Published online Dec 25, 2023. doi: 10.5527/wjn.v12.i5.132
Peer-review started: May 19, 2023
First decision: July 19, 2023
Revised: July 26, 2023
Accepted: September 26, 2023
Article in press: September 26, 2023
Published online: December 25, 2023
Processing time: 216 Days and 21.2 Hours
Randomized controlled trials (RCTs) of direct oral anticoagulants (DOACs) included a low proportion of atrial fibrillation (AF) patients with chronic kidney disease (CKD), and suggested that DOACs are safe and effective in patients with mild-to-moderate CKD. In a metanalysis of RCTs and observational studies, DOACs were associated with better efficacy (vs warfarin) in early CKD and had similar efficacy and safety profiles in patients with stages IV-V CKD. But few studies have provided data on the safety and effectiveness of each DOAC vs warfarin in patients with stage III CKD. The effectiveness and safety of DOACs in those patients are still subject to debate.
To assess and compare the effectiveness and safety of apixaban and rivaroxaban vs warfarin in this patient population.
A cohort of patients with an inpatient or outpatient code for AF and stage III CKD who were newly prescribed apixaban and rivaroxaban was created using the administrative databases from the Quebec province of Canada between 2013 and 2017. The primary effectiveness outcome was a composite of ischemic stroke, systemic embolism, and death, whereas the primary safety outcome was a composite of major bleeding within a year of DOAC vs warfarin initiation. Treatment groups were compared in an under-treatment analysis using inverse probability of treatment weighting and Cox proportional hazards.
A total of 8899 included patients filled out a new oral anticoagulation therapy claim; 3335 for warfarin and 5564 for DOACs. Compared with warfarin, 15 mg and 20 mg rivaroxaban presented a similar effectiveness and safety composite risk. Apixaban 5.0 mg was associated with a lower effectiveness composite risk [Hazard ratio (HR) 0.76; 95% confidence interval (CI): 0.65-0.88] and a similar safety risk (HR 0.94; 95%CI: 0.66-1.35). Apixaban 2.5 mg was associated with a similar effectiveness composite (HR 1.00; 95%CI: 0.79-1.26) and a lower safety risk (HR 0.65; 95%CI: 0.43-0.99. Although, apixaban 5.0 mg was associated with a better effectiveness (HR 0.76; 95%CI: 0.65-0.88), but a similar safety risk profile (HR 0.94; 95%CI: 0.66-1.35). The observed improvement in the effectiveness composite for apixaban 5.0 mg was driven by a reduction in mortality (HR 0.61; 95%CI: 0.43-0.88).
In comparison with warfarin, rivaroxaban and apixaban appear to be effective and safe in AF patients with stage III CKD.
Core Tip: Compared to warfarin, rivaroxaban and apixaban appear to be effective and safe in atrial fibrillation patients with stage III chronic kidney disease (CKD) in real world. Rivaroxaban 15 mg and 20 mg presented a similar effectiveness and safety composite risk. However, apixaban 2.5 mg might even have a better safety profile than warfarin, while apixaban 5.0 mg might have a better effectiveness profile than warfarin, to a reduction in deaths. Appropriately sized randomized controlled trials are needed to confirm these findings in stage III CKD patients.
- Citation: Perreault S, Boivin Proulx LA, Lenglet A, Massy ZA, Dorais M. Effectiveness and safety of apixaban and rivaroxaban vs warfarin in patients with atrial fibrillation and chronic kidney disease. World J Nephrol 2023; 12(5): 132-146
- URL: https://www.wjgnet.com/2220-6124/full/v12/i5/132.htm
- DOI: https://dx.doi.org/10.5527/wjn.v12.i5.132
Patients with chronic kidney disease (CKD) often develop atrial fibrillation (AF) at a rate of more than twice that of the general population[1-3]. Because patients with both AF and CKD have a greater risk of systemic embolism and bleeding events, an effective therapy is challenging[4-6]. For patients with non-valvular AF (NVAF) requiring oral anticoagulation therapy (OAC), medical evidence suggests treatment with a direct oral anticoagulant (DOAC) over warfarin , including patients with stage I-IV CKD[7]. Despite these recommendations, warfarin remains the OAC of choice for most AF patients [8] as well as AF patients with moderate to severe CKD[9].
Although the randomized controlled trials (RCTs) of DOACs included a low proportion of AF patients with CKD, the results suggested that DOACs are safe and effective in patients with mild-to-moderate CKD (stages I-III CKD, using Cockcroft-Cault formula)[10-13]. In a metanalysis of observational studies and RCTs, DOACs were found to be more effective (vs warfarin) in early CKD and had similar efficacy and safety profiles in patients with CKD stages IV-V as well as patients on dialysis[14]. Recent population-based studies of AF patients with CKD have also examined the effectiveness and safety of DOACs vs warfarin[15-22]. However, few of these studies examined the safety and effectiveness of individual DOACs vs warfarin, nor did they examine the impact of varying doses in patients with stage III CKD with respect to stroke, systemic embolic events, major bleeding, or death[16,23]. Therefore, we attempted to evaluate and compare the efficacy and safety of various DOACs, including low-dose rivaroxaban (15 mg once per day), standard-dose rivaroxaban (20 mg once per day), low-dose apixaban (2.5 mg twice per day), and standard-dose apixaban (5.0 mg twice per day) vs warfarin in AF patients with stage III CKD.
We analyzed several Quebec health care claims databases, in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines[24]. The need for informed consent was waived by the local institutional research committee (University of Montreal, Montreal, Quebec, Canada). The study protocol complied with the ethical guidelines of the 1975 declaration of Helsinki and was approved by the institutional research committee of the University of Montreal.
We assembled a cohort of inpatients or outpatients using the Med-Echo administrative databases (hospital discharge reports), medical services of the Régie de l’Assurance Maladie du Québec (RAMQ), and RAMQ public drug plans, all databases administered by the RAMQ[25-28]. The databases were linked via encrypted health insurance numbers. Information from these databases provided a complete picture of hospital admissions, medical services, and medication used, if the patient was still living in the Quebec province.
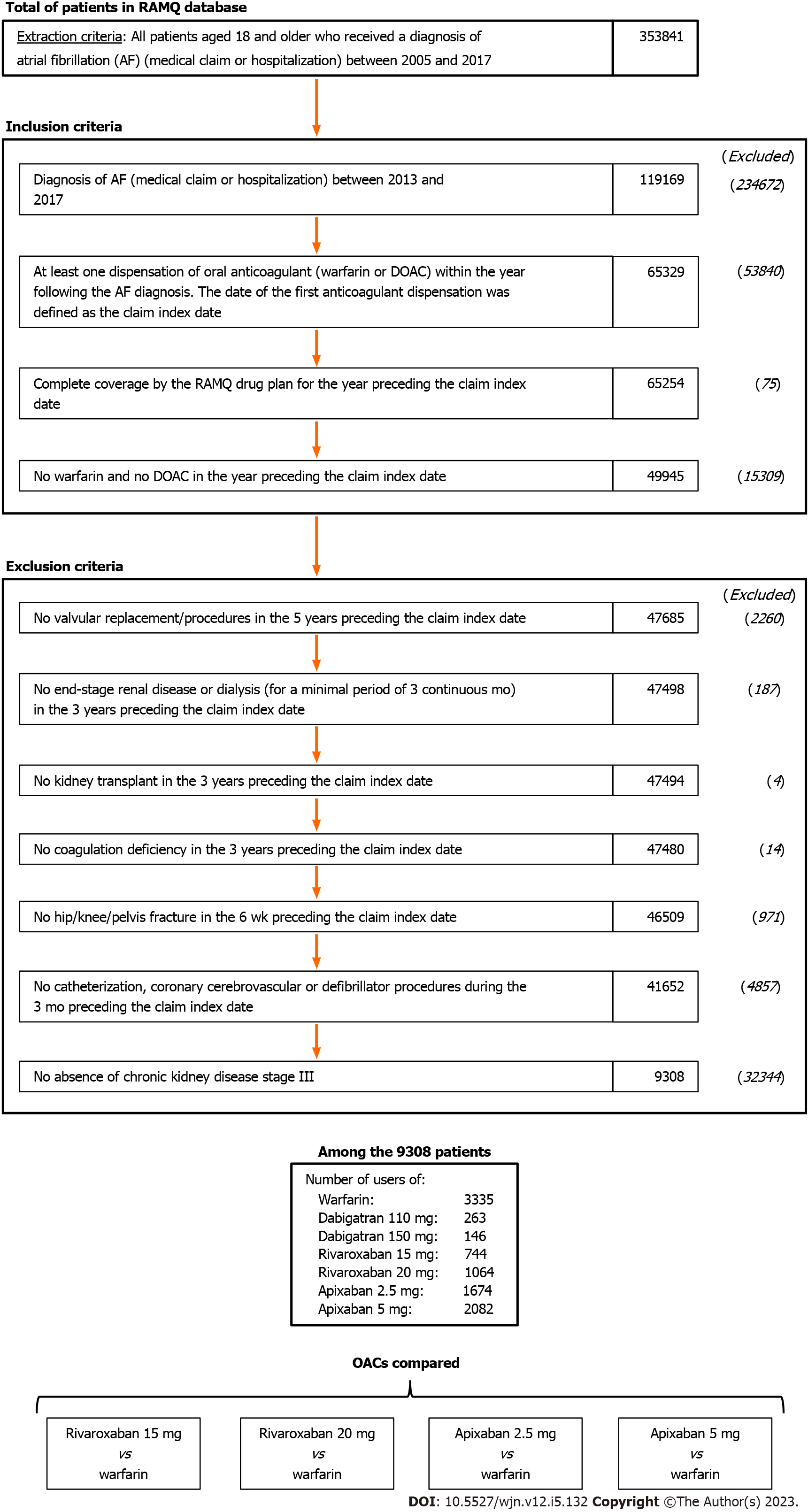
We identified adult patients with AF from January 1, 2013, to December 31, 2017. AF was detected by searching for the international classification of diseases, 9th revision (ICD-9) codes 427.3, 427.31, or 427.32, or the international classification of diseases, 10th revision (ICD-10) code I48[29,30]. The first instance of AF coding was used to determine eligibility. The cohort was subsequently restricted to patients who filled a new prescription for rivaroxaban (15 mg or 20 mg once daily), apixaban (2.5 or 5.0 mg twice daily) or warfarin within a year of AF diagnosis. Few patients had a new prescription of either dabigatran or edoxaban, so they were not included in our analysis. The date of the first OAC claim was defined as the date of cohort entry. New OAC users were defined as those not exposed to any OACs in the year prior to the claim index date. Patients were also required to have had pharmacy coverage for at least 12 mo and enrollment in a drug health insurance plan for at least one year before cohort entry.
