Published online Jul 25, 2021. doi: 10.5527/wjn.v10.i4.59
Peer-review started: March 21, 2021
First decision: May 6, 2021
Revised: May 11, 2021
Accepted: July 23, 2021
Article in press: July 23, 2021
Published online: July 25, 2021
Processing time: 137 Days and 14.4 Hours
Chronic kidney disease (CKD) is a common medical condition that is increasing in prevalence. Existing published evidence has revealed through regression analyses that several clinical characteristics are associated with mortality in CKD patients. However, the predictive accuracies of these risk factors for mortality have not been clearly demonstrated.
To demonstrate the accuracy of mortality predictive factors in CKD patients by utilizing the area under the receiver operating characteristic (ROC) curve (AUC) analysis.
We searched Ovid MEDLINE, EMBASE, and the Cochrane Library for eligible articles through January 2021. Studies were included based on the following criteria: (1) Study nature was observational or conference abstract; (2) Study populations involved patients with non-transplant CKD at any CKD stage severity; and (3) Predictive factors for mortality were presented with AUC analysis and its associated 95% confidence interval (CI). AUC of 0.70-0.79 is considered acceptable, 0.80-0.89 is considered excellent, and more than 0.90 is considered outstanding.
Of 1759 citations, a total of 18 studies (n = 14579) were included in this systematic review. Eight hundred thirty two patients had non-dialysis CKD, and 13747 patients had dialysis-dependent CKD (2160 patients on hemodialysis, 370 patients on peritoneal dialysis, and 11217 patients on non-differentiated dialysis modality). Of 24 mortality predictive factors, none were deemed outstanding for mortality prediction. A total of seven predictive factors [N-terminal pro-brain natriuretic peptide (NT-proBNP), BNP, soluble urokinase plasminogen activator receptor (suPAR), augmentation index, left atrial reservoir strain, C-reactive protein, and systolic pulmonary artery pressure] were identified as excellent. Seventeen predictive factors were in the acceptable range, which we classified into the following subgroups: predictors for the non-dialysis population, echocardiographic factors, comorbidities, and miscellaneous.
Several factors were found to predict mortality in CKD patients. Echocardiography is an important tool for mortality prognostication in CKD patients by evaluating left atrial reservoir strain, systolic pulmonary artery pressure, diastolic function, and left ventricular mass index.
Core Tip: Although the current evidence has shown that several clinical factors are associated with mortality in chronic kidney disease (CKD), the accuracy of mortality prediction has not been clearly demonstrated. Our systematic review of studies that reported prognostic mortality factors using area under the receiver operating characteristic curve analysis in CKD patients provides an accuracy measurement. A total of 18 studies were identified. Eight hundred thirty two patients had non-dialysis CKD, and 13747 patients had end-stage kidney disease. Of 24 predictive factors, none were considered outstanding for mortality prediction. A total of seven predictive factors were identified as excellent. Our review summarizes the current accuracy of prognostic factors for CKD mortality.
- Citation: Hansrivijit P, Chen YJ, Lnu K, Trongtorsak A, Puthenpura MM, Thongprayoon C, Bathini T, Mao MA, Cheungpasitporn W. Prediction of mortality among patients with chronic kidney disease: A systematic review. World J Nephrol 2021; 10(4): 59-75
- URL: https://www.wjgnet.com/2220-6124/full/v10/i4/59.htm
- DOI: https://dx.doi.org/10.5527/wjn.v10.i4.59
Chronic kidney disease (CKD) is defined by the presence of kidney damage (such as hematuria or structural abnormalities), an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2, or albuminuria of greater than 30 mg in 24 h with duration of more than 3 mo[1]. The prevalence of CKD ranges from 8% to 16% of the population worldwide[2,3]. CKD is more prevalent in low- and middle-income countries compared with high-income countries[4]. With the increasing prevalence of hypertension and diabetes, it is projected that CKD prevalence in adults over 30-years-old will increase from 14.4% in 2020 to 16.7% in 2030[5]. Moreover, in the general population of the United States, the average GFR decline rate is approximately 1 mL/min per 1.73 m2 per year[6,7] with a lifetime risk of developing CKD (as defined by eGFR less than 60 mL/min per 1.73 m2) of more than 50%[8].
Progression of CKD has been associated with significantly increased adverse clinical outcomes, such as end-stage kidney disease (ESKD), dialysis dependence, cardiovascular events, and all-cause mortality[9,10]. Tonelli et al[11] conducted a meta-analysis of 38 studies that included over 1.3 million participants. They showed that the absolute risk for death increased exponentially with decreasing kidney function[11]. Several risk factors have been identified to predict mortality in CKD patients. One meta-analysis demonstrated that age, diabetes, previous cardiovascular disease, adiponectin, and C-reactive protein are all risk factors for mortality in dialysis patients[12]. Alternatively, an analysis of the Cleveland Clinic CKD Registry of 621 CKD patients with age > 65 years showed that increasing age, congestive heart failure, absence of arteriovenous fistula, and lack of nephrology care prior to dialysis initiation were significant risk factors for increased 1-year mortality after dialysis initiation[13]. However, data on mortality risk factors for non-dialysis dependent CKD patients are limited.
Nearly all of the available evidence has reported their findings using regression analysis. Thus, the accuracy of mortality prediction has not been clearly demonstrated. In this study, we conducted a systematic review of studies that utilized associated area under the receiver operating characteristic (ROC) curve (AUC) analysis to report predictive factors for mortality in CKD patients. This method allows the reader to assess the predictive accuracy of distributional models in comparison with regression analyses[14]. Our findings would help guide the future design of an accurate mortality risk calculator for CKD patients.
This manuscript follows the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis)[15] statement as well as MOOSE (Meta-analysis of Observational Studies in Epidemiology)[16] guidelines. A systematic search was conducted through Ovid MEDLINE, EMBASE, and the Cochrane Library from each respective database inception to January 2021. The domain for the systematic search on Cochrane Library included PubMed, EMBASE, ClinicalTrials.gov, and International Clinical Trial Registry Platform (ICTRP). The following search terms were used: (“chronic kidney disease” OR “CKD” OR “end-stage kidney disease” OR “ESKD” OR “end-stage renal disease” OR “ESRD”) AND (“mortality” OR “death” OR “survival”) AND (“area under curve” OR “AUC”). The detailed search strategy for each database is summarized in Supplementary material, Appendix 1. No language restrictions were applied during the systematic search.
The eligibility of each study was determined by the following inclusion criteria: (1) The study nature was observational or conference abstract; (2) Study populations involved patients with non-transplant CKD at any CKD stage severity; and (3) Predictive factors for mortality were presented with AUC analysis and its associated 95% confidence interval (CI). Case reports, case series, review articles, or articles concerning pediatric patients were excluded. Study eligibility was independently evaluated by two investigators (Hansrivijit P and Chen YJ). Disagreements were resolved by mutual consensus among all authors. The quality of each study was appraised using the Newcastle-Ottawa quality scale which assesses six components: (1) representativeness of the subjects, (2) ascertainment of the exposure, (3) demonstration of the outcome of interest was not present at the start of study, (4) assessment of outcome, (5) follow-up duration period was long enough for the outcome to occur, and (6) adequate follow-up duration.
The titles and abstracts of all references were screened (Hansrivijit P and Chen YJ) prior to proceeding with full-text review. The full-text of the selected screened articles were then reviewed to determine their eligibility for inclusion into the systematic review. A standardized data collection form was invented to extract the following information from included study: first author’s name, year of publication, country of origin, study design, subject(s), sample size, age, male sex, AUC and its 95%CI, and follow-up duration. Full articles or conference abstracts that reported the AUC without 95%CI were excluded. An AUC of 1.0 represents the ideal predictor with a 100% sensitivity and 0% false positive rate. Thus, in this mortality model, factors with AUC closer to 1.0 represented a better predictor for mortality, with AUC of 0.70-0.79 considered acceptable, 0.80-0.89 considered excellent, and more than 0.90 considered outstanding[17,18].
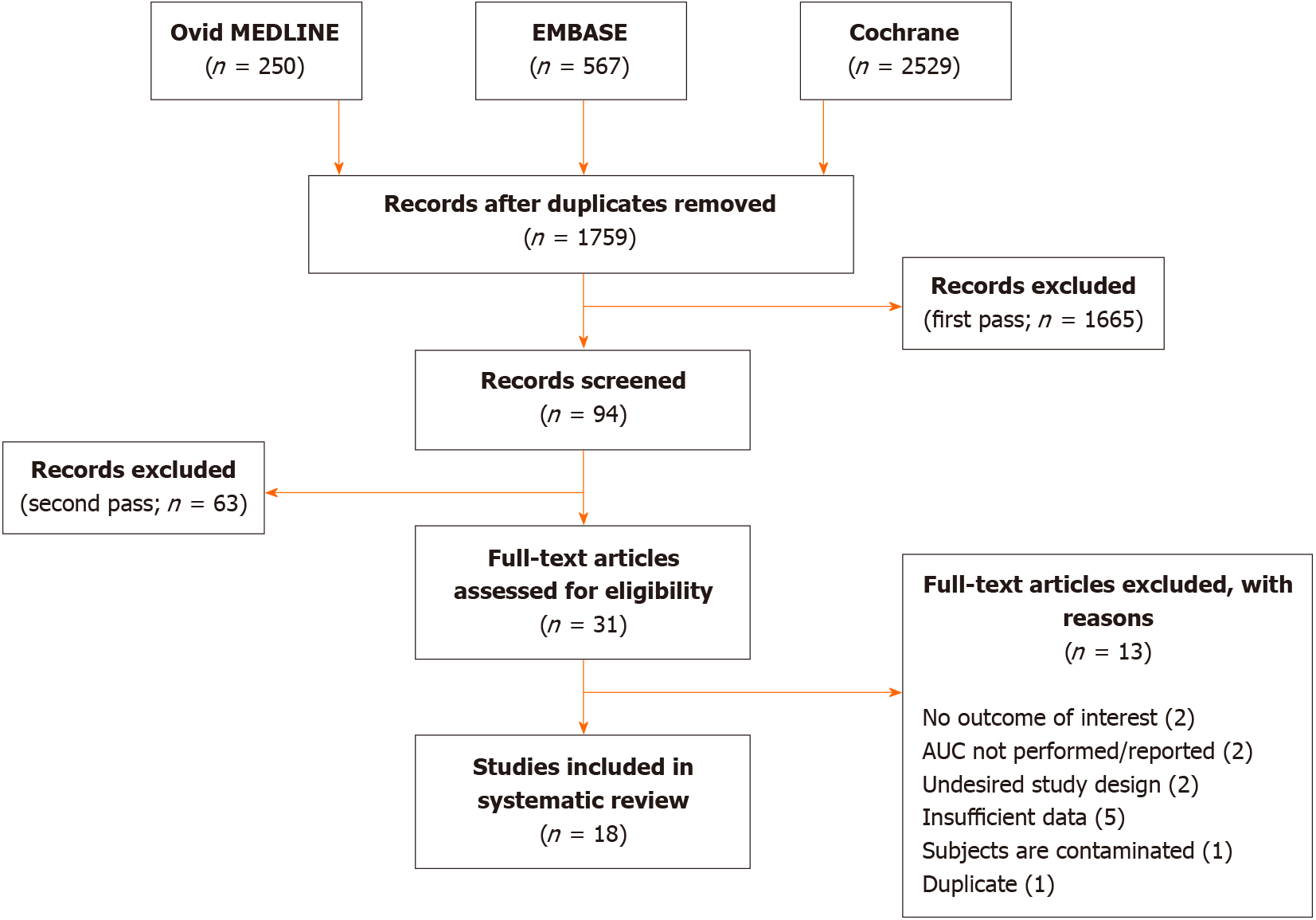
A total of 1759 citations underwent three passes of article screening. After duplicates were removed, another 1665 irrelevant articles were excluded after the title and abstract were screened. The methods and results of the remaining 94 articles were then evaluated for eligibility in accordance with the inclusion criteria. Only 31 articles passed this stage to undergo full-text review. A final total of 18 studies from 2004 to 2021 consisting of 14579 subjects were included in this systematic review. Figure 1 provides a flowchart of the literature search and study selection. All studies were observational in nature (retrospective in 33.3% and prospective in 66.7%). The median age was 62.0 (± 4.1) years, and 57.3% were male. The median duration of follow-up was 2.0 (± 1.1) years. Table 1 describes the included study characteristics and results. The risk of bias assessment is shown in Supplementary Table 1.
| Ref. | Country | Study type | n | Subject | Age, yr | Male, % | AUC for mortality (95%CI) | Follow-up |
| Dara et al[19], 2004 | United States | Retrospective | 476 | HD patients | 66 | 58 | APACHE III on hospital mortality 0.76 (0.66-0.84) and 30-d mortality 0.78 (0.68-0.86); SOFA score on hospital mortality 0.65 (0.55-0.75) and 30-d mortality 0.66 (0.55-0.76) | - |
| Miskulin et al[20], 2004 | United States | Prospective | 1779 | Outpatient dialysis patients | 62 | 53 | ICED 0.72 (0.69-0.75); CCI 0.67 (0.65-0.70); Wright- Khan indices 0.68 (0.65-0.70); Davies indices 0.68 (0.65-0.70) | 1 yr |
| Wang et al[21], 2007 | Hong Kong | Prospective | 238 | ESRD on continuous PD | 55.7 | 51.3 | cTnT 0.774 (0.706-0.841); High sensitivity CRP 0.691 (0.614-0.768 | 3 yr |
| Sun et al[22], 2008 | China | Prospective | 217 | HD patients | 65.4 | 62.2 | BNP on CV events 0.61 (0.57-0.70); NT-proBNP on CV events 0.83 (0.75-0.91) | 2 yr |
| Selim et al[23], 2010 | Macedonia | Prospective | 125 | HD patients | 48.8 | - | BNP > 1200 pg/mL on CV mortality 0.612 (0.473-0.750); NT-proBNP > 10000 pg/mL on CV mortality 0.747 (0.677-0.816) | 2 yr |
| Breidthardt et al[24], 2011 | Switzerland | Retrospective | 113 | HD patients | 67.9 | 60 | BNP: all-cause mortality 0.70 (0.60-0.81); BNP: CV mortality 0.82 (0.73-0.90) | 735 d |
| Tomaszuk-Kazberuk et al[25], 2011 | Poland | Prospective | 39 | HD patients | 59 | 63.8 | Perfusion defects on MCE for death 0.752 (0.582-0.878) | 3 yr |
| Chiang et al[26], 2016 | Taiwan | Prospective | 132 | ESRD patients on PD | 53.7 | 47.0 | Short-term detrended fluctuation analysis (DFAα1) < 0.95-0.761 (0.617-0.905) | 34 mo |
| Sato et al[27], 2017 | Japan | Retrospective | 302 | HD patients | 63.4 | 65.4 | Neutrophil-lymphocyte ratio for 1-yr mortality 0.791 (0.602-0.980) | 1 yr |
| Wang et al[28], 2018 | China | Prospective | 300 | CKD 3-5 | 60 | 53.6 | UPCR for mortality 0.78 (0.72-0.84); eGFR for mortality 0.75 (0.69-0.81); Mitral E/E’ ratio for mortality 0.74 (0.67-0.80); LV mass index for mortality 0.72 (0.65-0.79); LVEF for mortality 0.50 (0.42-0.58) | 68 mo |
| Wlazeł et al[29], 2018 | Poland | Prospective | 64 | HD patients | 66 | 66 | SuPAR 0.84 (0.7-0.94) | 3 yr |
| Danial et al[30], 2019 | Malaysia | Retrospective | 160 | CKD 3-5 with adverse drug reaction | - | 57.5 | Mortality risk score model 0.789 (0.700-0.878) | Few weeks (until end date of ADRs) |
| Jagadeswaran et al[31], 2019 | India | Prospective | 129 | Pre-dialysis CKD 3-5 | 50.6 | 51.2 | Malnutrition inflammation score for mortality 0.709 (0.604-0.815) | 36 mo |
| Mukai et al[32], 2019 | Sweden | Prospective | 261 | CKD 5 (both dialysis and non-dialysis) | 56 | 66 | Skin autofluorescence for all-cause mortality 0.78 (0.71-0.86); Augmentation index for all-cause mortality 0.81 (0.75-0.87) | 25 mo |
| Pladys et al[33], 2020 | France | Retrospective | 9052 | ESRD on dialysis | 68.4 | - | Rennes score model 1(REIN and SNDS data) 0.789 (0.761–0.816); Model 2 (REIN data only) 0.794 (0.768-0.821); Wright classification 0.631 (0.621-0.639); Modified CCI 0.703 (0.689-0.716) | 1 yr |
| Shahidi et al[34], 2020 | Iran | Retrospective | 824 | HD patients | 57.9 | 61.8 | Hemoglobin change on mortality 0.61 (0.56-0.65) | 24.08 mo |
| Gan et al[35], 2021 | Australia | Prospective | 243 | CKD 3-4 | 65.6 | 62.6 | Left atrial reservoir strain for CV death 0.84 (0.76-0.90) | 3.9 yr |
| Rroji et al[36], 2021 | Albania | Prospective | 125 | ESRD on dialysis | 52.4 | 60.0 | CRP on CV mortality 0.80 (0.73-0.92); Left ventricular mass index on CV mortality 0.76 (0.65-0.88); Ratio of early mitral inflow velocity/annular diastolic velocity (E/E’) on CV mortality 0.75 (0.64-0.86); Systolic pulmonary artery pressure on CV mortality 0.80 (0.68-0.92); Pulse pressure on CV mortality 0.72 (0.60-0.84) | 2 yr |
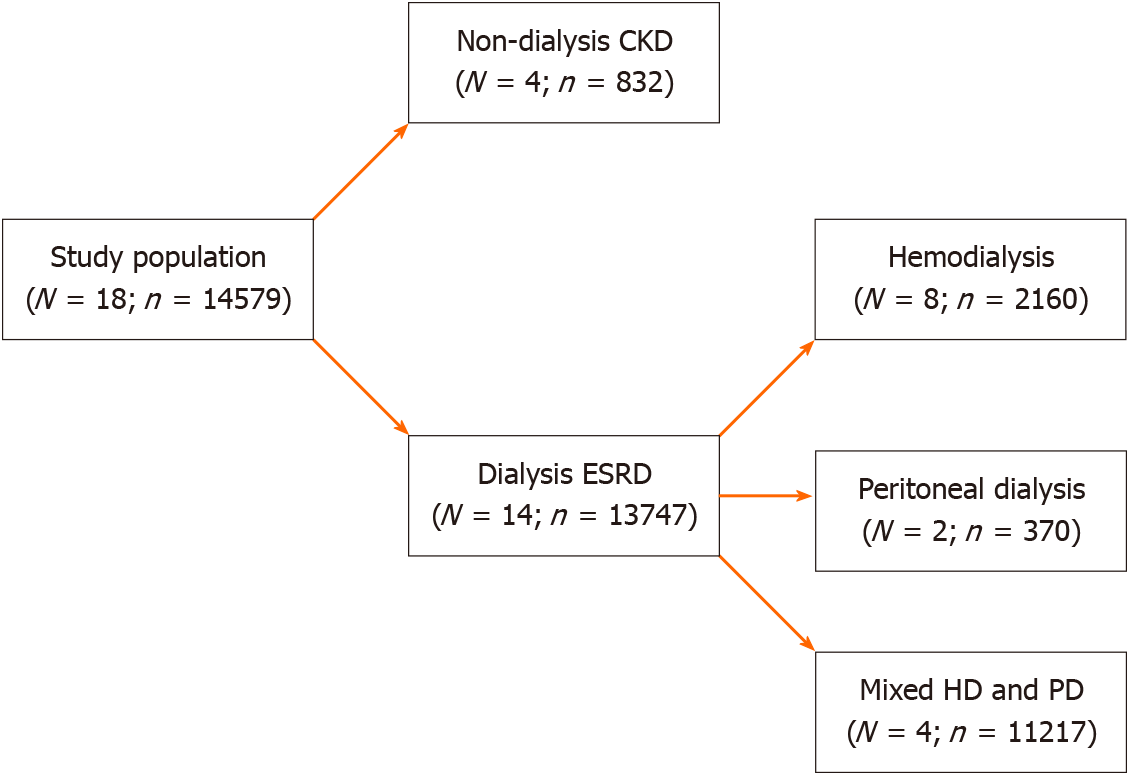
Of 14579 patients from 18 studies, 832 patients had non-dialysis CKD, and 13,747 patients had dialysis-dependent CKD. Among studies that included dialysis-dependent CKD patients, 2160 patients required hemodialysis (HD) and 370 patients required peritoneal dialysis (PD). The remaining 11217 patients (from four studies) had been composed of a non-differentiated mixed population of HD and PD patients. Figure 2 illustrates the study population classification.
A total of 24 predictive factors for mortality were identified. We classified these factors into three categories depending on their AUCs. None had an AUC greater than 0.90, which is considered outstanding for mortality prediction. The majority (n = 17) were in the acceptable range (AUCs 0.70-0.79). A total of seven predictive factors were identified as excellent (AUCs 0.80-0.89). Table 2 shows the predictive factors for mortality based on the population studied.
| AUC range | Interpretation | Predictive factors (AUC) | Subjects | Ref. |
| ≥ 0.90 | Outstanding | - | - | - |
| 0.80-0.89 | Excellent | NT-proBNP (0.83) | HD | Sun et al[22] |
| BNP (0.82) | HD | Breidthardt et al[24] | ||
| SuPAR (0.84) | HD | Wlazeł et al[29] | ||
| Augmentation index (0.81) | ND, HD, PD | Mukai et al[32] | ||
| Left atrial reservoir strain (0.84) | ND | Gan et al[35] | ||
| CRP (0.80) | HD, PD | Rroji et al[36] | ||
| Systolic PAP (0.80) | HD, PD | Rroji et al[36] | ||
| 0.70-0.79 | Acceptable | APACHE III (0.76) | HD | Dara et al[19] |
| ICED (0.72) | HD, PD | Miskulin et al[20] | ||
| cTnT (0.77) | PD | Wang et al[21], | ||
| NT-proBNP > 10000 pg/mL (0.75) | HD | Sun et al[22] | ||
| MCE perfusion defect (0.75) | HD | Tomaszuk-Kazberuk et al[25] | ||
| DFA1 < 0.95 (0.76) | PD | Chiang et al[26] | ||
| Neutrophil/lymphocyte ratio (0.79) | HD | Sato et al[27] | ||
| UPCR (0.78) | ND | Wang et al[28] | ||
| eGFR (0.75) | ND | Wang et al[28] | ||
| Mitral E/E’ ratio (0.74, 0.75) | ND, HD, PD | Wang et al[28] and Rroji et al[36] | ||
| LV mass index (0.72, 0.76) | ND, HD, PD | Wang et al[28] and Rroji et al[36] | ||
| Mortality risk score (0.79) | ND | Danial et al[30] | ||
| Malnutrition inflammation score (0.71) | ND | Jagadeswaran et al[31] | ||
| Skin autofluorescence (0.78) | ND, HD, PD | Mukai et al[32] | ||
| Rennes score (0.79) | HD, PD | Pladys et al[33] | ||
| Modified CCI (0.70) | HD, PD | Pladys et al[33] | ||
| Pulse pressure (0.72) | HD, PD | Rroji et al[36] |
N-terminal pro-brain natriuretic peptide (NT-proBNP) and brain natriuretic peptide (BNP) were found to be excellent predictors of mortality in dialysis patients[22,24]. In the general population, numerous factors such as age, female sex, and obesity can influence NT-proBNP level separate from heart failure[37]. BNP is eliminated by three main mechanisms: enzymatic metabolism, clearance through receptors, and urinary excretion[22]. Both BNP and NT-proBNP can be elevated in dialysis patients due to poor renal clearance, concomitant heart disease, heart failure, and volume expansion[38,39]. The mechanisms underlying the link between elevated BNP and NT-proBNP with increased mortality have not been fully established. One study suggested that chronic HD induces recurrent transient episodes of myocardial ischemia that result in myocardial stunning, hibernation, and remodeling[40]. Burton et al[40] described in this study that the decline in cardiac function and cardiac stunning were correlated with increasing BNP levels. These myocardial changes likely have a causative pathophysiologic role in the high cardiovascular complication rates and deaths in HD patients.
Soluble urokinase plasminogen activator receptor (suPAR) and C-reactive protein (CRP) were also excellent predictors for mortality in dialysis patients[29,36]. SuPAR is a proteolytic cleavage product of the glycosyl-phosphatidylinositol anchor of the urokinase plasminogen activator receptor expressed on immune and endothelial cells[41]. The concentration of suPAR is low in healthy individuals, but it becomes elevated in the presence of infection or inflammatory diseases[41]. Observational studies of dialysis patients have shown that higher suPAR levels are detected in patients that have severe angiographic coronary artery disease[29,42] or after cardiovascular events, both of which are associated with significantly increased cardiovascular death[43]. Increased suPAR levels are also associated with end-organ fibrosis, such as cirrhosis[44,45] and focal segmental glomerulosclerosis[46]. Similarly, CRP is a nonspecific acute phase reactant that can be elevated in the setting of infection or inflammatory processes[47]. Higher CRP levels are associated with pulmonary hypertension, which could represent crosstalk between inflammatory cells and pulmonary wall components[36,48,49]. Moreover, a population-based study demonstrated that CRP is independently associated with elevated parathyroid hormone levels (PTH) and increased mortality[50]. Elevated PTH in CKD patients can lead to renal osteodystrophy and accelerated vascular calcification. The associations between suPAR, CRP, and increased death suggest that these markers show promise for use as standard CKD mortality predictors in the future.
Echocardiography has been shown as a useful tool in the assessment of mortality risk in CKD patients. Gan et al[35] have demonstrated that impaired left atrial reservoir strain (LASr) is the best predictor of adverse cardiovascular outcomes in CKD stage 3-4 patients. In this assessment, the left atrium is divided into a total of 12 segments: six segments each from the apical four- and two-chamber views. LASr is measured from the average peak systolic strain from the 12 segments[35]. Impaired LASr is defined as ≤ 23%, based on a previously reported lower limit of reference value[51]. A few hypotheses have been postulated to link impaired LASr and mortality in the CKD population. First, the left atrium may be more predictive for mortality because it is a thin-walled chamber and may exhibit earlier alterations before the left ventricle[51]. Second, alterations of LASr irrespective of left ventricular (LV) function have been noted in heart failure with preserved ejection fraction[52], further highlighting it as a more sensitive marker. The underlying mechanisms for reduced LASr are believed to be a consequence of atrial fibrosis, which could be accelerated in the setting of CKD due to the attendant high systemic inflammatory state[53,54]. These potential pathophysiologic mechanisms may explain the significance of reduced LASr as an early indicator for poor outcomes in non-dialysis CKD patients.
Rroji et al[36] added that systolic pulmonary artery pressure (PAP) is predictive for mortality in dialysis patients. Other studies have concordantly shown that pulmonary hypertension (PH) confers a significant mortality risk in dialysis patients[55,56]. The prevalence of PH increases with CKD severity[55], and several studies have proposed mechanisms for the association between PH and CKD for both non-dialysis and dialysis patients. Arteriovenous fistulas for dialysis access and exposure to bio-incompatible dialysis membranes are a few of these factors that could increase the risk for PH in CKD patients[48,57]. The latter causes neutrophil activation that subsequently migrates to the lungs, resulting in increased pulmonary inflammation and vascular resistance[57]. For non-dialysis CKD patients, CKD itself can pathophysiologically directly incite pulmonary circulatory dysfunction and remodel
Augmentation index (AIx) was predictive of mortality in CKD patients[32]. AIx was derived from the arterial pulse wave analysis using a tonometry-based SphygmoCor device[32]. Augmentation pressure (AP) is defined as the maximum systolic pressure subtracted from the inflection point pressure (the merging of incident and the reflected wave). AIx is defined as AP divided by pulse pressure (PP), presented as a percentage. This index represents arterial stiffness, which could be a result of various patho
Non-dialysis population: Several predictive factors for mortality have been identified among non-dialysis CKD participants. Urine protein creatinine ratio (UPCR), eGFR, mortality risk score and malnutrition inflammation score (MIS) have been demonstrated to be predictive of mortality in this patient population[28,30,31]. It is thus not surprising that Wang et al[21] reported that UPCR and eGFR are predictive of mortality among CKD patients. The presence of proteinuria in CKD patients has been widely associated with an increased risk of CKD progression and death[58]. Similarly, eGFR decline represents progression of CKD severity, which independently confers a significantly increased mortality risk[59,60]. Renal interstitial fibrosis/scarring and tubular atrophy are consistent with GFR decline and degree of proteinuria[58]. Along with these changes, the tubular epithelial cells are stimulated to synthesize reactive oxygen species, which further attracts inflammatory cells and interstitial myofibroblasts, leading to more fibrosis and scarring[58]. These interstitial changes are associated with CKD progression, which could indirectly increase mortality[58]. Thus, early interventions aiming to prevent proteinuria and CKD progression are essential for reducing mortality in CKD patients.
In 2019, Danial et al[30] developed a mortality risk score for CKD patients. In this study, the authors investigated mortality from adverse drug reactions (ADRs) that specifically occurred in CKD patients. The mortality risk prediction model included the following variables: (1) History of heart disease; (2) Dyslipidemia; (3) Electrolyte imbalance; (4) Psychotic agents; (5) Creatine kinase; (6) Total number of medications; and (7) Conservative management (how the treatment was provided to subjects with ADR)[30]. The authors noted that these clinical factors are easily obtained in clinical practice, and this score allowed preemptive identification of CKD patients at risk of ADR-associated mortality. These patients may thus have a significant benefit with early clinical intervention and medication adjustment. However, this model has not been proven to be predictive of mortality outside the scope of ADR, and consequently, its applicability in general CKD patients may be limited.
MIS is another mortality risk predictor tool for both pre-dialysis and dialysis-dependent CKD patients. The MIS consists of ten elements obtained from the past medical history, physical examination, body mass index, and laboratory indices[61]. In 2001, Kalantar-Zadeh et al[61] demonstrated that the MIS correlated with morbidity and mortality in maintenance HD patients. Later, Jagadeswaran et al[31] applied MIS to a pre-dialysis Indian population and found that MIS had a 56.5% sensitivity and 81% specificity (AUC 0.709; 95%CI: 0.604-0.815) for mortality prediction during a 36-mo follow-up. The mortality risk increased by 13.7% for each additional point in the MIS. These studies demonstrate the importance of malnutrition prevention for both pre-dialysis and dialysis-dependent CKD patients.
Echocardiographic factors: The ratios of early inflow velocity/annular diastolic velocity (E/E’) through the mitral valve and the LV mass index are predictive of mortality in CKD patients, regardless of dialysis status[28,36]. Mitral E/E’ ratio may be a marker of LV diastolic dysfunction[62] or a predictor of pulmonary hypertension[36]. Diastolic dysfunction has been associated with increased mortality in various CKD and non-CKD patient populations[63-65]. Similarly, an increased LV mass index represents LV hypertrophy, a well-established predictor for heart failure and death in CKD patients[66]. The 2013 European Society of Hypertension/European Society of Cardiology guidelines recommend performing echocardiography to refine cardiovascular risk assessment in hypertensive patients[67]. However, there are no recommendations specifically for CKD patients, despite studies showing an increased prevalence of LV hypertrophy with advancing CKD[68]. Our study further adds to the available evidence supporting echocardiography for mortality risk stratification in CKD patients. It should be highlighted that a combination of echocardiographic findings should be utilized to predict mortality in CKD patients, rather than isolated findings. This concept is supported by Tripepi et al[69] where the measurement of LV mass index in isolation does not provide significant prognostic value for CKD patients.
Real-time myocardial contrast echocardiography (MCE) is a non-invasive tool for assessment of myocardial ischemia in HD patients[70]. During this procedure, intravenous contrast is utilized to assess myocardial perfusion adequacy, which is defined as homogenous enhancement in > 50% of myocardial wall thickness for each segment[25]. One study showed that MCE and coronary angiography resulted in equal accuracy for anticipating combined cardiovascular endpoints and death[25]. The authors concluded that MCE is a safe and uncomplicated test that can aid in the selection of candidates for coronary revascularization. However, this data was limited to only HD patients. The utility of MCE should be confirmed in large multi-center studies of non-dialysis CKD patients.
Comorbidities: Comorbidities may serve as significant predictors for mortality in dialysis patients. Miskulin et al[20] investigated the index of coexisting disease (ICED) as an accurate mortality predictor in dialysis patients. In this study, ICED had greater discriminatory ability than other instruments. ICED is comprised of 19 medical conditions and 11 physical impairments; these are classified into four and three severity levels, respectively[20]. Another unique feature of ICED is the assessment of physical limitations, which has been shown as a strong prognostic factor in dialysis patients[71].
The Rennes comorbidity score and modified Charlson Comorbidity Index (CCI) have also been demonstrated to be predictive for mortality among dialysis patients[33]. The Rennes comorbidity score consists of seven components: Age, albumin level, cardiac, respiratory, and hepatic diseases, active malignancy, and walking disability[33]. Similarly, the modified CCI consists of 16 matrices composed of a wide range of medical comorbidities found in the original CCI plus patient’s age[33]. Pladys et al[33] found that the original CCI (without including patient’s age) had a low ability to predict 1-year mortality. Surprisingly, the Rennes score resulted in a very impressive ability to predict 1-year mortality among dialysis patients despite its inclusion of only seven variables[33]. Altogether, we conclude that specific comorbidities are crucial in determining mortality risk among dialysis patients. Not unexpectantly, accuracy is improved by the consideration of several high-yield comorbidities rather than relying solely on one particular comorbidity.
Miscellaneous: Acute physiologic assessment and chronic health evaluation (APACHE) III is an acceptable predictor for hospital, and 30-d mortality among HD patients admitted to the intensive care unit (ICU)[19]. The APACHE III prognostic system was invented in 1991 and has been validated in several patient populations[72]. The APACHE III is calculated from clinical data obtained during the first 24 h of ICU admission. It consists of several components, including the primary reason for ICU admission, age, sex, race, preexisting comorbidities, and location prior to ICU admission[72]. Although several modifications have been made to the original APACHE score, only APACHE III has confirmed accuracy for mortality prediction in critically ill HD patients. Dara et al[19] compared the performance of APACHE III with the SOFA score. They found that APACHE III outperformed SOFA score for mortality prediction in critically ill HD patients. In our systematic review, this was the only study that focused on ICU patients.
The prognostic value of serological biomarkers, such as cardiac troponin T (cTnT) and NT-proBNP, for mortality in dialysis patients has been shown in observational studies[21,22]. As noted earlier, these biomarkers highlight the association between cardiac remodeling and death in dialysis patients. cTnT has been shown to predict mortality in PD patients independent of inflammation, residual renal function, and cardiac hypertrophy[21]. This was supported by the recent Netherland Cooperative Study on the Adequacy of Dialysis report, where the predictive power of cTnT for mortality is superior to other risk factors in a mixed population of HD and PD patients[73]. We suggest the use of cTnT for its prognostic value in at least PD patients due to its ease of testing.
Pre-dialysis neutrophil-lymphocyte ratio (NLR) is a novel and strong short-term predictor for all-cause mortality in HD patients[27]. NLR has been shown to have predictive utility in numerous disease states, including acute myocardial infarction and autoimmune disease[74,75]. NLR represents systemic inflammation, nutritional status, and atherosclerosis, which are all prevalent in CKD patients[76,77]. One single-center Japanese cohort revealed that NLR is superior to other generic biomarkers for 1-year survival among patients with ESKD due to diabetic nephropathy[27]. Although the usefulness of NLR has been widely studied in CKD patients, more data on its accuracy in diverse CKD patients are needed.
Skin autofluorescence (SAF) has been studied in healthy and uremic individuals[78-80]. SAF is predictive of microvascular disease progression, cardiovascular events, and nonspecific adverse clinical outcomes in diabetic and CKD patients regardless of dialysis status[79,81-84]. SAF is measured by illuminating approximately 1 cm2 of skin surface with a 300-420 nm light source. SAF is calculated as the ratio between the emission light and reflected excitation light, multiplied by 100[32]. It is speculated that SAF represents the accumulation of advanced glycation end-products that cause collagen and elastin cross-linking, which can translate into increased arterial stiffness[85-87]. In 2019, Mukai et al[32] found that SAF in CKD patients is predictive of adverse clinical outcomes, including death. This study supports the association between SAF, arterial stiffness, and increased mortality in CKD patients.
PP is the difference between systolic and diastolic blood pressure. Rroji et al[36] demonstrated that PP has a significant impact on dialysis patient survival. In their study, patients with pulmonary hypertension had significantly higher PP compared with patients without pulmonary hypertension. The underlying mechanism between increased PP and death in CKD is not fully understood. However, we postulate that increased PP, either from a reduction of diastolic blood pressure or increase of systolic pressure, could increase morbidity and mortality due to reduced coronary perfusion pressure, elevated systemic vascular resistance, or decreased vascular compliance[88]. However, the predictive value of PP in CKD patients needs to be demonstrated in a larger study.
Detrended fluctuation analysis (DFA) of heart rate dynamics is another prognostic marker for PD patients. In recent years, heart rate variability derived from beat-to-beat heart rate dynamic monitors has been used as a surrogate marker of autonomic modulation in an effort to predict patient outcomes[89-91]. DFA is a scaling analysis method to represent the correlation properties of a signal[92]. This form of analysis permits the detection of long-range correlation embedded in non-stationary time series[93]. To date, a few studies have demonstrated that DFA provides significant information on risk of cardiovascular events in heart failure and acute coronary syndrome patients[94,95]. In 2016, Chiang and colleagues showed that lower short-term DFA was predictive of total mortality in PD patients (median follow-up duration of 34 mo)[26]. They were the first study to measure and associate autonomic dysregulation with clinical outcomes in ESRD patients. Subsequent studies have shown consistent data obtained from the general population, where reduced DFA was associated with increased mortality via sudden cardiac death[96]. DFA shows potential promise in CKD patients since ESRD is associated with an overactive sympathetic nervous system[97]. This dysregulation results in an amplification of intracellular cyclic AMP (cAMP), which leads to an increase of the action potentials in the sinoatrial node[98]. These pathophysiological changes can be detected in the altered beat-to-beat variability[99]. However, DFA is a relatively new method of clinical analysis, and its application in other patient populations such as HD and non-dialysis CKD remains to be elucidated.
Our study has several limitations that should be considered. First, the predictive factors reported in each study were not homogenous. They varied from each study due to study design and patient population. Because of this limitation, a meta-analysis, subgroup analysis, and test of homogeneity could not be performed. Second, all included studies were observational in nature, making them susceptible to selection bias. However, we minimized this bias by conducting the risk of bias assessment. Third, the pooled sample size for PD patients remained relatively small compared to non-dialysis CKD and HD patients. Generalization of our research findings to the PD population should be cautiously performed. Nonetheless, the findings from our research could be applied towards the design of future prospective studies with the goal of developing a prognostication scoring system for mortality in CKD patients. To date, the outstanding factors (defined by AUC ≥ 0.90) for mortality prediction in CKD patients have yet to be discovered.
Several factors were identified to provide accurate predictions of mortality in CKD patients. Echocardiography is an important tool for mortality prognostication in CKD patients by evaluating LASr, systolic PAP, mitral E/E’ ratio, and LV mass index. NT-proBNP, BNP, suPAR, CRP, and AIx are excellent factors in mortality prediction among CKD patients.
Chronic kidney disease (CKD) is a common medical condition that is increasing in prevalence. Understanding the accuracy of mortality risk factors in CKD patients could mitigate death.
Evidence has shown that several clinical factors are associated with mortality in CKD patients using regression analyses. However, the accuracy of these mortality predictive factors has not been clearly demonstrated.
To establish the accuracy of mortality predictive factors among CKD patients by utilizing the area under the receiver operating characteristic curve (AUC) analysis.
Ovid MEDLINE, EMBASE, and the Cochrane Library were searched for eligible articles through January 2021. Only studies that reported their mortality predictive factors with AUC and 95% confidence interval were included. These factors were classified as acceptable, excellent, or outstanding based on their AUC.
Of 1759 citations, a total of 18 studies (n = 14579) were included in the systematic review. Eight hundred thirty two patients had non-dialysis CKD, and 13747 patients had dialysis-dependent CKD (2160 hemodialysis, 370 peritoneal dialysis, and 11217 undifferentiated modalities of dialysis). Of 24 predictive factors, none were considered outstanding for mortality prediction. A total of seven predictive factors (N-terminal pro-brain natriuretic peptide, brain natriuretic peptide, soluble urokinase plasminogen activator receptor, augmentation index, left atrial reservoir strain, C-reactive protein, and systolic pulmonary artery pressure) were identified as excellent. Seventeen predictive factors were in the acceptable range, which we classified into the following subgroups: predictors for the non-dialysis population, echocardiographic factors, comorbidities, and miscellaneous.
This study determined several mortality risk factors for CKD patients that were deemed acceptable or excellent. Echocardiography is an important tool for mortality prognostication in CKD patients.
The results of this study provide a preliminary perspective on the importance of identifying better prognostic factors for mortality in CKD patients. There is a lack of predictive risk factors with an AUC greater than 0.90. Currently identified mortality risk factors can be combined to create a risk calculator for CKD patients, which could be subsequently validated in future research.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu JM, Patel J S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2017;7:1-59. Kidney Int Suppl (2011). 2017;7:e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 255] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 2. | Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3736] [Cited by in RCA: 3547] [Article Influence: 197.1] [Reference Citation Analysis (0)] |
| 3. | Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3153] [Cited by in RCA: 2866] [Article Influence: 238.8] [Reference Citation Analysis (0)] |
| 4. | Mills KT, Xu Y, Zhang W, Bundy JD, Chen CS, Kelly TN, Chen J, He J. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88:950-957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 588] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 5. | Hoerger TJ, Simpson SA, Yarnoff BO, Pavkov ME, Ríos Burrows N, Saydah SH, Williams DE, Zhuo X. The future burden of CKD in the United States: a simulation model for the CDC CKD Initiative. Am J Kidney Dis. 2015;65:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 6. | Peralta CA, Vittinghoff E, Bansal N, Jacobs D Jr, Muntner P, Kestenbaum B, Lewis C, Siscovick D, Kramer H, Shlipak M, Bibbins-Domingo K. Trajectories of kidney function decline in young black and white adults with preserved GFR: results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Kidney Dis. 2013;62:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Grams ME, Rebholz CM, Chen Y, Rawlings AM, Estrella MM, Selvin E, Appel LJ, Tin A, Coresh J. Race, APOL1 Risk, and eGFR Decline in the General Population. J Am Soc Nephrol. 2016;27:2842-2850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 8. | Grams ME, Chow EK, Segev DL, Coresh J. Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis. 2013;62:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 9. | van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium; van der Velde M, Levey AS, de Jong PE, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 713] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 10. | Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, Jafar T, Jassal SK, Landman GW, Muntner P, Roderick P, Sairenchi T, Schöttker B, Shankar A, Shlipak M, Tonelli M, Townend J, van Zuilen A, Yamagishi K, Yamashita K, Gansevoort R, Sarnak M, Warnock DG, Woodward M, Ärnlöv J; CKD Prognosis Consortium. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 462] [Cited by in RCA: 643] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 11. | Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1213] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 12. | Ma L, Zhao S. Risk factors for mortality in patients undergoing hemodialysis: A systematic review and meta-analysis. Int J Cardiol. 2017;238:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 13. | Saeed F, Arrigain S, Schold JD, Nally JV Jr, Navaneethan SD. What are the Risk Factors for One-Year Mortality in Older Patients with Chronic Kidney Disease? Nephron. 2019;141:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Grund B, Sabin C. Analysis of biomarker data: logs, odds ratios, and receiver operating characteristic curves. Curr Opin HIV AIDS. 2010;5:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47172] [Article Influence: 2948.3] [Reference Citation Analysis (0)] |
| 16. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 16795] [Article Influence: 671.8] [Reference Citation Analysis (0)] |
| 17. | Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5:1315-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 2557] [Article Influence: 170.5] [Reference Citation Analysis (0)] |
| 18. | Hosmer Jr DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression, Third Edition. John Wiley & Sons, 2013. |
| 19. | Dara SI, Afessa B, Bajwa AA, Albright RC. Outcome of patients with end-stage renal disease admitted to the intensive care unit. Mayo Clin Proc. 2004;79:1385-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Miskulin DC, Martin AA, Brown R, Fink NE, Coresh J, Powe NR, Zager PG, Meyer KB, Levey AS; Medical Directors; Dialysis Clinic, Inc. Predicting 1 year mortality in an outpatient haemodialysis population: a comparison of comorbidity instruments. Nephrol Dial Transplant. 2004;19:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Wang AY, Lam CW, Wang M, Chan IH, Goggins WB, Yu CM, Lui SF, Sanderson JE. Prognostic value of cardiac troponin T is independent of inflammation, residual renal function, and cardiac hypertrophy and dysfunction in peritoneal dialysis patients. Clin Chem. 2007;53:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Sun L, Sun Y, Zhao X, Xu C, Chen D, Li L, Ma Y, Rong S, Mei C. Predictive role of BNP and NT-proBNP in hemodialysis patients. Nephron Clin Pract. 2008;110:c178-c184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Selim G, Stojceva-Taneva O, Gelev S, Stojcev N, Dzekova P, Trajcevska L, Asani A, Busletic I, Pavleska S, Sikole A. A multi-biomarker approach for the prediction of cardiovascular mortality in hemodyalisis patients. International Journal of Artificial Organs. Conference: 37th Annual European Society for Artificial Organs, ESAO Congress. Skopje Macedonia. 72/74 via Friuli, 20135 Milan, Italy: Wichtig editore, 2010. |
| 24. | Breidthardt T, Kalbermatter S, Socrates T, Noveanu M, Klima T, Mebazaa A, Mueller C, Kiss D. Increasing B-type natriuretic peptide levels predict mortality in unselected haemodialysis patients. Eur J Heart Fail. 2011;13:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Tomaszuk-Kazberuk A, Sobkowicz B, Malyszko J, Malyszko JS, Hirnle T, Dobrzycki S, Mysliwiec M, Musial WJ. Real-time myocardial contrast echocardiography as a useful tool to select candidates for coronary revascularization among patients with end-stage renal disease - a 3-year follow-up study. Adv Med Sci. 2011;56:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Chiang JY, Huang JW, Lin LY, Chang CH, Chu FY, Lin YH, Wu CK, Lee JK, Hwang JJ, Lin JL, Chiang FT. Detrended Fluctuation Analysis of Heart Rate Dynamics Is an Important Prognostic Factor in Patients with End-Stage Renal Disease Receiving Peritoneal Dialysis. PLoS One. 2016;11:e0147282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Sato H, Takeuchi Y, Matsuda K, Kagaya S, Saito A, Fukami H, Ojima Y, Nagasawa T. Pre-Dialysis Neutrophil-Lymphocyte Ratio, a Novel and Strong Short-Term Predictor of All-Cause Mortality in Patients With Diabetic Nephropathy: Results From a Single-Center Study. Ther Apher Dial. 2017;21:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Wang AYM, Wu HH, Cai Q, Wong HKS, Lu X. Kidney and cardiac parameters-which are more important in predicting circulatory congestion and mortality risk in CKD Insights from a 5-year prospective analysis. J Am Soc Nephrol. 2018;29:416-417. |
| 29. | Wlazeł RN, Szadkowska I, Bartnicki P, Rośniak-Bąk K, Rysz J. Clinical and prognostic usefulness of soluble urokinase plasminogen activator receptor in hemodialysis patients. Int Urol Nephrol. 2018;50:339-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Danial M, Hassali MA, Meng OL, Kin YC, Khan AH. Development of a mortality score to assess risk of adverse drug reactions among hospitalized patients with moderate to severe chronic kidney disease. BMC Pharmacol Toxicol. 2019;20:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Jagadeswaran D, Indhumathi E, Hemamalini AJ, Sivakumar V, Soundararajan P, Jayakumar M. Inflammation and nutritional status assessment by malnutrition inflammation score and its outcome in pre-dialysis chronic kidney disease patients. Clin Nutr. 2019;38:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 32. | Mukai H, Svedberg O, Lindholm B, Dai L, Heimbürger O, Barany P, Anderstam B, Stenvinkel P, Qureshi AR. Skin autofluorescence, arterial stiffness and Framingham risk score as predictors of clinical outcome in chronic kidney disease patients: a cohort study. Nephrol Dial Transplant. 2019;34:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Pladys A, Vigneau C, Raffray M, Sautenet B, Gentile S, Couchoud C, Bayat S. Contribution of medico-administrative data to the development of a comorbidity score to predict mortality in End-Stage Renal Disease patients. Sci Rep. 2020;10:8582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Shahidi S, Amooshahi M, Shekl Abadi E, Shekl Abadi R, Faghih N, Feizi A. SAT-221 Association of blood cell count, iron indices and hemoglobin level with mortality in hemodialysis patients. Kidney Int Rep. 2020;5:S94-S95. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Gan GCH, Kadappu KK, Bhat A, Fernandez F, Gu KH, Cai L, Byth K, Eshoo S, Thomas L. Left Atrial Strain Is the Best Predictor of Adverse Cardiovascular Outcomes in Patients with Chronic Kidney Disease. J Am Soc Echocardiogr. 2021;34:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 36. | Rroji M, Cafka M, Seferi S, Seiti J, Barbullushi M, Goda A. The potential effect of cardiac function on pulmonary hypertension, other risk factors, and its impact on survival in dialysis patients. Int Urol Nephrol. 2021;53:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Costello-Boerrigter LC, Boerrigter G, Redfield MM, Rodeheffer RJ, Urban LH, Mahoney DW, Jacobsen SJ, Heublein DM, Burnett JC Jr. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 329] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 38. | Goetze JP, Jensen G, Møller S, Bendtsen F, Rehfeld JF, Henriksen JH. BNP and N-terminal proBNP are both extracted in the normal kidney. Eur J Clin Invest. 2006;36:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Haller C, Zehelein J, Remppis A, Müller-Bardorff M, Katus HA. Cardiac troponin T in patients with end-stage renal disease: absence of expression in truncal skeletal muscle. Clin Chem. 1998;44:930-938. [PubMed] |
| 40. | Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 517] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 41. | Thunø M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27:157-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 213] [Reference Citation Analysis (0)] |
| 42. | Eapen DJ, Manocha P, Ghasemzadeh N, Patel RS, Al Kassem H, Hammadah M, Veledar E, Le NA, Pielak T, Thorball CW, Velegraki A, Kremastinos DT, Lerakis S, Sperling L, Quyyumi AA. Soluble urokinase plasminogen activator receptor level is an independent predictor of the presence and severity of coronary artery disease and of future adverse events. J Am Heart Assoc. 2014;3:e001118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 43. | Meijers B, Poesen R, Claes K, Dietrich R, Bammens B, Sprangers B, Naesens M, Storr M, Kuypers D, Evenepoel P. Soluble urokinase receptor is a biomarker of cardiovascular disease in chronic kidney disease. Kidney Int. 2015;87:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Koch A, Voigt S, Kruschinski C, Sanson E, Dückers H, Horn A, Yagmur E, Zimmermann H, Trautwein C, Tacke F. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care. 2011;15:R63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 45. | Berres ML, Schlosser B, Berg T, Trautwein C, Wasmuth HE. Soluble urokinase plasminogen activator receptor is associated with progressive liver fibrosis in hepatitis C infection. J Clin Gastroenterol. 2012;46:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 648] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 47. | Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004;279:48487-48490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 1007] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 48. | Kawar B, Ellam T, Jackson C, Kiely DG. Pulmonary hypertension in renal disease: epidemiology, potential mechanisms and implications. Am J Nephrol. 2013;37:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Huertas A, Tu L, Humbert M, Guignabert C. Chronic inflammation within the vascular wall in pulmonary arterial hypertension: more than a spectator. Cardiovasc Res. 2020;116:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 50. | Cheng SP, Liu CL, Liu TP, Hsu YC, Lee JJ. Association between parathyroid hormone levels and inflammatory markers among US adults. Mediators Inflamm. 2014;2014:709024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 51. | Morris DA, Takeuchi M, Krisper M, Köhncke C, Bekfani T, Carstensen T, Hassfeld S, Dorenkamp M, Otani K, Takigiku K, Izumi C, Yuda S, Sakata K, Ohte N, Tanabe K, Osmanoglou E, Kühnle Y, Düngen HD, Nakatani S, Otsuji Y, Haverkamp W, Boldt LH. Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: multicentre study. Eur Heart J Cardiovasc Imaging. 2015;16:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 52. | von Roeder M, Rommel KP, Kowallick JT, Blazek S, Besler C, Fengler K, Lotz J, Hasenfuß G, Lücke C, Gutberlet M, Schuler G, Schuster A, Lurz P. Influence of Left Atrial Function on Exercise Capacity and Left Ventricular Function in Patients With Heart Failure and Preserved Ejection Fraction. Circ Cardiovasc Imaging. 2017;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 53. | Freed BH, Shah SJ. Stepping Out of the Left Ventricle's Shadow: Time to Focus on the Left Atrium in Heart Failure With Preserved Ejection Fraction. Circ Cardiovasc Imaging. 2017;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Rao AK, Djamali A, Korcarz CE, Aeschlimann SE, Wolff MR, Stein JH. Left atrial volume is associated with inflammation and atherosclerosis in patients with kidney disease. Echocardiography. 2008;25:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Walther CP, Nambi V, Hanania NA, Navaneethan SD. Diagnosis and Management of Pulmonary Hypertension in Patients With CKD. Am J Kidney Dis. 2020;75:935-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 56. | Tang M, Batty JA, Lin C, Fan X, Chan KE, Kalim S. Pulmonary Hypertension, Mortality, and Cardiovascular Disease in CKD and ESRD Patients: A Systematic Review and Meta-analysis. Am J Kidney Dis. 2018;72:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 57. | Bolignano D, Rastelli S, Agarwal R, Fliser D, Massy Z, Ortiz A, Wiecek A, Martinez-Castelao A, Covic A, Goldsmith D, Suleymanlar G, Lindholm B, Parati G, Sicari R, Gargani L, Mallamaci F, London G, Zoccali C. Pulmonary hypertension in CKD. Am J Kidney Dis. 2013;61:612-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 58. | Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet. 2017;389:1238-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1654] [Cited by in RCA: 2424] [Article Influence: 303.0] [Reference Citation Analysis (0)] |
| 59. | Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 2006;69:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 403] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 60. | Patel AD, Ibrahim M, Swaminathan RV, Minhas IU, Kim LK, Venkatesh P, Feldman DN, Minutello RM, Bergman GW, Wong SC, Singh HS. Five-year mortality outcomes in patients with chronic kidney disease undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv. 2017;89:E124-E132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38:1251-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 658] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 62. | Obokata M, Reddy YNV, Borlaug BA. Diastolic Dysfunction and Heart Failure With Preserved Ejection Fraction: Understanding Mechanisms by Using Noninvasive Methods. JACC Cardiovasc Imaging. 2020;13:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 63. | Tennøe AH, Murbræch K, Andreassen JC, Fretheim H, Garen T, Gude E, Andreassen A, Aakhus S, Molberg Ø, Hoffmann-Vold AM. Left Ventricular Diastolic Dysfunction Predicts Mortality in Patients With Systemic Sclerosis. J Am Coll Cardiol. 2018;72:1804-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 64. | Jentzer JC, Anavekar NS, Mankad SV, Khasawneh M, White RD, Barsness GW, Rabinstein AA, Kashani KB, Pislaru SV. Echocardiographic left ventricular diastolic dysfunction predicts hospital mortality after out-of-hospital cardiac arrest. J Crit Care. 2018;47:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 65. | Lassen MCH, Sengeløv M, Qasim A, Jørgensen PG, Bruun NE, Olsen FJ, Fritz-Hansen T, Gislason G, Biering-Sørensen T. Ratio of Transmitral Early Filling Velocity to Early Diastolic Strain Rate Predicts All-Cause Mortality in Heart Failure with Reduced Ejection Fraction. J Card Fail. 2019;25:877-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Dubin RF, Deo R, Bansal N, Anderson AH, Yang P, Go AS, Keane M, Townsend R, Porter A, Budoff M, Malik S, He J, Rahman M, Wright J, Cappola T, Kallem R, Roy J, Sha D, Shlipak MG; CRIC Study Investigators. Associations of Conventional Echocardiographic Measures with Incident Heart Failure and Mortality: The Chronic Renal Insufficiency Cohort. Clin J Am Soc Nephrol. 2017;12:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 67. | Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F; Task Force Members. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3425] [Cited by in RCA: 3344] [Article Influence: 304.0] [Reference Citation Analysis (0)] |
| 68. | Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, Dries D, Xie D, Chen J, He J, Anderson A, Go AS, Shlipak MG; Chronic Renal Insufficiency Cohort (CRIC) Study Group. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol. 2012;23:1725-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 69. | Tripepi G, D'Arrigo G, Mallamaci F, London G, Tangri N, Hsu JY, Feldman HI, Zoccali C. Prognostic values of left ventricular mass index in chronic kidney disease patients. Nephrol Dial Transplant. 2021;36:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Pérez de Isla L, Rodrigo JL, Almería C, Pérez Ferro M, Serra V, Zamorano JL. Myocardial contrast echocardiography in coronary artery disease. Eur J Echocardiogr. 2004;5 Suppl 2:S11-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 71. | McClellan WM, Anson C, Birkeli K, Tuttle E. Functional status and quality of life: predictors of early mortality among patients entering treatment for end stage renal disease. J Clin Epidemiol. 1991;44:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 127] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 72. | Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2591] [Cited by in RCA: 2719] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 73. | Havekes B, van Manen JG, Krediet RT, Boeschoten EW, Vandenbroucke JP, Dekker FW; NECOSAD Study Group. Serum troponin T concentration as a predictor of mortality in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2006;47:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Ouellet G, Malhotra R, Penne EL, Usvya L, Levin NW, Kotanko P. Neutrophil-lymphocyte ratio as a novel predictor of survival in chronic hemodialysis patients. Clin Nephrol. 2016;85:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 75. | Uslu AU, Küçük A, Şahin A, Ugan Y, Yılmaz R, Güngör T, Bağcacı S, Küçükşen S. Two new inflammatory markers associated with Disease Activity Score-28 in patients with rheumatoid arthritis: neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Int J Rheum Dis. 2015;18:731-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 76. | Turkmen K, Ozcicek F, Ozcicek A, Akbas EM, Erdur FM, Tonbul HZ. The relationship between neutrophil-to-lymphocyte ratio and vascular calcification in end-stage renal disease patients. Hemodial Int. 2014;18:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 77. | Agarwal R, Light RP. Patterns and prognostic value of total and differential leukocyte count in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:1393-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 78. | Meerwaldt R, Graaff R, Oomen PHN, Links TP, Jager JJ, Alderson NL, Thorpe SR, Baynes JW, Gans ROB, Smit AJ. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47:1324-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 552] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 79. | Meerwaldt R, Hartog JW, Graaff R, Huisman RJ, Links TP, den Hollander NC, Thorpe SR, Baynes JW, Navis G, Gans RO, Smit AJ. Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J Am Soc Nephrol. 2005;16:3687-3693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 291] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 80. | den Hollander NC, Mulder DJ, Graaff R, Thorpe SR, Baynes JW, Smit GP, Smit AJ. Advanced glycation end products and the absence of premature atherosclerosis in glycogen storage disease Ia. J Inherit Metab Dis. 2007;30:916-923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 81. | Lutgers HL, Graaff R, Links TP, Ubink-Veltmaat LJ, Bilo HJ, Gans RO, Smit AJ. Skin autofluorescence as a noninvasive marker of vascular damage in patients with type 2 diabetes. Diabetes Care. 2006;29:2654-2659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 82. | McIntyre NJ, Fluck RJ, McIntyre CW, Taal MW. Skin autofluorescence and the association with renal and cardiovascular risk factors in chronic kidney disease stage 3. Clin J Am Soc Nephrol. 2011;6:2356-2363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 83. | Jiang J, Chen P, Chen J, Yu X, Xie D, Mei C, Xiong F, Shi W, Zhou W, Liu X, Sun S, Zhang P, Yang X, Zhang Y, Liang X, Zhang Z, Lin Q, Yu Y, Miyata T, Tian J, Liang M, Luo W, Xu X, Hou F. Accumulation of tissue advanced glycation end products correlated with glucose exposure dose and associated with cardiovascular morbidity in patients on peritoneal dialysis. Atherosclerosis. 2012;224:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 84. | Meerwaldt R, Lutgers HL, Links TP, Graaff R, Baynes JW, Gans RO, Smit AJ. Skin autofluorescence is a strong predictor of cardiac mortality in diabetes. Diabetes Care. 2007;30:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 219] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 85. | Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003;21:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 453] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 86. | Pischetsrieder M. Chemistry of glucose and biochemical pathways of biological interest. Perit Dial Int. 2000;20 Suppl 2:S26-S30. [PubMed] |
| 87. | Sims TJ, Rasmussen LM, Oxlund H, Bailey AJ. The role of glycation cross-links in diabetic vascular stiffening. Diabetologia. 1996;39:946-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 179] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 88. | Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, Tendera M, Tavazzi L, Bhatt DL, Steg PG; CLARIFY Investigators. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet. 2016;388:2142-2152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 321] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 89. | Oikawa K, Ishihara R, Maeda T, Yamaguchi K, Koike A, Kawaguchi H, Tabata Y, Murotani N, Itoh H. Prognostic value of heart rate variability in patients with renal failure on hemodialysis. Int J Cardiol. 2009;131:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 90. | Mylonopoulou M, Tentolouris N, Antonopoulos S, Mikros S, Katsaros K, Melidonis A, Sevastos N, Katsilambros N. Heart rate variability in advanced chronic kidney disease with or without diabetes: midterm effects of the initiation of chronic haemodialysis therapy. Nephrol Dial Transplant. 2010;25:3749-3754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 91. | Seely AJ, Macklem PT. Complex systems and the technology of variability analysis. Crit Care. 2004;8:R367-R384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 255] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 92. | Peng CK, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos. 1995;5:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2664] [Cited by in RCA: 1682] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 93. | Hu K, Ivanov PC, Chen Z, Carpena P, Stanley HE. Effect of trends on detrended fluctuation analysis. Phys Rev E Stat Nonlin Soft Matter Phys. 2001;64:011114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 398] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 94. | Ho KK, Moody GB, Peng CK, Mietus JE, Larson MG, Levy D, Goldberger AL. Predicting survival in heart failure case and control subjects by use of fully automated methods for deriving nonlinear and conventional indices of heart rate dynamics. Circulation. 1997;96:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 286] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 95. | Huikuri HV, Mäkikallio TH, Peng CK, Goldberger AL, Hintze U, Møller M. Fractal correlation properties of R-R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation. 2000;101:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 394] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 96. | Mäkikallio TH, Huikuri HV, Mäkikallio A, Sourander LB, Mitrani RD, Castellanos A, Myerburg RJ. Prediction of sudden cardiac death by fractal analysis of heart rate variability in elderly subjects. J Am Coll Cardiol. 2001;37:1395-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 146] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 97. | Vonend O, Rump LC, Ritz E. Sympathetic overactivity--the Cinderella of cardiovascular risk factors in dialysis patients. Semin Dial. 2008;21:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 98. | Converse RL Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 817] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 99. | Tulppo MP, Kiviniemi AM, Hautala AJ, Kallio M, Seppänen T, Mäkikallio TH, Huikuri HV. Physiological background of the loss of fractal heart rate dynamics. Circulation. 2005;112:314-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 170] [Article Influence: 8.5] [Reference Citation Analysis (0)] |