Published online Nov 25, 2021. doi: 10.5527/wjn.v10.i6.109
Peer-review started: May 9, 2021
First decision: June 6, 2021
Revised: August 19, 2021
Accepted: September 15, 2021
Article in press: September 15, 2021
Published online: November 25, 2021
Processing time: 197 Days and 16.9 Hours
Hema-Plus, a recombinant human erythropoietin (rHuEPO) or epoetin alfa has shown effectiveness in correction of anemia in Thai population in clinical practice. This study was aimed to demonstrate efficacy and safety under the evidence-based approach.
To evaluate the efficacy and safety of rHuEPO (Hema-Plus) for treatment of anemia over 12 wk in Thai patients with Stage V chronic kidney disease (CKD) on peritoneal dialysis (PD).
This study was an open-label, multi-center study to enroll 30 CKD patients identified to start PD with hemoglobin (Hb) less than 9.5 g/dL, serum ferritin more than 100 ng/mL, serum transferrin saturation more than or equal to 20% and who had not previously received epoetin. Patients with conditions that could increase the risk of adverse effects from study participation or interfere with study outcomes, were using concomitant androgens or had secondary hyperparathyroidism were excluded. All eligible patients started Hema-Plus by SC injection at 4000 IU once or twice weekly (week 0) and with follow-up at weeks 2, 4, 8, and 12. Dosage adjustment could be done to achieve Hb level of 11-12 g/dL. Primary end point was mean change in Hb level from baseline to end of treatment (week 12). Safety was assessed throughout the study. Quality of life (QoL) was assessed using KDQOL-36.
All 30 enrolled patients completed the study. Mean (standard deviation) Hb at baseline (week 0) to the end of 12 wk was significantly increased from 7.39 (1.29) g/dL to 11.15 (1.73) g/dL (paired t-test, P value < 0.001). Overall change of Hb means from baseline over the other 4 visits was statistically significantly increased (repeated measure ANOVA, P value < 0.001). Ten out of 39 adverse events (AEs) were serious. Two serious AEs were probably related to study medication by investigators’ assessment. At week 12, the QoL scores in all domains were significantly increased from baseline.
Hema-Plus administered for 12 wk for treatment of anemia in patients on PD effectively increased Hb levels with acceptable safety profile.
Core Tip: This was an open-label, single-arm, prospective, multicenter study of Hema-Plus®, a recombinant human erythropoietin (rHuEPO) for management of anemia in 30 Thai chronic kidney disease patients who started peritoneal dialysis with hemoglobin (Hb) lower than 9.5 g/dL and who had not previously received epoetin. The results showed that the rHuEPO could significantly increase Hb from baseline throughout the 12-wk treatment duration with acceptable safety profile. The quality of life (QoL) scores at week 12 assessed using KDQOL-36 were significantly increased from baseline in all domains.
- Citation: Chuengsaman P, Narenpitak S, Sritippayawan S. Efficacy and safety of recombinant human erythropoietin (Hema-Plus®) for management of anemia in Thai patients on peritoneal dialysis. World J Nephrol 2021; 10(6): 109-121
- URL: https://www.wjgnet.com/2220-6124/full/v10/i6/109.htm
- DOI: https://dx.doi.org/10.5527/wjn.v10.i6.109
Anemia is a common condition among patients with chronic kidney disease (CKD). This anemia is an almost inevitable feature of CKD, especially end-stage renal disease because the kidneys play an important role in the production of erythropoietin which supports erythropoiesis. Renal anemia begins in early stages of CKD and worsens as it progresses[1,2]. When anemia is left untreated the consequences can be severe. The more dangerous impacts include cardiovascular disease, worsening angina symptoms, left ventricular hypertrophy (LVH) and congestive heart failure. These abnormalities reduce the quality of life (QoL), interfere with rehabilitation and decrease survival in patients with CKD[1,3-5].
The primary etiology of anemia associated with CKD is insufficient renal production of the erythropoietin. Additionally, other factors contributing to anemia in CKD include iron deficiency, hyperparathyroidism, acute and chronic inflammatory disease, and shortened red blood cell survival[4,6]. The treatment of anemia in patients with CKD provides the following known beneficial effects: A reduced requirement for blood transfusions and associated complications, improved QoL, decreased hospitalizations and reduced overall healthcare costs[4,5].
The use of erythropoiesis-stimulating agents (ESAs) for treatment of renal anemia can relieve the anemia symptoms and improve QoL[7,8]. Recombinant human erythropoietin (rHuEPO) or epoetin alfa is generally well tolerated. In patients with chronic renal failure the adverse events (AEs) reported from a clinical point of view include hypertension, increased blood pressure, seizures, thrombotic vascular events, headache, increased heart rate, nausea, vomiting, diarrhea, pyrexia, hypercalcemia, hyperkalemia, hypersensitivity reaction (rash, urticarial, anaphylactic reaction, angio-edema), and increased incidence of thrombotic vascular events. A rare, but serious complication related to epoetin is antibody-mediated pure red cell aplasia (PRCA)[8,9]. When treated with ESA, patients with anemia and CKD may encounter hyporesponsiveness. Many factors contribute to ESA hyporesponsiveness such as iron deficiency, non-adherence to iron or ESA therapy, acute/chronic blood loss, infection, inflammation, inadequate dialysis, aluminum overload, malignancy, chronic conditions, hyperparathyroidism, and PRCA[3,10]. Practice guidelines suggest evaluation for specific causes of hyporesponsiveness when the Hb level is inappropriately low for the ESA dose administered[7,11].
Hema-Plus is a rHuEPO. The product was approved for marketing in the manufacturing country in 1998. To date, the product has been registered in more than 20 countries. The product was approved for marketing in Thailand in September 2012. This study was proposed to evaluate the efficacy and safety of the product in the management of anemia in patients on peritoneal dialysis (PD) and also the QoL of those patients.
This was an open-label, single-arm, prospective, multicenter, phase IV study to evaluate efficacy and safety of Hema-Plus® in correcting anemia among CKD patients receiving PD. The study was conducted at three dialysis centers offering continuous ambulatory PD (CAPD) services in Thailand, i.e., Banphaeo Hospital, Udon Thani Hospital, and Siriraj Hospital.
Patients underwent PD catheter implantation following normal practice at each study site. Following the break-in period, eligible patients commenced PD and started a 12-wk treatment of Hema-Plus® on the same day. The efficacy and safety of the study drug along with the impact on patients’ QoL were assessed.
The study was conducted in accordance with International Conference on Harmonization–Good Clinical Practice Guideline and the Declaration of Helsinki. The protocol was approved by the independent ethics committee at each participating site, prior to the study initiation. All patients gave written informed consent. The study was registered on the Thai Clinical Trial Registry (TCTR), URL http://www.thaiclinicaltrials.org/ (TCTR ID: 20140128002). The study adhered to The Strengthening of the Reporting of Observational Studies in Epidemiology guidelines[12].
Eligibility was assessed during a 2-wk screening period. CKD patients aged 18–70 years who were indicated to start PD based on estimated glomerular filtration rate (eGFR) of less than 15 mL/min/1.73 m2, and had hemoglobin (Hb) < 9.5 g/dL, serum ferritin > 100 ng/mL and serum transferrin saturation (TSAT) ≥ 20% were enrolled in the study. The eGFR was calculated by using the CKD epidemiology collaboration equation[13].
Patients who previously received epoetin or any investigational drugs (within 3 mo of screening) or had blood transfusion (within 7 d prior to screening) or received concurrent treatment with either androgenic agents or immunosuppressive therapy were considered ineligible. This study also excluded patients with the following conditions: Poorly controlled hypertension or ischemic heart disease; congestive heart failure; infection or inflammatory disease (within 4 wk prior to screening); secondary hyperparathyroidism; history of seizure; previous malignant tumor or residual tumor after anticancer therapy; active bleeding or history of bleeding disorders; infections with HIV or hepatitis B or C viruses; signs, symptoms and laboratory assessment associated with erythropoietin-resistant bone marrow diseases such as aplastic anemia, myelodysplastic syndrome, myelofibrosis, myelophthisis anemia and PRCA; known hypersensitivity to products derived from mammalian cells or containing human albumin; pregnant or breastfeeding; and women with childbearing potential unwilling to use an effective birth control method.
Participants were treated with Hema-Plus® (rHuEPO), either in the form of sterile vials or prefilled syringes, via self-administered subcutaneous injections at 4000 IU/dose once or twice weekly, as per the usual practice of each participating site. In order to achieve or maintain the Hb level within the target range of 11–12 g/dL[14], dose adjustment was permitted at the discretion of the investigator. The trial treatment period was 12 wk with the first dose was given at week 0 (baseline). It was recommended to give all patients oral supplementation of iron, vitamin B12 and/or folic acid.
A physical examination, a complete blood count and concomitant medications review was conducted at every visit (weeks 0, 2, 4, 8 and 12). AEs and serious AEs (SAEs) were assessed throughout the study and during each visit, using the following scale of severity—mild, moderate and severe. Analyses of serum ferritin, TSAT and blood chemistry were performed at weeks 4, 8 and 12. Dialysis adequacy was evaluated anytime during weeks 4 and 8. The QoL was assessed by interview at initial screening and end of the study (week 12), using the Thai language version of kidney disease QoL instrument (KDQOL-36)[15,16]. If erythropoietin-induced, antibody-mediated PRCA was suspected, an anti-erythropoietin antibodies test could be performed.
All efficacy end points were assessed at the end of study treatment (week 12), with the primary end point being mean Hb level at week 12. The secondary efficacy end points were the percentage of patients achieving target Hb level, mean percentage change from baseline of Hb level and the QoL scores. The safety end point was assessed through frequency and percentages of AEs, and SAEs throughout the treatment period.
All statistical analyses were performed in the intention-to-treat population. The sample size estimation in this study was based on changes of Hb from 8.0 ± 1.3 g/dL at baseline to 10.5 ± 1.6 g/dL at week 12 in a previous study[17] and from 7.1 ± 1.14 g/dL at baseline to 10.1 ± 1.49 g/dL at week 12 in another study[18], in which hemodialysis patients were studied, and a change of hematocrit from 23.8 ± 3.8 g/dL at baseline to 33.7 ± 4.8 g/dL at week 12 among PD patients in a study by Nissenson et al[19]. With a type I error of 0.05 allowed and a power of 80% required, a sample size of 15 to 27 patients was required. With a 10% lost to follow-up, this study proposed a sample of 30 patients.
Mean Hb level and mean QoL scores at week 12 were compared with the respective data at week 0 using paired t-tests. Repeated measure ANOVA with multiple pair-wise comparisons with Benferroni-Holm step down adjustment was employed to determine the changes of mean Hb level at each visit compared with their respective previous visits. All statistical analyses were performed by a qualified statistician using SPSS version 24 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.). For missing data, the last observation carried forward imputation method was employed. A two-sided P value for significance of less than 0.05 was applied for all statistical analyses in this study.
Out of 58 patients screened, 37 were enrolled and received at least one dose of the study drug. Seven patients were discontinued from the study and excluded from efficacy analysis due to ineligibility since enrollment. All patients who received at least one dose of the study drug were included in safety analysis (n = 37), and the thirty patients who met all inclusion criteria and did not meet any exclusion criteria were included in efficacy analysis (n = 30).