We also excluded patients with a code for any condition or procedure that might have impacted the choice of OAC and duration of treatment at discharge: Cardiac valve replacement or valve procedures in the five years before cohort entry; end-stage CKD (meaning being on dialysis), kidney transplant, dialysis, or coagulation deficiency in the three years before cohort entry; medical procedures (including cardiac catheterization, stent, coronary artery bypass grafting, cerebrovascular, or defibrillator) in the three months before cohort entry; deep vein thrombosis or orthopedic surgery in the six months before cohort entry.
Lastly, the cohort was restricted to patients with stage III CKD by using the algorithm 2 to identify CKD G3-5ND, and then applying the exclusion of CKD G4-5ND by using the algorithm 3 (as defined by a composite variable covering the ICD code, drug use, and consultations with a nephrologist, as identified in the administrative databases). The composite variable has been validated, with reference to medical chart reviews of older adults with CKD [the algorithm used for estimated glomerular filtration rate (eGFR) definition], and has presented good positive predictive values[31].
Treatment with an OAC was checked against the prescription fulfillment dates and the number of days of medication supplied for each fill. Exposure to treatment was considered in all analyses. We consider a gap of less than 30 d between the end of a treatment period and a new fill corresponded to continuous treatment. Patients were censored when they discontinued a treatment, switched to another OAC, or to another dose level. Allowing a gap in treatment of up to 30 d is reasonable because of the DOACs’ short half-life. Taking this definition into account, the adherence rate over the 12-mo assessment period was at least 92% for all included patients. The patient’s OAC exposure and censored status were updated every 30 d.
The primary effectiveness outcome was a composite of ischemic stroke or systemic embolism (SE) and all-cause mortality. The primary safety outcome was a composite of major bleeding, defined as either intracranial hemorrhage, gastrointestinal bleeding, or major bleeding from other sites. The individual components of the safety and effectiveness outcomes were evaluated in a secondary analysis.
We identified the outcomes by screening the ICD-9 or ICD-10 codes for the primary diagnosis on inpatient claims (Supplementary Table 1). In earlier validation studies, these codes performed relatively well and gave positive predictive values of over 80%[32,33].
We documented demographic variables upon cohort entry and determined the associated morbidities from the inpatient and outpatient ICD-9 and ICD-10 diagnostic codes recorded in the three years preceding the cohort entry[30-32]. Next, we used the patients’ characteristics and associated comorbidities to calculate the CHADS2 score (Supplementary Tables 2 and 3)[34] and the modified HAS-BLED score (Supplementary Tables 2 and 4). The comorbidity burden was scored with the Charlson-Deyo Comorbidity Index[35,36]. A frailty score was also calculated from the modified elders risk assessment in the two years preceding cohort entry[37,38]. Lastly, we assessed all drug prescriptions filled in the two weeks preceding the cohort entry.
Descriptive statistics were used to summarize the demographic and clinical characteristics of patients, according to the type of OAC used. The follow-up periods and the level of adherence were reported as the mean with standard deviation (SD) or the median with interquartile range. The adherence to treatment in the year of follow-up was calculated by dividing the total number of days of treatment by 365. When the dispensing periods overlapped, the full length of each filled claim was accounted for, and the start date of the second claim was shifted to the end of the previous claim.
For the main analyses of the primary effectiveness and safety composites in an on-treatment, we used an inverse probability of treatment weighting (IPTW) approach to account for differences in patient characteristics between treatment groups[39,40]. Four IPTW cohorts were created: (1) Rivaroxaban 15 mg vs warfarin; (2) rivaroxaban 20 mg vs warfarin; (3) apixaban 2.5 mg vs warfarin; and (4) apixaban 5.0 mg vs warfarin. We then used a multivariable logistic regression model to estimate the observed probability (according to propensity score matching) of being in the treatment group (rivaroxaban 15 mg, rivaroxaban 20 mg, apixaban 2.5 mg, and apixaban 5.0 mg), based on all the baseline covariates, and the impact of temporal trends accounted for in the analysis by including the date of cohort entry in the IPTW matching. By approximating the randomization used in RCTs, the IPTW approach establishes a pseudo-population, balances the treatment groups according to the covariates included in the model, and thus minimizes the impact of confounding biases in observational studies. All weights were stabilized by multiplying the IPTW weight by the marginal probability of being in the treatment group. Descriptive statistics were also used to summarize the baseline characteristics of each IPTW cohort. For baseline characteristics, only absolute standardized differences of 10% or more between the unadjusted cohort and the IPTW-adjusted cohort were considered meaningful[39]. We reported the outcomes per 100 person-years for each treatment in each IPTW population. Hazard ratios (HRs) with 95%CIs associated were estimated using Cox proportional hazards models for each of the four IPTW cohorts described above.
Patients were censored at the time of enrollment if they were in a non-governmental drug coverage plan, admitted to a long-term care facility, admitted to the hospital (for more than two weeks), or in the case of a safety or effectiveness endpoint or death (whichever occurred first). The patient’s OAC exposure and censored status were updated every 30 d.
For the sensitivity analyses of the primary effectiveness and safety composites, we first estimated Cox proportional HRs for outcomes in an intent-to-treat analyses in which we removed the censoring criteria of drug discontinuation or switching, so that all patients were followed up for 365 d unless they were censored for another reason. We used an IPTW approach to account for differences in patient characteristics between treatment groups. We reported the outcomes per 100 person-years for each treatment in each IPTW population. HRs and 95%CIs associated were estimated using Cox proportional hazards models for each of the four IPTW cohorts described above.
Secondly, we provided a negative control outcomes analyses using the risk of diabetes complications (primary code of hospitalization (ICD-9: 250.1-250.9, 357.2, 366.41; ICD-10: E10-E14 excluding E10.9, E11.9, E12.9, E13.0, E14.9). Lastly, we calculated an E-value to assess the impact of unmeasured confounding[41]. The E-value indicates how strongly an unmeasured confounder would have to be associated with use of apixaban 2.5 mg, or apixaban 5.0 mg vs warfarin and the outcomes to reduce the observed effect to the null, depending on the measured covariates. All analyses were performed using SAS software (version 9.4, SAS Institute, Cary, NC). A biomedical statistician performed statistical review of the study.
