Published online May 25, 2022. doi: 10.5527/wjn.v11.i3.96
Peer-review started: June 2, 2021
First decision: July 31, 2021
Revised: August 15, 2021
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: May 25, 2022
Processing time: 355 Days and 22.2 Hours
Kidney disease (KD) is characterized by the presence of elevated oxidative stress, and this is postulated as contributing to the high cardiovascular morbidity and mortality in these individuals. Chronic KD (CKD) is related to high grade inflammatory condition and pro-oxidative state that aggravates the progression of the disease by damaging primary podocytes. Liposoluble vitamins (vitamin A and E) are potent dietary antioxidants that have also anti-inflammatory and antiapoptotic functions. Vitamin deficits in CKD patients are a common issue, and multiple causes are related to them: Anorexia, dietary restrictions, food cooking methods, dialysis losses, gastrointestinal malabsorption, etc. The potential benefit of retinoic acid (RA) and α-tocopherol have been described in animal models and in some human clinical trials. This review provides an overview of RA and α tocopherol in KD.
Core Tip: Oxidative stress in patients with kidney disease (KD) is an important risk factor for cardiovascular disease. Vitamin A and E are important antioxidants with many roles in health and KD. High levels of vitamin A may have adverse health effects but higher levels of vitamin E have been associated with a lower overall mortality. Exogenous administration of these vitamins to patients with KD have shown controversial results.
- Citation: Rojo-Trejo MH, Robles-Osorio ML, Sabath E. Liposoluble vitamins A and E in kidney disease. World J Nephrol 2022; 11(3): 96-104
- URL: https://www.wjgnet.com/2220-6124/full/v11/i3/96.htm
- DOI: https://dx.doi.org/10.5527/wjn.v11.i3.96
Kidney disease (KD) is characterized by the presence of elevated oxidative stress and this is postulated to contributing to the high cardiovascular mortality in these individuals. Liposoluble vitamins (vitamins A and E) are potent dietary antioxidants that also have anti-inflammatory and antiapoptotic functions. Antioxidant therapies have been extensively used to decrease oxidative stress and cardiovascular disease (CVD) risk. In the kidneys, the beneficial effects of retinoic acid (RA) have been reported in multiple disease models, such as glomerulosclerosis, renal fibrosis, and acute kidney injury (AKI).
Vitamin E has a myriad of cellular effects, such as decreasing the synthesis of pro-inflammatory molecules and oxidative stress response, inhibiting the nuclear factor-kappaB (NF-kB) pathway, regulating cell cycle, and inhibiting the expression of pro-apoptotic factors that can have a positive impact on KD. The aim of this review is to present an overview about the impact of liposoluble vitamins on KD.
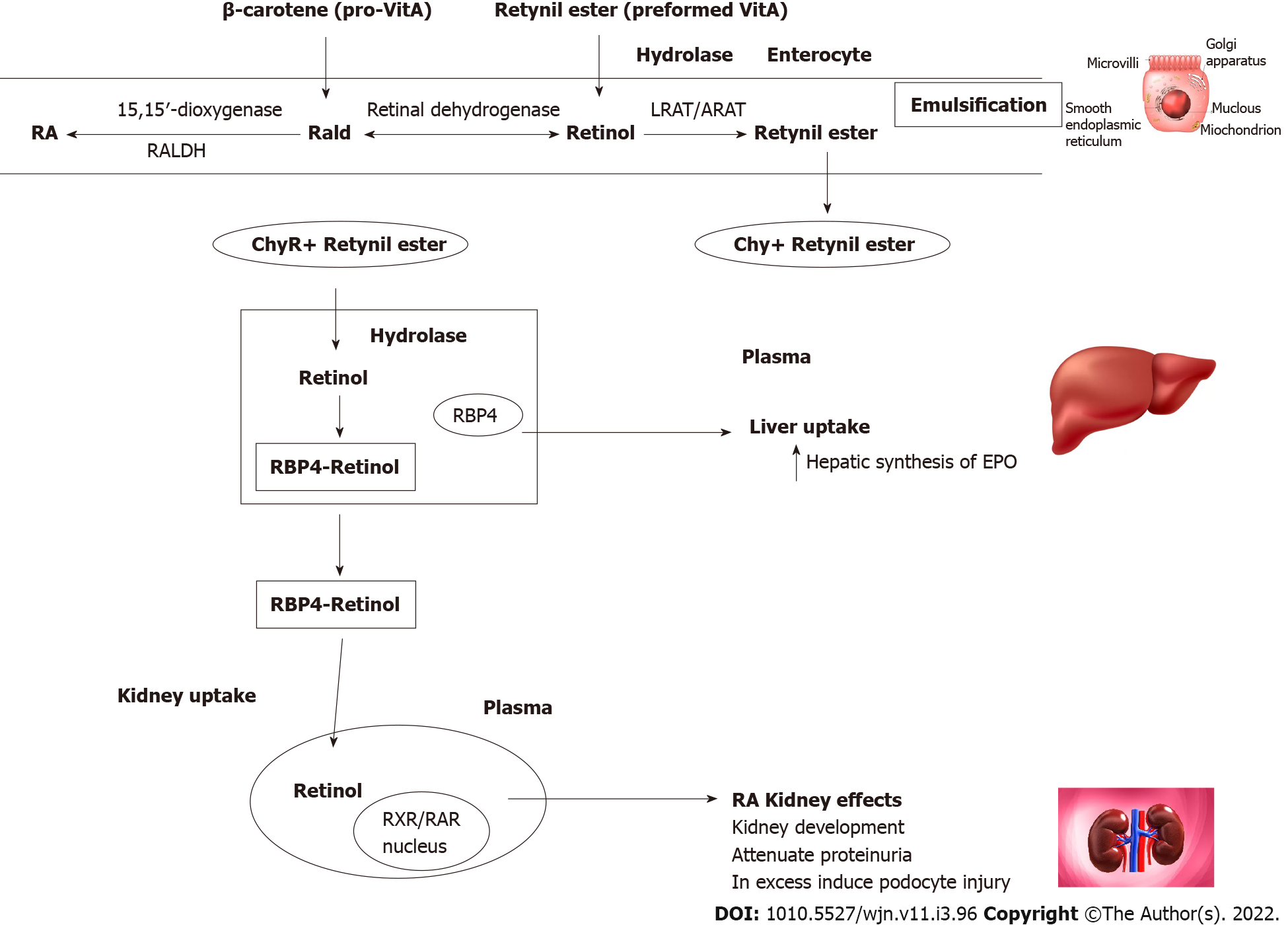
Vitamin A, is the name of a group of fat-soluble retinoids, including retinol and retinyl-esters that are essential for human survival; vitamin A is available into the human diet by intake of either food containing preformed vitamin A (e.g., red meats) or carotenoids (e.g., carrots and green leafy vegetables).
Retinoids are vital for human health and play a crucial role in the regulation of nocturnal vision, reproduction, immune function, and cell differentiation[1,2]. Recent advances in the study of retinoids metabolism have highlighted their importance in adipose tissue biology, glucose metabolism, and bone mineralization[3,4].
Most actions of retinol are mediated by its metabolite all-trans (AT)RA, which is synthesized intracellularly in target tissues from retinol[5]. Retinol is stored primarily as retinyl ester in the hepatic stellate cells, and to a lesser extent, in adipose tissue and other extrahepatic sites.
Retinoids regulate a number of physiological processes and through regulating the expression of over 500 genes; retinoids bind to nuclear receptors called RA receptors and retinoid X receptors, which themselves are DNA-binding transcriptional regulators and members of the nuclear hormone receptor family[6].
The liver plays a central role in vitamin A physiology. The retinol-binding protein 4 (RBP4) is secreted from the liver to bind and transport vitamin A to extrahepatic target tissues for intracellular ATRA synthesis. The primary physiological role of RBP4 is to guarantee a constant and continuous supply of retinol to peripheral tissues despite fluctuations in dietary vitamin A intake[7,8].
The kidney plays a key role in vitamin A homeostasis; findings of kinetic studies have revealed that approximately 50% of the circulating retinol pool originates in the kidneys. Retinol is filtered through the glomerular barrier and is then taken up in the proximal tubule by the endocytic receptor megalin; kidney-specific megalin deletion in mice, increases the urinary excretion of retinol and RBP4; in these mice, the syntheses of hepatic retinol and retinyl esters is reduced. These findings suggests a more complex role of the kidney in retinoid homeostasis[9]. More than 99% of retinol is reabsorbed by the proximal renal tubule; RBP4 has been identified as a very sensitive biomarker for proximal tubular cells dysfunction[10].
Patients with impaired renal function have been reported to have high circulating levels of retinol and RBP4, possibly due to a combination of decreased retinol-RBP4 complex clearance, reduced conversion of retinol to ATRA, and tissue accumulation of RBP4[11]. Dialysis patients have elevated serum levels of retinol and RBP4[12].
Increased RBP4 concentrations has been associated with an increased risk for osteoporosis, heart disease, and dyslipidemia. Furthermore, many studies have demonstrated an important link of RBP4 with adiposity, insulin resistance, and type II diabetes[4,13,14]. Interestingly, ATRA has been shown to be inversely associated with CVD and mortality in dialysis patients[12].
The most important food sources of vitamin A are liver, fish liver oil, dairy products (butter, milk, etc.), egg yolk, dark green leafy vegetables, and deeply colored yellow/orange vegetables and fruits[15]. The recommended dietary allowance for men and women is 900 and 700 μg retinol activity equivalents/d, respectively[16].
The Kidney Disease Outcomes Quality Initiative guideline no recommends routinely vitamin A supplementation (grade opinion), and there are no studies about the nutritional requirements in chronic KD (CKD) population[17]. There is no information about dietary recommendations in the pediatric population with CKD.
There are only a few studies that have evaluated vitamin A intake in CKD and dialysis subjects. In a cross-sectional study of 91 hemodialysis patients, only 23% of individuals covered vitamin A dietary recommendation[18]. As most sources of vitamin A have high potassium and phosphorous contents, the intake of vitamin A may be limited in advanced stages of CKD. Cooking techniques used to lower potassium in foods affect carotene concentration; boiling decreases up to 20%-30% of carotene content after 30 min, thereby making it more difficult to achieve adequate vitamin intake[19].
Vitamin A and its metabolites have a pivotal role during prenatal development, and vitamin A status is critical for the fetus. Maternal vitamin A deficiency is associated with preterm delivery, fetal death, or major congenital malformations in the offspring[20]. Studies in rodents suggest that retinol availability is essential in order to have an adequate renal development. Fetal retinol crosses the placental barrier from the maternal circulation and is converted to ATRA in peripheral tissues. Vitamin A deficiency has been associated in pregnant rats with mild renal hypoplasia in term fetuses; and the addition of ATRA to fetal rat kidneys cultured ex vivo accelerates new nephron formation[21-23].
The expression of the proto-oncogene c-ret, which plays an essential role in renal organogenesis, is modulated by retinoid environment. This indicates that the control of nephron mass by vitamin A may partly be mediated by the tyrosine kinase receptor ret, and this receptor modulates the ureteric bud branching morphogenesis[21].
In a cohort of 9-13 years old children in Nepal whose mothers participated in a randomized controlled trial of vitamin A supplementation before, during, and after pregnancy, the rate of hypertension or microalbuminuria did not differ by supplement group[24]. In conclusion, adequate vitamin A supply is crucial in determining final nephron numbers, and whether these findings have a prime role in the further development of CKD or hypertension is still controversial[25].
The glomerular filtration barrier consists of three layers: Fenestrated endothelial cells, glomerular basement membrane, and podocytes. Podocytes are specialized epithelial cells, whose major function is regulation of the glomerular filtration. Podocyte injury is implicated in many glomerular diseases including focal segmental glomerular sclerosis, diabetic KD, and human immunodeficiency virus (HIV)-associated nephropathy; loss of podocytes contributes to progressive KD as these cells have a low proliferative capacity. Research on podocytes and retinoids has been the subject of recent excellent reviews[26,27]. The pleiotropic effects of retinoids in animal models of KD are shown in Table 1. In HIV-1-transgenic mice, ATRA inhibits proliferation and induces differentiation in podocytes through cAMP/PKA activation[28].
| Drug | Animal model/disease/n | Outcome | |
| Animal | |||
| atRA | anti-Thy1.1 model rats | Mesangioproliferative glomerulonephritis | RA limits glomerular proliferation, glomerular lesions, and albuminuria. Marked reduction in renal TGF-β1. Reduction RAS activity[29] |
| atRA | HIV-1–transgenic mice | HIV associated kidney disease | atRA inhibits proliferation and induces differentiation in podocytes through RAR-mediated cAMP/PKA activation[28] |
| atRA | Streptozotocin-induced diabetic rats | Diabetic kidney disease | atRA decreases MCP-1 urinary excretion. Decreases proteinuria[34] |
| Tamibarotene | Male C57BL/6 mice | Unilateral ureteral obstruction | Inhibits the accumulation of fibrocytes and alleviates renal fibrosis mediated by IL-17A[64] |
| atRA | Atg5flox/flox:Cagg-Cre mice | Cisplatin nephrotoxicity | RA activates autophagy and alleviates cisplatin acute kidney injury[37] |
| atRA | Male rats | Unilateral ureteral obstruction | ATRA treatment can increase the angiopoitin-1 and decrease interstitial fibrosis[65] |
| Human | |||
| Isotretinoin | FSGS; MCD (shase II study) | 12 (only 6 completed the study) | No complete or partial remission at 6 mo (clinicaltrials.gov) |
| Tamibarotene | Lupus nephritis (phase II study) | 20 | Not published |
Retinoid treatment of rats with experimental mesangioproliferative glomerulonephritis causes a significant reduction in albuminuria, inflammation, and cell proliferation. Retinoids have been demonstrated to induce a marked reduction in renal transforming growth factor (TGF)-β1 and TGF receptor II expression[29]. NF-κB and nitric oxide synthase expression are reduced in mesangial cells after ATRA administration[30]. Renin-angiotensin system activity is also reduced[31]. Retinoids restore injured podocytes that regulate the transition of parietal epithelial cells to podocytes in rat models of glomerular inflammation (Figure 1)[32].