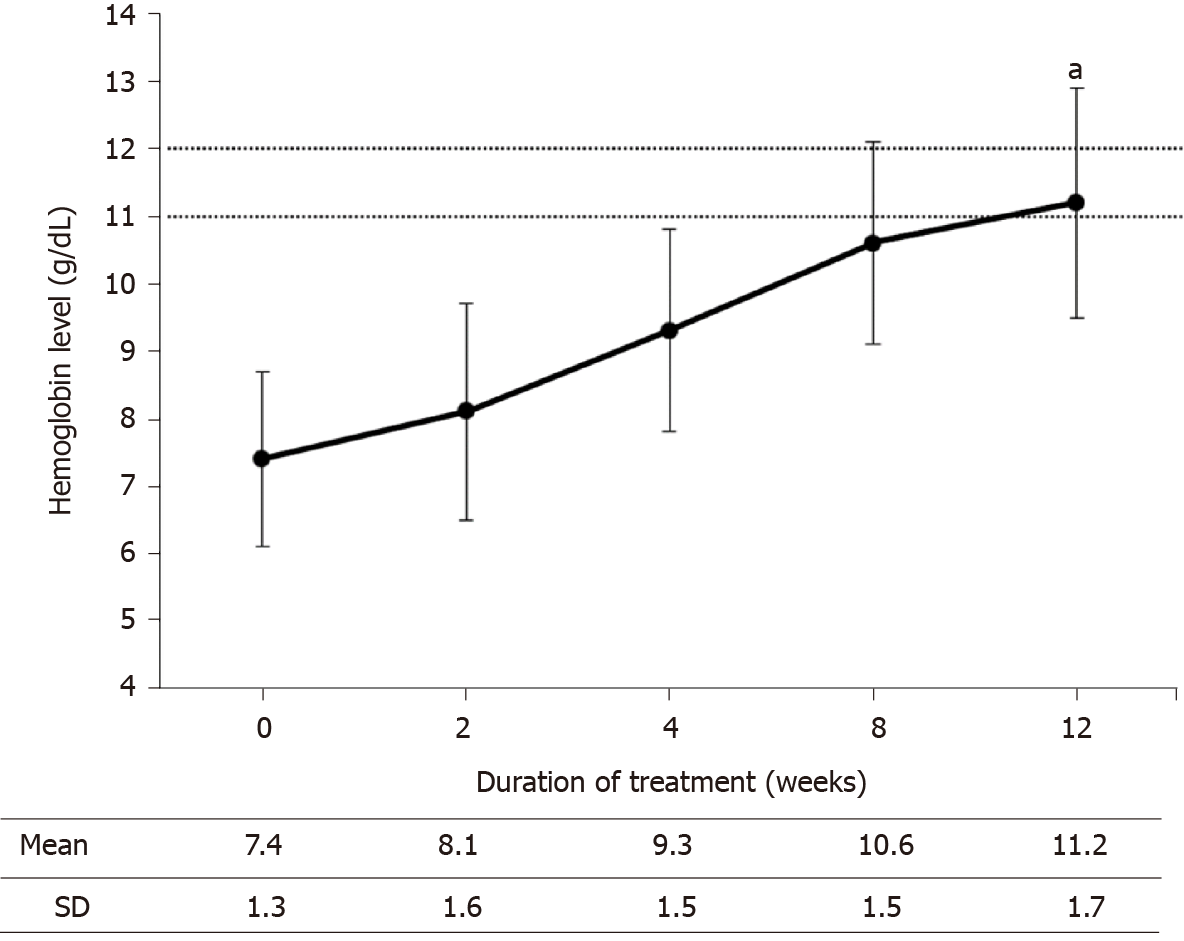
The 30 eligible patients completed the 12-wk treatment with Hema-Plus® (Figure 1). Of these, 18 (60%) were male. The mean age of patients was 48.8 years [standard deviation (SD) = 13.6] with the mean Hb level of 7.4 g/dL (SD = 1.3). Baseline characteristics are further described in Table 1. The average dose received by patients was 6466.7 IU/wk (SD = 1899; range: 3333.3–11666.7), equivalent to 117.6 IU/kg/week (SD = 42.2; range: 58.4–269.8).
| Characteristics | ||
| Sex—No. of patients (%) | ||
| Male | 18.0 | (60.0) |
| Female | 12.0 | (40.0) |
| Age (years)1 | 48.8 | (13.6) |
| Weight (kg)1 | 55.9 | (11.1) |
| TSAT (%)2 | 39.6 | (23.0) |
| Ferritin (ng/mL)2 | 931.2 | (592.7) |
| Hemoglobin (g/dL)1 | 7.4 | (1.3) |
| Hematocrit (%)1 | 22.2 | (4.0) |
| Reticulocyte count (%)1 | 1.1 | (0.9) |
| eGFR (ml/min/1.73 m2 | 6.2 | (3.3) |
| BUN (mg%)2 | 77.1 | (34.2) |
| Creatinine (mg%)2 | 10.0 | (4.5) |
| Albumin (g%)2 | 3.8 | (0.6) |
| Vitamin B12 (pg/mL)2,4 | 922.3 | (422.9) |
| Folic acid (ng/mL)2,4 | ||
| RBC folate (n = 19) | 8192.8 | (4785.0) |
| Serum folate (n = 10) | 102.2 | (217.4) |
| C-reactive protein (mg/L)2 | 10.7 | (29.5) |
At the end of the study, Hb levels of all participants increased from baseline. The mean Hb level at week 12 was significantly higher than that of week 0 [11.2 g/dL (SD = 1.7) vs 7.4 g/dL (SD = 1.3); P < 0.001], corresponding to a percentage change of 53.8% (SD = 26.7; range: 8.2–134.6). Elevation of Hb levels was observed throughout the study period (Figure 2). When the Hb levels at each visit were compared with those of the prior visit, the increases in mean Hb levels were found to be significant (Table 2).
| Comparison | Hb change1 | P value | Significance criteria |
| Week 0 vs Week 2 | 0.7 (0.2) | 0.0003 | 0.0250 |
| Week 2 vs Week 4 | 1.3 (0.2) | < 0.0001 | 0.0167 |
| Week 4 vs Week 8 | 1.3 (0.2) | < 0.0001 | 0.0125 |
| Week 8 vs Week 12 | 0.6 (0.2) | 0.0060 | 0.0500 |
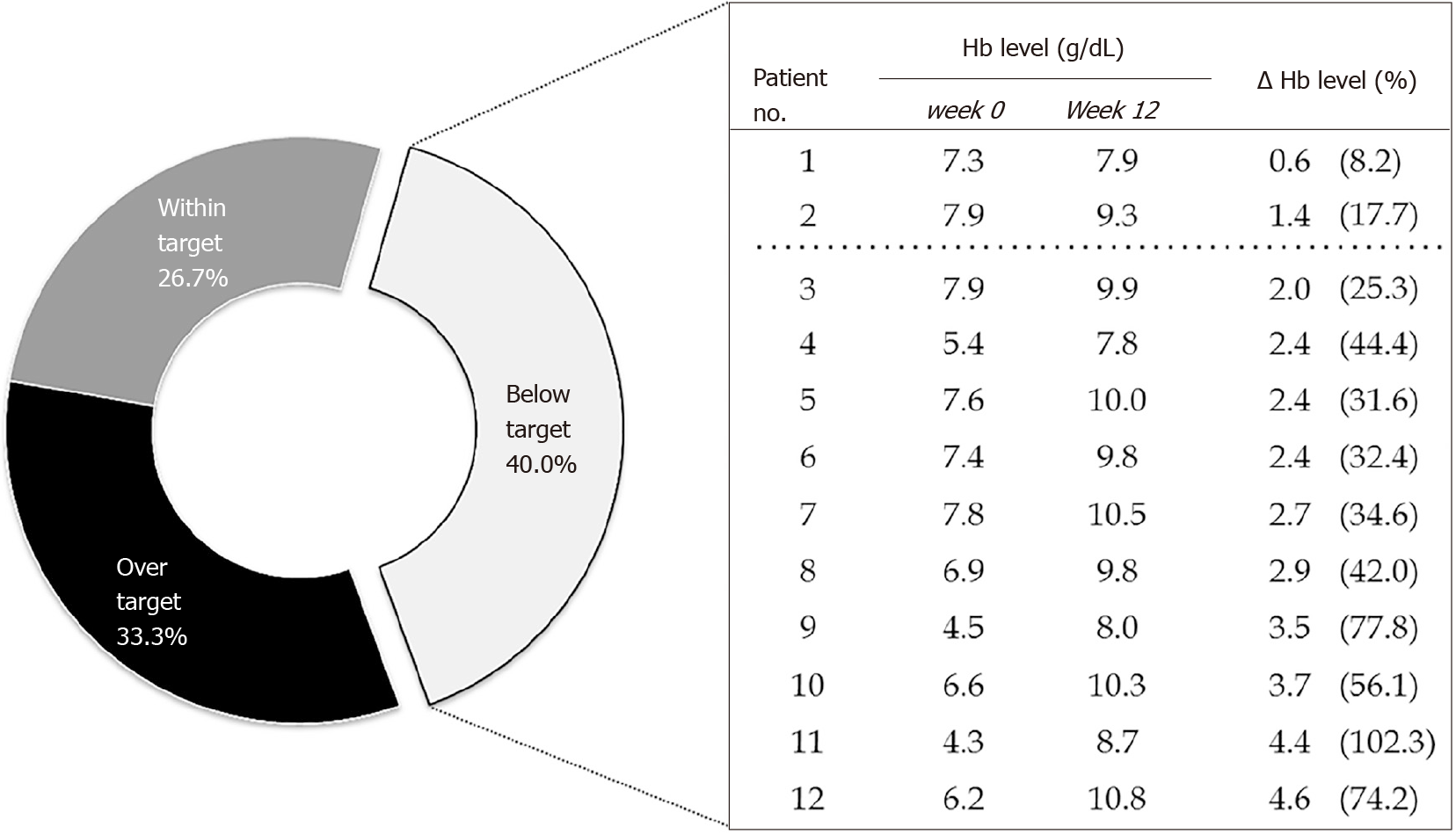
Overall, 18 participants (60%) had achieved the Hb levels within or exceeding the targets upon completion of the study drug treatment. Of these, 8 (26.7%) had their Hb levels within the target range of 11–12 g/dL; while, the Hb levels were greater than 13 g/dL in 10 participants (33.3%). Ten out of 12 patients with below-the-target Hb levels achieved increases in Hb concentrations of equal or greater than 2 g/dL (Figure 3). This is in line with the kidney disease: Improving global outcomes (KDIGO) clinical practice guideline[20] which recommends the increase of Hb level at the rate of 1–2 g/dL per month. Therefore, those patients were considered as responding to the treatment.
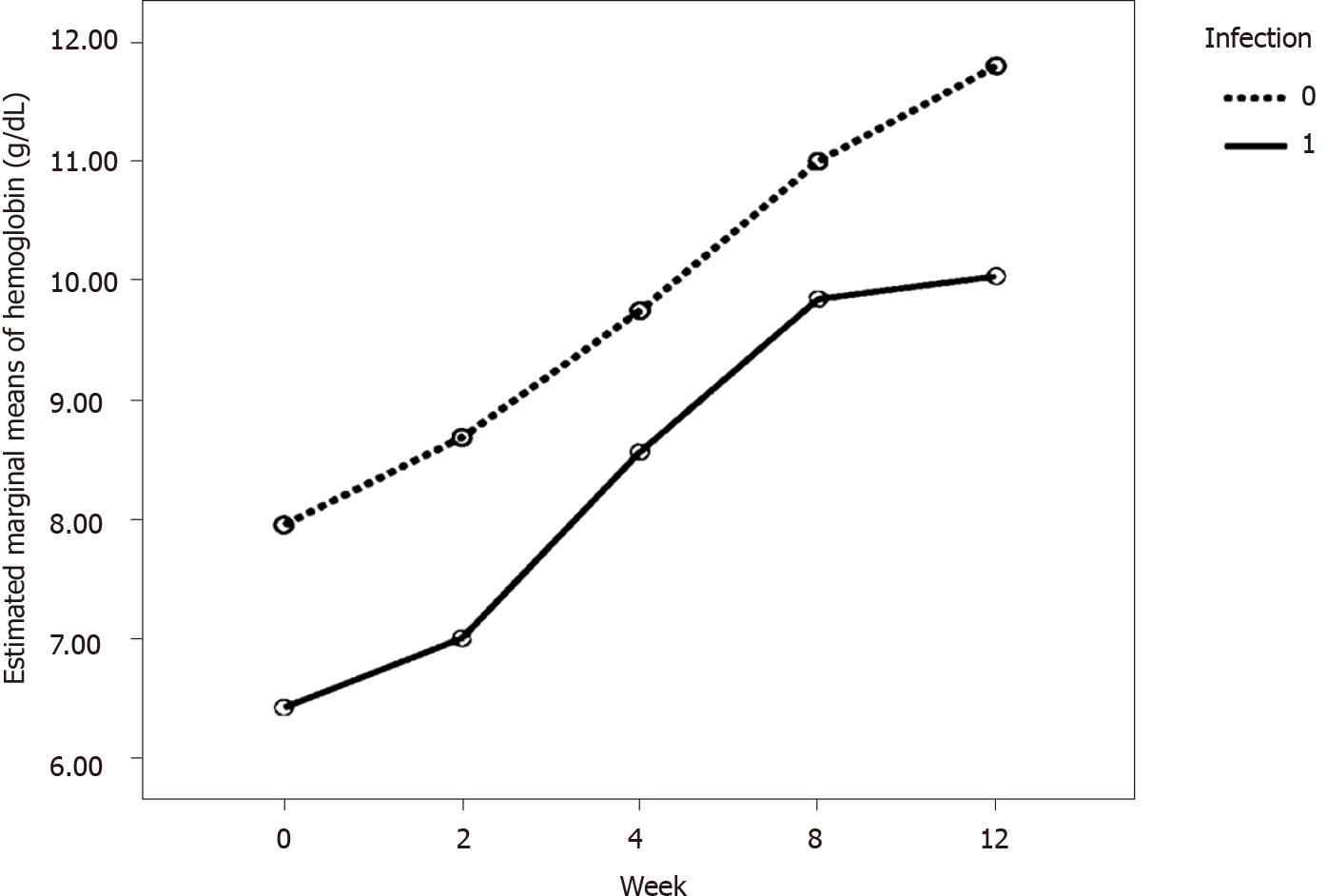
We performed additional analysis to compare Hb levels at baseline and week 12 by the factors that could affect the ESA response, i.e. thalassemia, infection, hemolysis, blood loss, hyperparathyroidism, low blood folic acid, and iron deficiency. However, only infection had a certain variation, i.e. 36.67% of the 30 patients having infection (i.e. exit site infection, peritonitis, pneumonia, and cellulitis), which was adequate to test for statistical significance. Table 3 shows frequency of patients with factors potentially affecting ESA response. Repeated measure ANOVA was used to compare Hb levels between patients with and without infection. Overall, it was found that infection was significantly associated with different Hb level (P value = 0.001). Patients with infection had a significantly lower Hb level at baseline, week 2, week 4, week 8 and week 12 (P value = 0.001, 0.004, 0.034, 0.035 and 0.005, respectively) when compared to the patients without infection. Table 4 presents the comparisons of Hb at each visit by infection status and Figure 4 shows estimated marginal means of Hb levels from baseline to week 12 by infection status using repeated measure ANOVA.
| Factors potentially affecting ESA response | n (%) |
| Thalassemia | 1 (3.33) |
| Infection | 11 (36.67) |
| Hemolysis | 0 |
| Blood loss | 0 |
| Hyperparathyroidism | 1 (3.33) |
| Low blood folic acid | 0 |
| Iron deficiency | 3 (10.00) |
| Visit | Hemoglobin levels (g/dL), mean (SD) | P value | ||
| Infection (n = 11) | Non-infection (n = 19) | |||
| Visit 2 (week 0; baseline) | 6.41(1.48) | 7.95 (0.75) | 0.001a | 0.001b |
| Visit 3 (week 2) | 7.00 (1.51) | 8.68 (1.33) | 0.004a | |
| Visit 4 (week 4) | 8.56 (1.53) | 9.75 (1.33) | 0.034a | |
| Visit 5 (week 8) | 9.85 (1.40) | 11.00 (1.36) | 0.035a | |
| Visit 6 (week 12) | 10.04 (1.73) | 11.80 (1.39) | 0.005a | |
Treatment with Hema-Plus® improved the patients’ health-related QoL as determined by the KDQOL-36™ survey (Table 5). At week 12, there were significant increases in KDQOL-36 scores from baseline in all aspects evaluated-physical and mental health as well as symptoms and burden of the kidney disease.
| Parameter | Week 0 | Week 12 | ∆ | P value1 | |||
| SF-12 scores | |||||||
| Physical health composite | 36.8 | (8.2) | 41.5 | (9.8) | 4.8 | (8.5) | 0.004 |
| Mental health composite | 41.5 | (9.6) | 48.4 | (8.4) | 6.9 | (10.7) | 0.001 |
| Burden of kidney disease | 34.4 | (28.4) | 46.0 | (31.3) | 11.7 | (25.0) | 0.016 |
| Symptoms/problems of kidney disease | 65.6 | (19.6) | 79.4 | (13.6) | 13.8 | (20.4) | 0.001 |
| Effects of kidney disease | 63.0 | (18.2) | 76.3 | (18.4) | 13.3 | (23.6) | 0.004 |
Overall, 39 AEs were reported from 18 patients (out of 37 patients included in the safety population) during the study period (Table 6). Most of those (37/39) were deemed very unlikely to be related to the study drug. All reported AEs were mild (30/39) to moderate (7/39), except one case of volume overload which was classified as a severe event, but very unlikely to be related to the study drug. A total of 10 SAEs occurred in 7 patients. Of these, two occurrences of hypertensive urgency were deemed to be probably related to the study drug. The most frequently reported AEs, occurring in more than 10% of the patients, were exit site infection/inflammation and edema.
| Adverse events | No. of patients (%) | No. of events |
| Total | 18 (48.65) | 39 |
| Blood and lymphatic disorders | ||
| Bicytopenia1 | 1 (2.70) | 1 |
| Gastrointestinal disorders | ||
| Diarrhea | 1 (2.70) | 1 |
| Nausea | 1 (2.70) | 1 |
| Infections and infestations | ||
| Exit site infection/inflammation | 6 (16.22) | 6 |
| Pneumonia1 | 2 (5.40) | 2 |
| Eosinophilic peritonitis | 2 (5.40) | 2 |
| Injury, poisoning and procedural complications | ||
| Closed fractures of femur1 | 1 (2.70) | 1 |
| Accidental tear small bowel1 | 1 (2.70) | 1 |
| Investigations | ||
| Hyperkalemia | 1 (2.70) | 1 |
| Hypokalemia | 1 (2.70) | 1 |
| Hyperphosphatemia | 1 (2.70) | 1 |
| Metabolism and nutrition disorders | ||
| Hypoglycemia | 2 (5.40) | 2 |
| Musculoskeletal and connective tissue disorders | ||
| Acute arthritis | 1 (2.70) | 1 |
| Nervous system disorders | ||
| Headache | 1 (2.70) | 1 |
| Dizziness | 1 (2.70) | 1 |
| Renal and urinary disorders | ||
| Volume overload1,2 | 1 (2.70) | 1 |
| Respiratory, thoracic and mediastinal disorders | ||
| Chest discomfort | 1 (2.70) | 1 |
| Bronchospasm | 1 (2.70) | 1 |
| Pleural effusion1 | 1 (2.70) | 1 |
| Skin and subcutaneous tissue disorders | ||
| Edema | 4 (10.81) | 4 |
| Paleness | 1 (2.70) | 2 |
| Cellulitis | 1 (2.70) | 1 |
| Itching from dry skin | 1 (2.70) | 1 |
| Surgical and medical procedures | ||
| Catheter malfunction/ malposition3 | 2 (5.40) | 2 |
| Vascular disorder | ||
| Hypertensive urgency1,4 | 2 (5.40) | 2 |
None of the AEs led to discontinuation or modification of the study treatment. Most events (32/39), including all SAEs, were improved or recovered. There was no new or unexpected safety signals observed.
The treatment of anemia in patients with CKD with ESAs has provided the following known beneficial effects: A reduced requirement for blood transfusions and the associated complications, improvement of heart failure symptoms and regression of left ventricular hypertrophy, improved QoL, decreased hospitalizations and reduction of overall healthcare costs[4,5,21].
It is difficult to predict dose requirements of ESA in individual patients who have high comorbidities, diabetes, other disorders, and inflammation. Hb should be increased slowly between 1.0–2.0 g/dL (10–20 g/L) per month during the initial ESA therapy (correction phase), so as to avoid adverse effects like hypertension, seizures, vascular access thrombosis, and possible cardiovascular events. However, it is recommended that the starting dose of epoetin alpha should be 20–50 IU/kg weight thrice every week with Hb target of 11.0-12.0 g/dL. Dose adjustment should be determined by the patient’s Hb level, the target Hb level, the observed rate of increase in Hb level and clinical circumstances[20,22].
Results from the present study showed that subcutaneously administered Hema-Plus at the doses and frequencies used in this patient population for treatment of anemia in CKD patients with PD significantly increased Hb levels over 12 wk. More than half of the study patients (60%) had Hb levels within (26.67%) or higher (33.33%) than the target range (11-12 g/dL)[1] at week 12 with percentages of change ranging from 8.22% to 134.62%. In addition, there were significant improvements in the QoL scores in all domains between baseline and week 12. The previous studies about QoL in PD patients have also shown that anemia was a significant factor in lowering the patients’ QoL[23-25]. Access to medicines is a significant factor to ensure healthy lives and promote well-being of all people of all ages[26]. The Hema-Plus, which is a locally made recombinant human erythropoietin (rHuEPO) with lower cost, might help Thai people to gain more accessibility to epoetin alfa in Thailand.
This present study showed that the patients with infection had a significantly lower Hb level when compared to the patients without infection. This result was consistent with several studies that have indicated that markers of inflammation have an association with decreased response to EPO[27]. In this present study, only infection had a certain variation which was adequate to test for statistical significance. There are a lot of factors that influence the response to rHuEPO to keep in mind[27], such as iron deficiency[28-30], oxidative stress[31-33], hyperparathyroidism[34], and angiotensin-converting enzyme inhibitors[35,36].
In terms of safety, epoetin alpha is generally well tolerated. The most frequently reported AEs from studies of epoetin alpha in phase I trials are headache, polycythemia, tiredness, common cold, diarrhea, nausea, stomach pains, chest pressure sensation, back pain, leg pain, and dizziness[37]. In this present study there were 38 AEs experienced by patients exposed to Hema-Plus treatment for 12 wk. The majority of AEs were mild to moderate with 1 AE (volume overload) which was severe in intensity. The highest rate of AE was exit site infection/inflammation, followed by edema, catheter malfunction, pneumonia, hypoglycemia, and hypertensive urgency, respectively. All of the AEs were very unlikely to be related to study drug, except two SAEs (hypertensive urgency) which were deemed to be probably related to Hema-Plus according to investigators assessment. A multicenter study[38] analyzed the effectiveness of administering relatively high doses of rHuEPO subcutaneously in 41 patients CAPD by giving once every week dosing for 8 wk (initial phase) followed by once every 2 wk dosing for 12 wk (maintenance phase). The highest dose of rHuEPO within the initial 8 wk was 12000 IU once per week and within the maintenance phase was 12000 IU twice weekly. The results from this study showed that administration of the relatively high doses of rHuEPO was safe and potentially an effective administration plan for the correction of renal anemia. However, there were 2 patients who developed treatable hypertension with mild headache in the study[38]. Therefore, blood pressure should be closely monitored when patients are receiving high dose high frequency of rHuEPO. In the present study, there was no PRCA, a rare but serious complication related to epoetin[7,8], reported during the 12-wk administration of the study drug.
Our study had some limitations that require attention. This study was an open-label single group study which did not directly compare with other epoetin alfa medications. The study design of this study intended to evaluate efficacy and safety during the correction phase of the study drug, and did not include maintenance therapy. The study treatment was 12 wk in which the long-term safety might not be concluded. In addition, a large numbers of subjects were required to study PRCA event because the PRCA incidence rates were very low[39]. The longer period study is suggested to determine whether use of Hema-Plus could maintain the Hb level with acceptable safety profile.
In conclusion, this study demonstrates that Hema-Plus administered for 12 wk was effective in correcting anemia in CKD patients undergoing PD with significantly improved QoL. The safety profile was acceptable and also looked comparable to epoetin alfa.
The Hema-Plus is a locally made recombinant human erythropoietin (rHuEPO) that may help Thai people to gain more accessibility to epoetin alfa in Thailand due to lower costs.
There are no publicly available studies of Hema-Plus in Thai chronic kidney disease (CKD) patients on peritoneal dialysis (PD). The results from this study may be used as an evidence and information for considering to use Hema-Plus, locally made rHuEPO in these patients.
This study aimed to evaluate the efficacy and safety of Hema-Plus for treatment of anemia during correction phase in Thai patients with Stage V CKD on PD.
An open-label, multi-center study of Hema-Plus in patients was used to assess the efficacy, i.e., mean change in hemoglobin (Hb) level from baseline, safety, and changes in quality of life (QoL) after receiving the study drug for 12 wk.
Efficacy analysis comprised 30 patients. At the end of 12 wk-treatment, mean Hb was statistically significantly increased from baseline. Two hypertensive urgency events were observed probably related to study medication. QoL scores were significantly increased from baseline.
Hema-Plus administered for 12 wk for treatment of anemia in CKD patients on PD effectively could increase Hb levels, and QoL with acceptable safety profile.
This study results could be used as an evidence for efficacy and safety information of the locally made rHuEPO, Hema-Plus for anemia correction phase. Longer study duration is suggested to ensure long-term safety and efficacy.
Thanks to Mr. Stephen Pinder a medical-English specialist for English proofreading of this manuscript. We are also graceful to Siriraj Hospital, Mahidol University for permission to use the validated KDQOL-36 in Thai Kidney Disease Patient Questionnaire in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Urology and nephrology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Techane T S-Editor: Fan JR L-Editor: A P-Editor: Wu RR
| 1. | Locatelli F, Del Vecchio L. Optimizing the management of renal anemia: challenges and new opportunities. Kidney Int Suppl. 2008;S33-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Segal GM, Eschbach JW, Egrie JC, Stueve T, Adamson JW. The anemia of end-stage renal disease: hematopoietic progenitor cell response. Kidney Int. 1988;33:983-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Mara NB. Anemia in Patients With Chronic Kidney Disease. Dia Spec. 2008;12. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Lankhorst CE, Wish JB. Anemia in renal disease: diagnosis and management. Blood Rev. 2010;24:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Evans RW, Rader B, Manninen DL. The quality of life of hemodialysis recipients treated with recombinant human erythropoietin. Cooperative Multicenter EPO Clinical Trial Group. JAMA. 1990;263:825-830. [PubMed] |
| 6. | Potasman I, Better OS. The role of secondary hyperparathyroidism in the anemia of chronic renal failure. Nephron. 1983;33:229-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Locatelli F, Aljama P, Bárány P, Canaud B, Carrera F, Eckardt KU, Hörl WH, Macdougal IC, Macleod A, Wiecek A, Cameron S; European Best Practice Guidelines Working Group. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004;19 Suppl 2:ii1-i47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 236] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Perlman RL, Finkelstein FO, Liu L, Roys E, Kiser M, Eisele G, Burrows-Hudson S, Messana JM, Levin N, Rajagopalan S, Port FK, Wolfe RA, Saran R. Quality of life in chronic kidney disease (CKD): a cross-sectional analysis in the Renal Research Institute-CKD study. Am J Kidney Dis. 2005;45:658-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 271] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 9. | Taylor and Francis Group. Hema-Plus 2000 IU, 4000 IU, 10000 IU Package Insert 2011. [cited 10 April 2021]. Available from: https://www.taylorfrancis.com/chapters/mono/10.1201/9781482295467-17/iu-iu-andrew-peacock. |
| 10. | Johnson DW, Pollock CA, Macdougall IC. Erythropoiesis-stimulating agent hyporesponsiveness. Nephrology. 2007;321. [RCA] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | KDOQI. ; National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am J Kidney Dis. 2006;47:S11-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 370] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 12. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3667] [Cited by in RCA: 6822] [Article Influence: 620.2] [Reference Citation Analysis (0)] |
| 13. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20319] [Cited by in RCA: 20043] [Article Influence: 1252.7] [Reference Citation Analysis (0)] |
| 14. | KDOQI. KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:471-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 457] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 15. | Thaweethamcharoen T, Srimongkol W, Noparatayaporn P, Jariyayothin P, Sukthinthai N, Aiyasanon N, Kitisriworapan P, Jantarakana K, Vasuvattakul S. Validity and Reliability of KDQOL-36 in Thai Kidney Disease Patient. Value Health Reg Issues. 2013;2:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Hays RD, Kallich JD, Mapes DL, Coons S, Amin N, Carter WB, Kamberg C. Kidney Disease Quality of Life Short Form (KDQOL-SF ™), Version 1.3: A Manual for Use and Scoring. Santa Monica, CA: RAND Corporation; 1997. |
| 17. | Thanakitcharu P, Siriwiwatanakul N. Hemoglobin response and influence on left ventricular hypertrophy after 24-week treatment of a biosimilar epoetin-alfa in hemodialysis patients with anemia. J Med Assoc Thai. 2007;90:2574-2586. [PubMed] |
| 18. | Besarab A, Salifu MO, Lunde NM, Bansal V, Fishbane S, Dougherty FC, Beyer U; Ba16285 Study Investigators. Efficacy and tolerability of intravenous continuous erythropoietin receptor activator: a 19-week, phase II, multicenter, randomized, open-label, dose-finding study with a 12-month extension phase in patients with chronic renal disease. Clin Ther. 2007;29:626-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Nissenson AR, Korbet S, Faber M, Burkart J, Gentile D, Hamburger R, Mattern W, Schreiber M, Swartz R, Van Stone J. Multicenter trial of erythropoietin in patients on peritoneal dialysis. J Am Soc Nephrol. 1995;5:1517-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Chapter 3: Use of ESAs and other agents**Excluding iron which is discussed in Chapter 2. to treat anemia in CKD. Kidney Int Suppl. 2012;2:299-310. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Eschbach JW, Abdulhadi MH, Browne JK, Delano BG, Downing MR, Egrie JC, Evans RW, Friedman EA, Graber SE, Haley NR. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med. 1989;111:992-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 493] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 22. | Locatelli F, Del Vecchio L. Erythropoiesis-stimulating agents in renal medicine. Oncologist. 2011;16 Suppl 3:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Lew SQ, Piraino B. Quality of life and psychological issues in peritoneal dialysis patients. Semin Dial. 2005;18:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Furuland H, Linde T, Ahlmén J, Christensson A, Strömbom U, Danielson BG. A randomized controlled trial of haemoglobin normalization with epoetin alfa in pre-dialysis and dialysis patients. Nephrol Dial Transplant. 2003;18:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 153] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Liu H, Yao Y, Cao Y, Yang X, Huang B, Han X, Ren C. Anemia management trends in patients on peritoneal dialysis in the past 10 years. Int J Clin Exp Med. 2015;8:18050-18057. [PubMed] |
| 26. | Singh PK. Make medicines accessible for all. [cited 10 April 2021]. Available from: https://www.who.int/southeastasia/news/opinion-editorials/detail/make-medicines-accessible-for-all 2017. |
| 27. | Kwack C, Balakrishnan VS. Managing erythropoietin hyporesponsiveness. Semin Dial. 2006;19:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | DeVita MV, Frumkin D, Mittal S, Kamran A, Fishbane S, Michelis MF. Targeting higher ferritin concentrations with intravenous iron dextran lowers erythropoietin requirement in hemodialysis patients. Clin Nephrol. 2003;60:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Aronoff GR. Safety of intravenous iron in clinical practice: implications for anemia management protocols. J Am Soc Nephrol. 2004;15 Suppl 2:S99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 30. | Fishbane S, Galgano C, Langley RC Jr, Canfield W, Maesaka JK. Reticulocyte hemoglobin content in the evaluation of iron status of hemodialysis patients. Kidney Int. 1997;52:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 140] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Himmelfarb J. Uremic toxicity, oxidative stress, and hemodialysis as renal replacement therapy. Semin Dial. 2009;22:636-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Roob JM, Khoschsorur G, Tiran A, Horina JH, Holzer H, Winklhofer-Roob BM. Vitamin E attenuates oxidative stress induced by intravenous iron in patients on hemodialysis. J Am Soc Nephrol. 2000;11:539-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 134] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Kato A, Odamaki M, Hishida A. Blood 8-hydroxy-2'-deoxyguanosine is associated with erythropoietin resistance in haemodialysis patients. Nephrol Dial Transplant. 2003;18:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Goicoechea M, Gomez-Campdera F, Polo JR, Tejedor A, Ruiz MA, Vazquez I, Verde E, Valderrabano F. Secondary hyperparathyroidism as cause of resistance to treatment with erythropoietin: effect of parathyroidectomy. Clin Nephrol. 1996;45:420-421. [PubMed] |
| 35. | Albitar S, Genin R, Fen-Chong M, Serveaux MO, Bourgeon B. High dose enalapril impairs the response to erythropoietin treatment in haemodialysis patients. Nephrol Dial Transplant. 1998;13:1206-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Azizi M, Rousseau A, Ezan E, Guyene TT, Michelet S, Grognet JM, Lenfant M, Corvol P, Ménard J. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-seryl-aspartyl-lysyl-proline. J Clin Invest. 1996;97:839-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 256] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 37. | Strengers PFW, Twuijver Ev. Chapter 33-Blood, blood components, plasma, and plasma products. In: Aronson JK, editor. Side Effects of Drugs Annual: Elsevier; 2009: 527-546. |
| 38. | Nomoto Y, Kawaguchi Y, Kubota M, Tagawa H, Kubo K, Ogura Y, Shoji T, Kawada Y, Koshikawa S, Mimura N. A multicenter study with once a week or once every two weeks high-dose subcutaneous administration of recombinant human erythropoietin in continuous ambulatory peritoneal dialysis. Perit Dial Int. 1994;14:56-60. [PubMed] |
| 39. | McKoy JM, Stonecash RE, Cournoyer D, Rossert J, Nissenson AR, Raisch DW, Casadevall N, Bennett CL. Epoetin-associated pure red cell aplasia: past, present, and future considerations. Transfusion. 2008;48:1754-1762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |