janssens SP, Belohlávek J, Borleffs CJW, Dobreanu D, Langkilde AM, Bengtsson O, Petersson M, McMurray JJV, Solomon SD, Vaduganathan M. Association of Dapagliflozin vs Placebo With Individual Kansas City Cardiomyopathy Questionnaire Components in Patients With Heart Failure With Mildly Reduced or Preserved Ejection Fraction: A Secondary Analysis of the DELIVER Trial. JAMA Cardiol. 2023;8:684-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |