Peer-review started: June 12, 2017
First decision: July 20, 2017
Revised: August 3, 2017
Accepted: September 16, 2017
Article in press: September 17, 2017
Published online: November 12, 2017
Processing time: 144 Days and 13.9 Hours
To assess the real-world effectiveness and cost of simeprevir (SMV), and/or sofosbuvir (SOF)-based therapy for chronic hepatitis C virus (HCV) infection.
The real-world performance of patients treated with SMV/SOF ± ribavirin (RBV), SOF/RBV, and SOF/RBV with pegylated-interferon (PEG) were analyzed in a consecutive series of 508 patients with chronic HCV infection treated at a single academic medical center. Patients with genotypes 1 through 4 were included. Rates of sustained virological response - the absence of a detectable serum HCV RNA 12 wk after the end of treatment [sustained virological response (SVR) 12] - were calculated on an intention-to-treat basis. Costs were calculated from the payer’s perspective using Medicare/Medicaid fees and Redbook Wholesale Acquisition Costs. Patient-related factors associated with SVR12 were identified using multivariable logistic regression.
SVR12 rates were as follows: 86% (95%CI: 80%-91%) among 178 patients on SMV/SOF ± RBV; 62% (95%CI: 55%-68%) among 234 patients on SOF/RBV; and 78% (95%CI: 68%-86%) among 96 patients on SOF/PEG/RBV. Mean costs-per-SVR12 were $174442 (standard deviation: ± $18588) for SMV/SOF ± RBV; $223003 (± $77946) for SOF/RBV; and $126496 (± $31052) for SOF/PEG/RBV. Among patients on SMV/SOF ± RBV, SVR12 was less likely in patients previously treated with a protease inhibitor [odds ratio (OR): 0.20, 95%CI: 0.06-0.56]. Higher bilirubin (OR: 0.47, 95%CI: 0.30-0.69) reduced the likelihood of SVR12 among patients on SOF/RBV, while FIB-4 score ≥ 3.25 reduced the likelihood of SVR12 (OR: 0.18, 95%CI: 0.05-0.59) among those on SOF/PEG/RBV.
SVR12 rates for SMV and/or SOF-based regimens in a diverse real-world population are comparable to those in clinical trials. Treatment failure accounts for 27% of costs.
Core tip: To our knowledge, this study is the largest real-world investigation of outcomes in patients with chronic hepatitis C virus infection with genotypes 1-4 being treated with simeprevir and/or sofosbuvir-containing regimens that has been conducted in a single center. We provide compelling real-world data in a large (n = 508), diverse population of patients, showing that the effectiveness of these regimens is comparable to that seen in multicenter clinical trials. Further, our unique cost analysis reveals that the cost-per-sustained virological response of simeprevir- and/or sofosbuvir-based therapy is lower than telaprevir-based triple therapy, likely due to higher rates of cure and lower rates of adverse events.
- Citation: Bichoupan K, Tandon N, Crismale JF, Hartman J, Del Bello D, Patel N, Chekuri S, Harty A, Ng M, Sigel KM, Bansal MB, Grewal P, Chang CY, Leong J, Im GY, Liu LU, Odin JA, Bach N, Friedman SL, Schiano TD, Perumalswami PV, Dieterich DT, Branch AD. Real-world cure rates for hepatitis C virus treatments that include simeprevir and/or sofosbuvir are comparable to clinical trial results. World J Virol 2017; 6(4): 59-72
- URL: https://www.wjgnet.com/2220-3249/full/v6/i4/59.htm
- DOI: https://dx.doi.org/10.5501/wjv.v6.i4.59
Treatment options for patients with chronic hepatitis C virus (HCV) infection are expanding rapidly. Data from clinical trials indicate that newer regimens have reduced side effects compared to dual therapy with pegylated interferon (PEG) and ribavirin (RBV) and higher sustained virological response (SVR) rates[1-10]. SVR is equivalent to a virological cure, and is currently defined as the absence of detectable HCV RNA in blood 12 wk after the end-of-treatment (EOT). SVR at 12 wk (SVR12) has supplanted SVR at 24 wk as the standard endpoint[11]. SVR12 is associated with reduced rates of liver-related and all-cause mortality, even among patients with advanced liver disease[12-14]. Additional benefits include improvements in quality of life, as well as decreased healthcare utilization[15].
As most patients can be treated safely with newer interferon-free direct-acting antiviral (DAA) regimens, current AASLD/IDSA guidelines recommend treating all patients with chronic HCV, except those with life expectancies too short for HCV cure to be considered beneficial[16]. These recommendations, along with birth cohort screening of baby boomers and direct-to-consumer advertising, have created a significant public demand for treatment[17]. Comparative data about the clinical and economic effectiveness of new regimens are needed to inform discussions about costs and to allow selection of the best option for each patient.
The first HCV NS3/4A protease inhibitors (PIs), telaprevir (TVR) and boceprevir (BOC), were used in combination with PEG and RBV. These triple therapy regimens had a high burden of adverse events and high costs-per-SVR, as well as cumbersome dosing regimens[2-5,18,19]. Simeprevir (SMV) was approved by the United States Food and Drug Administration (United States FDA) in 2013 for the treatment of genotype (GT) 1 HCV. Used in combination with PEG and RBV, it was at least as effective in achieving SVR as TVR and BOC in large randomized trials, but it reduced the pill burden and improved tolerability[9,10,20]. In 2014, the United States FDA approved sofosbuvir (SOF), a nucleotide analog NS5B polymerase inhibitor with activity against GT 1-6. Depending upon GT and prior treatment history, it was initially used either in combination with PEG/RBV, with SMV ± RBV, or with RBV alone. SVR rates with these SOF-containing regimens ranged from 56% to over 90% in registration trials[6-8]. SOF is now used most commonly in fixed-dose combination with NS5A inhibitors, including ledipasvir and velpatasvir[21].
We previously established that the cost-per-SVR of TVR-based triple therapy in clinical practice approached $200000-far higher than projections based on results of randomized clinical trials[19]. In the present study, we examine the clinical and economic performance of regimens containing SMV and/or SOF in a consecutive series of 508 patients and identify risk factors associated with treatment success (SVR12) or failure. SMV remains an important option for patients with resistance associated substitutions (RASs) to NS5A inhibitors, and in liver transplantation recipients[22-24]. Prior studies assessing outcomes of SMV- and/or SOF-containing regimens in clinical practice were limited to patients with GT 1 HCV[25-29]. Other recent studies assessing real-world outcomes of SOF-based dual- or triple-therapy have focused on patients with a single genotype[30,31]. Here we offer a comprehensive examination of real-world outcomes of three different treatment regimens across genotypes 1-4.
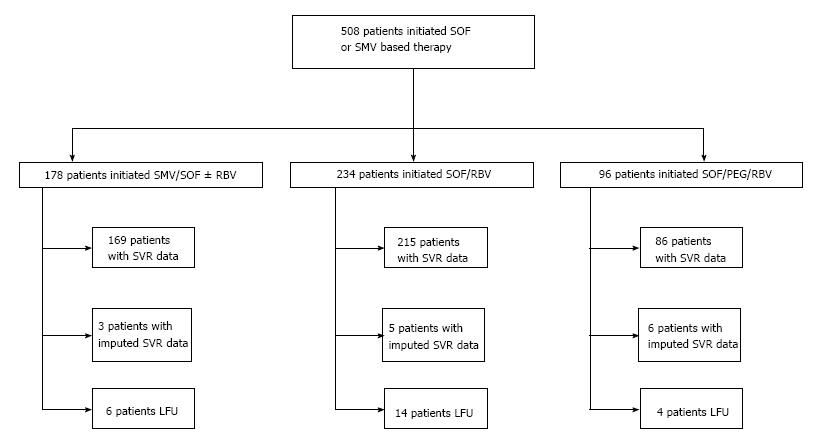
Data were collected on a consecutive series of 508 patients with chronic HCV infection who started treatment with a SMV- and/or SOF-containing regimen between December 2013 and June 2014 at the Mount Sinai Medical Center in New York City. Patients with HCV GT 1, 2, 3, and 4 were included in the study. Subjects were identified using two complementary methods: (1) healthcare providers compiled lists of patients meeting inclusion criteria; and (2) the Mount Sinai Data Warehouse, a database integrating multiple electronic health record platforms, was queried to identify all patients with any history of International Classification of Diseases, Ninth Revision (ICD-9) diagnostic codes for chronic HCV infection (070.54) and a prescription order for SMV or SOF. The combined list was validated by manual chart review. Patients on the following regimens were included: SMV/SOF ± RBV, SOF/RBV, and SOF/PEG/RBV. All patients received at least one dose of SMV and/or SOF. Patients who had undergone liver transplantation or who had HIV/HCV co-infection were excluded from this study; however, data on HIV/HCV co-infected patients are published elsewhere[32]. Choice of the HCV treatment regimen, duration of treatment, and adverse event (AE) management, including the use of erythropoietin, were at the discretion of the provider. Data on demographics, HCV kinetics, clinical laboratory tests, office visits, medications, AE management, and other aspects of medical care, including past use of PIs (TVR or BOC) were collected. Providers were notified of patients who were lost to follow-up (LFU), but there was no systematic method for contacting patients who did not complete SVR12 testing.
HCV viral load was measured using a real-time polymerase chain reaction assay (COBAS AmpliPrepCOBAS Taqman HCV Test version 2.0; Roche Molecular Diagnostics, Pleasanton, CA), which defines a HCV viral load below 15 IU/mL as “undetectable”. Breakthrough and relapse were defined as the achievement of undetectable HCV RNA during treatment, followed by the detection of HCV RNA during treatment, or after treatment was completed or stopped, respectively. Advanced fibrosis/cirrhosis was defined as a FIB-4 score ≥ 3.25 (24). SVR12 was defined as an undetectable HCV RNA at least 12 wk after the EOT. The study was conducted in accordance with the Helsinki agreement, with approval of the Mount Sinai Institutional Review Board (GCO 10-0032).
The cost of care was calculated for each patient based on Medicare and Medicaid fee schedules as described in our previous study[19], and included laboratory tests, physician fees, and AE management. Costs of HCV medications were derived from the Red Book Wholesale Acquisition Costs, accessed in December 2014: SOF, $1000/d; SMV, $790/d; RBV, $15.56/d; PEG, $672/wk. Costs are expressed in 2014 United States dollars.
Descriptive statistics were used to analyze the baseline characteristics of the cohort of 508 patients who initiated SMV- and/or SOF-based therapy and to compare these characteristics to those of a cohort of patients who initiated treatment with TVR- or BOC-based regimens at the Mount Sinai Medical Center[19].
SVR12 rates and costs were calculated on an intention-to-treat (ITT) basis for the entire population of 508 patients who initiated SMV- and/or SOF-based therapy and for patients on each of the three treatment regimens. Treatment outcome (SVR12 or non-SVR12) was imputed for 14/508 (2.75%) patients (3 on SMV/SOF ± RBV, 5 patients on SOF/RBV, and 6 on SOF/PEG/RBV) who lacked SVR12 data, but who had an undetectable viral load at EOT and/or at 4 wk after EOT, based on the average SVR12 rate for patients on the same regimen. For patients receiving SOF/RBV, each genotype was analyzed separately because of the varying SVR12 rates of different genotypes[6,33]. A subgroup analysis was carried out on 130 patients receiving SMV/SOF ± RBV who had no prior exposure to PI therapy and did not have Childs-Pugh B or C cirrhosis, similar to the study group in the COSMOS trial[34].
Costs were calculated as mean and standard deviation (SD). The cost-per-SVR12 and its SD were calculated by determining the mean and SD of total cost of care (medications, adverse event costs, laboratory fees, and care provider fees) and dividing it by the SVR12 rate. A one-way sensitivity analysis was conducted to determine the impact of the costs of medications on the cost-per-SVR12, with the prices of HCV medications varied from 50% to 100% to reflect possible drug discount rates.
For the 470 patients with confirmed SVR12 data, univariable and multivariable logistic regression were used to identify factors associated with SVR12 and generate forest plots. Unless otherwise indicated, multivariable models retained variables with a P-value < 0.05. In a fully-adjusted model, all variables were included except those that exhibited collinearity.
To compare values between groups, t-tests were used for normally distributed continuous variables and Mann-Whitney U tests for non-normally distributed variables or costs. χ2 or Fisher’s exact tests were used for categorical variables. A P-value < 0.05 was considered significant. R software and Microsoft Excel were used for statistical analysis.
Table 1 shows the characteristics of all 508 patients and those of patients on each regimen: 178 (35%) received SMV/SOF ± RBV, 234 (46%) received SOF/RBV, and 96 (19%) received SOF/PEG/RBV. Of patents treated with SMV/SOF ± RBV, 99% were GT 1, compared with 87% of patients treated with PEG/RBV/SOF and 44% treated with SOF/RBV. The remaining distribution of HCV GTs in each treatment group is displayed in Table 1. The median age was 60 years [interquartile range (IQR): 54-64], 71 (14%) were black, 183 (37%) were female, and 204 (40%) were naïve to previous HCV treatment, while 18% had failed TVR or BOC treatment in the past. Over half (54%) had a FIB-4 score ≥ 3.25, indicating advanced fibrosis/cirrhosis (METAVIR F3-F4). The median FIB-4 score was 3.54 (IQR: 1.73-6.72), consistent with the likelihood that most patients had advanced fibrosis/cirrhosis.
| Total | SMV/SOF ± RBV | SOF/RBV | SOF/PEG/RBV | |
| Continuous: median (IQR)/categorical: n (%) | ||||
| n | 508 | 178 | 234 | 96 |
| Age, yr | 60 (54-64) | 61 (57-65) | 60 (54-65) | 56 (50-62) |
| Race, black, n (%) | 71 (14) | 27 (15) | 23 (10) | 21 (22) |
| HCV genotype, n (%) | ||||
| 1 | 362 (71) | 177 (99.4) | 102 (44) | 83 (87) |
| 2 | 69 (14) | 0 (0) | 69 (29) | 0 (0) |
| 3 | 52 (10) | 0 (0) | 52 (22) | 0 (0) |
| 4 | 25 (5) | 1 (0.6) | 11 (5) | 13 (14) |
| Gender, female | 183 (37) | 67 (39) | 87 (39) | 29 (31) |
| BMI, kg/m2 | 27.7 (24.7-30.8) | 27.5 (24.5-30.2) | 27.9 (2.6-31.0) | 27.7 (25.1-31.1) |
| Diabetes, n (%) | 111 (22) | 29 (16) | 59 (25) | 23 (24) |
| Naïve to treatment, n (%) | 204 (40) | 51 (29) | 114 (49) | 39 (41) |
| PI failure, n (%) | 89 (18) | 48 (27) | 18 (8) | 23 (24) |
| HCV viral load, log U/mL | 6.15 (5.61-6.58) | 6.28 (5.78-6.74) | 6.05 (5.43-6.50) | 6.13 (5.63-6.53) |
| Hemoglobin, g/dL | 13.8 (12.6-15.1) | 13.9 (12.8-15.1) | 13.5 (12.4-14.7) | 14.7 (13.3-15.4) |
| Platelet, × 103/μL | 143 (90-195) | 146 (94-193) | 125 (71-183) | 180 (125-209) |
| ALT, U/L | 63 (39-105) | 72 (45-119) | 59 (37-99) | 60 (37-101) |
| AST, U/L | 62 (38-99) | 70 (40-113) | 63 (38-101) | 48 (33-83) |
| Total Bilirubin, mg/L | 0.70 (0.50-1.10) | 0.70 (0.50-1.00) | 0.8 (0.5-1.5) | 0.60 (0.40-0.83) |
| Albumin, g/dL | 4.0 (3.5-4.4) | 4.10 (3.70-4.40) | 3.8 (3.2-4.3) | 4.20 (3.80-4.45) |
| FIB-4 score | 3.54 (1.73-6.72) | 3.66 (1.90-5.99) | 4.74 (1.91-9.89) | 2.09 (1.46-3.85) |
| FIB-4 ≥ 3.25, n (%) | 267 (54) | 97 (56) | 137 (61) | 33 (34) |
To investigate how the real-world population of patients seeking and receiving treatment may be changing, characteristics of 508 patients initiating treatment with SMV- and/or SOF-based regimens (December 2013 until June 2014) were compared to those of 223 patients who initiated treatment with BOC- and TVR-based regimens during the previous era, May 2011 until December 2011 (Table 2). The group treated with SMV- and/or SOF-based regimens was significantly older (P < 0.01), had a higher percentage with a FIB-4 score ≥ 3.25 (P = 0.02), and lower hemoglobin levels (P < 0.01). A subset analysis of treatment naïve patients indicated that patients on a SMV- or SOF-based treatment regimen had significantly lower albumin than treatment naïve patients receiving BOC and/or TVR (P = 0.04, Supplementary Table 1). The greater age and more advanced liver disease of the cohort on SMV- and SOF-based regimens likely reflects both the aging of HCV-infected population and the higher potency and tolerability of the newer regimens, which allow patients with advanced liver disease to be treated with a greater probability of success.
| SMV- and/or SOF-containing regimens | TVR- or BOC-containing regimens | P-value | |
| Continuous: median (IQR)/categorical: n (%) | |||
| n | 508 | 223 | |
| Age, yr | 60 (54-64) | 57 (51-61) | < 0.011 |
| Race, black, n (%) | 71/508 (14) | 41/223 (18) | 0.132 |
| Gender, female, n (%) | 183/508 (37) | 79/223 (35) | 0.892 |
| BMI, kg/m2> | 27.7 (24.7-30.8) | 27.1 (24.5-30.7) | 0.651 |
| Diabetes, n (%) | 111/508 (22) | 48/223 (22) | 0.892 |
| Naïve to treatment, n (%) | 204/508 (40) | 68/223 (31) | 0.012 |
| HCV viral load, log IU/mL | 6.15 (5.61-6.58) | 6.31 (5.89-6.66) | < 0.013 |
| Hemoglobin, g/dL | 13.8 (12.6-15.1) | 14.3 (13.1-15.3) | < 0.011 |
| Platelet, × 103/μL | 143 (90-195) | 152 (107-195) | 0.193 |
| ALT, U/L | 63 (39-105) | 67 (44-106) | 0.133 |
| AST, U/L | 62 (38-99) | 62 (39-104) | 0.753 |
| Albumin, g/dL | 4.0 (3.5-4.4) | 4.2 (3.9-45) | < 0.011 |
| FIB-4 score | 3.54 (1.73-6.72) | 2.65 (1.77-5.60) | 0.063 |
| FIB-4 ≥ 3.25, n (%) | 267/508 (54) | 98/221 (44) | 0.032 |
Of the 508 patients who started treatment, the outcome (SVR12 or non-SVR12) was known with certainty for 470 patients who completed SVR12 testing, and it was imputed for 14 patients who completed EOT or SVR4 testing (see Methods and Figure 1). Twenty-four patients (5%) initiated treatment but lacked EOT data. Their baseline characteristics were compared to those of the other 484 patients, and no significant differences were found (Supplementary Table 2). In the ITT analysis of SVR12 rates, 136 (27%) patients were considered to fail treatment. This number included 16 patients with a null response to treatment, 91 who relapsed after an EOT response (including 61 patients treated with SOF/RBV, 15 treated with SMV/SOF ± RBV, and 15 treated with SOF/PEG/RBV), one patient with a virological breakthrough (treated with SOF/RBV), three who died, two with imputed failure, and 24 who were LFU. SVR12 rates and corresponding 95% confidence intervals (CIs) are presented in Table 3. The overall SVR12 rate was 73% (95%CI: 69%-77%). It was 86% (95%CI: 80%-91%) among patients on SMV/SOF ± RBV, 62% (95%CI: 55%-68%) among patients on SOF/RBV, and 78% (95%CI: 68%-86%) among patients on SOF/PEG/RBV. Among patients treated with SMV/SOF ± RBV in the “COSMOS-like” cohort (which excluded patients who had previously failed a PI and/or had Child-Pugh class B or C cirrhosis), the SVR12 rate was 90% (95%CI: 83%-94%). This is similar to the SVR12 rate in the COSMOS study, which was 92% for patients with METAVIR scores F0-2 and 94% for patients with METAVIR scores F3-4[34]. SVR12 rates varied by GT for patients treated with SOF/RBV, and ranged from 44% (95%CI: 34%-54%) for GT 1 to 83% (95%CI: 71%-90%) for GT 2 (Table 3). A comparison between SVR12 rates with regards to GT was not statistically feasible in the group receiving SMV/SOF ± RBV as only one patient in this group was infected with GT 4. SVR12 rates did not differ significantly between patients with GT 1 and GT 4 HCV in the group treated with SOF/PEG/RBV.
| SVR12 rates | ||
| SVR/total (%) | 95%CI, % | |
| All treatments | 372/508 (73) | 69-77 |
| SMV/SOF ± RBV | 153/178 (86) | 80-91 |
| “COSMOS-like" cohort | 117/130 (90) | 83-94 |
| SOF/RBV | 144/234 (62) | 55-68 |
| Genotype | ||
| 1 | 45/102 (44) | 34-54 |
| 2 | 57/69 (83) | 71-90 |
| 3 | 35/52 (67) | 53-79 |
| 4 | 7/11 (64) | 32-88 |
| SOF/PEG/RBV | 75/96 (78) | 68-86 |
The total cost of care from the payer’s perspective was determined for all 508 patients (including the 24 LFU patients). The analysis included costs of HCV medications, laboratory tests, physician fees, and adverse event management. Table 4 presents the total costs as well as costs-per-SVR for each regimen. The total cost of care for the 508 patients was $68.29 million. Treatment of the 136/508 (27%) patients who failed therapy accounted for $18.23 million (27%) of these costs. Adverse event management accounted for only about $289371 (0.4%) of costs[19].
| HCV medications ($) | Adverse Event costs ($) | Lab fees ($) | Provider fees ($) | Total cost of care ($) | 1SVR12 rate (%) | Cost-per-SVR ($) | |
| SMV/SOF ± RBV | 26379909 | 65231 | 89947 | 154488 | 26689574 | 153/178 (86) | 174442 (18588) |
| SOF/RBV genotype | 31616725 | 143770 | 136353 | 215584 | 32112432 | 144/234 (62) | 223003 (77946) |
| 1 | 15723055 | 106274 | 59942 | 94435 | 15983705 | 45/102 (44) | 355193 (98493) |
| 2 | 5736955 | 32477 | 37540 | 61136 | 5868109 | 57/69 (83) | 102949 (21346) |
| 3 | 8279942 | 5019 | 31914 | 49381 | 8366257 | 35/52 (67) | 239036 (48831) |
| 4 | 1876773 | 6956 | 10633 | 1894362 | 7/11 (64) | 270623 (124) | |
| SOF/PEG/RBV | 9275858 | 80370 | 48929 | 82045 | 9487202 | 75/96 (78) | 126469 (31052) |
Costs-per-SVR were calculated on an ITT basis by dividing the total costs by the SVR12 rate. As shown in Table 4, the cost-per-SVR was $174442 (SD ± $18588) for SMV/SOF ± RBV; $223003 (± $77946) for SOF/RBV; and $126469 (± $31052) for SOF/PEG/RBV. The cost-per-SVR when drug costs were discounted by 50% were $88,233 (± $9188), $113223 (± $39282), and $64657 (± $16002) for SMV/SOF ± RBV, SOF/RBV, and SOF/PEG/RBV, respectively. The cost-per-SVR for SMV/SOF ± RBV was compared to the cost-per-SVR for patients treated with TVR-based regimens at our center. The median cost-per-SVR for TVR-based regimens was $189322 (IQR: $143558-$211296) and the median cost-per-SVR for SMV/SOF ± RBV was $177975 (IQR: $176455-$178138), which was significantly different (P = 0.02) according to the Mann-Whitney U test.
Univariable and multivariable logistic regression were used to identify factors associated with SVR12 for the 470 patients with confirmed SVR12 test results. Data are presented separately for the 3 regimens: SMV/SOF ± RBV (Table 5), SOF/RBV (Table 6), and SOF/PEG/RBV (Table 7). Among patients on SMV/SOF ± RBV, in a multivariable model that retained variables with a P-value below 0.05, SVR12 was less likely in patients with a history of failed PI treatment (OR: 0.20, 95%CI: 0.06-0.56, P = 0.01). Factors associated with SVR12 in patients treated with SMV/SOF ± RBV were also examined in a fully-adjusted model that retained all variables except those that exhibited collinearity with other variables (Table 8). In this model, SVR12 was less likely among patients with a history of PI failure (OR: 0.12, 95%CI: 0.02-0.52, P < 0.01), higher baseline bilirubin (OR: 0.31, 95%CI: 0.08-0.86, P = 0.04), and a higher viral load (OR: 0.21, 95%CI: 0.05-0.70, P = 0.04). RBV use was not significantly associated with SVR12 (OR: 0.64, 95%CI: 0.09-3.78, P = 0.63); however, patients treated with SMV/SOF with RBV were more likely to have a history of PI failure (P < 0.01), reflecting a tendency to prescribe RBV for patients with less favorable treatment characteristics (Supplementary Table 3).
| SMV/SOF ± RBV | Univariable | Multivariable | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age, per yr | 1.01 | 0.96-1.06 | 0.73 | |||
| Race, black | 0.43 | 0.15-1.45 | 0.14 | |||
| Gender, female | 1.69 | 0.61-5.44 | 0.34 | |||
| BMI, per kg/m2 | 0.97 | 0.87-1.08 | 0.55 | |||
| Diabetes, n (%) | 0.64 | 0.21-2.42 | 0.47 | |||
| Naïve to treatment | 7.96 | 1.57-145.37 | 0.04 | |||
| PI Failure | 0.23 | 0.08-0.61 | < 0.01 | 0.2 | 0.06-0.56 | < 0.01 |
| Ribavirin | 0.78 | 0.29-2.03 | 0.61 | |||
| HCV viral load, per log IU/mL | 0.61 | 0.26-1.26 | 0.22 | |||
| Hemoglobin, per g/dL | 1.17 | 0.92-1.48 | 0.18 | |||
| Platelets, per 103/µL | 1 | 0.99-1.01 | 0.19 | |||
| ALT, per U/L | 1 | 0.99-1.01 | 0.37 | |||
| AST, per U/L | 1 | 0.99-1.01 | 0.89 | |||
| Total bilirubin, per mg/dL | 0.56 | 0.29-1.06 | 0.06 | 0.52 | 0.28-1.02 | 0.06 |
| Albumin, per g/dL | 1.82 | 0.72-4.45 | 0.19 | |||
| FIB-4 ≥ 3.25 | 0.66 | 0.22-1.83 | 0.44 | |||
| SOF/RBV | Univariable | Multivariable | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age, per yr | 0.98 | 0.95-1.01 | 0.14 | |||
| Race, black | 0.33 | 0.13-0.80 | 0.02 | |||
| Gender, female | 1.96 | 1.08-3.65 | 0.03 | |||
| BMI, per kg/m2 | 0.96 | 0.90-1.01 | 0.15 | |||
| Diabetes | 0.95 | 0.50-1.83 | 0.87 | |||
| Naïve to treatment | 1.24 | 0.71-2.19 | 0.45 | |||
| PI failure | 0.33 | 0.11-0.89 | 0.03 | |||
| HCV viral load, per log IU/mL | 0.80 | 0.56-1.11 | 0.2 | |||
| HCV genotype | ||||||
| 1 | Ref | Ref | Ref | Ref | Ref | Ref |
| 2 | 7.24 | 0.57-1.29 | < 0.01 | 4.66 | 2.06-11.42 | < 0.01 |
| 3 | 3.29 | 1.55-7.37 | < 0.01 | 2.76 | 1.22-6.59 | 0.02 |
| 4 | 2.03 | 0.57-8.21 | 0.28 | 1.91 | 0.51-8.06 | 0.35 |
| Hemoglobin, per g/dL | 1.11 | 0.95-1.32 | 0.17 | |||
| Platelet, per 103/μL | 1.01 | 1.01-1.02 | < 0.01 | |||
| ALT, per U/L | 1 | 0.99-1.00 | 0.1 | |||
| AST, per U/L | 0.99 | 0.99-1.00 | < 0.01 | |||
| Total bilirubin, per mg/dL | 0.37 | 0.24-0.55 | < 0.01 | 0.47 | 0.30-0.69 | < 0.01 |
| Albumin, per g/dL | 3.15 | 1.98-5.19 | < 0.01 | |||
| FIB-4 ≥ 3.25 | 0.23 | 0.12-0.45 | < 0.01 | |||
| SOF/PEG/RBV | Univariable | Multivariable | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age, per yr | 0.99 | 0.94-1.04 | 0.67 | |||
| Race, black | 0.98 | 0.30-3.86 | 0.98 | |||
| Gender, female | 2.31 | 0.67-10.79 | 0.22 | |||
| BMI, per kg/m2 | 0.95 | 0.83-1.09 | 0.42 | |||
| Diabetes, n (%) | 1.15 | 0.35-4.48 | 0.83 | |||
| Naïve to treatment | 7.72 | 1.98-51.36 | < 0.01 | 7.01 | 1.69-48.27 | 0.02 |
| PI failure | 1.06 | 0.32-4.16 | 0.92 | |||
| HCV viral load, log IU/mL | 1.07 | 0.55-1.92 | 0.83 | |||
| HCV genotype | ||||||
| 1 | Ref | Ref | Ref | |||
| 4 | 1.42 | 0.33-9.83 | 0.67 | |||
| Hemoglobin, per g/dL | 1309 | 0.76-1.55 | 0.64 | |||
| Platelets, per 103/μL | 1.01 | 0.99-1.02 | 0.14 | |||
| ALT, per U/L | 0.99 | 0.98-1.01 | 0.39 | |||
| AST, per U/L | 0.98 | 0.96-0.99 | < 0.01 | |||
| Total bilirubin, per mg/dL | 0.18 | 0.04-0.73 | 0.02 | |||
| Albumin, per g/dL | 3.50 | 1.21-11.04 | 0.03 | |||
| FIB-4 ≥ 3.25 | 0.16 | 0.05-0.50 | < 0.01 | 0.18 | 0.05-0.59 | < 0.01 |
| SMV/SOF ± RBV | Univariable | Multivariable | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age, per yr | 1.01 | 0.96-1.06 | 0.73 | 0.97 | 0.87-1.06 | 0.55 |
| Race, black | 0.43 | 0.15-1.45 | 0.14 | 0.66 | 0.11-4.90 | 0.66 |
| Gender, female | 1.69 | 0.61-5.44 | 0.34 | 0.41 | 0.08-1.94 | 0.26 |
| BMI, per kg/m2 | 0.97 | 0.87-1.08 | 0.55 | 1.02 | 0.88-1.20 | 0.75 |
| Diabetes, n (%) | 0.64 | 0.21-2.42 | 0.47 | |||
| Naïve to treatment | 7.96 | 1.57-145.37 | 0.04 | |||
| PI failure | 0.23 | 0.08-0.61 | < 0.01 | 0.12 | 0.02-0.52 | < 0.01 |
| Ribavirin | 0.78 | 0.29-2.03 | 0.61 | 0.64 | 0.09-3.78 | 0.63 |
| HCV viral load, per log IU/mL | 0.61 | 0.26-1.26 | 0.22 | 0.21 | 0.05-0.70 | 0.02 |
| Hemoglobin, g/dL | 1.17 | 0.92-1.48 | 0.18 | |||
| Platelet, per 103/μL | 1 | 0.99-1.01 | 0.19 | 1.01 | 0.99-1.02 | 0.35 |
| ALT, per U/L | 1 | 0.99-1.01 | 0.37 | 1.01 | 0.99-1.02 | 0.41 |
| AST, per U/L | 1 | 0.99-1.01 | 0.89 | |||
| Total Bili, per mg/dL | 0.56 | 0.29-1.06 | 0.06 | 0.31 | 0.08-0.86 | 0.04 |
| Albumin, per g/dL | 1.82 | 0.72-4.45 | 0.19 | |||
| FIB-4 ≥ 3.25 | 0.66 | 0.22-1.83 | 0.44 | 0.89 | 0.10-6.29 | 0.92 |
Among patients on SOF/RBV, SVR12 was less likely among patients with a higher baseline total bilirubin level (OR: 0.37, 95%CI: 0.24-0.55, P < 0.01) and more likely among patients infected by GT 2 HCV (OR: 4.66, 95% CI: 2.06-11.42, P < 0.01) or GT 3 HCV (OR: 2.76, 95%CI: 1.22-6.59, P = 0.02) compared to GT 1. There was no difference between GT 4 and GT 1 (Table 6). Among patients on SOF/PEG/RBV, SVR12 was more likely among patients who were naïve to treatment (OR: 7.01, 95%CI: 1.69-48.27, P = 0.02) and less likely among patients with a FIB-4 score ≥ 3.25 (OR: 0.18, 95%CI: 0.05-0.59, P < 0.01; Table 7).
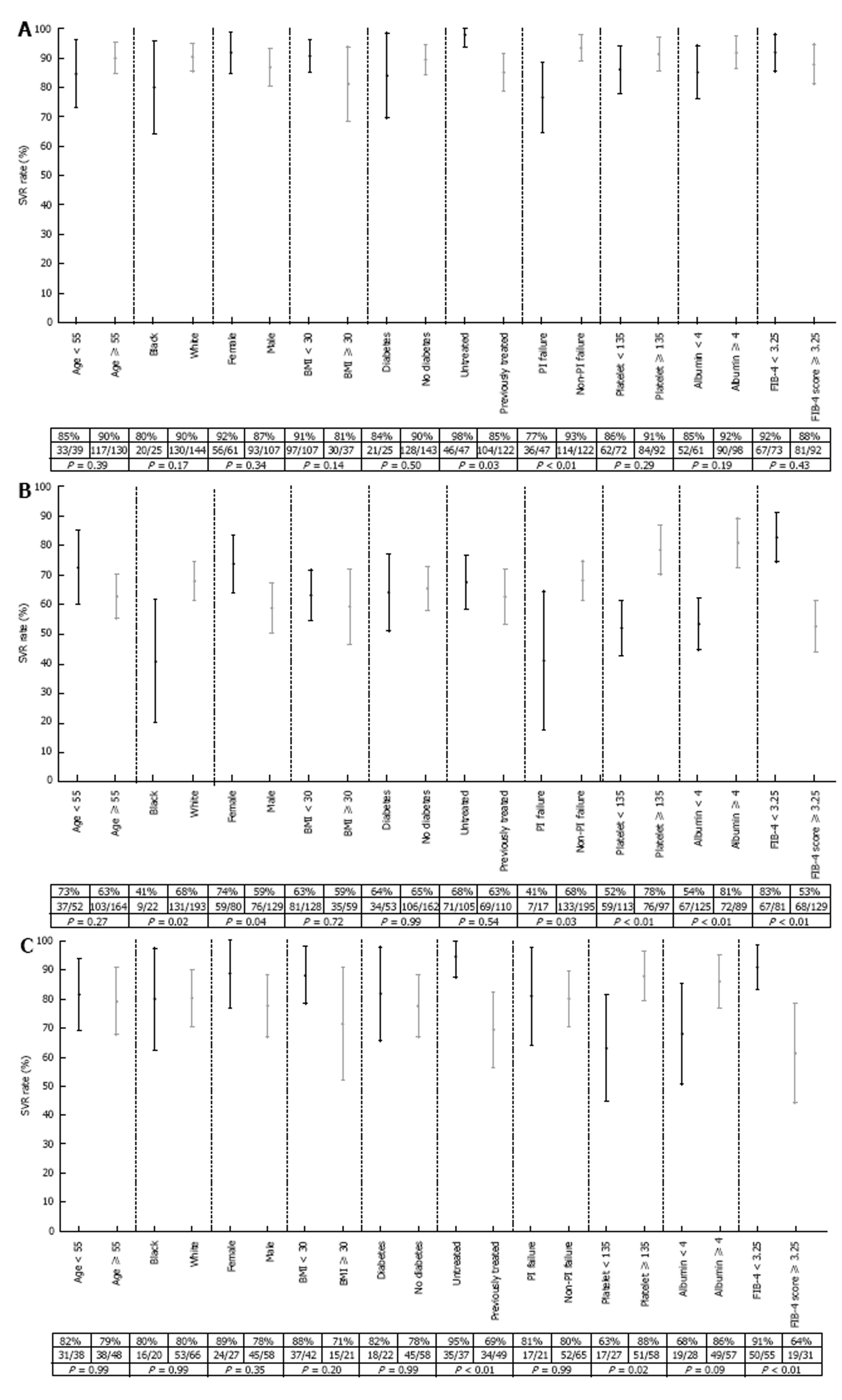
Figure 2 presents forest plots of SVR12 rates and 95%CIs of various subgroups of the 470 patients on each of the three regimens. Of note: Among patients on SMV/SOF ± RBV, the SVR12 rate was 77% (36/47) among patients who previously failed PI treatment, compared to 93% (114/122) among patients without a history of PI failure, P < 0.01 (Figure 2A). SVR12 was also significantly lower among patients with advanced fibrosis/cirrhosis as noted by a FIB-4 score ≥ 3.25 who were treated with either SOF/RBV (83% vs 53%, P < 0.01, Figure 2B) or SOF/PEG/RBV (91% vs 61%, P < 0.01, Figure 2C).
HCV treatment is evolving at a rapid pace, and timely data are needed regarding the clinical and economic performance of current and emerging medical therapies. This study provides information about the largest consecutive series of patients treated at a single center in the United States with regimens containing SMV and/or SOF that has been reported thus far. Importantly, we examined outcomes in patients infected with GTs 1-4, while other large studies of SMV- and/or SOF-based regimens in the United States were limited to patients with single GTs[25-27,29-31]. This study provides data about the effectiveness of various regimens when used in real-world clinical practice in a diverse patient population. Fourteen percent of the cohort was African-American, over half (54%) likely had advanced fibrosis/cirrhosis as determined by FIB-4 score ≥ 3.25, and 60% had previously failed treatment, including 17% that failed prior treatment with PIs.
Compared to the group of patients treated at our center with first generation PIs, the group treated with SMV- and/or SOF-based regimens was significantly older and included a higher percentage of patients with advanced fibrosis/cirrhosis. These differences are consistent with the aging of the baby-boomer cohort, which comprises over 75% of patients infected with HCV in the United States[35]. The greater tolerability and effectiveness of the newer therapies allow patients with more advanced liver disease to achieve an SVR12, causing a shift in the demographic profile of patients receiving treatment. The higher probability of cure and reduced side effect profile may also may encourage a greater number of patients to seek treatment[36,37].
Whereas real-world SVR12 rates with TVR- and BOC-containing regimens were lower than in large registration trials[18,38], the SVR12 rates in this study generally accord with results obtained in formal trials. Among the 508 patients who began therapy, SVR12 rates calculated on an ITT basis were 86% for SMV/SOF ± RBV, 62% for SOF/RBV, and 78% for SOF/PEG/RBV. For comparison, in registration trials, SVR12 rates for SMV/SOF ± RBV ranged from 83%-97%[7,8]; for SOF/RBV, they ranged from 56%-97%[6]; and for SOF/PEG/RBV, they ranged from 80%-90%[6]. The relatively low overall SVR12 rate for SOF/RBV in our population likely reflects the fact that 48% (113/234) of patients treated with SOF/RBV had GT 1 or GT 4 HCV. Published data show that SVR12 rates may be lower for these genotypes, especially in the setting of advanced liver disease[33]. Patients with GT 3 HCV also had a relatively lower rate of SVR12 at 67%; this is similar to the SVR12 rate seen in another recent study assessing real-world rates of SVR12 in patients with GT3 HCV, where the SVR12 rate was 69.4%[39]. In contrast, patients in our study with GT 2 HCV who were treated with SOF/RBV had an SVR12 rate of 83% (95%CI: 71%-90%), again consistent with the high rate of response to SOF/RBV for GT 2 as published in the literature. Among patients treated with either SMV/SOF ± RBV or SOF/PEG/RBV, GT did not significantly impact SVR12 rates.
Multivariable logistic regression identified factors associated with lower SVR12 rates, helping to define patients who may benefit from alternative treatment strategies. Among all treatment regimens examined, the presence of more advanced liver disease was negatively associated with achieving an SVR12. These findings accord with another recently published study assessing treatment outcomes among patients with GT4 HCV treated with SOF/RBV or SOF/PEG/RBV, where those with advanced liver disease were less likely to achieve SVR12[31]. The observation that more advanced liver disease was associated with treatment failure across all three regimens is noteworthy because advanced liver disease is becoming increasingly prevalent in patients with HCV infection[40,41]. This underscores the urgency of efforts to screen patients for HCV infection and transition them into care in order to minimize liver disease progression and obtain the maximal benefits from HCV therapies.
Cost of HCV eradication has become a major concern for the general public and the medical community[42]. We previously analyzed costs of TVR-based triple therapy and found that TVR, which at the time cost $4606/wk, accounted for the majority of the expenses[19]. The costs of both SMV ($5530/wk) and SOF ($7000/wk) are higher than TVR. In part because of this increased drug cost, data from a recently published study suggested that cost-per-SVR was relatively constant when comparing SMV/SOF ± RBV with TVR-based triple therapy[28]. In contrast, our data suggest that the median cost-per-SVR for SMV/SOF ± RBV is significantly lower than that of TVR-based treatment, likely because of a shorter duration of treatment, reduced adverse event management costs, and higher SVR12 rates compared with TVR-based regimens. Heterogeneity in the demographic makeup and percentage of patients with advanced fibrosis/cirrhosis in study populations may account for the discordant results.
Interestingly, SOF/RBV had the highest mean cost-per-SVR, $223003. This may be related to the reduced effectiveness of this regimen in patients with GT 1 and GT4 HCV[6,33]. Among patients with GT 2 HCV treated with SOF/RBV, the mean cost-per-SVR was the lowest for any regimen, $102949 (SD ± $21346). In addition to GT, the stage of liver fibrosis may affect the choice and duration of treatment and therefore cost. For instance, AASLD/IDSA guidelines suggest treating patients with compensated GT 1 HCV cirrhosis for 24 wk with the combination of SMV/SOF with or without RBV, compared to 12 wk for those without cirrhosis[16]. This regimen would therefore be more expensive in patients with cirrhosis than in those without.
While DAAs remain expensive, it is hoped that the increasing number of treatment options and increased competition will drive costs down. This may especially be important in emerging economies[43]. In addition to occupying a place in the global market, SMV will likely play an important role in specific settings, including the treatment of HCV after liver transplantation, where it has been used successfully without RBV with SVR12 rates ranging from 78%-88%[24,26,44,45]. SMV may also play an important role in patients with a history of failed NS5A inhibitor therapy. Approximately 5%-15% of patients may fail therapy with regimens containing NS5A inhibitors such as ledipasvir, elbasvir, or daclatasvir. These treatment failures often occur in patients with HCV RASs, some of which may confer cross-resistance for multiple drugs within this class[46]. In patients who fail NS5A therapy, treatment with SMV/SOF can result in an SVR12 rate of 88%[22]. RAS testing is becoming more common, as it is recommended by AASLD guidelines prior to initiation of therapy with elbasvir/grazoprevir[47]. While the newest NS5A inhibitor, velpatasvir (used in fixed-dose combination with SOF) may be impacted less by the presence of pretreatment RASs, this regimen remains expensive and may not be accessible to all patients[21]. More precise targeting of therapy may improve patient outcomes and reduce costs.
The strengths of this study include the large number of patients who consecutively initiated therapy, as well as the diversity of the cohort, which was comprised of a racially heterogeneous population with varying stages of liver fibrosis, treatment history, and HCV GT. Importantly, costs were based on data from individual patients, as opposed to aggregate outcomes or projections, and are thus more reflective of costs incurred by payers.
Despite these strengths, our study group was not large enough to delineate all the factors that may impact SVR12 rates. Further, ITT analyses included treatment outcomes (SVR12/non-SVR12) that were imputed in 14 (2.75%) patients; however, any minor artifactual elevation in the SVR12 rate that occurred because of this imputation was likely more than off-set by the assumption that all 24 LFU patients failed therapy. Finally, costs in this study were only calculated from the payer’s perspective, rather than from a patient or societal perspective.
In conclusion, this study provides the largest single-center consecutive series of patients treated with SMV- and/or SOF-based regimens in a diverse population. Rates of SVR12 were high, and generally comparable to those seen in registration trials. Cost-per-SVR was dependent upon the drug regimen, and was influenced by patient and HCV-specific factors. Patients with more advanced liver disease were less likely to achieve SVR12.
Treatment options for patients with chronic hepatitis C virus (HCV) infection are expanding rapidly. The first direct-acting antiviral drugs for HCV, telaprevir (TVR) and boceprevir (BOC), were used in combination with pegylated interferon (PEG) and ribavirin (RBV). These drugs led to enhanced rates of sustained virological response (SVR), but had high rates of adverse events, cumbersome dosing regimens, and high costs-per-SVR due to low SVR rates in clinical practice. Newer regimens based on the NS5B inhibitor sofosbuvir (SOF) have greater tolerability, easier dosing, and higher SVR rates, but remain costly. Comparative data regarding the clinical and economic effectiveness of new regimens are necessary to optimize selection of a treatment regimen for each patient. This paper analyzes the clinical effectiveness and cost of simeprevir (SMV)- and/or SOF-based regimens in a large, diverse real-world patient population among patients infected with HCV genotypes 1-4.
Efforts to screen patients within the baby-boomer cohort for chronic HCV infection, direct-to-consumer advertising, and an increased drive to by the World Health Organization to eliminate viral hepatitis by 2030 are allowing a greater number of patients to receive care. Understanding the real-world effectiveness and cost of various HCV treatment regimens can help providers optimize therapy for their patients, especially in resource-poor areas that may not have access to the newest and most costly treatment regimens. Prior studies have addressed these questions among patients within more homogenous populations, with respect to both ethnic diversity and HCV genotype. Here, the authors assess effectiveness and cost an ethnically and genotypically diverse patient population.
The authors offer an analysis of HCV cure rates using three treatment regimens: SMV/SOF ± RBV, SOF/RBV, and SOF/PEG/RBV. Cure rates with these regimens are comparable to those seen in clinical trials. The authors describe factors associated with a lower likelihood of SVR, which include the presence of more advanced liver disease and prior failure of TVR- or BOC-based triple therapy. Importantly, despite SMV and SOF being more expensive medications than telaprevir or boceprevir, the cost-per-SVR was significantly lower than that which was seen using TVR-based triple therapy.
These data will be useful to providers when selecting SMV- and/or SOF-based therapy for patients, especially when newer and more expensive direct-acting antiviral therapy is not available. A similar cost analysis could be performed using newer drugs, utilizing the data presented in this study as a comparator.
Sustained virological response - the absence of detectable HCV RNA in the blood at 12 wk after completion of treatment. Direct-acting antiviral (DAA) drugs - a class of oral drugs that directly inhibit HCV viral replication, which includes NS3/4A protease inhibitors (PIs) such as TVR, BOC, SMV, and grazoprevir; NS5B polymerase inhibitors, including SOF and dasabuvir; and NS5A inhibitors, including ledipasvir, velpatasvir elbasvir, daclatasvir, and ombitasvir.
From the clinical point of view, this study reports valuable results and gives clue to clinicians to properly manage chronic HCV infection in patients.
Manuscript source: Invited Manuscript
Specialty type: Virology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Farshadpour F, Roohvand F S- Editor: Cui LJ L- Editor: A E- Editor: Lu YJ
| 1. | Asselah T, Boyer N, Saadoun D, Martinot-Peignoux M, Marcellin P. Direct-acting antivirals for the treatment of hepatitis C virus infection: optimizing current IFN-free treatment and future perspectives. Liver Int. 2016;1:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 187] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 2. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1861] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 3. | Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1214] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 4. | Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, DiNubile MJ, Sniukiene V, Brass CA, Albrecht JK, Bronowicki JP; SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1980] [Article Influence: 141.4] [Reference Citation Analysis (0)] |
| 5. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1308] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 6. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1325] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 7. | Kwo P, Gitlin N, Nahass R, Bernstein D, Etzkorn K, Rojter S, Schiff E, Davis M, Ruane P, Younes Z. Simeprevir plus sofosbuvir (12 and 8 weeks) in hepatitis C virus genotype 1-infected patients without cirrhosis: OPTIMIST-1, a phase 3, randomized study. Hepatology. 2016;64:370-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 8. | Lawitz E, Matusow G, DeJesus E, Yoshida EM, Felizarta F, Ghalib R, Godofsky E, Herring RW, Poleynard G, Sheikh A. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: A phase 3 study (OPTIMIST-2). Hepatology. 2016;64:360-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 9. | Forns X, Lawitz E, Zeuzem S, Gane E, Bronowicki JP, Andreone P, Horban A, Brown A, Peeters M, Lenz O. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology. 2014;146:1669-1679.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 10. | Manns M, Marcellin P, Poordad F, de Araujo ES, Buti M, Horsmans Y, Janczewska E, Villamil F, Scott J, Peeters M. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 291] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 11. | Yoshida EM, Sulkowski MS, Gane EJ, Herring RW Jr, Ratziu V, Ding X, Wang J, Chuang SM, Ma J, McNally J, Stamm LM, Brainard DM, Symonds WT, McHutchison JG, Beavers KL, Jacobson IM, Reddy KR, Lawitz E. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology. 2015;61:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 12. | Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 652] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 13. | Simmons B, Saleem J, Heath K, Cooke GS, Hill A. Long-Term Treatment Outcomes of Patients Infected With Hepatitis C Virus: A Systematic Review and Meta-analysis of the Survival Benefit of Achieving a Sustained Virological Response. Clin Infect Dis. 2015;61:730-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 14. | Tada T, Kumada T, Toyoda H, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Kitabatake S, Yama T, Tanaka J. Viral eradication reduces all-cause mortality in patients with chronic hepatitis C virus infection: a propensity score analysis. Liver Int. 2016;36:817-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Smith-Palmer J, Cerri K, Valentine W. Achieving sustained virologic response in hepatitis C: a systematic review of the clinical, economic and quality of life benefits. BMC Infect Dis. 2015;15:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 16. | AASLD-IDSA. When and in Whom to Initiate HCV Therapy. Recommendations for testing, managing, and treating hepatitis C. Available from: http://www.hcvguidelines.org/full-report/when-and-whom-initiate-hcv-therapy. |
| 17. | Westergaard RP, Stockman LJ, Hyland HA, Guilfoyle SM, Fangman JJ, Vergeront JM. Provider Workforce Assessment in a Rural Hepatitis C Epidemic: Implications for Scale-up of Antiviral Therapy. J Prim Care Community Health. 2015;6:215-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Vo KP, Vutien P, Akiyama MJ, Vu VD, Ha NB, Piotrowski JI, Wantuck J, Roytman MM, Tsai N, Cheung R. Poor Sustained Virological Response in a Multicenter Real-Life Cohort of Chronic Hepatitis C Patients Treated with Pegylated Interferon and Ribavirin plus Telaprevir or Boceprevir. Dig Dis Sci. 2015;60:1045-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Bichoupan K, Martel-Laferriere V, Sachs D, Ng M, Schonfeld EA, Pappas A, Crismale J, Stivala A, Khaitova V, Gardenier D. Costs of telaprevir-based triple therapy for hepatitis C: $189,000 per sustained virological response. Hepatology. 2014;60:1187-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Reddy KR, Zeuzem S, Zoulim F, Weiland O, Horban A, Stanciu C, Villamil FG, Andreone P, George J, Dammers E. Simeprevir versus telaprevir with peginterferon and ribavirin in previous null or partial responders with chronic hepatitis C virus genotype 1 infection (ATTAIN): a randomised, double-blind, non-inferiority phase 3 trial. Lancet Infect Dis. 2015;15:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Weisberg IS, Jacobson IM. A pangenotypic, single tablet regimen of sofosbuvir/velpatasvir for the treatment of chronic hepatitis C infection. Expert Opin Pharmacother. 2017;18:535-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Hézode C, Chevaliez S, Scoazec G, Soulier A, Varaut A, Bouvier-Alias M, Ruiz I, Roudot-Thoraval F, Mallat A, Féray C. Retreatment with sofosbuvir and simeprevir of patients with hepatitis C virus genotype 1 or 4 who previously failed a daclatasvir-containing regimen. Hepatology. 2016;63:1809-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | O’Leary JG, Fontana RJ, Brown K, Burton JR Jr, Firpi-Morell R, Muir A, O’Brien C, Rabinovitz M, Reddy R, Ryan R, Shprecher A, Villadiego S, Prabhakar A, Brown RS Jr. Efficacy and safety of simeprevir and sofosbuvir with and without ribavirin in subjects with recurrent genotype 1 hepatitis C postorthotopic liver transplant: the randomized GALAXY study. Transpl Int. 2017;30:196-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Pungpapong S, Aqel B, Leise M, Werner KT, Murphy JL, Henry TM, Ryland K, Chervenak AE, Watt KD, Vargas HE. Multicenter experience using simeprevir and sofosbuvir with or without ribavirin to treat hepatitis C genotype 1 after liver transplant. Hepatology. 2015;61:1880-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 25. | Sulkowski MS, Vargas HE, Di Bisceglie AM, Kuo A, Reddy KR, Lim JK, Morelli G, Darling JM, Feld JJ, Brown RS. Effectiveness of Simeprevir Plus Sofosbuvir, With or Without Ribavirin, in Real-World Patients With HCV Genotype 1 Infection. Gastroenterology. 2016;150:419-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 26. | Pillai AA, Wedd J, Norvell JP, Parekh S, Cheng N, Young N, Spivey JR, Ford R. Simeprevir and Sofosbuvir (SMV-SOF) for 12 Weeks for the Treatment of Chronic Hepatitis C Genotype 1 Infection: A Real World (Transplant) Hepatology Practice Experience. Am J Gastroenterol. 2016;111:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Yee BE, Nguyen NH, Jin M, Lutchman G, Lim JK, Nguyen MH. Lower response to simeprevir and sofosbuvir in HCV genotype 1 in routine practice compared with clinical trials. BMJ Open Gastroenterol. 2016;3:e000056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Langness JA, Tabano D, Wieland A, Tise S, Pratt L, Harrington LA, Lin S, Ghuschcyan V, Nair KV, Everson GT. Curing Chronic Hepatitis C: A Cost Comparison of the Combination Simeprevir Plus Sofosbuvir vs. Protease-Inhibitor-Based Triple Therapy. Ann Hepatol. 2017;16:366-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Dolatimehr F, Karimi-Sari H, Rezaee-Zavareh MS, Alavian SM, Behnava B, Gholami-Fesharaki M, Sharafi H. Combination of sofosbuvir, pegylated-interferon and ribavirin for treatment of hepatitis C virus genotype 1 infection: a systematic review and meta-analysis. Daru. 2017;25:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Satsangi S, Mehta M, Duseja A, Taneja S, Dhiman RK, Chawla Y. Dual treatment with sofosbuvir plus ribavirin is as effective as triple therapy with pegylated interferon plus sofosbuvir plus ribavirin in predominant genotype 3 patients with chronic hepatitis C. J Gastroenterol Hepatol. 2017;32:859-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Elsharkawy A, Fouad R, El Akel W, El Raziky M, Hassany M, Shiha G, Said M, Motawea I, El Demerdash T, Seif S. Sofosbuvir-based treatment regimens: real life results of 14 409 chronic HCV genotype 4 patients in Egypt. Aliment Pharmacol Ther. 2017;45:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Del Bello D, Cha A, Sorbera M, Bichoupan K, Levine C, Doyle E, Harty A, Patel N, Ng M, Gardenier D. Real-World Sustained Virologic Response Rates of Sofosbuvir-Containing Regimens in Patients Coinfected With Hepatitis C and HIV. Clin Infect Dis. 2016;62:1497-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Osinusi A, Meissner EG, Lee YJ, Bon D, Heytens L, Nelson A, Sneller M, Kohli A, Barrett L, Proschan M. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA. 2013;310:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 247] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 34. | Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, DeJesus E, Pearlman B, Rabinovitz M, Gitlin N. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 597] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 35. | Sayiner M, Wymer M, Golabi P, Ford J, Srishord I, Younossi ZM. Presence of hepatitis C (HCV) infection in Baby Boomers with Medicare is independently associated with mortality and resource utilisation. Aliment Pharmacol Ther. 2016;43:1060-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Norton BL, Voils CI, Timberlake SH, Hecker EJ, Goswami ND, Huffman KM, Landgraf A, Naggie S, Stout JE. Community-based HCV screening: knowledge and attitudes in a high risk urban population. BMC Infect Dis. 2014;14:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Zeremski M, Dimova RB, Zavala R, Kritz S, Lin M, Smith BD, Zibbell JE, Talal AH. Hepatitis C virus-related knowledge and willingness to receive treatment among patients on methadone maintenance. J Addict Med. 2014;8:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Mauss S, Böker K, Buggisch P, Christensen S, Hofmann WP, Schott E, Pfeiffer-Vornkahl H, Alshuth U, Hüppe D. Real-life experience with first generation HCV protease inhibitor therapy in Germany: The prospective, non-interventional PAN cohort. Z Gastroenterol. 2015;53:644-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Wehmeyer MH, Ingiliz P, Christensen S, Hueppe D, Lutz T, Simon KG, Schewe K, Boesecke C, Baumgarten A, Busch H. Real-world effectiveness of sofosbuvir-based treatment regimens for chronic hepatitis C genotype 3 infection: results from the multicenter German hepatitis C cohort (GECCO-03). J Med Virol. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Younossi ZM, Otgonsuren M, Henry L, Arsalla Z, Stepnaova M, Mishra A, Venkatesan C, Hunt S. Inpatient resource utilization, disease severity, mortality and insurance coverage for patients hospitalized for hepatitis C virus in the United States. J Viral Hepat. 2015;22:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Galbraith JW, Donnelly JP, Franco RA, Overton ET, Rodgers JB, Wang HE. National estimates of healthcare utilization by individuals with hepatitis C virus infection in the United States. Clin Infect Dis. 2014;59:755-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Reau NS, Jensen DM. Sticker shock and the price of new therapies for hepatitis C: Is it worth it? Hepatology. 2014;59:1246-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Andrieux-Meyer I, Cohn J, de Araújo ES, Hamid SS. Disparity in market prices for hepatitis C virus direct-acting drugs. Lancet Glob Health. 2015;3:e676-e677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 44. | Crittenden NE, Buchanan LA, Pinkston CM, Cave B, Barve A, Marsano L, McClain CJ, Jones CM, Marvin MR, Davis EG. Simeprevir and sofosbuvir with or without ribavirin to treat recurrent genotype 1 hepatitis C virus infection after orthotopic liver transplantation. Liver Transpl. 2016;22:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Jackson WE, Hanouneh M, Apfel T, Alkhouri N, John BV, Zervos X, Zein NN, Hanouneh IA. Sofosbuvir and simeprevir without ribavirin effectively treat hepatitis C virus genotype 1 infection after liver transplantation in a two-center experience. Clin Transplant. 2016;30:709-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Poveda E, Wyles DL, Mena A, Pedreira JD, Castro-Iglesias A, Cachay E. Update on hepatitis C virus resistance to direct-acting antiviral agents. Antiviral Res. 2014;108:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 47. | AASLD. AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. Available from: http://www.hcvguidelines.org. |