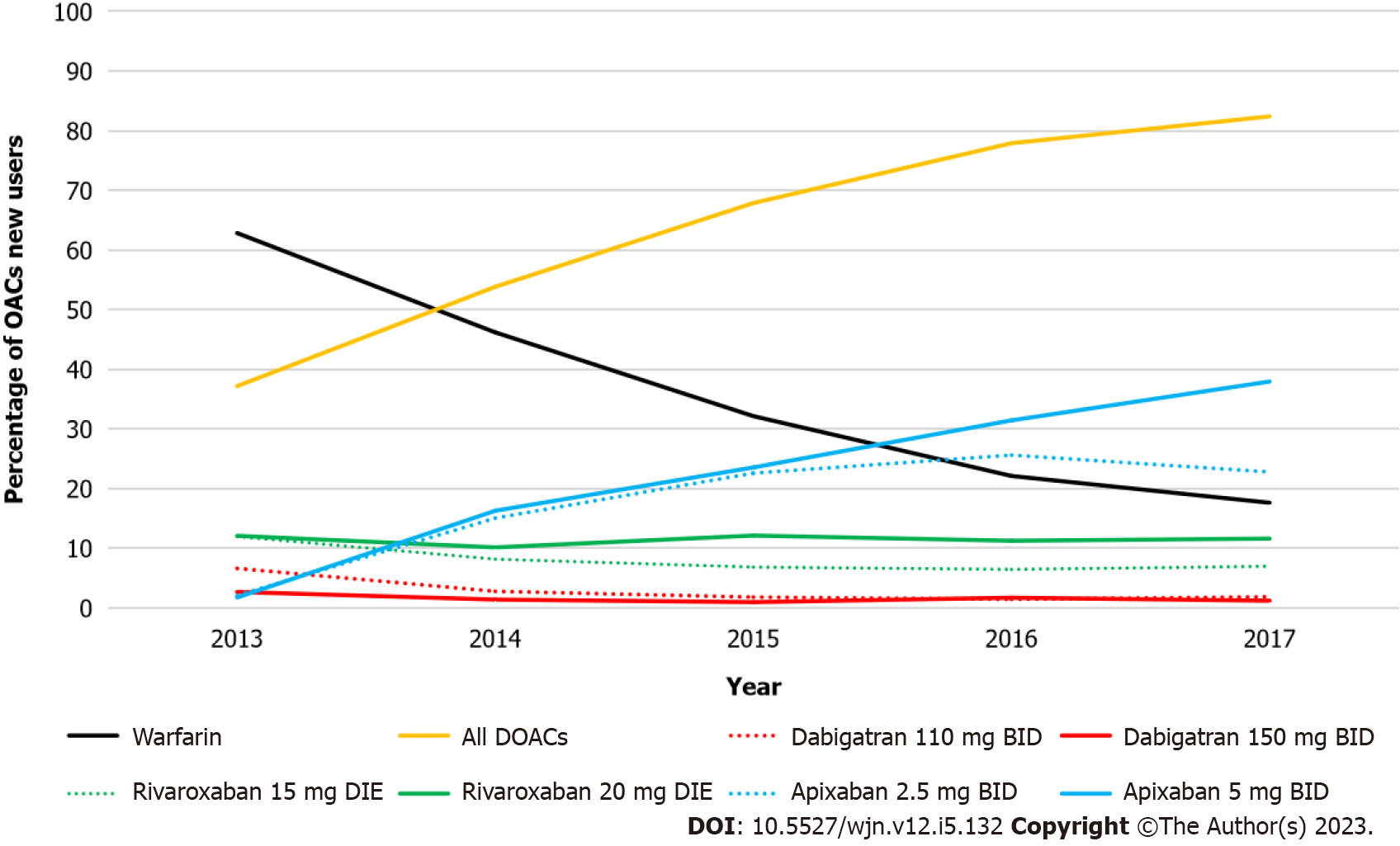
A total of 8899 included AF patients with stage III CKD filled a new OAC prescription: 3335 for warfarin, 744 for the 15-mg dose of rivaroxaban, 1064 for 20-mg rivaroxaban, 1674 for 2.5-mg apixaban, and 2082 for 5.0-mg apixaban (Figure 1). The frequency of warfarin prescriptions decreased over time and was associated with a concomitant increase in DOAC prescription (Figure 2). As of 2017, apixaban 5.0 mg was the most commonly initiated drug.
The patients’ unadjusted characteristics are summarized in Supplementary Tables 5-8. Compared with warfarin users, rivaroxaban 15 mg users were slightly younger (mean ± SD age: 83.0 ± 8.5 vs 82.6 ± 7.8, respectively) and had a lower mean ± SD Charlson-Deyo Comorbidity index (6.1 ± 3.4 vs 5.3 ± 3.5, respectively), a lower mean ± SD CHADS2 score (3.1 ± 1.2 vs 2.8 ± 1.2, respectively) and a lower mean ± SD HAS-BLED score of 3.6 ± 1.3 vs 3.2 ± 1.3, respectively. Compared with users of warfarin, rivaroxaban 20 mg users were younger (mean ± SD age: 83.0 ± 8.5 vs 74.2 ± 9.2, respectively) and had a lower mean ± SD Charlson-Deyo Comorbidity index (6.1 ± 3.4 vs 4.7 ± 3.5, respectively), a lower mean ± SD CHADS2 score (3.1 ± 1.2 vs 2.3 ± 1.2, respectively), and a lower mean ± SD HAS-BLED score (3.6 ± 1.3 vs 2.7 ± 1.3, respectively). Compared with warfarin users, apixaban 2.5 mg users were older (mean ± SD age: 83.0 ± 8.5 vs 86.5 ± 6.3, respectively), had a lower mean ± SD Charlson-Deyo Comorbidity index (6.1 ± 3.4 vs 5.4 ± 3.3, respectively), a similar mean ± SD CHADS2 score of 3.1 ± 1.2 vs 3.0 ± 1.1, respectively), and a similar mean ± SD HAS-BLED score of 3.6 ± 1.3 vs 3.3 ± 1.3, respectively. And, compared with users of warfarin, apixaban 5.0 mg users were also younger (mean ± SD age: 83.0 ± 8.5 vs 78.0 ± 8.4, respectively), and had a lower mean ± SD Charlson-Deyo Comorbidity index (6.1 ± 3.4 vs 5.1 ± 3.5, respectively), a lower mean ± SD CHADS2 score (3.1 ± 1.2 vs 2.6 ± 1.2, respectively), and a lower mean ± SD HAS-BLED score (3.6 ± 1.3 vs 3.0 ± 1.3, respectively). As shown in Table 1, demographic and clinical characteristics of cohorts of new OAC users with stage III CKD after IPTW from 2013 to 2017 are well balanced.
