Peer-review started: May 7, 2020
First decision: July 25, 2020
Revised: August 7, 2020
Accepted: August 24, 2020
Article in press: August 24, 2020
Published online: September 25, 2020
Processing time: 140 Days and 4.9 Hours
The pandemic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has garnered the attention of scientists worldwide in the search for an effective treatment while also focusing on vaccine development. Several drugs have been used for the management of coronavirus disease 2019 (COVID-19), which has affected many hospitals and health centers worldwide. Statistically significant results are lacking on the effectiveness of the experimented drugs in reducing COVID-19 morbidity or mortality, as there are very few published randomized clinical trials. Despite this, the literature offers some material for study and reflection. This opinion review attempts to address three burning questions on COVID-19 treatment options. (1) What kind of studies are currently published or ongoing in the treatment of patients with COVID-19? (2) What drugs are currently described in the literature as options of treatment for patients affected by the infection? And (3) Are there specific clinical manifestations related to COVID-19 that can be treated with a customized and targeted therapy? By answering these questions, we wish to create a summary of current COVID-19 treatments and the anti-COVID-19 treatments proposed in the recent clinical trials developed in the last 3 mo, and to describe examples of clinical manifestations of the SARS-CoV-2 infection with a cause-related treatment.
Core Tip: The pandemic spread of coronavirus disease 2019 (COVID-19) has led to the need to standardize a therapeutic approach in order to offer the same indications for all patients admitted to the hospital admissions for severe acute respiratory syndrome coronavirus 2 infection. However, no specific drug or drug regimen has been approved for treatment. This opinion review describes the recent literature on this topic and summarizes the treatment strategies currently in use for COVID-19 related complications.
- Citation: Di Franco S, Alfieri A, Petrou S, Damiani G, Passavanti MB, Pace MC, Leone S, Fiore M. Current status of COVID-19 treatment: An opinion review. World J Virol 2020; 9(3): 27-37
- URL: https://www.wjgnet.com/2220-3249/full/v9/i3/27.htm
- DOI: https://dx.doi.org/10.5501/wjv.v9.i3.27
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, defined as coronavirus disease 2019 (COVID-19), was originally discovered and identified as the cause of numerous viral pneumonia cases occurring in Wuhan (Hubei Province, China)[1]. It has now spread worldwide with recent epidemiological data reporting 18614177 infected and 702642 deaths[2]. In the context of a growing global health emergency, medical professionals demand a need for up-to-date guidelines for the management and treatment of this novel infection. Currently, there are multiple approved treatments (drugs, monoclonal antibodies, vaccines) for COVID-19. According to the WHO Interim Guidance on the 2019 coronavirus, early treatment is suggested if patients have confirmed diagnosis with mild symptoms associated with co-morbidities, increased risk of mortality, or moderate-severe clinical manifestations. Due to the swab time delay for confirmation of results, in the presence of a strong suggestive clinical presentation it is reasonable to start antiviral treatment as soon as possible[3]. Due to the severity of clinical symptoms and no statistically significant recommended treatment regimen, experimental use of a drug not yet approved may be necessary to improve patients’ outcomes. To get more oriented among the numerous options of treatments proposed, this opinion review will summarize and clarify the role of each drug that has been used against COVID-19 in the clinical practice and those now under scientific examination.
The treatments proposed in the literature for COVID-19 are mainly based on the results of retrospective or observational studies, making it more difficult to hypothesize evidence-based therapies. Due to the need for more reliable data, the number of ongoing clinical trials are increasing. According to the International Clinical Trial Registry Platform database there are 1918 reported studies, with 1744 ongoing on COVID-19 patients[4]. Of these studies, 1661 are specific for the treatment of SARS-CoV-2 infection.
The most common classes of drugs used include antimalarial drugs, immunomodulators, convalescent plasma (CP), antiretrovirals, antibacterial drugs, lipid-lowering medications, anticoagulants and recently ivermectin. In this context, a large volume of data will soon be available and provide valuable novel recommendations regarding pathogenesis, treatment and prognosis[5].
There is currently no therapy for COVID-19 infection whose efficacy has been proven. Nonetheless due to the current global crisis, it is crucial to be able to formulate an effective therapeutic strategy based on the evidence existing in the literature. From the analysis of all of the clinical trials designed on COVID-19 infection, we found a wide number of drugs employed in a multimodal treatment.
Hydroxychloroquine, an old anti-malaria drug, displayed the ability to inhibit coronavirus replication in vitro. Real-life data are currently discordant in recognizing its anti-SARS-CoV-2 claimed effect[6,7]. Since the virus was found to utilize the cell surface receptor angiotensin-converting enzyme 2 (ACE2) expressed in the lung, heart, kidney, and intestine[8], it has been hypothesized that hydroxychloroquine may also interfere with ACE2 receptor glycosylation, thus preventing SARS-CoV-2 binding to target cells[9]. In addition, hydroxychloroquine can inhibit the acidification of lysosomes and endosomes, interfering with the fusion process of the virus with the host[10]. The results of the Phase 3 clinical trial NCT: 04315948 may clarify the role of hydroxychloroquine in COVID-19 patients’ prognosis.
Chloroquine alone or in combination with remdesivir and/or tocilizumab (under investigation by the clinical trial NCT: 04303507) may be effective against COVID-19 despite the more dangerous side effects than compared to hydroxychloroquine[11].
Among the drugs that seem to possess immunomodulatory benefits and reduce SARS-CoV-2 cell penetration, there are statins (the most prescribed ones are the atorvastatin 20 mg/d or an equivalent dose of rosuvastatin 40 mg/d). Statins act by reducing chemokine release, adhesion molecules, and modulating T-cell activity. Rosuvastatin, in particular, appears to have direct antiviral properties by binding and inhibiting the active site of the main protease enzyme (Mpro) of SARS-CoV-2. In a retrospective analysis Zhan et al[12] found that statin treatment among 13981 patients with COVID-19 was associated with a lower risk of all-cause mortality. Furthermore, the addition of ACE inhibitors or angiotensin II receptor blockers did not affect statin-associated outcomes in the studied cohort[12].
Remdesivir is a nucleoside analogue with a promising virus-inhibitory effect. It exhibits in vitro antiviral activity against coronaviruses[13] and in vivo has been shown to curb severe acute respiratory syndrome caused by coronavirus infection[14]. The drug can also inhibit viral replication interfering with the nascent viral-RNA chain resulting in its premature termination[15]. In a Phase 1 clinical trial the security and pharmacological effects of remdesivir were assessed[16]. Recently, in patients with severe COVID-19 receiving remdesivir clinical improvement was observed in 68% of cases (36 of 53 patients)[17]. However in a randomized, double-blind, placebo-controlled, multicenter trial, remdesivir was not associated with statistically significant clinical benefits[18]. Ongoing clinical trials (NCT: 04292899 and 04292730) should provide additional data on its effectiveness.
Azithromycin has shown in vitro antiviral activity against SARS-CoV-2, documented in literature at dosages similar to those used to treat bacterial pneumonia[19,20]. The mechanism of action is not well understood. It is believed to interfere with the acidification processes of lysosomes and endosomes[21] or amplification of the antiviral action of interferon in the host[22]. The use of azithromycin in combination with chloroquine/hydroxychloroquine has been described in the treatment of COVID-19 but the available clinical data is derived from retrospective, observational or uncontrolled studies[23,24]. The randomized telemedicine-based trial NCT: 04332107, now in Phase 3, may elicit further information.
Lopinavir and ritonavir are protease inhibitors used in HIV infections. Their use in combination allows the increase in half-life of lopinavir by enzymatic induction[25]. It has demonstrated in vitro antiviral activity for SARS-CoV[26] and MERS-CoV through inhibition of the 3-chymotrypsin-like protease[27]. Currently, there is no statistically significant evidence of its efficacy against SARS-CoV-2 in vitro. The studies available on the use of the lopinavir/ritonavir combination for the treatment of COVID-19 are mainly reports or retrospective studies, making it difficult to evaluate its effectiveness. In a randomized, controlled, open-label Chinese trial, no benefit was observed with lopinavir-ritonavir treatment[28]. There are several ongoing clinical trials. Among them NCT: 02735707 in its recruiting phase, is structured to compare the administration of lopinavir-ritonavir with no antiviral treatment.
Tocilizumab and sarilumab are monoclonal antibodies directed against the interleukin 6 (IL-6) receptor in which COVID-19 appears to target in the severe inflammatory process and cytokine storm causing critical damage to the lungs and other organs[29,30]. Tocilizumab appears to be a viable treatment strategy in COVID-19 patients with risk of developing cytokine storm[30]. Studies supporting this thesis are mainly case-reports and retrospective analyses. There are a few randomized clinical trials (RCTs) in development (ChiCTR: 200002976, EuCTR: 2020-001110-38 NCT: 04320615) for the evaluation of the efficacy and safety of Tocilizumab, alone or in combination, in the treatment of severe pneumonia in COVID-19 hospitalized patients. Sarilumab is currently being studied in a multicenter Phase 2-3 study for the treatment of severe forms of COVID-19 (NCT: 04315298).
Anakinra is another monoclonal antibody used in the treatment of patients in critical condition. (NCT: 04330638). By blocking the IL-1 receptor, the drug could help reduce the cytokine storm triggered by the virus[31].
The monoclonal antibody eculizumab, which prevents the cleavage of the C5 fraction of the complement in the C5a and C5b, could also reduce this cytokine cascade. Currently, an encouraging case series has been published on the topic by Diurno et al[32] (2020) and we are looking forward to the results NCT: 04288713.
Among the immunomodulatory drugs with a possible action in reducing cytokine storm, colchicine has also been used. It is a non-selective inhibitor of NLRP3 inflammasome, and inhibitor of microtubule polymerization and leukocyte infiltration[33]. The COLCORONA trial is now ongoing in the recruiting phase (NCT: 04322682), while the GRECCO-19 study (NCT: 04326790) of 189 patients has recruited the necessary samples[34].
Several studies have analyzed the role of corticosteroids. Such drugs could theoretically act as immunomodulators. Wang et al[35] completed a randomized controlled trial, albeit with few patients, on the use of methylprednisolone highlighting that the short-term administration of the drug could be beneficial. In February 2020, Villar et al[36] published a randomized clinical study (NCT: 01731795) of 277 patients that defined the usefulness of the early administration of dexamethasone in reducing days of endotracheal intubation and overall mortality. Although these studies seem encouraging, further evidence of efficacy is needed[36].
In addition, CP has frequently been used as supplement therapy. It is a classic adaptive immunotherapy that was successfully and safely used in the treatment of infections caused by viruses similar to SARS-CoV-2[37], such as SARS, MERS, and in the 2009 H1N1 pandemic[38,39]. Data from the meta-analysis conducted By Mair-Jenkins et al[40] reported that this treatment can reduce the mortality of patients with COVID-19 especially if administered early to the onset of symptoms. The limitation of this treatment is the scarce availability of donor plasma considering that only recovered COVID-19 patients with neutralizing antibody titers above 1:640 are considered good plasma donors. Once the plasma is collected from donors, it is adequately treated and then infused into clinically symptomatic patients. A single 200 mL transfusion of CP is generally well tolerated and followed by improvement of the clinical symptoms. There is a subsequent increase of oxyhemoglobin saturation within 3 d and a rapid neutralization of the viremia[41]. The clinical trial NCT: 04321421 would clarify the usefulness of this treatment.
Among the integrative treatments, vitamin C infusion may produce an increase in the synthesis of norepinephrine and vasopressin[42], reduce cytokine levels[43], and prevent neutrophil activation and trap formation promoting vascular injury[44]. Its role is being investigated by the clinical trial NCT: 04264533.
Due to the increased incidence of thrombo-inflammation and hypercoagulability related to COVID-19, enoxaparin which inhibits factor Xa and thrombin is frequently present in almost all the clinical practice protocols of treatment[45]. The clinical trial identified as NCT: 04367831 is investigating the role of enoxaparin in COVID-19 and is currently ongoing.
There has also been an increase in tumor necrosis factor alpha (TNF-α) and IL-17 in peripheral blood samples of COVID-19 patients, but evidence in customized treatment is still lacking. Encouraging results arrived from real-life data in a large cohort of psoriatic patients on biologic agents. They demonstrated an increased risk of infection rates but without an increased risk of intensive care unit hospitalization or death[46]. Two trials were registered in the Chinese Clinical Trial Registry (ChiCTR2000030089, ChiCTR2000030703) that evaluate the potential use of adalimumab (anti-TNF-α) and ixekizumab (anti-IL-17) in the armamentarium to treat severe COVID-19 patients. Apremilast, a phosphodiesterase type 4 inhibitor, was a candidate treatment because it demonstrated inhibition of neutrophil, monocyte and lymphocyte migration during lung inflammation and decreased pro-inflammatory cytokine production[47].
A wide array of drugs used in current clinical practice but not yet approved and investigated by more than three ongoing clinical trials are presented in Table 1. Among these drugs is Ivermectin, an FDA-approved anti-parasitic. This drug showed to have broad-spectrum anti-viral activity only in vitro[48], and results of a Phase 1 study are absolutely needed before using ivermectin. There are not enough data to support a recommendation for its use in a higher-than-approved dosage.
| Therapeutic agent | Mechanism of action | Ongoing trials, n | Associations | Suggested dosage | Route of administration | Principal side effects | Ref. | NCT identifier |
| Hydroxychloroquine | Changes the pH of endosomes, prevents viral entry, transport and post-entry replication | 224 | Azithromycin, tocilizumab, lopinavir-ritonavir | 200 mg BID or TID (10 d) | Oral-intravenous | Retinal toxicity, QT prolongation, nausea | [6-10] | NCT04315948 |
| Chloroquine | Increases the endosomal pH interfering with the process of virus/cell fusion | 225 | Remdesivir, tocilizumab | 2.5 g (3 d) | Oral-intravenous | Retinal toxicity, QT prolongation, nausea | [11] | NCT04303507 |
| Convalescent plasma | Adaptive immunotherapy (neutralizing antibody tiers above 1:640) | 129 | Remdesivir, Interferon-alpha, oseltamivir, antibacterial and antifungal frugal drugs, methylprednisolone | 200 ml single dose | Intravenous | Evanescent facial red spot | [37-41] | NCT04321421 |
| Lopinavir/Ritonavir | Inhibition of the HIV protease/inhibition of CitP450-iso3A4 and augmented plasmatic concentration of lopinavir | 67 | Hydroxychloroquine, azithromycin, dexamethasone | 200 mg/50 mg BID | Oral | Gastrointestinal upset, augmented plasmatic concentration of colchicine | [25-28] | NCT02735707 |
| And HGAM-CoA reductase inhibitors | ||||||||
| Azithromycin | Prophylaxis of bacterial super-infection | 59 | Hydroxychloroquine, tocilizumab, atovaquone | 500 mg | Oral-intravenous | QT prolongation | [19-24] | NCT04332107 |
| Tocilizumab | Monoclonal antibody which targets the IL-6 receptor | 50 | lopinavir-ritonavir, remdesivir, chloroquine, hydroxychloroquine | Dosing according to weight range | Intravenous | Runny or stuffy nose, sinus pain, sore throat, headache, gastrointestinal upset, urinary tract infection | [30] | NCT04320615 |
| Ivermectin | Suppression of SARS-CoV-2 viral replication in cell cultures (in vitro) | 30 | Hydroxychloroquine | 600 mcg/kg | Oral | Tiredness, loss of energy, stomach pain, loss of appetite, nausea, vomiting, diarrhea, dizziness | [48] | NCT04381884 |
| Dutasteride | ||||||||
| Azithromycin | ||||||||
| Proxalutamide | ||||||||
| Statin | Reduces chemokine release, adhesion molecules, and modulating T cell activity | 23 | Standard of care; colchicine + rosuvastatin | 20 mg/d atorvastatin Rosuvastatin 40 mg/d or equivalent | Oral | rabdomiolisis | [12] | NCT04472611 |
| Remdesivir | Nucleotide analogue that is incorporated into the nascent viral RNA chain resulting in its premature termination | 20 | Hydroxychloroquine, chloroquine, tocilizumab, convalescent plasma | 200 mg 1st day – 100 mg (10 d) | Intravenous | Phlebitis, constipation, headache, ecchymosis, nausea, pain in extremities | [13-18] | NCT04292899 |
| Methylprednisolone | Immunosuppression against cytokine storm | 17 | Siltuximab, tacrolimus | 40 mg BID (5 d) - f 1-2 mg/kg/d (5-7 d) | Oral-intravenous | Headache, nausea, weight gain, excitement, infections | [35] | NCT04323592 |
| Sarilumab | Monoclonal antibody which targets the IL-6 receptor | 17 | Not available | 400 mg or 200mg single dose | Intravenous | Neutropenia, increased ALT, injection site redness, upper respiratory infections, nasal congestion, sore throat, urinary tract infections, thrombocytopenia | [29] | NCT04315298 |
| Colchicine | Non-selective inhibitor of NLRP3 inflammasome, inhibitor of microtubule polymerization and leukocyte infiltration | 17 | Not available | 0.5 mg per os (BID) for 3 d - then once daily for the last 27 d | Oral-intravenous | Gastrointestinal upset, low blood cells count and rhabdomyolysis | [33-34] | NCT04322682 |
| Heparin | Inhibition of Xa factor and thrombin | 15 | Methylprednisolone | Dosed to target activated partial thromboplastin time (aPTT) between 1.5-2.0 times the normal value | Subcutaneous injection | Reduced creatinine clearance | [45] | NCT04485429 |
| Anakinra | Monoclonal antibody which targets the IL-1 receptor | 11 | Siltuximab or tocilizumab (single i.v. injection) | 1 injection a day (max 28 d) | Subcutaneous injection | Gastrointestinal upset, headache, joint pain, flu symptoms, redness-bruising-pain in the injection site | [30,31] | NCT04330638 |
| Dexamethasone | Immunosuppression against cytokine storm | 10 | Not available | 20 mg/d (5 d) then 10 mg/d (5 d) | Intravenous | Headache, weight gain, excitement, infections | [36] | NCT04325061 |
| Enoxaparin | Inhibition of Xa factor and thrombin | 5 | Not available | 4000 UI/d or 100 UI/kg | Subcutaneous injection | Skin irritation in injection site, bleeding, heparin-induced thrombocytopenia, fatigue, fever | [45] | NCT04367831 |
| Eculizumab | Monoclonal antibody which targets C5 inhibiting its cleavage in C5a and C5b | 3 | Hydroxychloroquine, lopinavir-ritonavir, ceftriaxone, vitamin C | 3600 mg/wk (8-22 wk) | Intravenous | Fever, headache, nausea and vomiting, body aches, confusion, increased sensitivity to light, stiffness | [32] | NCT04288713 |
| Vitamin C | Antioxidant, increases the synthesis of norepinephrine and vasopressin, attenuate increases in cytokine levels | 3 | All mentioned drugs | 12 g/12 h (7 d) or 50 mg/kg/6 h (4 d) | Oral-intravenous | Gastrointestinal upset | [42-44] | NCT04264533 |
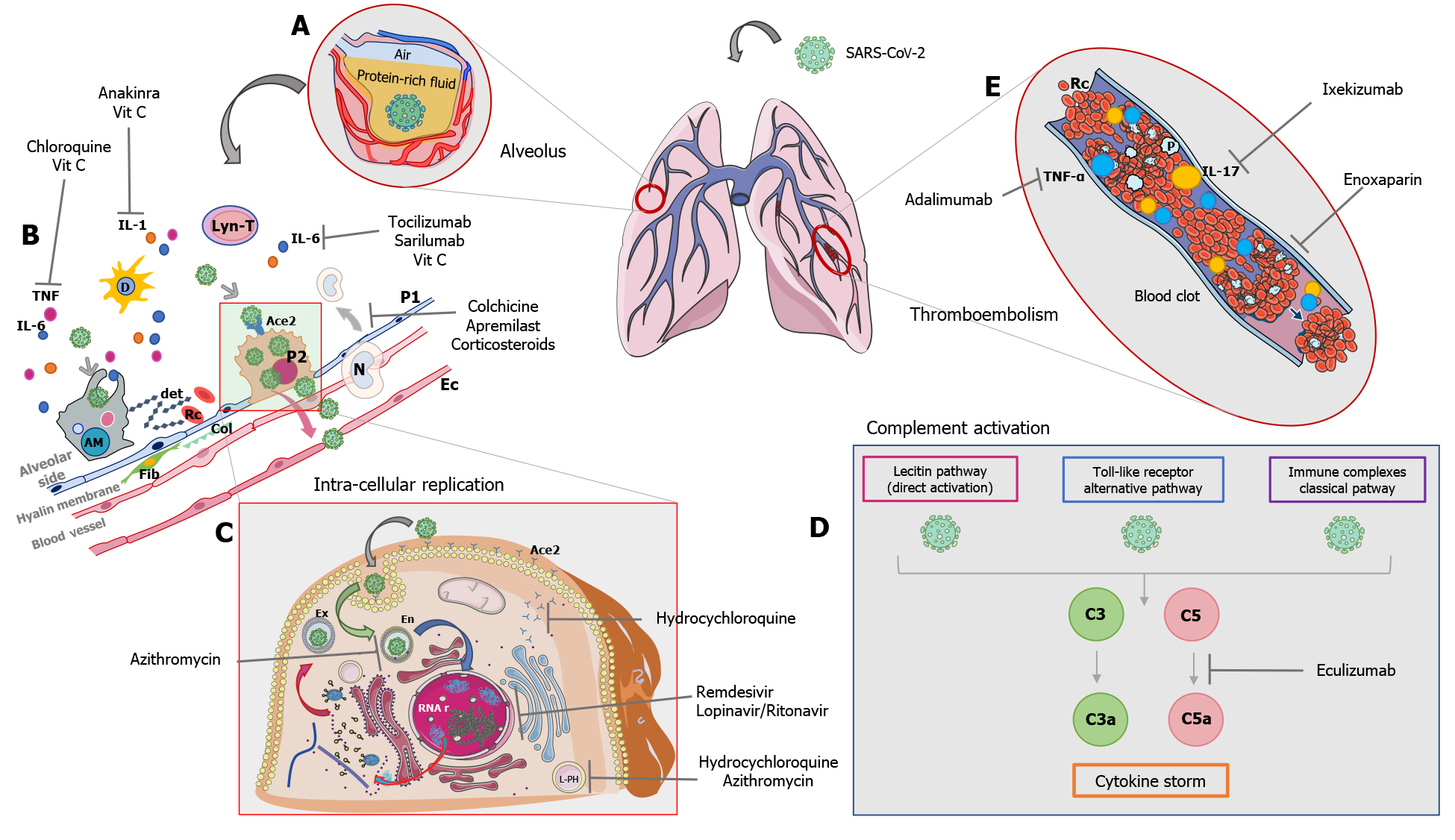
The pathogenesis of the damage induced by the SARS-CoV-2 virus is presently being analyzed. There are two clinical manifestations that are most frequently found in infected patients, namely respiratory failure and systemic coagulopathy. Currently it appears, according to a report by Magro et al[49], that the multiple district damage induced by the infection is caused by a hyper-activation of the complement system and exacerbation of the cytokine cascade. On histological samples of patients’ lung and skin tissue who died from COVID-19, there was a discernable deposit of C5b9, C4d, and the mannose-binding lectin-associated serine protease 2. These proteins are the residual products of complement activation[49]. Once the host’s barriers are overcome, the virus stimulates the innate immunity and enters the cell by binding to the ACE2 receptor. It goes on to destroy the endothelial cells of all organs whose cells express the ACE2 receptor widely. The same cell destruction increases tissue permeability and facilitates the systemic release of the virus. This results in hyperproduction of interleukins (cytokine storm and intracellular activation of the inflammasome) and hypercoagulability with diffuse thrombosis in the microcirculation.
Due to the unproven efficacy of antiviral drugs alone, there is strong reason to believe it useful to administer other drug treatments to facilitate meaningful recovery. Drugs such as eculizumab, which act by blocking the cleavage/activation of complement factor C5; tocilizumab, sarilumab, and anakinra which block the interleukin receptors by limiting the cytokine cascade; colchicine which acts by interfering with the inflammasome NLRP3; vitamin C which may reduce the activation of neutrophils and stimulate the endogenous production of vasopressors; and enoxaparin which assists in the prevention and treatment of hypercoagulation thrombosis. The mechanisms of drug action according to cell damage, complement activation and cytokine storm are described in Figure 1.
Although a short period of time has passed since the novel coronavirus was initially described, several treatment options have been introduced. To simplify the current therapeutic armamentarium, Table 1 summarizes the most investigated options for the treatment of COVID-19 in decreasing order by number of ongoing RCTs. Nonetheless there is still no proven evidence based therapeutic plan that can offer the best survival chance to patients infected. The inconsistent results presented in the literature on the treatment of SARS-CoV-2, is likely due to the lack of well-controlled studies with an adequate sample size. A meticulous understanding of the pathophysiology and immunological response of the host is also still necessary. Additional data are required to provide a proper risk stratification for patients and an adequate place in therapy of current investigational options.
Manuscript source: Invited manuscript
Specialty type: Virology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang L S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14118] [Article Influence: 2823.6] [Reference Citation Analysis (1)] |
| 2. | World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report – 199 - 6 Aug 2020: World Health Organization; 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200806-covid-19-sitrep-199.pdf?sfvrsn=6b9d262d_2. |
| 3. | World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, 28 January 2020. Contract No: WHO/nCoV/Clinical/2020.3 Available from: https://apps.who.int/iris/handle/10665/330893. |
| 4. | World Health Organization. International Clinical Trials Registry Platform (ICTRP). Consulted on May 2020. Available from: https://apps.who.int/trialsearch/. |
| 5. | Bragazzi NL, Dai H, Damiani G, Behzadifar M, Martini M, Wu J. How Big Data and Artificial Intelligence Can Help Better Manage the COVID-19 Pandemic. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 6. | Gendelman O, Amital H, Bragazzi NL, Watad A, Chodick G. Continuous hydroxychloroquine or colchicine therapy does not prevent infection with SARS-CoV-2: Insights from a large healthcare database analysis. Autoimmun Rev. 2020;19:102566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | McKee DL, Sternberg A, Stange U, Laufer S, Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol Res. 2020;157:104859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 353] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 8. | Wang PH, Cheng Y. Increasing Host Cellular ReceptorâAngiotensin-Converting Enzyme 2 (ACE2) Expression by Coronavirus may Facilitate 2019-nCoV Infection. 2020 Preprint. Available from: bioRxiv. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;55:105938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 704] [Cited by in RCA: 673] [Article Influence: 134.6] [Reference Citation Analysis (0)] |
| 10. | Zhou D, Dai SM, Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020;75:1667-1670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 321] [Cited by in RCA: 344] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 11. | Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4289] [Cited by in RCA: 4564] [Article Influence: 912.8] [Reference Citation Analysis (0)] |
| 12. | Zhang XJ, Qin JJ, Cheng X, Shen L, Zhao YC, Yuan Y, Lei F, Chen MM, Yang H, Bai L, Song X, Lin L, Xia M, Zhou F, Zhou J, She ZG, Zhu L, Ma X, Xu Q, Ye P, Chen G, Liu L, Mao W, Yan Y, Xiao B, Lu Z, Peng G, Liu M, Yang J, Yang L, Zhang C, Lu H, Xia X, Wang D, Liao X, Wei X, Zhang BH, Zhang X, Yang J, Zhao GN, Zhang P, Liu PP, Loomba R, Ji YX, Xia J, Wang Y, Cai J, Guo J, Li H. In-Hospital Use of Statins Is Associated with a Reduced Risk of Mortality among Individuals with COVID-19. Cell Metab. 2020;32:176-187.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 394] [Cited by in RCA: 363] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 13. | Al-Tawfiq JA, Al-Homoud AH, Memish ZA. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med Infect Dis. 2020;34:101615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 14. | Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I, Bannister R, Park Y, Babusis D, Clarke MO, Mackman RL, Spahn JE, Palmiotti CA, Siegel D, Ray AS, Cihlar T, Jordan R, Denison MR, Baric RS. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1011] [Cited by in RCA: 1126] [Article Influence: 160.9] [Reference Citation Analysis (0)] |
| 15. | Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R, Ray AS, Cihlar T, Siegel D, Mackman RL, Clarke MO, Baric RS, Denison MR. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio. 2018;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 920] [Cited by in RCA: 997] [Article Influence: 142.4] [Reference Citation Analysis (0)] |
| 16. | World Health Organization. WHO R&D Blueprint – Ad-hoc Expert Consultation on clinical trials for Ebola Therapeutics. 11 October 2018. Available from: https://www.who.int/ebola/drc-2018/treatments-approved-for-compassionate-use-update/en/. |
| 17. | Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D'Arminio Monforte A, Ismail S, Kato H, Lapadula G, L'Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020;382:2327-2336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1926] [Cited by in RCA: 1884] [Article Influence: 376.8] [Reference Citation Analysis (0)] |
| 18. | Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2337] [Cited by in RCA: 2485] [Article Influence: 497.0] [Reference Citation Analysis (0)] |
| 19. | Touret F, Gilles M, Barral K, Nougairède A, Decroly E, de Lamballerie X, Coutard B. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. 2020 Preprint. Available from: bioRxiv. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Andreani J, Le Bideau M, Duflot I, Jardot P, Rolland C, Boxberger M, Wurtz N, Rolain JM, Colson P, La Scola B, Raoult D. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb Pathog. 2020;145:104228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 21. | Tyteca D, Van Der Smissen P, Mettlen M, Van Bambeke F, Tulkens PM, Mingeot-Leclercq MP, Courtoy PJ. Azithromycin, a lysosomotropic antibiotic, has distinct effects on fluid-phase and receptor-mediated endocytosis, but does not impair phagocytosis in J774 macrophages. Exp Cell Res. 2002;281:86-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Li C, Zu S, Deng YQ, Li D, Parvatiyar K, Quanquin N, Shang J, Sun N, Su J, Liu Z, Wang M, Aliyari SR, Li XF, Wu A, Ma F, Shi Y, Nielsevn-Saines K, Jung JU, Qin FX, Qin CF, Cheng G. Azithromycin Protects against Zika virus Infection by Upregulating virus-induced Type I and III Interferon Responses. Antimicrob Agents Chemother. 2019;63:e00394-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 23. | Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Tissot Dupont H, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3279] [Cited by in RCA: 3272] [Article Influence: 654.4] [Reference Citation Analysis (0)] |
| 24. | Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Sevestre J, Mailhe M, Doudier B, Aubry C, Amrane S, Seng P, Hocquart M, Eldin C, Finance J, Vieira VE, Tissot-Dupont HT, Honoré S, Stein A, Million M, Colson P, La Scola B, Veit V, Jacquier A, Deharo JC, Drancourt M, Fournier PE, Rolain JM, Brouqui P, Raoult D. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med Infect Dis. 2020;34:101663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 512] [Cited by in RCA: 477] [Article Influence: 95.4] [Reference Citation Analysis (0)] |
| 25. | Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, Spahn JE, Bauer L, Sellers S, Porter D, Feng JY, Cihlar T, Jordan R, Denison MR, Baric RS. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1216] [Cited by in RCA: 1154] [Article Influence: 230.8] [Reference Citation Analysis (0)] |
| 26. | Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, Kao RY, Poon LL, Wong CL, Guan Y, Peiris JS, Yuen KY; HKU/UCH SARS Study Group. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1117] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 27. | de Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, van Nieuwkoop S, Bestebroer TM, van den Hoogen BG, Neyts J, Snijder EJ. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875-4884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 539] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 28. | Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3386] [Cited by in RCA: 3626] [Article Influence: 725.2] [Reference Citation Analysis (0)] |
| 29. | Lu CC, Chen MY, Lee WS, Chang YL. Potential therapeutic agents against COVID-19: What we know so far. J Chin Med Assoc. 2020;83:534-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 30. | Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1228] [Cited by in RCA: 1217] [Article Influence: 243.4] [Reference Citation Analysis (0)] |
| 31. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6746] [Article Influence: 1349.2] [Reference Citation Analysis (0)] |
| 32. | Diurno F, Numis FG, Porta G, Cirillo F, Maddaluno S, Ragozzino A, De Negri P, Di Gennaro C, Pagano A, Allegorico E, Bressy L, Bosso G, Ferrara A, Serra C, Montisci A, D'Amico M, Schiano Lo Morello S, Di Costanzo G, Tucci AG, Marchetti P, Di Vincenzo U, Sorrentino I, Casciotta A, Fusco M, Buonerba C, Berretta M, Ceccarelli M, Nunnari G, Diessa Y, Cicala S, Facchini G. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24:4040-4047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 179] [Reference Citation Analysis (0)] |
| 33. | Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1426] [Cited by in RCA: 3152] [Article Influence: 630.4] [Reference Citation Analysis (0)] |
| 34. | Deftereos SG, Siasos G, Giannopoulos G, Vrachatis DA, Angelidis C, Giotaki SG, Gargalianos P, Giamarellou H, Gogos C, Daikos G, Lazanas M, Lagiou P, Saroglou G, Sipsas N, Tsiodras S, Chatzigeorgiou D, Moussas N, Kotanidou A, Koulouris N, Oikonomou E, Kaoukis A, Kossyvakis C, Raisakis K, Fountoulaki K, Comis M, Tsiachris D, Sarri E, Theodorakis A, Martinez-Dolz L, Sanz-Sánchez J, Reimers B, Stefanini GG, Cleman M, Filippou D, Olympios CD, Pyrgakis VN, Goudevenos J, Hahalis G, Kolettis TM, Iliodromitis E, Tousoulis D, Stefanadis C. The Greek study in the effects of colchicine in COvid-19 complications prevention (GRECCO-19 study): Rationale and study design. Hellenic J Cardiol. 2020;61:42-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 35. | Wang Y, Jiang W, He Q, Wang C, Wang B, Zhou P, Dong N, Tong Q. Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, China. 2020 Preprint. Available from: medRxiv. [DOI] [Full Text] |
| 36. | Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, Aguilar G, Alba F, González-Higueras E, Conesa LA, Martín-Rodríguez C, Díaz-Domínguez FJ, Serna-Grande P, Rivas R, Ferreres J, Belda J, Capilla L, Tallet A, Añón JM, Fernández RL, González-Martín JM; dexamethasone in ARDS network. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 766] [Article Influence: 153.2] [Reference Citation Analysis (0)] |
| 37. | Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20:398-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 672] [Cited by in RCA: 678] [Article Influence: 135.6] [Reference Citation Analysis (0)] |
| 38. | Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, Chan P, Wong KC, Leung CB, Cheng G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 693] [Cited by in RCA: 687] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 39. | Ko JH, Seok H, Cho SY, Ha YE, Baek JY, Kim SH, Kim YJ, Park JK, Chung CR, Kang ES, Cho D, Müller MA, Drosten C, Kang CI, Chung DR, Song JH, Peck KR. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther. 2018;23:617-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 217] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 40. | Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, Makki S, Rooney KD, Nguyen-Van-Tam JS, Beck CR; Convalescent Plasma Study Group. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 755] [Cited by in RCA: 689] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 41. | Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, Zhou M, Chen L, Meng S, Hu Y, Peng C, Yuan M, Huang J, Wang Z, Yu J, Gao X, Wang D, Yu X, Li L, Zhang J, Wu X, Li B, Xu Y, Chen W, Peng Y, Hu Y, Lin L, Liu X, Huang S, Zhou Z, Zhang L, Wang Y, Zhang Z, Deng K, Xia Z, Gong Q, Zhang W, Zheng X, Liu Y, Yang H, Zhou D, Yu D, Hou J, Shi Z, Chen S, Chen Z, Zhang X, Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020;117:9490-9496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1251] [Cited by in RCA: 1314] [Article Influence: 262.8] [Reference Citation Analysis (0)] |
| 42. | Carr AC, Shaw GM, Fowler AA, Natarajan R. Ascorbate-dependent vasopressor synthesis: a rationale for vitamin C administration in severe sepsis and septic shock? Crit Care. 2015;19:418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 175] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 43. | Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med. 2020;8:433-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 44. | Fowler AA 3rd, Truwit JD, Hite RD. Morris PE, DeWilde C, Priday A, Fisher B, Thacker LR 2nd, Natarajan R, Brophy DF, Sculthorpe R, Nanchal R, Syed A, Sturgill J, Martin GS, Sevransky J, Kashiouris M, Hamman S, Egan KF, Hastings A, Spencer W, Tench S, Mehkri O, Bindas J, Duggal A, Graf J, Zellner S, Yanny L, McPolin C, Hollrith T, Kramer D, Ojielo C, Damm T, Cassity E, Wieliczko A, Halquist M. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA. 2019;322:1261-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 594] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 45. | Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18:1559-1561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 470] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 46. | Damiani G, Pacifico A, Bragazzi NL, Malagoli P. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: Real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;e13475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 47. | Bridgewood C, Damiani G, Sharif K, Quartuccio L, McGonagle D. Rationale for use of PDE4 inhibition for severe inflammation in COVID-19 Pneumonia. 2020 Preprint. . [DOI] [Full Text] |
| 48. | Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1171] [Cited by in RCA: 1301] [Article Influence: 260.2] [Reference Citation Analysis (0)] |
| 49. | Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1635] [Cited by in RCA: 1597] [Article Influence: 319.4] [Reference Citation Analysis (1)] |