PATHOGENESIS OF ERECTILE DYSFUNCTION IN CRF
The pathogenesis of ED in CRF is often multifactorial and is primarily organic in origin. Factors to be considered include peripheral vascular disease, neurogenic abnormalities, hormonal disturbances and medications used for treatment of conditions associated with CRF. These physiological abnormalities may be supplemented by significant psychological stresses and abnormalities resulting from chronic illness and generalized changes in body function[4].
Vasculogenic factors
Vasculogenic factors can be divided into arterial insufficiency and veno-oclusive dysfunction (VOD). Arterial insufficiency is an important cause of ED in CRF. It is well known that patients with uremia on chronic hemodialysis have accelerated atherosclerosis affecting both large and small vessels and vasculogenic impotence could be suspected in these patients regardless of age[5]. Kaufman et al[6] found cavernous artery occlusive disease in 78% of uremic patients. This renal failure-associated vascular disease of the penis was found to occur independently of the presence of known systemic atherosclerotic vascular risk factors. Patients who underwent early treatment of the uremia by renal transplantation had vasculogenic ED only in the case of rejection of the renal transplant, suggesting that early renal transplantation may delay or prevent the development of the penile vasculopathy[6].
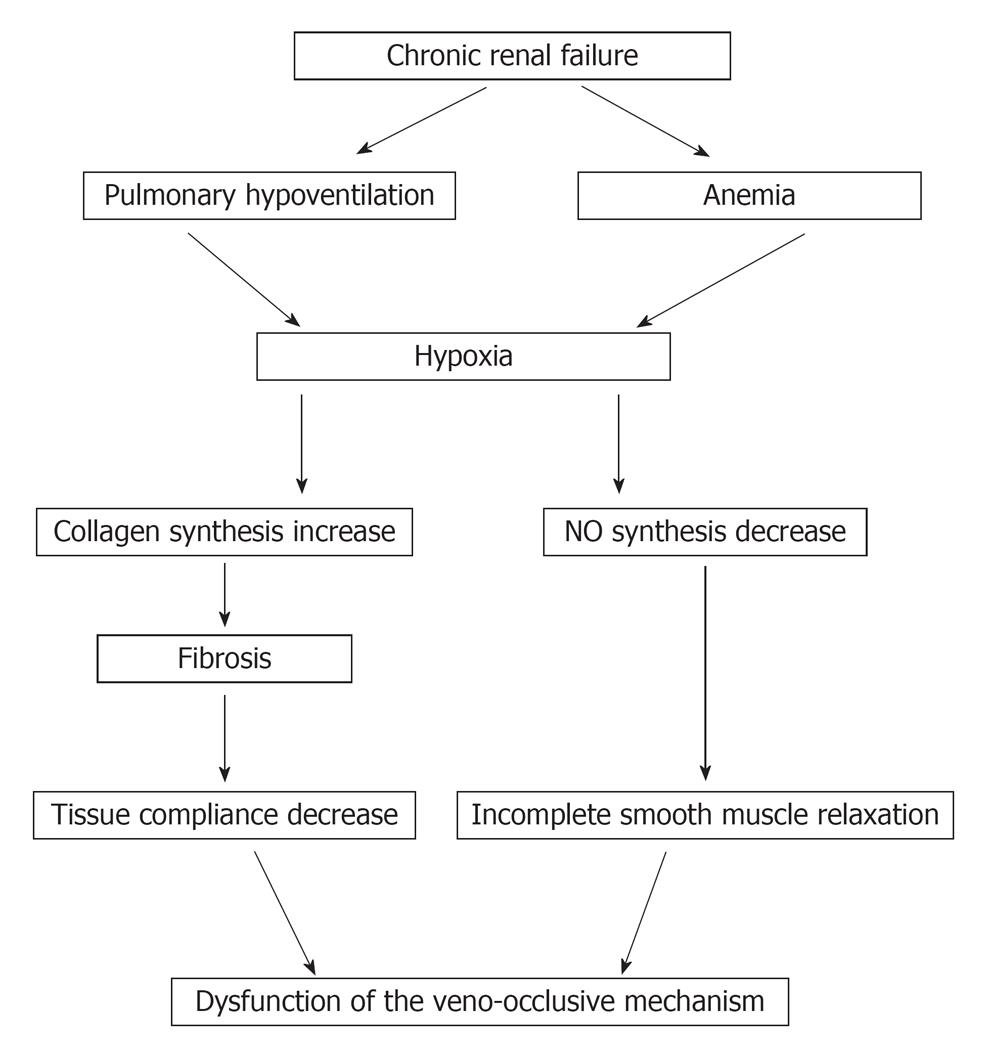
As a result of recent developments in the understanding of the physiology of ED, not only arterial inflow but also venous outflow abnormalities must be considered in the vascular assessment of ED. In one study VOD was found in 90% of impotent CRF patients[6]. The explanation of VOD is based on CRF associated hypoxia. Two distinct sources for the hypoxia in ESRD have been identified: a pulmonary-associated hypoxia based on hypoventilation and pulmonary microembolization[7] and an anemia-associated hypoxia based on diminished erythropoietin production[8]. Hypoxia has been shown to reduce nitric oxide synthase (NOS) mRNA expression and to decrease the activity of the enzyme NOS[9]. In addition, hypoxia has been found to increase transforming growth factor B1, which increases collagen synthesis with subsequent fibrosis and decrease tissue compliance. Such hypoxia-induced fibrotic structural changes in the erectile tissue may impair vascular compliance that would lead to VOD (Figure 1)[10].
Figure 1 Pathophysiology of chronic renal failure associated hypoxia in erectile dysfunction.
Neurogenic factors
Autonomic control of erectile smooth muscle tissue is critical in the maintenance of erectile function. Autonomic dysfunction is common in metabolic disorders, such as CRF. Impotence, postural dizziness, gastric fullness or delay in emptying, bowel dysfunction, and reduced sweating are the most common symptoms due to autonomic dysfunction[11]. Peripheral neuropathy is another cause of neurogenic ED in such group of patients[11].
Pharmacological factors
Some medications given to uremic patients may be implicated in the pathogenesis of ED. Several antihypertensive agents are known to cause sexual problems or exacerbate existing problems. However, not all classes of antihypertensive agents share the same risk of inducing sexual problems, and certain classes of antihypertensive agents tend to be associated with a higher prevalence of sexual dysfunction than others. A higher incidence of ED has been reported in some studies of diuretics, including spironolactone and thiazides, β blockers as well as centrally acting antiadrenergic agents, such as methyldopa and clonidine[12]. Few studies were found within the literature that evaluated the association between the existence of ED in CRF patients and the use of antihypertensive drugs. Rodger[13] showed that there was no relationship between the two phenomena. Cerqueira et al[1] found that ED was of equal frequency among patients using antihypertensive drugs and those that were not (26% vs 21%).
Histamine-2 receptor antagonists, particularly cimetidine, carry a high risk for causing ED due to increased plasma prolactin levels. More modern anti-ulcer drugs (e.g., proton pump inhibitors) do not appear to be associated with the same risk factor[14]. In addition, psychotropic drugs that may be used for treatment of depression can cause ED by influencing central nervous system mechanisms.
Psychological factors
The psychological impact of uremia has a significant role in ED in patients with CRF. Patients with uremia especially those on hemodialysis, have a significant incidence of psychiatric and depressive illnesses compared with the normal population[1,15]. The etiology of depression in patients with ESRD is usually associated with several losses. These include loss of kidney function, well being, place in family and workplace, financial resources, and of sexual function. Also the higher incidence of depression may be related to the problems of dependence on the machine, the problems of obtaining a kidney donor and worries about an uncertain future[15].
Depression may be difficult to diagnose in patients with ESRD. This is due, at least in part, to the overlap between the symptoms of depression and those of uremia. Symptoms of depression include sleep disturbance, depressed mood, appetite disturbances or weight change, problems in concentration, psychomotor agitation or depression, fatigue, feelings of guilt, worthlessness, loss of interest in life or pleasure, and recurrent thoughts of death or suicidal tendency[15].
There is a well established association between depression, its severity and ED. Procci et al[16] have identified a higher incidence of depressive episodes in patients on hemodialysis than in a normal population. Cerqueira and associates evaluated 119 pateints with CRF and on hemodialysis and found that of the patients who had depression, 34.8% had ED. When the regression analysis was performed, psycho-emotional factors persisted in the final model, showing a strong relationship with ED[1].
Endocrine factors
The kidney plays an integral role in endocrine function. In men with CRF, disturbances in the pituitary-gonadal axis can be detected with only moderate reductions in the GFR and progressively worsen as renal failure progresses. Total and free testosterone levels are typically reduced. Low testosterone levels are most probably caused by decreased testosterone production, although there is evidence for elevated metabolic clearance of testosterone in addition to decreased production[4].
Another proposed endocrinal cause of ED in patients with CRF is hyperprolactinemia which is identified in more than 50% of CRF patients on dialysis. Increased prolactin secretion in CRF may be related in part to the development of secondary hyperparathyroidism, depletion of total body zinc and to medications used in patients with CRF, such as methyldopa, digoxin, cimetidine and metoclopromide[4]. The mechanism by which increased levels of prolactin may cause ED is not well defined; an alteration in libido in such patients may be the cause of ED. On the other hand, some authors have shown that there is no correlation between ED and hyperprolactinemia in patients with CRF on hemodiaysis[1].
Other endocrinal abnormalities, especially diabetes mellitus, can strongly contribute to ED in patients with CRF. There are number of pathophysiological mechanisms that may explain the underlying etiology of diabetic ED. While neuropathy, endocrinopathy and vasculopathy are undoubtedly important, it is becoming increasingly evident that endothelial and smooth muscle function is disordered in diabetes and that this may be the most important factor for the majority of patients with diabetic ED[17].
Anemia
Anemia could contribute to the etiology of ED in men with ESRD because it worsens the poor general condition and causes asthenia in these patients. The treatment of anemia with recombinant erythropoietin in male renal failure patients has been reported to improve their sexual performance[18]. This, however, remains controversial and has led some authors to consider the use of erythropoietin to be inappropriate as a primary treatment[19].
Zinc deficiency
Zinc deficiency has also been suggested as a cause of ED. Uremic patients are often deficient in zinc, probably due to reduced dietary intake and/or zinc malabsorption. In some trials, supplemental zinc resulted in significant increases in potency, libido, and frequency of intercourse[20]. It is possible that normalization of total body zinc may also be effective in correcting uremic hyperprolactinemia and increased plasma testosterone[20,21]. Some studies support the use of zinc in treatment of ED in dialysis patients[22], although others showed the absence of a therapeutic effect of zinc in the treatment of ED in dialyzed patients[23]. Because of the difficulty in assessing true tissue zinc levels and the effect of these levels on erectile function, it cannot be concluded that serum or tissue zinc levels are directly responsible for ED and that zinc administration could be expected to produce improvement in erectile function.
EVALUATION OF ERECTILE DYSFUNCTION IN CRF
Evaluation should always begin with a systematic history and physical examination. A careful history to identify psychological factors, such as depression, must be carried out. A careful physical examination, including studies for the identification of peripheral neuropathy and vascular abnormalities is important. Hormonal studies, including testosterone, LH, FSH, and prolactin, should be performed. In patients in whom veno-occlusive incompetence or arterial abnormalities are suspected, penile Doppler ultrasound, pharmacocavernosometry, and pharmacocavernosography may be helpful, especially if a surgical intervention is planned. Communication between the nephrologist and transplant surgeon is essential, since transplantation may reverse many of the previously mentioned abnormalities of uremia, and initiation of surgical treatment may be delayed until after transplantation is carried out in some patients[24].
TREATMENT OF ED IN CRF
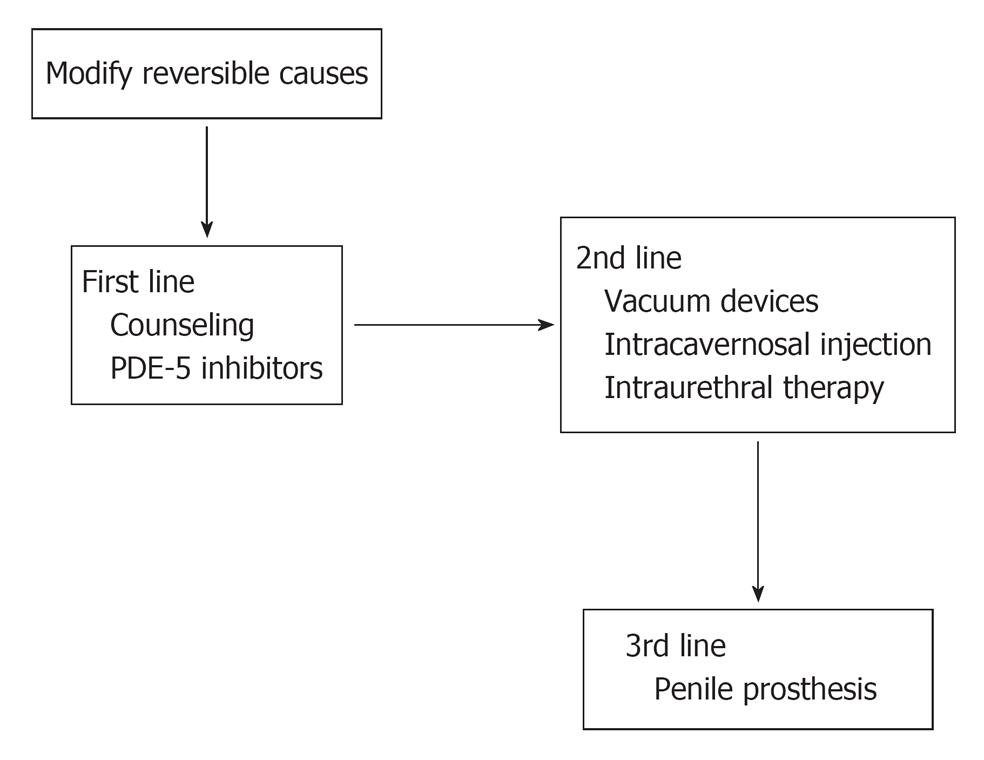
Treatment of ED in CRF patients must start with the determination and treatment of the underlying causes (Figure 2). The treatment of anemia with recombinant erythropoietin may improve the sexual performance[18]. If the ED is thought to be caused by β-blockers or centrally acting antihypertensives, these can be gradually changed to α-1adrenergic blockers or calcium channel blockers or angiotensin converting enzyme inhibitors.
Figure 2 Lines of treatment of erectile dysfunction in hemodialysis patients.
PDE: Phosphodiesterase.
Established guidelines recommend that patients with ED of suspected psychogenic origin should initially be offered sexual counseling. However the efficacy of psychotherapy is variable in the treatment of ED, also the acceptability of cost and the availability of qualified counselors may also limit the provision of such therapy[25]. Further lines of treatment of ED in ESRD can be classified as: 1st line [medical treatment which includes oral phosphodiesterase (PDE) -5 inhibitors and hormone regulation], 2nd line (intracavernosal injection, vacuum constriction device or alprostadil urethral suppository) and 3rd line (surgical treatment)[4].
Oral phosphodiesterase type 5 inhibitors
Since 1998 the Food and Drug Administration in the USA has approved three selective PDE type 5 inhibitors (PDE-5I): sildenafil, tadalafil, and vardenafil. Sildenafil citrate was the first PDE-5I introduced to the market. It is an orally active inhibitor of PDE-5; this agent therefore promotes erection by inhibiting the degradation of cGMP. cGMP in turn activates a specific protein kinase which blocks calcium channels and leads to decrease calcium influx. The consequence is a drop in cytosolic calcium concentrations and relaxation of the smooth muscle, thus facilitating the erectile response after sexual stimulation. Possible side effects include vasodilatory effects, such as headache, flushing, nasal congestion, systemic hypotension. Furthermore, the limited selectivity of sildenafil for retinal PDE-6 explains the “blue vision” effect[26].
In patients with CRF, the efficacy, safety and tolerability of sildenafil have been thoroughly reported[27-31], although strategies for the appropriate timing of drug intake in patients on dialysis programs has not been fully studied. Some authors reported the occurrence of transient hypotension after a 50-mg dose of sildenafil; as patients may remain relatively hypovolemic for some time after dialysis. They recommended that sildenafil should be used on days with no dialysis and that a smaller dose (25 mg) should be used initially[32].
Tadalafil and vardenafil are new orally administered potent PDE-5I which are more selective than sildenafil for PDE-6, and should not therefore be associated with the related visual disturbances that occur in some sildenafil-treated patients. Vardenafil, has the highest in vitro potency of all available PDE-5 inhibitors, and tadalafil, has a prolonged half-life that may enable couples to have non-programmed sexual activity. Data on the use of these drugs in CRF patients are still lacking. Only one clinical study showed the efficacy and safety of using vardenafil in treating ED in hemodiaysis patients[33].
Hormone regulation
Patients with deficient testosterone levels may be treated with testosterone replacement therapy, which may be beneficial in some cases. Most commonly, testosterone replacement therapy improves libido without significant impact on potency[34]. Recently, the efficacy of testosterone gel in the treatment of ED in hypogonadal hemodialysis patients has been showed in a cohort of 96 men with ESRD[35].
Pharmacological methods for decreasing hyperprolactinaemia also appear to be effective in some males with uremia-associated sexual dysfunction. Bromocriptine is uniformaly effective in normalizing prolactin levels in male dialysis patients with subsequent improvement in libido and potency[36]. However, the reported results are variable and the studies that have attempted to evaluate the effect of lowering prolactin levels on sexual dysfunction in male dialysis patients often suffer from methodological problems.
Vacuum constriction devices
These devices consist of an elastic band on a cylinder attached to a vacuum pump. The penis is placed in the cylinder and as air is pumped out, the vacuum draws blood into the corpora cavernosa. Once the penis is erect, the elastic band is slipped from the cylinder to the base of the penis, trapping the blood and maintaining the erection. Most manufacturers recommend that the constriction ring should be maintained for less than 30 min.
Lawrence et al[34] evaluated the effectiveness of vacuum tumescence therapy in male dialysis patients. Twenty-six patients used the vacuum constriction devices, with 19 (73.1%) having full correction of their ED. A further five predialysis patients used the devices, and all had correction of their ED. The authors concluded that vacuum tumescence therapy corrects ED in most patients[34].
Intracavernosal injection therapy
A wide variety of drugs are injected intracorporeally to induce an adequate erection, the most common of which are prostaglandin E1 (PGE1), papaverine hydrochloride and phentolamine mesylate. Patient and partner satisfaction is reported as 80%-85%. Side-effects are mainly local and include pain, haematoma or ecchymosis and priapism. Patients with ESRD have a coagulopathy, which is greater in those on hemodialysis and intracavernosal injections should, therefore, be used with caution[19]. Contraindications to therapy include poor eye sight, poor manual dexterity, coagulopathy and severe psychiatric disturbances.
Alprostadil urethral suppository
Alprostadil may be administered as an intraurethral gel suppository and reach the corpora cavernosa through direct venous communication. The single-use delivery system consists of an applicator with an alprostadil pellet in its tip. The patient inserts the applicator into the upright penis and rocks the applicator to separate the pellet[37]. Although the system has not been tested in patients with CRF, it has been effective in the treatment of ED arising from most other causes.
Surgical treatment
Penile prostheses should only be considered after failure of first and second-line therapies. These procedures should await renal transplantation, since many of men may improve their sexual function after transplantation. However penile prostheses can be successfully implanted without excessive risk of infection in patients with ED and ESRD[38].
EFFECT OF RENAL TRANSPLANTATION ON ERECTILE FUNCTION
Renal transplantation greatly improves the overall quality of life for patients with CRF and subsequently it may improve erectile function in a substantial number of renal transplant recipients (RTR), although its specific impact on this issue has been difficult to quantitate.
Some authors reported improvement of erectile function after renal transplantation[39,40]. The improvement of erectile function after transplantation is related to normalization of metabolic and hormonal function[41] and improvement of psychological problems in hemodilayzed patients[42]. On the other hand, others reported a high incidence(40% to 60% ) of ED among male RTR[43-45]. The etiology of ED in RTR is often multifactorial and several causal factors have been indicated, including anxiety, medication side effects, interference with penile vascularity, failure to resolve hormonal abnormalities or an underlying disease process, such as DM, hypercholesterolemia, hypertension and atherosclerosis, that has resulted in continued ED[44,45].