Copyright
©The Author(s) 2024.
World J Nephrol. Dec 25, 2024; 13(4): 98393
Published online Dec 25, 2024. doi: 10.5527/wjn.v13.i4.98393
Published online Dec 25, 2024. doi: 10.5527/wjn.v13.i4.98393
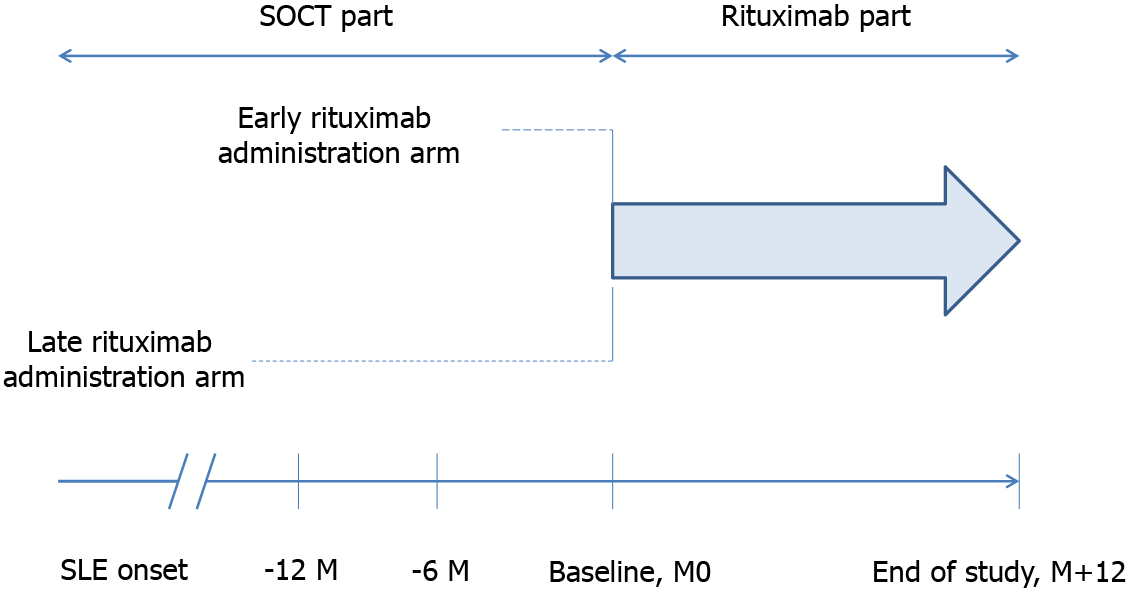
Figure 1 Study flow chart.
ERA: Early rituximab administration; LRA: Late rituximab administration.
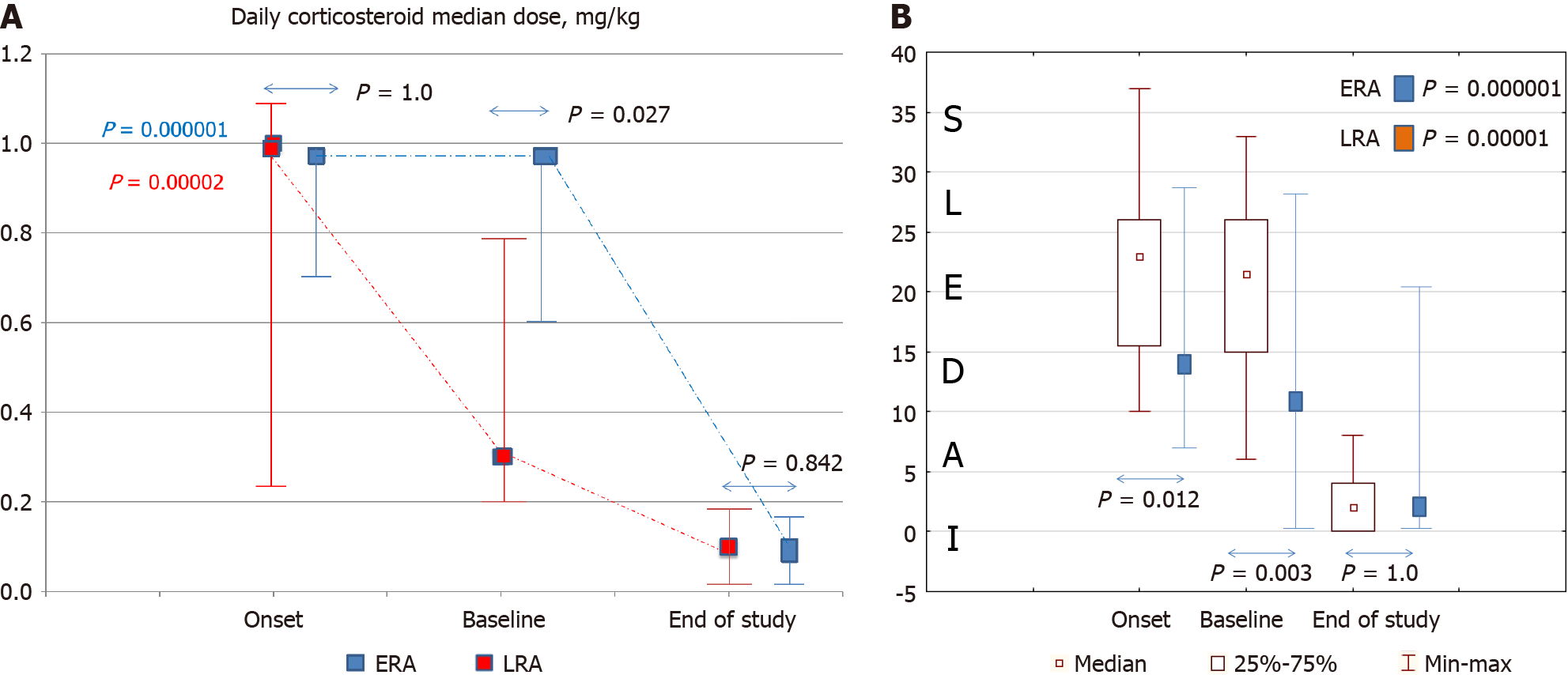
Figure 2 Outcomes after 12 months of treatment with early and late administration of rituximab BCD020 biosimilar.
A: Dynamics of corticosteroids; B: Dynamics of Systemic Lupus Erythematosus Disease Activity Index. ERA: Early rituximab administration; LRA: Late rituximab administration.
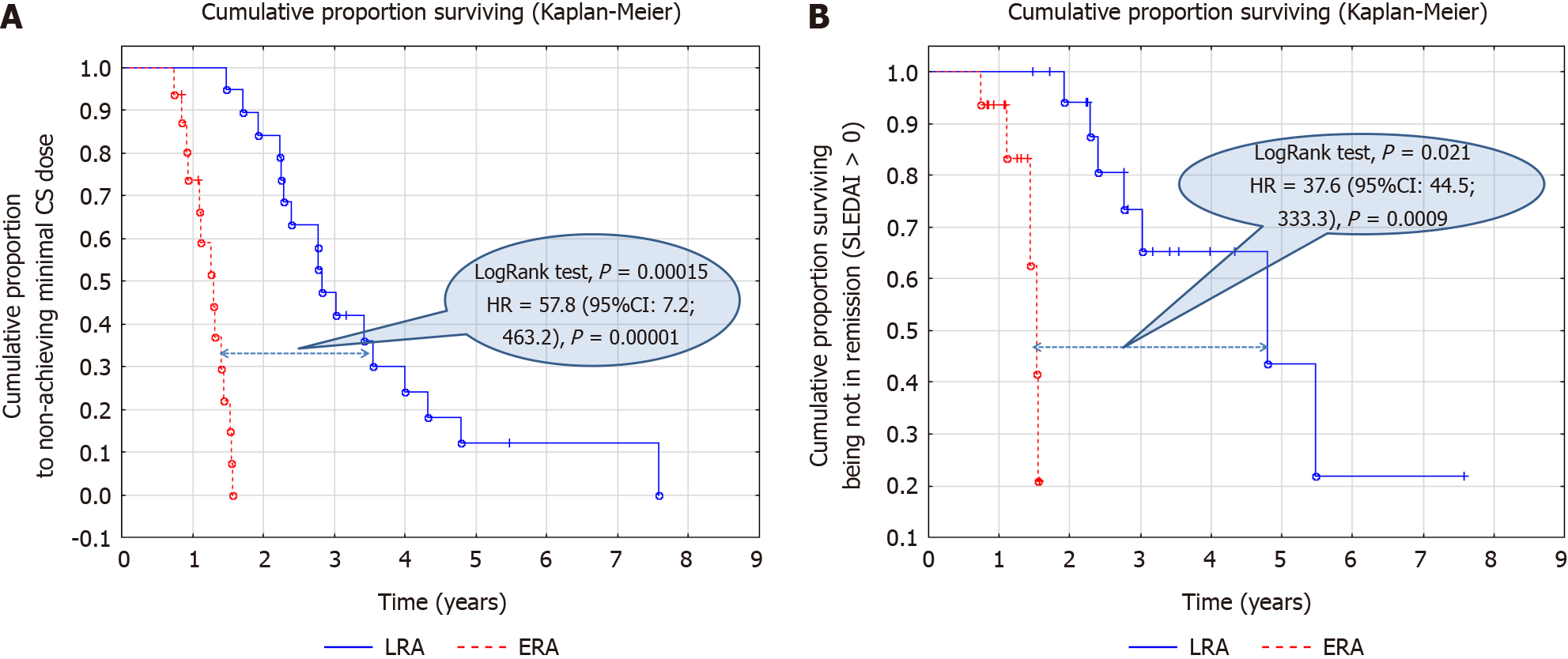
Figure 3 Long-lasting treatment outcomes of rituximab BCD020 biosimilar with early and late administration.
A: Probability of achieving low (≤ 0.2 mg/kg) corticosteroid dose in systemic lupus erythematosus patients with early and late rituximab administration; B: Probability of achieving remission (Systemic Lupus Erythematosus Disease Activity Index = 0) in systemic lupus erythematosus patients with early rituximab administration (ERA) and late rituximab administration (LRA).
- Citation: Kalashnikova E, Isupova E, Gaidar E, Lubimova N, Sorokina L, Chikova I, Kaneva M, Raupov R, Kalashnikova O, Aliev D, Gaydukova I, Kostik M. Outcomes of a 12-month course of early and late rituximab BCD020 biosimilar administration in juvenile systemic lupus erythematosus: A retrospective study. World J Nephrol 2024; 13(4): 98393
- URL: https://www.wjgnet.com/2220-6124/full/v13/i4/98393.htm
- DOI: https://dx.doi.org/10.5527/wjn.v13.i4.98393