| IPTW warfarin and rivaroxaban 15 mg | IPTW warfarin and rivaroxaban 20 mg | IPTW warfarin and apixaban 2.5 mg | IPTW warfarin and apixaban 5.0 mg | |||||
| Warfarin (n = 3335) | Rivaroxaban 15 mg (n = 744) | Warfarin (n = 3335) | Rivaroxaban 20 mg (n = 1064) | Warfarin (n = 3335) | Apixaban 2.5 mg (n = 1674) | Warfarin (n = 3335) | Apixaban 5.0 mg (n = 2082) | |
| Age (yr) | 82.9 ± 8.6 | 82.8 ± 7.7 | 80.1 ± 10.8 | 79.1 ± 8.2 | 84.3 ± 8.3 | 84.5 ± 7.2 | 80.4 ± 10.2 | 80.2 ± 7.8 |
| Female sex | 56.5 | 56.5 | 54.8 | 52.3 | 58.2 | 59.0 | 54.3 | 53.9 |
| CHA2DS2-VASc | 4.1 ± 1.4 | 4.1 ± 1.3 | 3.9 ± 1.5 | 3.9 ± 1.5 | 4.2 ± 1.4 | 4.2 ± 1.3 | 3.9 ± 1.5 | 3.8 ± 1.3 |
| CHADS2 score | 3.0 ± 1.2 | 3.0 ± 1.2 | 2.8 ± 1.3 | 2.9 ± 1.2 | 3.0 ± 1.2 | 3.0 ± 1.1 | 2.9 ± 1.3 | 2.8 ± 1.1 |
| HAS-BLED score | 3.5 ± 1.3 | 3.5 ± 1.3 | 3.3 ± 1.5 | 3.4 ± 1.4 | 3.5 ± 1.3 | 3.5 ± 1.2 | 3.3 ± 1.4 | 3.3 ± 1.2 |
| Charlson-Deyo Comorbidity Index | 5.9 ± 3.4 | 6.0 ± 3.6 | 5.7 ± 3.5 | 5.9 ± 3.6 | 5.9 ± 3.4 | 5.8 ± 3.2 | 5.7 ± 3.5 | 5.7 ± 3.4 |
| Frailty score | 18.6 ± 6.2 | 18.6 ± 5.9 | 17.7 ± 6.7 | 17.6 ± 6.3 | 18.8 ± 6.2 | 18.8 ± 5.8 | 17.7 ± 6.6 | 17.5 ± 6.1 |
| Comorbidities (including the index hospitalization and the three years prior to cohort entry) | ||||||||
| Hypertension | 86.2 | 86.5 | 83.9 | 85.2 | 86.9 | 86.9 | 84.5 | 84.0 |
| Coronary artery disease | 64.7 | 65.1 | 62.0 | 62.7 | 64.1 | 62.5 | 61.2 | 58.4 |
| Acute myocardial infarction | 21.1 | 20.3 | 18.9 | 17.5 | 20.9 | 20.9 | 18.8 | 15.8 |
| Chronic heart failure | 56.2 | 56.7 | 53.4 | 54.2 | 56.1 | 56.6 | 53.3 | 53.6 |
| Cardiomyopathy | 7.9 | 8.3 | 7.7 | 8.7 | 7.4 | 7.1 | 8.2 | 7.8 |
| Other cardiac dysrhythmias | 18.8 | 19.0 | 17.8 | 16.4 | 19.2 | 19.4 | 18.2 | 18.0 |
| Valvular heart disease | 26.1 | 27.1 | 24.9 | 24.6 | 25.7 | 25.6 | 23.5 | 22.3 |
| Stroke/TIA | 16.9 | 17.2 | 16.0 | 17.3 | 16.8 | 16.1 | 15.9 | 15.0 |
| Peripheral vascular disease | 27.7 | 27.5 | 26.6 | 25.9 | 27.3 | 26.2 | 25.6 | 23.2 |
| Dyslipidemia | 54.4 | 53.3 | 54.2 | 55.8 | 53.9 | 54.0 | 55.5 | 53.8 |
| Diabetes | 45.1 | 46.1 | 46.4 | 49.9 | 43.1 | 42.7 | 47.3 | 46.9 |
| Major bleeding | 38.5 | 38.3 | 37.0 | 39.3 | 38.3 | 37.0 | 36.2 | 34.8 |
| Major intracranial bleeding | 3.7 | 3.7 | 3.5 | 5.7 | 3.9 | 3.5 | 4.2 | 3.8 |
| Major gastrointestinal bleeding | 8.3 | 9.5 | 7.9 | 7.7 | 8.7 | 8.1 | 7.9 | 7.9 |
| Other sites of major bleeding | 32.0 | 30.9 | 30.9 | 32.4 | 31.8 | 31.2 | 29.8 | 28.8 |
| Liver disease | 2.6 | 2.8 | 2.9 | 2.9 | 2.5 | 2.4 | 2.7 | 2.5 |
| Chronic obstructive pulmonary disease/asthma | 44.0 | 44.2 | 46.0 | 49.7 | 42.2 | 42.1 | 44.5 | 45.3 |
| Depression | 11.8 | 11.5 | 12.2 | 11.8 | 11.7 | 11.4 | 12.1 | 11.9 |
| Medical procedures (three years prior to cohort entry) | ||||||||
| Cardiac catheterization | 5.0 | 5.4 | 5.2 | 5.3 | 5.0 | 4.9 | 6.4 | 6.4 |
| Percutaneous coronary intervention-stent | 4.1 | 3.9 | 3.8 | 4.1 | 3.8 | 4.0 | 4.1 | 4.0 |
| Coronary artery bypass grafting | 0.6 | 0.5 | 0.8 | 0.7 | 0.5 | 0.5 | 1.2 | 1.2 |
| Implantable cardiac device | 0.1 | 0.1 | < 0.1 | < 0.1 | < 0.1 | 0.0 | < 0.1 | 0.0 |
| Medications (two weeks prior to cohort entry) | ||||||||
| Statin | 51.0 | 51.2 | 51.1 | 52.9 | 50.0 | 47.9 | 50.6 | 50.4 |
| Antiplatelet | 8.7 | 8.5 | 8.1 | 8.9 | 8.3 | 7.4 | 8.1 | 7.9 |
| Low-dose ASA | 35.3 | 35.5 | 34.8 | 35.2 | 35.6 | 34.9 | 33.9 | 33.4 |
| Proton pump inhibitors | 49.7 | 49.6 | 47.8 | 46.9 | 50.0 | 49.1 | 46.4 | 44.7 |
| NSAIDs | 0.9 | 0.9 | 1.3 | 1.6 | 0.9 | 1.0 | 1.2 | 1.2 |
| Digoxin | 9.3 | 10.6 | 9.1 | 10.1 | 9.2 | 9.0 | 8.9 | 8.7 |
| Amiodarone | 9.6 | 9.3 | 8.6 | 6.1 | 9.3 | 9.8 | 8.8 | 8.0 |
| Antidepressants | 10.5 | 10.1 | 10.4 | 10.5 | 10.6 | 10.3 | 10.2 | 10.4 |
| Beta-blockers | 62.5 | 63.1 | 61.4 | 61.1 | 63.8 | 62.8 | 62.4 | 62.5 |
| Calcium channel blockers | 42.9 | 42.6 | 41.9 | 39.8 | 42.7 | 43.2 | 41.4 | 41.0 |
| Inhibitors of the renin-angiotensin system | 37.5 | 36.9 | 38.1 | 38.5 | 38.0 | 36.5 | 38.0 | 38.3 |
| Diuretics | 60.5 | 60.3 | 61.4 | 61.0 | 61.0 | 60.8 | 60.3 | 60.4 |
| Loop diuretics | 56.2 | 55.8 | 57.0 | 54.4 | 56.4 | 56.7 | 55.4 | 56.3 |
| Antidiabetics | 27.4 | 28.0 | 28.7 | 30.3 | 26.5 | 25.8 | 29.2 | 29.3 |
| Health medical services (one year prior to cohort entry) | ||||||||
| Consultations with specialist physicians | 1.2 ± 2.4 | 1.2 ± 1.9 | 1.2 ± 2.5 | 1.2 ± 2.3 | 1.2 ± 2.4 | 1.2 ± 2.2 | 1.2 ± 2.6 | 1.2 ± 1.8 |
| Consultations with family physicians | 1.3 ± 3.3 | 1.3 ± 2.8 | 1.2 ± 3.1 | 1.2 ± 2.5 | 1.3 ± 3.4 | 1.4 ± 3.0 | 1.2 ± 3.2 | 1.2 ± 2.6 |
| Emergency visits | 3.4 ± 3.1 | 3.4 ± 2.9 | 3.4 ± 3.4 | 3.5 ± 3.0 | 3.4 ± 3.1 | 3.5 ± 2.9 | 3.4 ± 3.3 | 3.3 ± 2.8 |
| Health hospital services (three years prior to cohort entry) | ||||||||
| All-cause hospital admission | 2.5 ± 2.1 | 2.5 ± 2.0 | 2.5 ± 2.4 | 2.6 ± 2.2 | 2.4 ± 2.0 | 2.5 ± 1.9 | 2.5 ± 2.3 | 2.4 ± 2.0 |
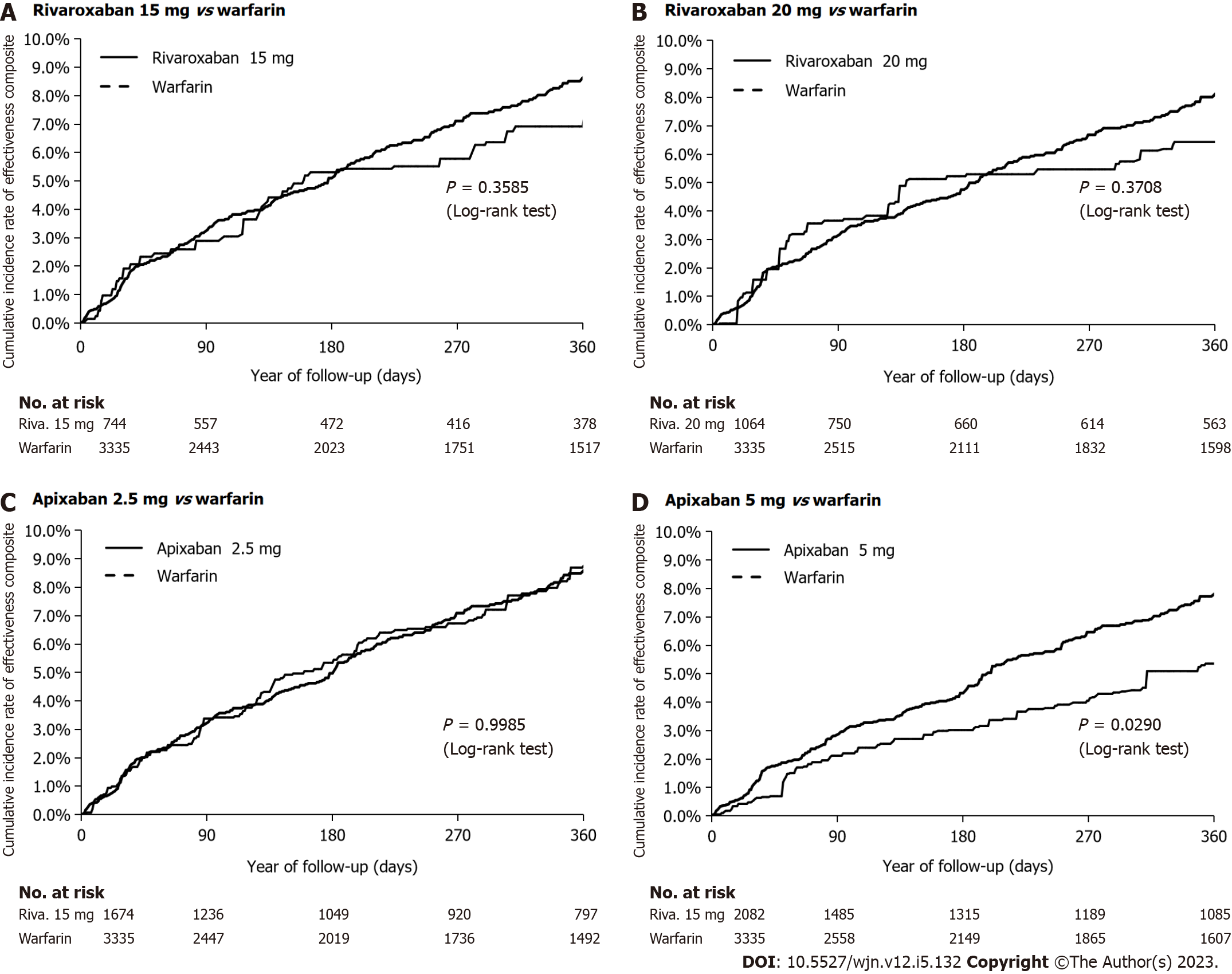
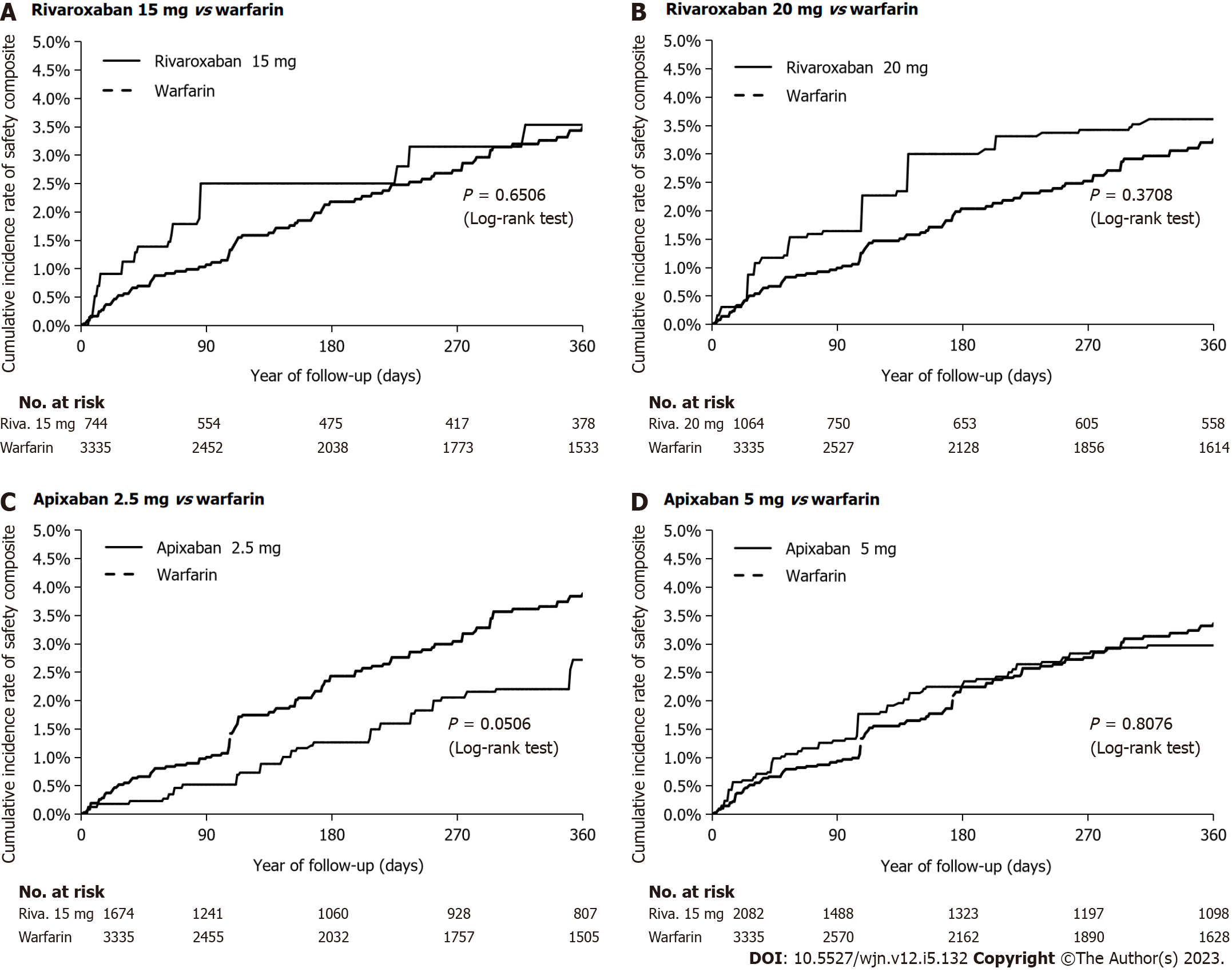
As shown in Table 1 and Supplementary Tables 5-8, there were no significant differences in baseline characteristics between the IPTW treatment groups. In Figures 3 and 4, we show the cumulative incidence curves for the effectiveness and safety composite outcomes in the IPTW in an on-treatment analysis. The follow-up times and levels of adherence are shown in Supplementary Tables 9 and 10.
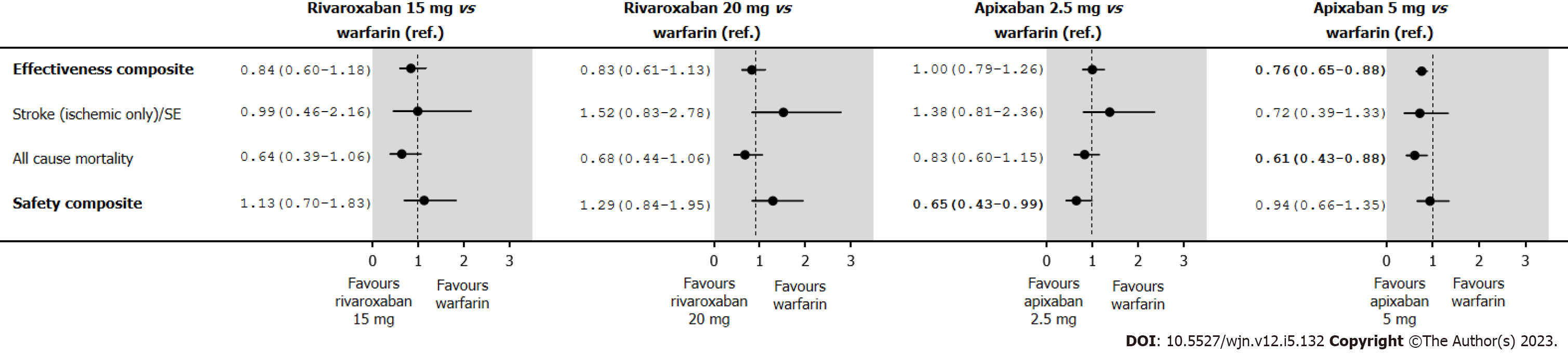
The annual rates and HRs for the primary analyses of the safety and effectiveness composites in the IPTW treatment groups in an on-treatment are shown in Supplementary Table 11. With warfarin as the reference group, we found rivaroxaban 15 mg and 20 mg had a similar effectiveness composite (HR 0.84; 95%CI: 0.60-1.18 and HR 0.83; 95%CI: 0.61-1.13, respectively) (Figure 5); and similar safety profile (HR 1.13; 95%CI: 0.70-1.83 and HR 1.29; 95%CI: 0.84-1.95, respectively). Apixaban 2.5 mg was similarly effective (HR 1.00; 95%CI: 0.79-1.26), but had a better safety profile (HR 0.65; 95%CI: 0.43-0.99), while apixaban 5.0 mg was associated with a better effectiveness (HR 0.76; 95%CI: 0.65-0.88), but a similar safety profile (HR 0.94; 95%CI: 0.66-1.35). A reduction in mortality (HR 0.61; 95%CI: 0.43-0.88) accounted for the observed improvement in the effectiveness composite for apixaban 5.0 mg.
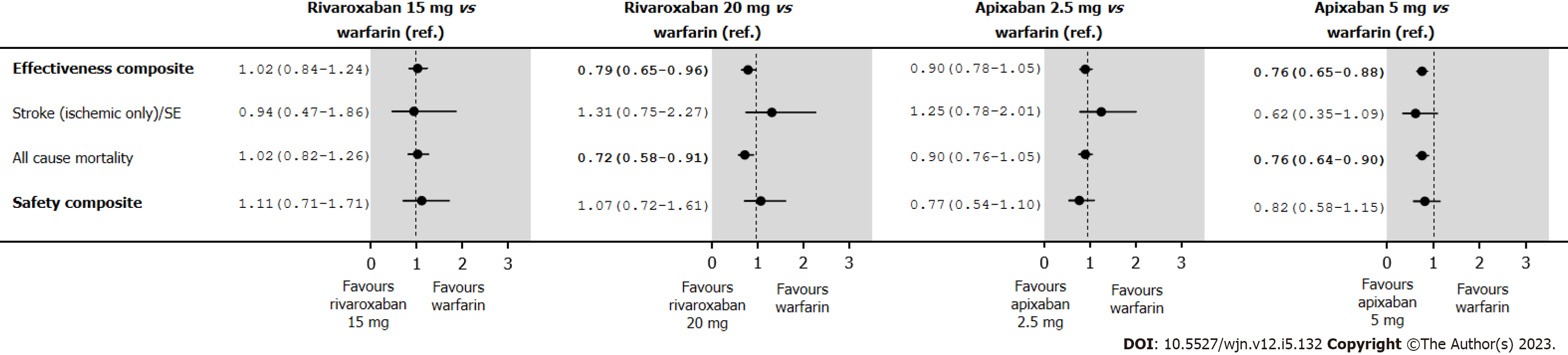
The annual rates and HRs for the analyses of the effectiveness and safety composites in the IPTW treatment groups in an intent-to-treat are shown in Supplementary Table 12. Under intent-to-treat analyses, rivaroxaban 20 mg presented a better effectiveness composite (HR 0.79; 95%CI: 0.65-0.96), and the observed improvement in the effectiveness composite was due to a reduction in mortality (HR 0.72; 95%CI: 0.58-0.91) (Figure 6). Those point estimates are in relation to those observed in the IPTW treatment groups in an on-treatment, and the level of significance is linked to an increase of the number of events, particularly among those in the warfarin group.
As shown in Table 2, warfarin and DOACs had a similar rate of hospitalization per 100 person-years for diabetes complications, with no significant HRs. As we expected, all groups had similar results. In Table 3, we found the E-value closest to boundary 1 for the effectiveness composite and apixaban 5.0 mg vs warfarin was 1.53; hence, we suspect an unmeasured confounder occurring 1.53 times more frequently in patients receiving apixaban 5.0 mg than in patients receiving warfarin, thus increasing the rate of safety composite events by a factor of 1.53. The high E-values indicate that the statistically significant results are robust with regards to unmeasured confounding factors.
| Incident rate of rivaroxaban 15 mg 100 PY (95%CI) | Incident rate of warfarin 100 PY (95%CI) | HR (95%CI)1 | P value | |
| Diabetes complications | 1.1 (0.2-2.0) | 1.1 (0.6-1.5) | 1.02 (0.40-2.60) | 0.96 |
| Incident rate of rivaroxaban 20 mg 100 PY (95%CI) | Incident rate of warfarin 100 PY (95%CI) | HR (95%CI)1 | P value | |
| Diabetes complications | 1.5 (0.6-2.4) | 1.0 (0.6-1.5) | 1.48 (0.72-3.06) | 0.29 |
| Incident rate of apixaban 2.5 mg 100 PY (95%CI) | Incident rate of warfarin 100 PY (95%CI) | HR (95%CI)1 | P value | |
| Diabetes complications | 0.8 (0.3-1.3) | 1.2 (0.8-1.7) | 0.66 (0.31-1.41) | 0.28 |
| Incident rate of apixaban 5.0 mg 100 PY (95%CI) | Incident rate of warfarin 100 PY (95%CI) | HR (95%CI) | P value | |
| Diabetes complications | 0.7 (0.2-1.1) | 1.4 (0.9-1.9) | 0.49 (0.24-1.02) | 0.06 |
| Hazard ratio (95%CI) | E value corresponding to the CI bound closest to 1 | E value for hazard ratio point estimate1 | |
| Apixaban 2.5 mg vs warfarin | |||
| Safety composite | 0.65 (0.43-0.99) | 1.11 | 2.45 |
| Apixaban 5.0 mg vs warfarin | |||
| Effectiveness composite | 0.76 (0.65-0.88) | 1.53 | 1.96 |
| All-cause mortality | 0.61 (0.43-0.88) | 1.53 | 2.66 |
The results of our cohort analysis provided several insights relevant to clinical practice. Firstly, DOAC prescription increased substantially over time, whereas warfarin prescription fell concomitantly. Nevertheless, over 10% of AF patients with stage III CKD were still being prescribed warfarin in 2017. Secondly, relative to warfarin, rivaroxaban appears to be safe and effective in AF patients with stage III CKD. Apixaban 2.5 mg might even have better safety profiles than warfarin; and for apixaban 5.0 mg, this difference in effectiveness was mainly driven by a reduction in deaths.
The increase in DOAC prescription is in line with the latest AF guidelines from the Canadian society of cardiology and European society of cardiology, which recommend DOAC therapy over warfarin for patients with NVAF and stage III CKD[7,42]. This recommendation is based on a sub-analysis of AF RCTs, which demonstrated that along with the DOACs’ logistic advantages vs dose-adjusted warfarin, these drugs are no worse or even better than warfarin for reducing the risk of AF-associated stroke or SE in AF patients with stage III CKD, with a lower or similar major bleeding risk[10-13]. A meta-analysis of RCTs and observational trials of AF patients with CKD showed that DOACs can provide a significant reduction in stroke/SE (HR 0.81; 95%CI: 0.68-0.97) and a nonsignificant reduction in major bleeding (HR 0.87; 95%CI: 0.69-1.05) in stage III CKD, when compared with warfarin[14].
Very little data exists regarding the effectiveness and safety of individual DOACs and the impact of various doses on patients with stage III CKD. Most of the existing data comes from observational studies[15-21]. Data from a sub-analysis of the Aristotle trial demonstrated that apixaban can effectively reduce the occurence of stroke, major bleeding, and mortality compared to that of warfarin among patients with impaired renal function (≤ 50 mL/min), when using creatinine-based estimates of GFR[13]. Wetmore et al[23] examined Medicare data from 22739 AF patients with stage III-IV CKD and found that apixaban reduced stroke/SE (HR 0.70; 95%CI: 0.51-0.96) and risk of major bleeding (HR 0.47; 95%CI: 0.37-0.59). Using electronic health record data, Fu et al[43] examined the safety and effectiveness of rivaroxaban vs warfarin in 555 stage III CKD AF patients and found a similar risk of stroke (HR 0.60; 95%CI: 0.23-1.56) and major bleeding (HR 0.73; 95%CI: 0.38-1.41). A subanalysis of the ROCKET-AF trial found that rivaroxaban 20 mg daily had a better efficacy profile in patients with a creatinine clearance (CrCl) of 50 mL/min or more but that rivaroxaban 15 mg daily had a similar efficacy profile in patients with a CrCl of 30-49 mL/min; the safety profile was similar for both CrCl categories[44]. Nonetheless, dose adjustment yielded results consistent with the overall trial, when compared with dose-adjusted warfarin[11]. Wetmore et al[23] found that in AF patients with stage III-IV CKD, rivaroxaban was associated with similar risks of stroke/SE (HR 0.80; 95%CI: 0.54-1.17) and major bleeding (HR 1.05; 95%CI: 0.85-1.30). However, the investigators did not report data on the effectiveness and safety of each dose level of DOAC vs warfarin in stage III CKD AF patients specifically.
Likewise, very few published studies have examined the impact of DOAC therapy vs warfarin on mortality, and also per specific dose. Makani et al[17] examined electronic health record data on 21733 AF patients with CKD and found that DOACs reduce the risk of all-cause mortality for all CKD classes. When examining individual DOACs in an on-treatment analysis, Wetmore et al[23] found a reduction in mortality for apixaban (HR 0.90; 95%CI: 0.84-0.96) but not for rivaroxaban (HR 0.95; 95%CI: 0.88-1.02) or dabigatran (HR 0.92; 95%CI: 0.84-1.01). These results might be explained by the fact that DOACs are associated with a lower incidence of renal adverse outcomes in patients with mild-to-moderate CKD, including declined renal function, a doubling in the serum creatinine level, or acute kidney injury[45]. Moreover, warfarin treatment is associated with an elevated risk of vascular and cardiac valve calcification[46-48], which in turn is associated with greater cardiovascular morbidity and mortality rates[49].
The present study has several strengths. First, it is one of the few large, real-world comparative studies of the effectiveness, safety, and mortality rates associated with individual DOACs and their dose levels vs warfarin. Second, we analyzed the single-payer health care claims database across the province of Quebec. Given that: (1) Most such clinical events result in an administrative claim, and (2) few patients in the province travel outside of Quebec for medical treatment, the study may likely have captured the vast majority of clinically significant events; which might not have been the case in previous single-hospital or single-insurer studies. Third, we performed IPTW cohorts by accounting for confounding effects in our primary analysis and we provided several sensitivity analyses.
Our study also had some limitations. First, observational studies of administrative data are subject to confounding bias by unadjusted factors, such as the severity of AF, the exact eGFR, the international normalized ratio, body weight, over-the-counter prescriptions, and ethnicity. Second, use of administrative claims depends on comprehensive, accurate coding and recording of all diagnoses, drugs, and procedures. Third, it might not be possible to generalize our results to younger patients, or patients treated with other DOACs (dabigatran and edoxaban). Fourth, the effect sizes for individual safety and effectiveness outcomes were small. Fifth, we could not use time spent in the therapeutic range to assess the appropriateness of warfarin dosing, since our database did not record the international normalized ratio. Finally, our study did not include exact eGFR values; however, we estimated eGFR using an algorithm known to be valid in older adults[31].
In this observational study of new OAC users with AF and stage III CKD, we found that rivaroxaban is safe and effective relative to warfarin but if CrCl is between 30-49 mL/min, we need to reduce the dose to 15 mg. Apixaban 2.5 mg might even have a better safety profile than warfarin, while apixaban 5.0 mg might have a better effectiveness profile than warfarin, including a reduction in deaths. Appropriately sized RCTs are needed to confirm these findings in stage III CKD patients.
The effectiveness and safety of apixaban and rivaroxaban in patients with atrial fibrillation (AF) and stage III chronic kidney disease (CKD) are not well established.
Few studies have evaluated the safety and efficacy of individual direct oral anticoagulants vs warfarin, nor have they established how dose selection impacts patients with AF and stage III CKD with respect to the incidence of stroke/systemic embolism (SE), major bleeding, and death.
We assessed and compared the effectiveness and safety of standard-dose rivaroxaban, low-dose rivaroxaban, standard-dose apixaban, and low-dose apixaban vs warfarin in a representative group of patients with AF and stage III CKD.
A cohort of new users of apixaban, rivaroxaban or warfarin in AF patients and stage III CKD was created using administrative databases. We defined the effectiveness as a composite of stroke, SE or death; safety was defined as a composite of major bleeding within 1-year of follow-up. Comparisons were under treatment analysis using inverse probability of treatment weighting and Cox models.
Rivaroxaban 15 mg and 20 mg were associated with a similar efficacy and safety composite risk vs warfarin. Apixaban 5.0 mg was linked with decreased effectiveness composite risk [hazard ratio (HR) 0.76; 0.65-0.88] and a similar safety risk (HR 0.94; 0.66-1.35), compared with apixaban 2.5 mg, which was associated with a similar effectiveness composite (HR 1.00; 0.79-1.26) and a lower safety risk (HR 0.65; 0.43-0.99).
This observational study of new users of rivaroxaban and apixaban find that both appear to be safe and effective compared to warfarin in patients with AF and stage III CKD. Apixaban 2.5 mg might even have a better safety profile than warfarin, while apixaban 5.0 mg might have a better effectiveness profile than warfarin, to a reduction in deaths.
The research perspective should be an appropriately sized randomized controlled trials to confirm these findings in AF patients with stage III CKD.
We sincerely appreciate the Régie d’Assurance Maladie du Québec and Quebec Health Ministry for their assistance with data management and the Commission d’accès à l’information for the authorization of the study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abrignani MG, Italy; Mehalingam V, India S-Editor: Qu XL L-Editor: A P-Editor: Yuan YY
| 1. | Ananthapanyasut W, Napan S, Rudolph EH, Harindhanavudhi T, Ayash H, Guglielmi KE, Lerma EV. Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Soliman EZ, Prineas RJ, Go AS, Xie D, Lash JP, Rahman M, Ojo A, Teal VL, Jensvold NG, Robinson NL, Dries DL, Bazzano L, Mohler ER, Wright JT, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Group. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC). Am Heart J. 2010;159:1102-1107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 338] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 3. | Baber U, Howard VJ, Halperin JL, Soliman EZ, Zhang X, McClellan W, Warnock DG, Muntner P. Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol. 2011;4:26-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 4. | Mitsuma W, Matsubara T, Hatada K, Imai S, Saito N, Shimada H, Miyazaki S. Clinical characteristics of hemodialysis patients with atrial fibrillation: The RAKUEN (Registry of atrial fibrillation in chronic kidney disease under hemodialysis from Niigata) study. J Cardiol. 2016;68:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Bonde AN, Lip GY, Kamper AL, Fosbøl EL, Staerk L, Carlson N, Torp-Pedersen C, Gislason G, Olesen JB. Renal Function and the Risk of Stroke and Bleeding in Patients With Atrial Fibrillation: An Observational Cohort Study. Stroke. 2016;47:2707-2713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Providência R, Marijon E, Boveda S, Barra S, Narayanan K, Le Heuzey JY, Gersh BJ, Gonçalves L. Meta-analysis of the influence of chronic kidney disease on the risk of thromboembolism among patients with nonvalvular atrial fibrillation. Am J Cardiol. 2014;114:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CC, Cox JL, Dorian P, Gladstone DJ, Healey JS, Khairy P, Leblanc K, McMurtry MS, Mitchell LB, Nair GM, Nattel S, Parkash R, Pilote L, Sandhu RK, Sarrazin JF, Sharma M, Skanes AC, Talajic M, Tsang TSM, Verma A, Verma S, Whitlock R, Wyse DG, Macle L; Members of the Secondary Panel. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Can J Cardiol. 2020;36:1847-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 392] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 8. | Marzec LN, Wang J, Shah ND, Chan PS, Ting HH, Gosch KL, Hsu JC, Maddox TM. Influence of Direct Oral Anticoagulants on Rates of Oral Anticoagulation for Atrial Fibrillation. J Am Coll Cardiol. 2017;69:2475-2484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 9. | Haas S, Camm AJ, Bassand JP, Angchaisuksiri P, Cools F, Corbalan R, Gibbs H, Jacobson B, Koretsune Y, Mantovani LG, Misselwitz F, Panchenko E, Ragy HI, Stepinska J, Turpie AG, Sawhney JP, Steffel J, Lim TW, Pieper KS, Virdone S, Verheugt FW, Kakkar AK; GARFIELD-AF Investigators. Predictors of NOAC versus VKA use for stroke prevention in patients with newly diagnosed atrial fibrillation: Results from GARFIELD-AF. Am Heart J. 2019;213:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Bohula EA, Giugliano RP, Ruff CT, Kuder JF, Murphy SA, Antman EM, Braunwald E. Impact of Renal Function on Outcomes With Edoxaban in the ENGAGE AF-TIMI 48 Trial. Circulation. 2016;134:24-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 218] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 11. | Fox KA, Piccini JP, Wojdyla D, Becker RC, Halperin JL, Nessel CC, Paolini JF, Hankey GJ, Mahaffey KW, Patel MR, Singer DE, Califf RM. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011;32:2387-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 424] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 12. | Hijazi Z, Hohnloser SH, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, Ezekowitz MD, Reilly PA, Siegbahn A, Yusuf S, Wallentin L. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation. 2014;129:961-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 13. | Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M, Keltai M, Lanas F, Lopes RD, Lopez-Sendon J, Granger CB, Wallentin L. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2012;33:2821-2830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 410] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 14. | Chen HY, Ou SH, Huang CW, Lee PT, Chou KJ, Lin PC, Su YC. Efficacy and Safety of Direct Oral Anticoagulants vs Warfarin in Patients with Chronic Kidney Disease and Dialysis Patients: A Systematic Review and Meta-Analysis. Clin Drug Investig. 2021;41:341-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Di Lullo L, Tripepi G, Ronco C, De Pascalis A, Barbera V, Granata A, Russo D, Di Iorio BR, Paoletti E, Ravera M, Fusaro M, Bellasi A. Safety and effectiveness of rivaroxaban and warfarin in moderate-to-advanced CKD: real world data. J Nephrol. 2018;31:751-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Siontis KC, Zhang X, Eckard A, Bhave N, Schaubel DE, He K, Tilea A, Stack AG, Balkrishnan R, Yao X, Noseworthy PA, Shah ND, Saran R, Nallamothu BK. Outcomes Associated With Apixaban Use in Patients With End-Stage Kidney Disease and Atrial Fibrillation in the United States. Circulation. 2018;138:1519-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 347] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 17. | Makani A, Saba S, Jain SK, Bhonsale A, Sharbaugh MS, Thoma F, Wang Y, Marroquin OC, Lee JS, Estes NAM, Mulukutla SR. Safety and Efficacy of Direct Oral Anticoagulants Versus Warfarin in Patients With Chronic Kidney Disease and Atrial Fibrillation. Am J Cardiol. 2020;125:210-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Schafer JH, Casey AL, Dupre KA, Staubes BA. Safety and Efficacy of Apixaban Versus Warfarin in Patients With Advanced Chronic Kidney Disease. Ann Pharmacother. 2018;52:1078-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Coleman CI, Kreutz R, Sood NA, Bunz TJ, Eriksson D, Meinecke AK, Baker WL. Rivaroxaban Versus Warfarin in Patients With Nonvalvular Atrial Fibrillation and Severe Kidney Disease or Undergoing Hemodialysis. Am J Med. 2019;132:1078-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 20. | Loo SY, Coulombe J, Dell'Aniello S, Brophy JM, Suissa S, Renoux C. Comparative effectiveness of novel oral anticoagulants in UK patients with non-valvular atrial fibrillation and chronic kidney disease: a matched cohort study. BMJ Open. 2018;8:e019638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Shin JI, Secora A, Alexander GC, Inker LA, Coresh J, Chang AR, Grams ME. Risks and Benefits of Direct Oral Anticoagulants across the Spectrum of GFR among Incident and Prevalent Patients with Atrial Fibrillation. Clin J Am Soc Nephrol. 2018;13:1144-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Liabeuf S, Laville SM, Bieber B, Tu C, Stengel B, Wong MMY, Calice da Silva V, Fliser D, Robinson BM, Pecoits-Filho R, Massy ZA. Prescription of Direct Oral Anticoagulants to Patients With Moderate-to-Advanced CKD: Too Little or Just Right? Kidney Int Rep. 2021;6:2496-2500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Wetmore JB, Roetker NS, Yan H, Reyes JL, Herzog CA. Direct-Acting Oral Anticoagulants Versus Warfarin in Medicare Patients With Chronic Kidney Disease and Atrial Fibrillation. Stroke. 2020;51:2364-2373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3667] [Cited by in RCA: 6835] [Article Influence: 621.4] [Reference Citation Analysis (0)] |
| 25. | Tamblyn R, Lavoie G, Petrella L, Monette J. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Québec. J Clin Epidemiol. 1995;48:999-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 415] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Eguale T, Winslade N, Hanley JA, Buckeridge DL, Tamblyn R. Enhancing pharmacosurveillance with systematic collection of treatment indication in electronic prescribing: a validation study in Canada. Drug Saf. 2010;33:559-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004;57:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 342] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 28. | Tamblyn R, Reid T, Mayo N, McLeod P, Churchill-Smith M. Using medical services claims to assess injuries in the elderly: sensitivity of diagnostic and procedure codes for injury ascertainment. J Clin Epidemiol. 2000;53:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 115] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Humphries KH, Jackevicius C, Gong Y, Svensen L, Cox J, Tu JV, Laupacis A; Canadian Cardiovascular Outcomes Research Team. Population rates of hospitalization for atrial fibrillation/flutter in Canada. Can J Cardiol. 2004;20:869-876. [PubMed] |
| 30. | Perreault S, Shahabi P, Côté R, Dumas S, Rouleau-Mailloux É, Feroz Zada Y, Provost S, Mongrain I, Dorais M, Huynh T, Kouz S, Diaz A, Blostein M, de Denus S, Turgeon J, Ginsberg J, Lelorier J, Lalonde L, Busque L, Kassis J, Talajic M, Tardif JC, Dubé MP. Rationale, design, and preliminary results of the Quebec Warfarin Cohort Study. Clin Cardiol. 2018;41:576-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Roy L, Zappitelli M, White-Guay B, Lafrance JP, Dorais M, Perreault S. Agreement Between Administrative Database and Medical Chart Review for the Prediction of Chronic Kidney Disease G category. Can J Kidney Health Dis. 2020;7:2054358120959908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Lambert L, Blais C, Hamel D, Brown K, Rinfret S, Cartier R, Giguère M, Carroll C, Beauchamp C, Bogaty P. Evaluation of care and surveillance of cardiovascular disease: can we trust medico-administrative hospital data? Can J Cardiol. 2012;28:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Levy AR, Tamblyn RM, Fitchett D, McLeod PJ, Hanley JA. Coding accuracy of hospital discharge data for elderly survivors of myocardial infarction. Can J Cardiol. 1999;15:1277-1282. [PubMed] |
| 34. | Andrade JG, Verma A, Mitchell LB, Parkash R, Leblanc K, Atzema C, Healey JS, Bell A, Cairns J, Connolly S, Cox J, Dorian P, Gladstone D, McMurtry MS, Nair GM, Pilote L, Sarrazin JF, Sharma M, Skanes A, Talajic M, Tsang T, Verma S, Wyse DG, Nattel S, Macle L; CCS Atrial Fibrillation Guidelines Committee. 2018 Focused Update of the Canadian Cardiovascular Society Guidelines for the Management of Atrial Fibrillation. Can J Cardiol. 2018;34:1371-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 35. | Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7532] [Cited by in RCA: 8637] [Article Influence: 261.7] [Reference Citation Analysis (0)] |
| 36. | D'Hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol. 1996;49:1429-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 773] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 37. | Crane SJ, Tung EE, Hanson GJ, Cha S, Chaudhry R, Takahashi PY. Use of an electronic administrative database to identify older community dwelling adults at high-risk for hospitalization or emergency department visits: the elders risk assessment index. BMC Health Serv Res. 2010;10:338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 38. | Fillion V, Sirois MJ, Gamache P, Guertin JR, Morin SN, Jean S. Frailty and health services use among Quebec seniors with non-hip fractures: a population-based study using adminsitrative databases. BMC Health Serv Res. 2019;19:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661-3679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1759] [Cited by in RCA: 2850] [Article Influence: 285.0] [Reference Citation Analysis (0)] |
| 40. | Allan V, Ramagopalan SV, Mardekian J, Jenkins A, Li X, Pan X, Luo X. Propensity score matching and inverse probability of treatment weighting to address confounding by indication in comparative effectiveness research of oral anticoagulants. J Comp Eff Res. 2020;9:603-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 41. | VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2080] [Cited by in RCA: 3464] [Article Influence: 433.0] [Reference Citation Analysis (0)] |
| 42. | Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3176] [Cited by in RCA: 6422] [Article Influence: 1605.5] [Reference Citation Analysis (0)] |
| 43. | Fu CM, Li LC, Lee YT, Wang SW, Hsu CN. Apixaban vs. Warfarin in Atrial Fibrillation Patients With Chronic Kidney Disease. Front Cardiovasc Med. 2021;8:752468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6519] [Cited by in RCA: 6901] [Article Influence: 492.9] [Reference Citation Analysis (2)] |
| 45. | Yao X, Tangri N, Gersh BJ, Sangaralingham LR, Shah ND, Nath KA, Noseworthy PA. Renal Outcomes in Anticoagulated Patients With Atrial Fibrillation. J Am Coll Cardiol. 2017;70:2621-2632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 46. | Koos R, Mahnken AH, Mühlenbruch G, Brandenburg V, Pflueger B, Wildberger JE, Kühl HP. Relation of oral anticoagulation to cardiac valvular and coronary calcium assessed by multislice spiral computed tomography. Am J Cardiol. 2005;96:747-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 47. | Shea MK, O'Donnell CJ, Hoffmann U, Dallal GE, Dawson-Hughes B, Ordovas JM, Price PA, Williamson MK, Booth SL. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr. 2009;89:1799-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 48. | Schurgers LJ, Aebert H, Vermeer C, Bültmann B, Janzen J. Oral anticoagulant treatment: friend or foe in cardiovascular disease? Blood. 2004;104:3231-3232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Elango K, Javaid A, Khetarpal BK, Ramalingam S, Kolandaivel KP, Gunasekaran K, Ahsan C. The Effects of Warfarin and Direct Oral Anticoagulants on Systemic Vascular Calcification: A Review. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |