Published online May 25, 2023. doi: 10.5527/wjn.v12.i3.66
Peer-review started: March 8, 2023
First decision: April 28, 2023
Revised: May 5, 2023
Accepted: May 17, 2023
Article in press: May 17, 2023
Published online: May 25, 2023
Processing time: 71 Days and 12.7 Hours
Anaplasmosis is a tick-borne disease with a range of clinical manifestations, from a flu-like illness with fever and myalgias to a severe systemic disease with multisystem organ failure. Although renal involvement is not a common presentation, there have been few cases reporting acute kidney injury from Anaplasmosis.
We present a 55-year-old female with anaplasmosis who developed acute kidney injury due to membranoproliferative glomerulonephritis (MPGN). The patient originally presented with cough and shortness of breath. She was admitted to the hospital with a diagnosis of community acquired pneumonia and received antibiotics. During the hospital course she developed severe acute renal failure. Initial serological work up didn’t provide any conclusive diagnosis. Hence, she underwent kidney biopsy which showed MPGN pattern suggesting autoimmune, multiple myeloma or infectious etiology. Extensive work up was undertaken which was negative for autoimmune diseases, vasculitis panel, paraproteinemias but tested positive for IgG anaplasma with high titers indicating Anaplasmosis.
Our case shows a unique presentation of severe acute renal failure from MPGN from tick borne illness. MPGN is usually seen with autoimmune diseases, hepatitis C virus infections, paraproteinemias. Hence, we suggest that tick borne illness should also be considered when evaluating acute renal failure cases in tick borne prevalent regions.
Core Tip: In areas endemic for tick borne illnesses, it is important to learn and understand common and rare presentations of tick borne illness. Our case is unique as it showed that tick borne illness can also cause severe renal failure by inciting Glomerulonephritis which is usually seen with other etiologies but very rarely with Anaplasmosis.
- Citation: Lathiya MK, Errabelli P, Mignano S, Cullinan SM. Infection related membranoproliferative glomerulonephritis secondary to anaplasmosis: A case report. World J Nephrol 2023; 12(3): 66-72
- URL: https://www.wjgnet.com/2220-6124/full/v12/i3/66.htm
- DOI: https://dx.doi.org/10.5527/wjn.v12.i3.66
Human granulocytic anaplasmosis (HGA) is a tickborne rickettsial disease caused by the bacterium Anaplasma phagocytophilum. It is a gram-negative intracellular bacterium transmitted by Ixodes scapularis tick. Typical symptoms of HGA include fever, malaise, headache, myalgias, and occasionally arthralgias[1]. We report a case that presented with cough, shortness of breath and acute kidney injury. Only 17 of the 110 reported patients of anaplasmosis had acute kidney injury[1]. To the best of our knowledge, this is the first report of a membranoproliferative glomerulonephritis (MPGN) nephritic pattern of acute kidney injury seen in a HGA infection.
A 55-year-old female presented to the Emergency Department with cough and shortness of breath that had persisted for three days.
The patients' medical history was pertinent for liver cirrhosis secondary to alcohol abuse, ascites requiring weekly paracentesis, chronic hyponatremia secondary to cirrhosis, chronic antibiotics for spontaneous bacterial peritonitis prophylaxis, and hypertension.
After evaluation, there was a concern for pneumonia, based on the basic laboratory tests and a chest x-ray. Her initial vitals were: 104 beats per minute heart rate, 28 per minute respiratory rate, 36.5 degrees Celsius temperature, 128/63 mmHg blood pressure, and 92%SpO2 on presentation to the emergency department. A complete physical examination revealed only significant rales in the left lower lung.
Her coronavirus disease 2019 polymerase chain reaction (PCR) resulted negative (Tables 1 and 2).
| Diagnostic Test | Normal value | Result |
| ANA | Negative | Negative |
| Serum DNA double-stranded antibody, IgG | < 30.0 (Negative) IU/mL | < 12.3 |
| Glomerular basement membrane IgG antibody | < 1.0 (Negative) U | < 0.2 |
| Serum myeloperoxidase antibody | < 0.4 (Negative) U | < 0.2 |
| Serum proteinase 3 antibody (PR3) | < 0.4 (Negative) U | < 0.2 |
| Serum complement C3 | 75-175 mg/dL | 42 |
| Serum complement C4 | 14-40 mg/dL | 8 |
| Serum haptoglobin | 30-200 mg/dL | 17 |
| Serum kappa free light chain | 0.3300-1.94 mg/dL | 9.28 |
| Serum lambda free light chain | 0.5700-2.63 mg/dL | 10.9 |
| Kappa/lambda FLC ratio | 0.2600-1.65 | 0.8514 |
| Serum protein electrophoresis | Negative monoclonal proteins | Negative monoclonal proteins |
| Serum antistrep-O titer | 0-530 IU/mL | 69 |
| Blood culture | Negative | Negative |
| Urine culture | Negative | Negative |
| SARS-CoV-2, PCR, rapid | Undetected | Undetected |
| EBV DNA, P | Undetected | Undetected |
| CMV DNA, P | Undetected | Undetected |
| Anaplasma phagocytophilum, PCR | Undetected | Undetected |
| Serum Anaplasma phagocytophilum antibody, IgG | < 1:64 titer | 1:512 |
| Ehrlichia chaffeensis, PCR | Negative | Negative |
| Ehrlichia chaffeensis (HME) antibody, IgG | < 1:64 | < 1:64 |
| Bartonella antibody panel, IgG and IgM | Negative | Negative |
| Serum Blastomyces antibody, EIA | Negative | Negative |
| Histoplasma antibody | Negative | Negative |
| HIV -1 Ab Screen, P | Negative | Negative |
| HIV-1 p24 Ag Screen, P | Negative | Negative |
| HIV Ag/Ab Screen, P | Negative | Negative |
| HIV-2 Ab Screen, P | Negative | Negative |
| Serum Mycoplasma antibody, IgM | Negative | Negative |
| Serum Mycoplasma antibody, IgG | Negative | Positive |
| Serum total hepatitis B core antibody | Negative | Negative |
| Diagnostic test | Normal value | At presentation | Hospital day 2 | Hospital day 6 | Hospital day 11 | Hospital day 15 | On discharge |
| Hemoglobin, g/dL | 11.6-15.0 | 8.9 | 8 | 9.1 | 9 | 6.8 | 7.3 |
| WBC count, ×109/L | 3.4-9.6 | 15.5 | 16.2 | 16.8 | 12.6 | 8.1 | 9.2 |
| Platelet count, ×109/L | 157-371 | 118 | 105 | 168 | 157 | 115 | 124 |
| Serum Urea Nitrogen, mg/dL | 45098 | 31 | 36 | 55 | 46 | 66 | 87 |
| Serum creatinine, mg/dL | 0.59-1.04 | 1.62 | 1.84 | 1.02 | 1.55 | 2.87 | 2.95 |
| Serum sodium | 135-145 mmol/L | 124 | 123 | 129 | 126 | 127 | 135 |
| Serum potassium | 3.6-5.2 mmol/L | 4.5 | 4.3 | 3.7 | 3.8 | 3.8 | 4 |
| Serum chloride | 98-107 mmol/L | 102 | 100 | 105 | 95 | 95 | 100 |
| Serum bicarbonate | 22-29 mmol/L | 11 | 11 | 10 | 20 | 20 | 20 |
| eGFR | ≥ 60 mL/min/BSA | 35 | 30 | 62 | 37 | 18 | 17 |
| Anion gap | 45122 | 11 | 12 | 12 | 9 | 12 | 15 |
| Serum calcium | 8.6-10.0 mg/dL | 7.7 | 7.3 | 7.8 | 7.2 | 7.3 | 7.6 |
| Serum albumin | 3.5-5.0 g/dL | 1.6 | 1.4 | 1.8 |
Chest x-ray showed bilateral patchy opacities and a subsequent computed tomography (CT) scan was performed, revealing pneumonia-like findings.
MPGN secondary to Anaplasmosis.
She was admitted to the hospital and started on empiric antibiotics for community-acquired pneumonia with cefepime and metronidazole. Her baseline creatinine was 0.6 to 1 mg/dL but during the hospital course she developed an acute kidney injury, with her creatinine reaching 1.6 mg/dL. Her acute kidney injury was thought to be caused by hepatorenal syndrome. She received albumin, midodrine, and octreotide.
On day 5, she underwent paracentesis due to underlying alcoholic liver cirrhosis with ascites. The paracentesis cultures were negative. On day 6, renal function recovered to baseline, but the leukocyte count remained persistently elevated, despite empirical treatment with cefepime and metronidazole. Her respiratory status did not improve, and a repeat chest CT was performed on day 7, revealing persistent bilateral opacities. Concurrently, her leukocytosis was worsening. Pulmonology was consulted and they have recommended diuresis to relieve the fluid overload and also recommended getting an echocardiogram. Her echocardiogram showed severe pulmonary hypertension, mild mitral regurgitation, and an ejection fraction of 60% without regional wall motion abnormalities.
During her hospital course, she developed anemia, with her hemoglobin dropping from 9 g/dL on day six to 6.8 g/dL on day fifteen. She was then transfused with two packed red blood cell (pRBC) units. The anemia evaluation revealed low haptoglobin, elevated lactate dehydrogenase, and a negative Coombs test. Peripheral blood smears lacked schistocytes but indicated that the patient had chronic normocytic anemia with elevated polychromasia in circulating nucleated erythrocytes.
Her renal function deteriorated again on day 11 with creatinine rising gradually to 2.9 mg/dL. Urinalysis revealed 11-20 white blood cell (WBC) per high-power field, more than 100 RBC, and a protein level of over 300, with a urine protein-to-creatinine ratio of 1.45. Serologies and a kidney biopsy were requested due to concerns over glomerulonephritis. Anti-neutrophil cytoplasmic antibody, serum protein electrophoresis, serum-free light chains, cryoglobulins, Anti streptolysin O antibody, and Legionella urine antigen tests were negative. Her serum C3 and C4 complement levels were both low, likely due to liver failure.
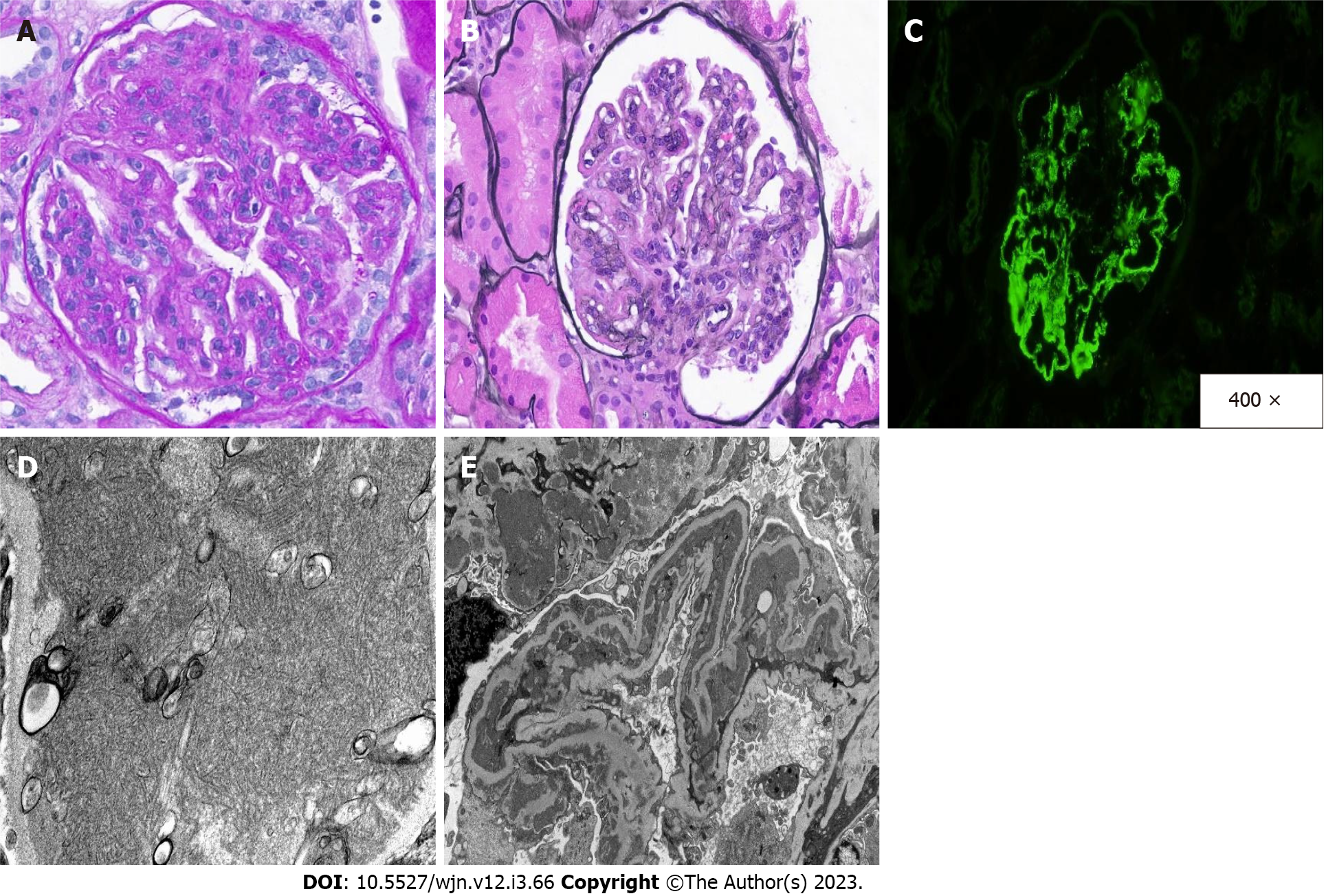
A kidney biopsy was performed. The biopsy revealed a MPGN pattern of injury. Immunofluorescence showed granular capillary wall deposits of IgA, IgG, and IgM, as well as C1q, kappa light chain, and lambda light chain, which are characteristic of immune-complex mediated MPGN with polyclonal deposits. Additionally, IgG subtype immunofluorescence was performed to exclude a monotypic antibody process. By subtyping, IgG subclasses were polytypic: IgG1, IgG2, and IgG3 were positive, and IgG4 was negative. By electron microscopy, the mesangial and peripheral capillary wall deposits were reiterated, but these electron-dense deposits showed an unusual fibrillary substructure (Figure 1). The differential diagnosis for such an injury pattern included autoimmune diseases, atypical infections, and hematologic processes, such as cryoglobulinemia. There was no evidence of an underlying autoimmune diseases, paraproteinemia, or cryoglobulinemia after a comprehensive evaluation.
As all her autoimmune tests and monoclonal protein studies came back negative, it was suggested that she should undergo a thorough investigation into infectious etiology. A repeat workup with urine cultures, blood cultures, human immunodeficiency virus (HIV) testing, viral hepatitis panel, Histoplasma antibodies, Blastomyces antibodies, Bartonella antibodies, and PCR for Epstein–Barr virus (EBV), Cytomegalovirus (CMV), Anaplasma, Methicillin-resistant Staphylococcus aureus, and streptococcal urine antigen were all negative. Her echocardiogram was negative for endocarditis. She had elevated Anaplasma antibody titers, IgG 1: 512. Infectious disease was consulted, and they advised starting doxycycline. She was started on doxycycline and was also started simultaneously on pulse steroids and prednisone taper as her kidney biopsy showed edema and inflammation in the renal parenchyma (Table 1).
But her kidney function continued to deteriorate. The patient was informed of the biopsy results and treatment options. She was not in need of dialysis immediately but informed her that she might need it if her renal function continued to worsen. With her other comorbidities, and after discussion, our patient decided to pursue comfort-only measures rather than aggressive treatment options and was discharged to home hospice.
Since the first reported case in Wisconsin in 1990, cases of HGA have steadily increased, rising from 348 cases in 2000 to 5655 cases in 2019 with a peak of 5762 cases in 2017. Anaplasmosis is commonly reported in the Upper Midwestern and Northeastern United States. However, only 88 case reports have been published thus far[1].
Tick bites are the most common mode of transmission for Anaplasmosis, mainly Ixodes ticks. The most common symptoms are fever, malaise, myalgias, headache, anorexia, and gastrointestinal symptoms such as diarrhea, nausea, vomiting, abdominal pain, cough, and occasionally a rash[1,2]. HGA symptoms and presentation are typically nonspecific and may be difficult to distinguish from other infections. Therefore, a high index of suspicion must be maintained. Diseases transmitted by the same vector, such as Lyme disease, Ehrlichiosis, and Babesiosis, are included in the differential diagnosis of Anaplasmosis; however, other zoonoses and non-vector-borne diseases should also be considered. Non-tick-borne infections, such as viral exanthems (human herpesvirus 6, EBV, enterovirus, adenovirus, and parvovirus B 19), and bacterial infections, are still considered in the differential diagnosis of anaplasmosis (Endocarditis, N. meningitides, N. gonorrhea, and secondary syphilis)[3,4]. Anaplasmosis is a clinical condition, however due to the nonspecific presentation, a broader workup to rule out other tick-borne diseases should be considered. Anaplasmosis causes mild anemia, thrombocytopenia, and leukopenia, as well as a mildly to moderately elevated liver function test[5]. A peripheral blood smear may show morulae within granulocytes, but this is not conclusive. The most conclusive tests are the indirect fluorescent antibody test and the polymerase chain reaction, which show a four-fold increase in IgG-specific antibodies and Anaplasma DNA in a whole blood sample, respectively[1].
Many complications of Anaplasmosis have been reported, which include end-organ damage such as acute kidney injury, rhabdomyolysis[2], multiorgan failure, non-traumatic splenic rupture, pancreatitis, secondary hemophagocytic lymphocytosis, and meningoencephalitis. Anaplasmosis has been linked to kidney disease, which is uncommon. In our case, the patient developed acute kidney injury due to MPGN from Anaplasmosis, which is very rare[6].
In this case, our patient was hospitalized with a diagnosis of community-acquired pneumonia and given antibiotics. During her hospitalization, she developed an acute kidney injury which we suspected was due to hepatorenal syndrome. Her kidney function initially improved with treatment, but it deteriorated again with a nephritic pattern of injury as evidenced by hematuria and proteinuria on urinalysis. In addition, her kidney biopsy revealed an unusual MPGN pattern of injury.
MPGN typically manifests in childhood, though it can manifest at any age. The clinical presentation and progression are extremely variable, ranging from benign to rapidly progressive. Thus, patients may exhibit asymptomatic, acute nephritic syndrome, nephrotic syndrome, or even rapidly progressive glomerulonephritis symptoms. In addition, the degree of kidney impairment varies based on the underlying lesions in the kidney, such that if a kidney biopsy reveals proliferative lesions, one is more likely to have a nephritic phenotype[3]; those with crescentic MPGN may present with rapidly progressive glomerulonephritis; and those with both repair and sclerosis are more likely to have a nephrotic phenotype. The MPGN pattern results from two underlying pathologies: (1) Immune complex-mediated; and (2) Complement-mediated. The immune-complex-mediated MPGN injury pattern is caused by circulating immunoglobulin or immune complexes, and etiologies include monoclonal gammopathy, autoimmune or rheumatologic disease, infection, or idiopathic[3]. This injury pattern is characterized by immune deposits that stain with either IgG or IgM predominance; typically complement staining is present, but not as strong as immunoglobulin staining. By contrast, complement-mediated MPGN—which encompasses C3 glomerulonephritis and dense deposit disease—typically will stain predominately with C3 and will typically have either genetic or acquired complement pathway alterations[3].
Monoclonal processes—such as proliferative glomerulonephritis with monoclonal deposits—can present with an MPGN pattern. However, unlike other immune-complex mediated MPGN, the immune complexes are monoclonal, not polyclonal, though an underlying B-cell/plasma cell clonal process is not always discovered or present[7]. In this case, immunofluorescence tests for both κ and λ light chains were positive, suggesting a polyclonal process. IgG subtype staining also showed polytypic distribution, further corroborating a polyclonal process[7,8]. Additionally, a normal κ:λ ratio and negative serum protein electrophoresis test for monoclonal proteins eliminated the need for a hematologic or oncologic evaluation.
However, infectious causes remained to be identified for MPGN-pattern nephritic renal injury. We performed a thorough infectious workup that included a viral hepatitis panel, Ehrlichia, Bartonella, Blastomyces, Histoplasma, HIV-1 and HIV-2 antibodies and antigens such as p24 and HIV Ag/Ab ratio, Anaplasmosis, Mycoplasma IgM and IgG, ascitic fluid studies, anti-strep-O titers, severe acute respiratory syndrome coronavirus 2, EBV, and CMV. Except for the anaplasmosis antibody, serum IgG 1:512 (normal IgG 1:64), which is 8 times higher than normal, all results were negative. This demonstrates that in our case, anaplasmosis is the underlying cause of the MPGN pattern nephritic etiology. Infectious disease was consulted, and a 14-d course of twice-daily doxycycline was given. She did not require dialysis and was started on high-dose prednisone with stabilization of her creatinine between 3.0 to 3.5 mg/dL and blood urea nitrogen between 130 and 140 mg/dL without significant uremic symptoms. Although she had anasarca, she did not require supplemental oxygen at the time of discharge. To control metabolic acidosis, sodium bicarbonate was added, and sevelamer was used for hyperphosphatemia. However, due to her current medical conditions and poor prognosis, the patient opted for comfort care, and she ultimately passed away.
Tick-borne diseases are relatively common, but early detection is essential. Due to obstacles such as the absence of objective, measurable evidence for these symptoms, it is difficult to determine whether a patient's complex of symptoms is the result of tick-borne disease or another cause. Due to these obstacles, there is a greater chance of overlooking tick-borne diseases as an etiology, which can result in complications and poor outcomes. Even though we reached a conclusion in our case and decided to initiate treatment, our patient had transitioned to comfort care measures only and declined further treatment. Due to the increasing prevalence of renal involvement and other complications in tick-borne diseases, it is crucial to diagnose and treat tick-borne diseases in their earliest and most prevalent stages.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koukoulaki M, Greece; Vlachopanos G, Greece S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Dumic I, Jevtic D, Veselinovic M, Nordstrom CW, Jovanovic M, Mogulla V, Veselinovic EM, Hudson A, Simeunovic G, Petcu E, Ramanan P. Human Granulocytic Anaplasmosis-A Systematic Review of Published Cases. Microorganisms. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 2. | Cho JM, Chang J, Kim DM, Kwak YG, Cho CR, Song JE. Human granulocytic anaplasmosis combined with rhabdomyolysis: a case report. BMC Infect Dis. 2021;21:1184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis--a new look at an old entity. N Engl J Med. 2012;366:1119-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 329] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 4. | Caster DJ, Summersgill JT, Paueksakon P, Massung RF, Shieh WJ, McLeish KR. Mixed cryoglobulinemia and secondary membranoproliferative glomerulonephritis associated with ehrlichiosis. CEN Case Rep. 2014;3:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Zhuo M, Calev H, Saunders SJ, Li J, Stillman IE, Danziger J. Acute Kidney Injury Associated With Human Granulocytic Anaplasmosis: A Case Report. Am J Kidney Dis. 2019;74:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | MacQueen D, Centellas F. Human Granulocytic Anaplasmosis. Infect Dis Clin North Am. 2022;36:639-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (1)] |
| 7. | Hemminger J, Nadasdy G, Satoskar A, Brodsky SV, Nadasdy T. IgG Subclass Staining in Routine Renal Biopsy Material. Am J Surg Pathol. 2016;40:617-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Howell DN, Gu X, Herrera GA. Organized deposits in the kidney and look-alikes. Ultrastruct Pathol. 2003;27:295-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |