Copyright
©2012 Baishideng.
World J Nephrol. Aug 6, 2012; 1(4): 100-105
Published online Aug 6, 2012. doi: 10.5527/wjn.v1.i4.100
Published online Aug 6, 2012. doi: 10.5527/wjn.v1.i4.100
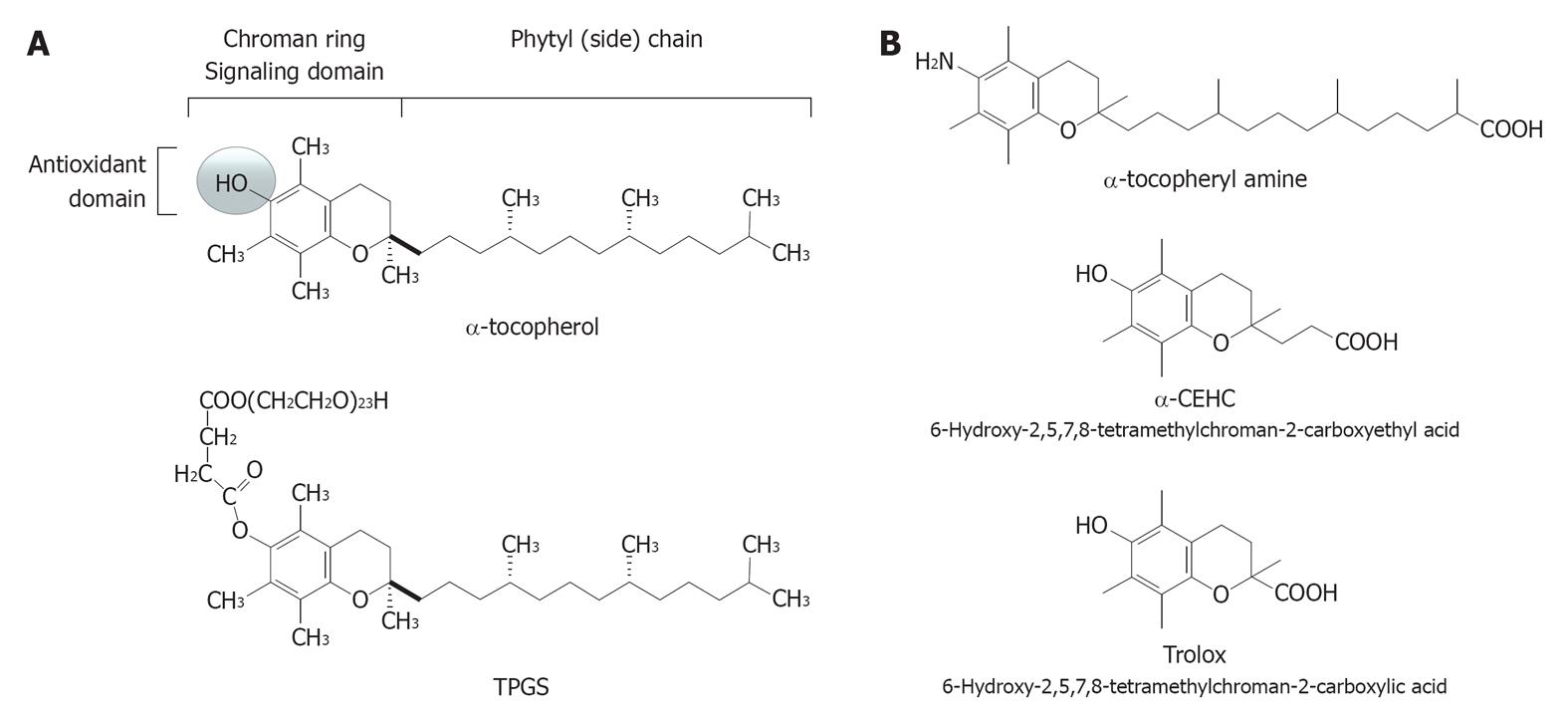
Figure 1 Molecular structure of some forms of vitamin E.
A: α-tocopherol (top) and its redox-silent synthetic analogue TPGS 1000 (bottom); B: Some examples of vitamin E analogues with antioxidant activity: the synthetic formsα-tocopherylamine (top) and Trolox (middle), and the hepatic short-chain metabolite α-CEHC (bottom).
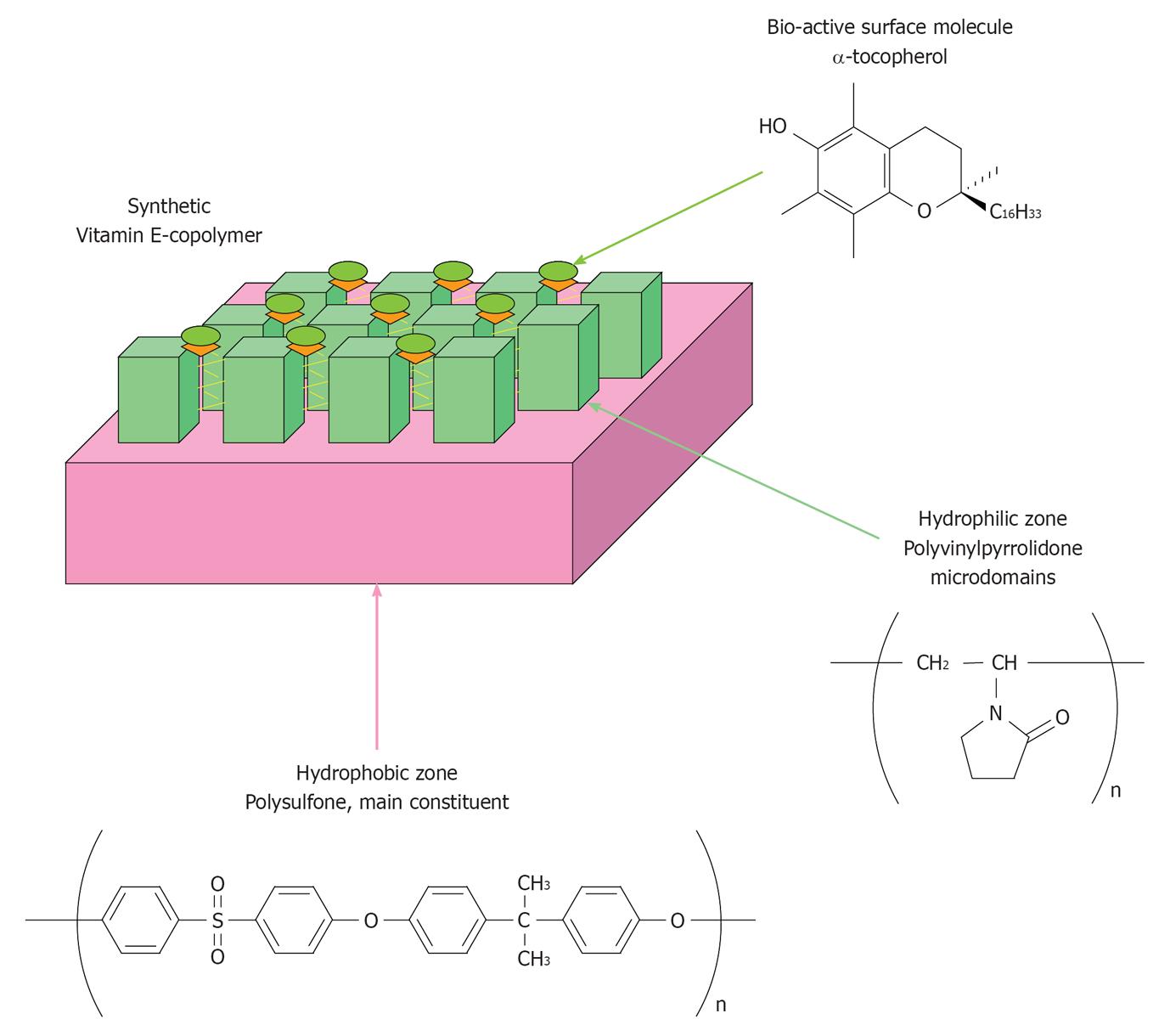
Figure 2 Schematic structure of the PS-PVP based copolymer functionalized with α-tocopherol.
Kindly provided by the R and D department of Ashai Medical, Tokyo, Japan.
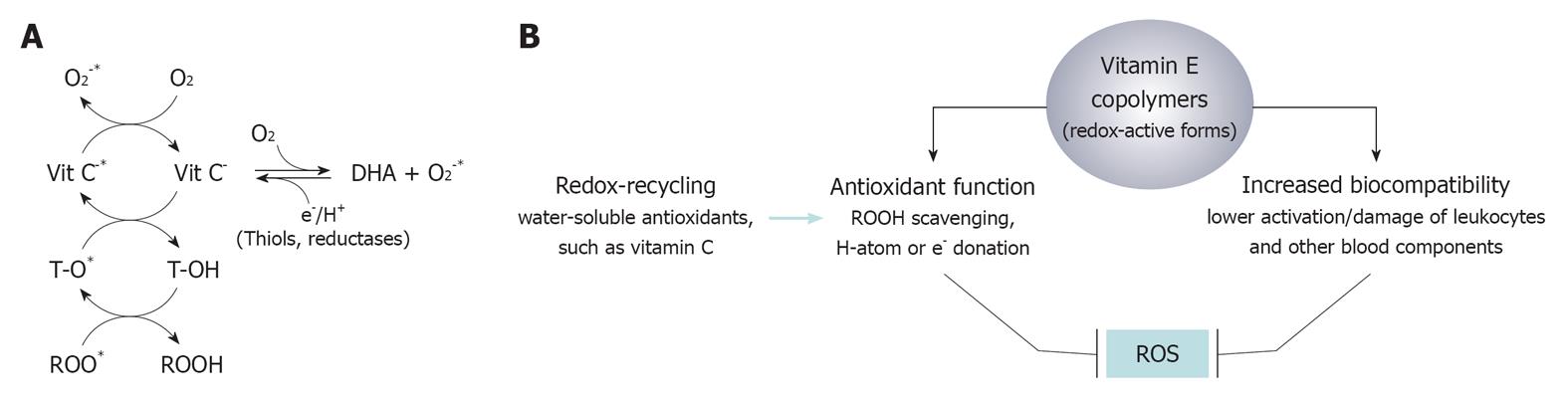
Figure 3 Coupled redox reaction of vitamin E and vitamin C (co-antioxidants) during the reduction (scavenging) of peroxyl radicals (A) and proposed scheme of the mechanisms that may lead vitamin E copolymers to control the flux of reactive oxygen species in the extracorporeal circulation (B).
ROS: Reactive oxygen species; Vit C: Ascorbic acid; Vit C-: Ascorbate anion; Vit C-*: Ascorbyl radical anion; DHA: Dehydroascorbate; O2: Molecular oxygen; O2-*: Superoxide anion; e-/H+: Electron/proton; T-OH: Tocopherol; T-O*: Tocopheryl radical; ROOH: Peroxyde (reduced form); ROO*: Peroxyl radical.
- Citation: Galli F. Vitamin E-derived copolymers continue the challenge to hemodialysis biomaterials. World J Nephrol 2012; 1(4): 100-105
- URL: https://www.wjgnet.com/2220-6124/full/v1/i4/100.htm
- DOI: https://dx.doi.org/10.5527/wjn.v1.i4.100