There are some reports of conspicuous clinical improvement in patients with lupus nephritis by using retinoid treatment[33]. In models of diabetic nephropathy, ATRA suppressed inflammatory changes and decreased proteinuria[34], and ATRA is significantly decreased in the cortex, which indicates that ATRA metabolism is markedly dysregulated in diabetic kidneys[35]. In Table 1 some postulated mechanisms of action of retinoid administration in animal models of KD and reported human clinical trials are described.
ATRA has been used therapeutically to reduce injury and fibrosis in models of AKI. ATRA signaling is activated in tubular epithelial cells and macrophages and reduces macrophage-dependent injury and fibrosis after AKI[36]. In models of cisplatin and contrast-induced AKI, retinoids activate autophagy, inhibit apoptosis, and decrease the oxidative status[37].
Erythropoietin (EPO) synthesis decreases in kidney failure, and some of the mechanisms proposed are the conversion of peritubular fibroblast into α-smooth muscle actin-expressing myofibroblasts, thereby losing their ability to secrete retinoids and EPO and defects in oxygen sensing[38]. Liver cells also synthesize EPO, and its contribution may increase when the kidneys are unable to maintain adequate levels for erythropoiesis[39]. ATRA is essential for hepatic production of EPO in early developmental stages and potentiates the EPO production through hypoxia-inducible factor signals and effectively improves renal anemia in mice[38].
The available evidence in cell cultures and animal models regarding the potential use of retinoids in the prevention and treatment of KD suggests that these compounds can effectively restore injured podocytes and decrease inflammation and interstitial fibrosis; however, a better understanding of retinoid signaling in renal cells is necessary to decreased toxicity and side effects of these compounds.
Vitamin E is a fat-soluble vitamin and the most abundant liposoluble antioxidant compound in the human body; α-tocopherol accounts for about 90% of the vitamin E activity in human tissues. Vitamin E is emulsified by the bile acids and absorbed in the form of micelles in the small intestine; α-tocopherol is mostly transported from the blood to the liver cells by chylomicrons, very low-density lipoproteins (LDL), and high-density lipoproteins (HDL)[40].
The specific α-tocopherol transfer protein (α-TTP) mediates the transport from the hepatic lysosomes into lipoproteins, whereas the excessive α-tocopherol and other forms of vitamin E are excreted in bile. The primary function of α-TTP is to maintain normal α-tocopherol concentrations in plasma and extrahepatic tissues. α-TTP is also expressed in the placenta, brain, spleen, lung, and kidney[41]. Besides the lipoprotein-lipase action, the delivery of α-tocopherol to tissues takes place by the uptake of lipoproteins throughout their corresponding receptors[42].
Vitamin E is present in various foods and oils such as nuts, seeds, vegetable oils, green leafy vegetables, and fortified cereals. The recommended dietary allowance for males and females aged ≥ 14 years is 15 mg daily (or 22 IU). In most countries, vitamin E deficiency is not prevalent and is usually associated with irregularities in the absorption of dietary fat. Previous studies have shown that subjects with CKD do not have the recommended micronutrient intake; however, the KDIGO nutritional guidelines do not recommend routine vitamin E supplementation[43].
Vitamin E localizes in the cell membrane and plays a key role in the regulation of redox interactions. Furthermore, it is considered one of the most important defenses against membrane lipid peroxidation and superoxide generation. It is the major antioxidant present in human lipoproteins, acts as a peroxyl-radical scavenger, and is a potent suppressor of LDL lipid oxidation; lipid oxidation has been implicated in chronic disease risk, including CVD and cancer[42,44]. Other important functions include the regulation of gene expression, improvement of immune response, inhibition of cell proliferation, and suppression of tumor angiogenesis[45]. In non-dialyzed and dialyzed CKD patients, plasma vitamin E levels are usually within the normal range; however, decreased α-tocopherol in red blood cell membranes of CKD subjects has been demonstrated[46].
Low levels of α-tocopherol in healthy subjects are associated with an increased risk for coronary artery disease[47], and higher intake has been shown to be protective; furthermore, recent studies suggest that higher α-tocopherol concentrations were related to a lower total mortality[48]. However, there is no information about tocopherol levels and mortality in CKD subjects, but some studies had been performed about vitamin E administration in this population.
Effects of vitamin E supplementation to ameliorate KD are controversial. The HOPE study found no beneficial effects of vitamin E administration on CVD mortality or renal complications[49]. Giannini et al[50] in a randomized trial in patients with Type 1 diabetes mellitus and persistent MA reported that vitamin E supplementation does not reduce albuminuria, but Khatami et al[51] found a significant decrease in urine protein excretion in T2 diabetic subjects.
The SPACE study performed in hemodialysis patients, found that high-dose α-tocopherol decreases the incidence of cardiovascular events but did not demonstrate a significant reduction in mortality[52]. Administration of α-tocopherol increases carboxy-ethyl-hydroxychromans with known potent anti-inflammatory and antioxidative properties[53], and a recent systematic review found that vitamin E administration reduces malondialdehyde in hyperactivity disorder (HD) patients; however, the effects on CVD or mortality were not particularly analyzed[54].
Vitamin E supplementation in HD subjects significantly improved the HDL function of cholesterol efflux capacity and in diabetic patients the endothelial function[55]. The use of vitamin E-coated dialyzer membranes may plausibly exert a site-specific scavenging effect on free radical species in synergy with reduced activation of neutrophils[56].
Vitamin E supplementation in CKD subjects is not recommended as has been shown to have no discernible effect on the overall mortality; one meta-analysis even demonstrated an increased mortality in healthy subjects who received a high dose of supplemented vitamin E[49,57]. Experimental and human clinical trials (Table 2) have demonstrated a role of vitamin E in preventing kidney injury. In the subtotal (5/6) nephrectomy remnant kidney model in the rat, α-tocopherol has the capacity to modulate both tubulointerstitial injury and glomerulosclerosis, inhibit the expression of TGF-β, and reduce plasma and kidney malondialdehyde concentration[58].
| Ref. | n | Dose | Inclusion criteria | Outcome |
| Mann et al[49] | 993 | 400 IU/d | 1.4 ≤ SCr ≤ 2.3 mg/dL. Plus CV disease or DM | Follow-up 4.5 yr. No apparent effect on CV outcomes |
| Giannini et al[50] | 10 | 1200 IU/d | Type 1 diabetes mellitus plus macroalbuminuria | Reduces markers of oxidative stress. No effect on MA |
| Khatami et al[51] | 60 | 1200 IU/d | Diabetic nephropathy | Decrease in protein/creatinine ratio. Reduction in inflammatory markers |
| Boaz et al[52] | 196 | 800 IU/d | Hemodialysis patients | Reduces CV disease |
| Himmelfarb et al[53] | 30 | 300 IU/d | 15 healthy subjects, 15 hemodialysis patients | Reduction on C reactive protein |
| Bergin et al[54] | Meta-analysis 16 papers | Reduction oxidative stress | ||
| Mune et al[55] | 40 | 300 mg/d | Hemodialysis subjects | Improvement in endothelial function |
Animal models have exhibited beneficial effects of vitamin E administration in the prevention of diabetic nephropathy by inhibition of the protein kinase C pathway and normalizing diacylglycerol cellular levels[59]. Tocotrienols are members of the vitamin E family with potent anti-oxidant activity; in db/db mice, T3β administration increased adiponectin levels and improved renal function[60].
Experimental immunoglobulin A nephropathy in rats is associated with increased renal oxidant injury, and dietary treatment with vitamin E has been reported to attenuate functional and structural changes[61]. The amelioration of renal injury by dietary α-tocopherol supplementation has also been observed in unilateral ureter obstruction[62] and puromycin aminonucleoside nephropathy[63]. There is still no robust evidence supporting the widespread use of vitamin E as a therapy for retarding chronic KD. Future studies with longer follow-up and larger sample size are necessary before any helpful recommendation.
RA and α-tocopherol have numerous cellular functions that can have an effect on kidney injury progression; however, further extensive research is needed before making clinical recommendations. Higher intake of natural carotenoids and tocopherols have been proven to have a beneficial impact on overall mortality, but supplementation with either of the two vitamins has not manifested any notable effect on the decrease in mortality of patients with CKD.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Society of Nephrology, 145569.
Specialty type: Urology and nephrology
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanaka H, Japan S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | D'Ambrosio DN, Clugston RD, Blaner WS. Vitamin A metabolism: an update. Nutrients. 2011;3:63-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 440] [Cited by in RCA: 378] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 2. | Rodriguez-Concepcion M, Avalos J, Bonet ML, Boronat A, Gomez-Gomez L, Hornero-Mendez D, Limon MC, Meléndez-Martínez AJ, Olmedilla-Alonso B, Palou A, Ribot J, Rodrigo MJ, Zacarias L, Zhu C. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog Lipid Res. 2018;70:62-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 531] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 3. | Blaner WS. Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol Ther. 2019;197:153-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 4. | Conaway HH, Henning P, Lerner UH. Vitamin a metabolism, action, and role in skeletal homeostasis. Endocr Rev. 2013;34:766-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Kedishvili NY. Retinoic Acid Synthesis and Degradation. Subcell Biochem. 2016;81:127-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Ziouzenkova O, Plutzky J. Retinoid metabolism and nuclear receptor responses: New insights into coordinated regulation of the PPAR-RXR complex. FEBS Lett. 2008;582:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Saeed A, Dullaart RPF, Schreuder TCMA, Blokzijl H, Faber KN. Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients. 2017;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 8. | Steinhoff JS, Lass A, Schupp M. Biological Functions of RBP4 and Its Relevance for Human Diseases. Front Physiol. 2021;12:659977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 9. | Raila J, Willnow TE, Schweigert FJ. Megalin-mediated reuptake of retinol in the kidneys of mice is essential for vitamin A homeostasis. J Nutr. 2005;135:2512-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Waikar SS, Bonventre JV. Biomarkers for the diagnosis of acute kidney injury. Nephron Clin Pract. 2008;109:c192-c197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Jing J, Isoherranen N, Robinson-Cohen C, Petrie I, Kestenbaum BR, Yeung CK. Chronic Kidney Disease Alters Vitamin A Homeostasis via Effects on Hepatic RBP4 Protein Expression and Metabolic Enzymes. Clin Transl Sci. 2016;9:207-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Kalousová M, Kubena AA, Kostírová M, Vinglerová M, Ing OM, Dusilová-Sulková S, Tesar V, Zima T. Lower retinol levels as an independent predictor of mortality in long-term hemodialysis patients: a prospective observational cohort study. Am J Kidney Dis. 2010;56:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Su Y, Huang Y, Jiang Y, Zhu M. The Association between Serum Retinol-Binding Protein 4 Levels and Cardiovascular Events in Patients with Chronic Kidney Disease. Lab Med. 2020;51:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Henze A, Frey SK, Raila J, Scholze A, Spranger J, Weickert MO, Tepel M, Zidek W, Schweigert FJ. Alterations of retinol-binding protein 4 species in patients with different stages of chronic kidney disease and their relation to lipid parameters. Biochem Biophys Res Commun. 2010;393:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. Biomarkers of Nutrition for Development (BOND)-Vitamin A Review. J Nutr. 2016;146:1816S-1848S. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 308] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 16. | Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press (US), 2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 146] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero JJ, Chan W, Fouque D, Friedman AN, Ghaddar S, Goldstein-Fuchs DJ, Kaysen GA, Kopple JD, Teta D, Yee-Moon Wang A, Cuppari L. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am J Kidney Dis. 2020;76:S1-S107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 1028] [Article Influence: 205.6] [Reference Citation Analysis (0)] |
| 18. | Luis D, Zlatkis K, Comenge B, García Z, Navarro JF, Lorenzo V, Carrero JJ. Dietary Quality and Adherence to Dietary Recommendations in Patients Undergoing Hemodialysis. J Ren Nutr. 2016;26:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Buratti S, Cappa C, Benedetti S, Giovanelli G. Influence of Cooking Conditions on Nutritional Properties and Sensory Characteristics Interpreted by E-Senses: Case-Study on Selected Vegetables. Foods. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Wiseman EM, Bar-El Dadon S, Reifen R. The vicious cycle of vitamin a deficiency: A review. Crit Rev Food Sci Nutr. 2017;57:3703-3714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 21. | Lelièvre-Pégorier M, Vilar J, Ferrier ML, Moreau E, Freund N, Gilbert T, Merlet-Bénichou C. Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int. 1998;54:1455-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 175] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Goodyer P, Kurpad A, Rekha S, Muthayya S, Dwarkanath P, Iyengar A, Philip B, Mhaskar A, Benjamin A, Maharaj S, Laforte D, Raju C, Phadke K. Effects of maternal vitamin A status on kidney development: a pilot study. Pediatr Nephrol. 2007;22:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Gilbert T, Merlet-Bénichou C. Retinoids and nephron mass control. Pediatr Nephrol. 2000;14:1137-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Stewart CP, Christian P, Katz J, Schulze KJ, Wu LS, LeClerq SC, Shakya TR, Khatry SK, West KP. Maternal supplementation with vitamin A or β-carotene and cardiovascular risk factors among pre-adolescent children in rural Nepal. J Dev Orig Health Dis. 2010;1:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Bhat PV, Manolescu DC. Role of vitamin A in determining nephron mass and possible relationship to hypertension. J Nutr. 2008;138:1407-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Mallipattu SK, He JC. The beneficial role of retinoids in glomerular disease. Front Med (Lausanne). 2015;2:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Chen A, Liu Y, Lu Y, Lee K, He JC. Disparate roles of retinoid acid signaling molecules in kidney disease. Am J Physiol Renal Physiol. 2021;320:F683-F692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | He JC, Lu TC, Fleet M, Sunamoto M, Husain M, Fang W, Neves S, Chen Y, Shankland S, Iyengar R, Klotman PE. Retinoic acid inhibits HIV-1-induced podocyte proliferation through the cAMP pathway. J Am Soc Nephrol. 2007;18:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Morath C, Dechow C, Lehrke I, Haxsen V, Waldherr R, Floege J, Ritz E, Wagner J. Effects of retinoids on the TGF-beta system and extracellular matrix in experimental glomerulonephritis. J Am Soc Nephrol. 2001;12:2300-2309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Na SY, Kang BY, Chung SW, Han SJ, Ma X, Trinchieri G, Im SY, Lee JW, Kim TS. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFkappaB. J Biol Chem. 1999;274:7674-7680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 187] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Dechow C, Morath C, Peters J, Lehrke I, Waldherr R, Haxsen V, Ritz E, Wagner J. Effects of all-trans retinoic acid on renin-angiotensin system in rats with experimental nephritis. Am J Physiol Renal Physiol. 2001;281:F909-F919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Zhang J, Pippin JW, Vaughan MR, Krofft RD, Taniguchi Y, Romagnani P, Nelson PJ, Liu ZH, Shankland SJ. Retinoids augment the expression of podocyte proteins by glomerular parietal epithelial cells in experimental glomerular disease. Nephron Exp Nephrol. 2012;121:e23-e37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Kinoshita K, Kishimoto K, Shimazu H, Nozaki Y, Sugiyama M, Ikoma S, Funauchi M. Successful treatment with retinoids in patients with lupus nephritis. Am J Kidney Dis. 2010;55:344-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Han SY, So GA, Jee YH, Han KH, Kang YS, Kim HK, Kang SW, Han DS, Han JY, Cha DR. Effect of retinoic acid in experimental diabetic nephropathy. Immunol Cell Biol. 2004;82:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Starkey JM, Zhao Y, Sadygov RG, Haidacher SJ, Lejeune WS, Dey N, Luxon BA, Kane MA, Napoli JL, Denner L, Tilton RG. Altered retinoic acid metabolism in diabetic mouse kidney identified by O isotopic labeling and 2D mass spectrometry. PLoS One. 2010;5:e11095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Chiba T, Skrypnyk NI, Skvarca LB, Penchev R, Zhang KX, Rochon ER, Fall JL, Paueksakon P, Yang H, Alford CE, Roman BL, Zhang MZ, Harris R, Hukriede NA, de Caestecker MP. Retinoic Acid Signaling Coordinates Macrophage-Dependent Injury and Repair after AKI. J Am Soc Nephrol. 2016;27:495-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 37. | Wu J, Zheng C, Wan X, Shi M, McMillan K, Maique J, Cao C. Retinoic Acid Alleviates Cisplatin-Induced Acute Kidney Injury Through Activation of Autophagy. Front Pharmacol. 2020;11:987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Katagiri N, Hitomi H, Mae SI, Kotaka M, Lei L, Yamamoto T, Nishiyama A, Osafune K. Retinoic acid regulates erythropoietin production cooperatively with hypoxia-inducible factors in human iPSC-derived erythropoietin-producing cells. Sci Rep. 2021;11:3936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | de Seigneux S, Lundby AK, Berchtold L, Berg AH, Saudan P, Lundby C. Increased Synthesis of Liver Erythropoietin with CKD. J Am Soc Nephrol. 2016;27:2265-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Packer L, Weber SU, Rimbach G. Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling. J Nutr. 2001;131:369S-373S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 316] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 41. | Herrera E, Barbas C. Vitamin E: action, metabolism and perspectives. J Physiol Biochem. 2001;57:43-56. [PubMed] |
| 42. | Azzi A, Stocker A. Vitamin E: non-antioxidant roles. Prog Lipid Res. 2000;39:231-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 289] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 43. | Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. 2014;72:76-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 569] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 44. | Miyazawa T, Burdeos GC, Itaya M, Nakagawa K, Miyazawa T. Vitamin E: Regulatory Redox Interactions. IUBMB Life. 2019;71:430-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 45. | Schindler R, Mentlein R. Flavonoids and vitamin E reduce the release of the angiogenic peptide vascular endothelial growth factor from human tumor cells. J Nutr. 2006;136:1477-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 46. | Uzum A, Toprak O, Gumustas MK, Ciftci S, Sen S. Effect of vitamin E therapy on oxidative stress and erythrocyte osmotic fragility in patients on peritoneal dialysis and hemodialysis. J Nephrol. 2006;19:739-745. [PubMed] |
| 47. | De Waart FG, Schouten EG, Stalenhoef AF, Kok FJ. Serum carotenoids, alpha-tocopherol and mortality risk in a prospective study among Dutch elderly. Int J Epidemiol. 2001;30:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Huang J, Weinstein SJ, Yu K, Männistö S, Albanes D. Relationship Between Serum Alpha-Tocopherol and Overall and Cause-Specific Mortality. Circ Res. 2019;125:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 49. | Mann JF, Lonn EM, Yi Q, Gerstein HC, Hoogwerf BJ, Pogue J, Bosch J, Dagenais GR, Yusuf S; HOPE Investigators. Effects of vitamin E on cardiovascular outcomes in people with mild-to-moderate renal insufficiency: results of the HOPE study. Kidney Int. 2004;65:1375-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 50. | Giannini C, Lombardo F, Currò F, Pomilio M, Bucciarelli T, Chiarelli F, Mohn A. Effects of high-dose vitamin E supplementation on oxidative stress and microalbuminuria in young adult patients with childhood onset type 1 diabetes mellitus. Diabetes Metab Res Rev. 2007;23:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Khatami PG, Soleimani A, Sharifi N, Aghadavod E, Asemi Z. The effects of high-dose vitamin E supplementation on biomarkers of kidney injury, inflammation, and oxidative stress in patients with diabetic nephropathy: A randomized, double-blind, placebo-controlled trial. J Clin Lipidol. 2016;10:922-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, Knecht A, Weissgarten Y, Brunner D, Fainaru M, Green MS. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet. 2000;356:1213-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 671] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 53. | Himmelfarb J, Kane J, McMonagle E, Zaltas E, Bobzin S, Boddupalli S, Phinney S, Miller G. Alpha and gamma tocopherol metabolism in healthy subjects and patients with end-stage renal disease. Kidney Int. 2003;64:978-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 54. | Bergin P, Leggett A, Cardwell CR, Woodside JV, Thakkinstian A, Maxwell AP, McKay GJ. The effects of vitamin E supplementation on malondialdehyde as a biomarker of oxidative stress in haemodialysis patients: a systematic review and meta-analysis. BMC Nephrol. 2021;22:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 55. | Mune M, Uto-Kondo H, Iteya I, Fujii Y, Ikeda S, Ikewaki K. Vitamin E supplementation improves high-densitiy lipoprotein and endothelial functions in end-stage kidney disease patients undergoing hemodialysis . Clin Nephrol. 2018;90:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Yang CC, Hsu SP, Wu MS, Hsu SM, Chien CT. Effects of vitamin C infusion and vitamin E-coated membrane on hemodialysis-induced oxidative stress. Kidney Int. 2006;69:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 57. | Miller ER 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1820] [Cited by in RCA: 1627] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 58. | Hahn S, Kuemmerle NB, Chan W, Hisano S, Saborio P, Krieg RJ Jr, Chan JC. Glomerulosclerosis in the remnant kidney rat is modulated by dietary alpha-tocopherol. J Am Soc Nephrol. 1998;9:2089-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Koya D, Lee IK, Ishii H, Kanoh H, King GL. Prevention of glomerular dysfunction in diabetic rats by treatment with d-alpha-tocopherol. J Am Soc Nephrol. 1997;8:426-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 153] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 60. | Dallner G, Bentinger M, Hussain S, Sinha I, Yang J, Schwank-Xu C, Zheng X, Swiezewska E, Brismar K, Valladolid-Acebes I, Tekle M. Dehydro-Tocotrienol-β Counteracts Oxidative-Stress-Induced Diabetes Complications in db/db Mice. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Trachtman H, Chan JC, Chan W, Valderrama E, Brandt R, Wakely P, Futterweit S, Maesaka J, Ma C. Vitamin E ameliorates renal injury in an experimental model of immunoglobulin A nephropathy. Pediatr Res. 1996;40:620-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Saborio P, Krieg RJ Jr, Kuemmerle NB, Norkus EP, Schwartz CC, Chan JC. Alpha-tocopherol modulates lipoprotein cytotoxicity in obstructive nephropathy. Pediatr Nephrol. 2000;14:740-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Trachtman H, Schwob N, Maesaka J, Valderrama E. Dietary vitamin E supplementation ameliorates renal injury in chronic puromycin aminonucleoside nephropathy. J Am Soc Nephrol. 1995;5:1811-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Li L, Luo R, Yang Y, Cheng Y, Ge S, Xu G. Tamibarotene inhibit the accumulation of fibrocyte and alleviate renal fibrosis by IL-17A. Ren Fail. 2020;42:1173-1183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Zhong Z, Li HY, Zhong H, Lin W, Lin S, Zhou T. All-trans retinoic acid regulating angiopoietins-1 and alleviating extracellular matrix accumulation in interstitial fibrosis rats. Ren Fail. 2021;43:658-663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |