Published online Aug 12, 2016. doi: 10.5501/wjv.v5.i3.125
Peer-review started: March 17, 2016
First decision: April 18, 2016
Revised: May 26, 2016
Accepted: July 11, 2016
Article in press: July 13, 2016
Published online: August 12, 2016
Processing time: 149 Days and 12.3 Hours
AIM: To characterize the circulating infectious bronchitis virus (IBV) strains in Egypt depending on the sequence of the spike-1 (S1) gene [hypervariable region-3 (HVR-3)] and to study the pathotypic features of these strains.
METHODS: In this work, twenty flocks were sampled for IBV detection using RRT-PCR and isolation of IBV in specific pathogen free (SPF) chicks during the period from 2010 to 2015. Partial sequencing and phylogenetic analysis of 400 bp representing the HVR-3 of the S1 gene was conducted. Pathotypic characterization of one selected virus from each group (Egy/Var-I, Egy/Var-II and classic) was evaluated in one day old SPF chicks. The chicks were divided into 4 groups 10 birds each including the negative control group. Birds were inoculated at one day by intranasal instillation of 105EID50/100 μL of IBV viruses [IBV-EG/1212B-2012 (Egy/Var-II), IBV/EG/IBV1-2011 (Egy/Var-I) and IBV-EG/11539F-2011 (classic)], while the remaining negative control group was kept uninfected. The birds were observed for clinical signs, gross lesions and virus pathogenicity. The real-time rRT-PCR test was performed for virus detection in the tissues. Histopathological examinations were evaluated in both trachea and kidneys.
RESULTS: The results revealed that these viruses were separated into two distinct groups; variant (GI-23) and classic (GI-1), where 16 viruses belonged to a variant group, including 2 subdivisions [Egy/Var-I (6 isolates) and Egy/Var-II (10 isolates)] and 4 viruses clustered to the classic group (Mass-like). IBV isolates in the variant group were grouped with other IBV strains from the Middle East. The variant subgroup (Egy/Var-I) was likely resembling the original Egyptian variant strain (Egypt/Beni-Suif/01) and the Israeli strain (IS/1494/2006). The second subgroup (Egy/Var-II) included the viruses circulating in the Middle East (Ck/EG/BSU-2 and Ck/EG/BSU-3/2011) and the Israeli strain (IS/885/00). The two variant subgroups (Egy/Var-I and Egy/Var-II) found to be highly pathogenic to SPF chicks with mortalities up to 50% than those of the classic group which was of low virulence (10% mortality). Pathogenicity indices were 25 (Egy/Var-II), 24 (Egy/Var-I) and 8 (classic); with clinical scores 3, 2 and 1 respectively.
CONCLUSION: These findings indicated that the recent circulating Egyptian IBVs have multiple heterogeneous origins in marked diversifying nature of their spread, with high pathotype in specific pathogen free chicks.
Core tip: Infectious bronchitis became enzootic in Egypt with frequent outbreaks of different variant in broiler chickens in spite of intensive vaccination programs used causing severe infections. These manuscripts discuss the prevalence of these different variants with pathotyping of these variants in specific pathogen free chicks.
- Citation: Zanaty A, Arafa AS, Hagag N, El-Kady M. Genotyping and pathotyping of diversified strains of infectious bronchitis viruses circulating in Egypt. World J Virol 2016; 5(3): 125-134
- URL: https://www.wjgnet.com/2220-3249/full/v5/i3/125.htm
- DOI: https://dx.doi.org/10.5501/wjv.v5.i3.125
Infectious bronchitis virus (IBV) is a highly contagious viral disease causing severe economic losses in the commercial poultry industry and is ubiquitous in most parts of the world. IBV targets primarily the upper respiratory tract; however the nephropathogenic strains have a predilection for the kidney of young chickens causing nephritis that can produce significant mortalities[1-3]. In layers and breeders, a decrease in egg production and egg quality has been documented[4].
IBV is a coronavirus of genus Gammacoronavirus; family Coronaviridae order Nidovirales[5]. IBV is an enveloped virus and has a linear positive sense non-segmented single-stranded RNA genome, approximately 27.6 kb in length. Four main structural proteins construct the IBV particles; namely the phosphorylated nucleoprotein (N), the membrane protein (M), the spike (S) glycoprotein and the small membrane protein (E). The S glycoprotein is proteolitically cleaved into 2 separate subunits; the S1 and S2 polypeptide[6]. The S1 subunit is attached to the viral envelope and is responsible for fusion of virus envelope and the host cell membrane. It carries virus-neutralizing and serotype-specific determinants that located in the hypervariable regions (HVRs) of the S1 subunit[7]. Furthermore, S1 reveals high sequence variability than S2 subunit[8]. Hence, the evolutionary characterization and detection of IBV is mainly targeting the analysis of the variable S1 gene or the expressed S1 protein[9].
IBV variants are distributed worldwide, there are more than 20 IBV serotypes differentiated globally[10]. Genomic insertions, deletions, point mutations, substitutions and RNA recombination of the S1 gene are associated with the emergence of new variants[5,11]. Different serotypes of newly evolved variants from chickens may cause partially efficacious vaccines or even vaccine breaks[12].
In Egypt, IBV strains continue to spread everywhere in the country, and have been isolated from both vaccinated and non-vaccinated flocks[13]. Different genotypes were isolated from poultry flocks and they were similar to Massachusetts, D3128, D274, D08880, 793B (4/91 and CR88), IS/885/00 and Egypt/Beni-Suef/01[14-16]. In 2011, two Egyptian strains, named Egy/Var-II (Ck/Eg/BSU-2, 3/2011), were reported as a new IBV variant resembling IS/885/00 strain according to sequence of the HVR-3[16]. Recently in 2016, depending on the full S1 sequence, Valastro et al[9] clustered the Egyptian variant strains in the GI-23 lineage which represents the unique wild-type cluster geographically confined to the middle East.
Herein, twenty chicken flocks suffering from IBV-like symptoms were genetically and phylogenetically analyzed based on the HVR-3 of S1 gene and compared to the previously isolated Egyptian viruses and others from neighboring countries along with common vaccine viruses used. In addition, pathotyping of three viruses was carried out to determine the pathogenic type of isolates. The resulting information will provide a guide for the matching level between field and vaccine viruses and that will help for optimal use of existing live vaccines and plan for future vaccine strategy.
Samples were collected from twenty broiler farms from 12 governorates in Egypt, showing mild-to-severe respiratory signs, in the period between 2010 and 2015 (Table 1). Samples were delivered to the Reference Laboratory for Quality control on Poultry production (RLQP), Egypt. The chickens were vaccinated with H120 strain of IBV at one day of age. Chickens showed respiratory symptoms such as gasping, coughing, sneezing and tracheal rales with white diarrhea in some cases. Necropsy showed mild to severe tracheitis with congested lung. In addition, birds were suffering from kidney lesions such as enlargement, congestion, and uroletheasis. The samples were collected as pooled homogenate from trachea and kidney. Further, the samples were prepared as 10% w/v suspensions in PBS (pH 7.4) and centrifuged at 3000 rpm for 10 min; the supernatants were then collected for further analysis.
| Isolate No. | Isolate name | Age of birds (d) | Governorate | GeneBank accession number | Phylogenetic group |
| 1 | IBV-EG/12773F(3)-2012 | 20 | Beni-Suef | KC608180 | Classic1 |
| 2 | IBV-EG/11539F-2011 | 23 | Kafr-El-Shikh | JQ839289 | Classic |
| 3 | IBV-EG/10643F(1-7)-2010 | 19 | Sharqia | KC608171 | Classic |
| 4 | IBV-EG/116F-1(1)-2011 | 30 | Gharbia | KC608176 | Classic |
| 5 | IBV-EG/11673F-2011 | 20 | Alexandria | KC608173 | Egy Var I |
| 6 | IBV-EG/11413F-2011 | 25 | Sharqia | KC608177 | Egy Var I |
| 7 | IBV-EG/10324F-2010 | 17 | Giza | KC608172 | Egy Var I |
| 8 | IBV-EG/1299B-2012 | 18 | Fayoum | KC608182 | Egy Var I |
| 9 | IBV-EG/1196F-2011 | 15 | Behira | KC608174 | Egy Var I |
| 10 | IBV/EG/IBV1-2011 | 25 | Suez | JQ839288 | Egy Var I |
| 11 | IBV-EG/1138F-4-2011 | 18 | Dakahlia | KC608175 | Egy Var II |
| 12 | IBV-EG/Qalyobia/121-2012 | 22 | Qaliobeya | KC608181 | Egy Var II |
| 13 | IBV-EG/1212B-2012 | 14 | Al Behira | JQ839287 | Egy Var II |
| 14 | IBV-EG/1293B-2012 | 21 | Fayoum | KC608178 | Egy Var II |
| 15 | IBV-EG/1262F(3)-2012 | 28 | Ismalia | KC608179 | Egy Var II |
| 16 | CH/EGYPT/13200F/2013 | 20 | Giza | KT832805 | Egy Var II |
| 17 | CH/EGYPT/13950F/2013 | 25 | Alexandria | KT832806 | Egy Var II |
| 18 | CH/EGYPT/14251F/2014 | 22 | Suez | KT832807 | Egy Var II |
| 19 | CH/EGYPT/141107F/2014 | 32 | Giza | KT832808 | Egy Var II |
| 20 | CH/EGYPT/15919F/2015 | 26 | Dakahlia | KT832809 | Egy Var II |
Viral RNA was extracted directly from the samples by using QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany), following the kit manufacturer’s instructions. The virus identification was confirmed by real-time reverse transcription-polymerase chain reaction (rRT-PCR) for the presence of the neucloprotein (NP) gene sequence of the IBV using Quantitect probe RT-PCR kit (Qiagen, Hilden, Germany), with specific primers and probe targeting the NP gene[17].
Viral isolation from trachea and kidney was performed according to Momayez et al[18]. Nine-eleven day old SPF chicken eggs were inoculated via the chorioallantoic route. Dead embryos were investigated for the presence of embryo stunting, curling, dwarfing, subcutaneous hemorrhage and ureate deposition in the mesonephros. The allantoic fluids from each sample were screened using rRT-PCR for further confirmation.
Positive virus screening was further tested using a specific primer set for the amplifications of the HVR-3 of the S1 gene using Qiagen one-step RT-PCR (Qiagen, Hilden, Germany), according to the manufacturer’s protocol[19]. Amplificates of 400 bp in size were excised and purified from gels using the QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany). The purified RT-PCR products were sequenced using Bigdye Terminator V3.1 cycle sequencing kit (Perkin-Elmer, Foster City, CA) and Applied Biosystems 3130 genetic analyzer (ABI, United States). Sequences similarities and relationships of the HVR-3 of the S1 gene from the 20 samples in this study were compared with previously published IBV vaccine and reference strains available in the public database (NCBI, United States). Amino acids phylogenetic tree was drawn for the sequenced isolates along with other vaccine and reference strains available in the GenBank database using MEGA version 6[20]. A comparative analysis of deduced amino acids and nucleotide sequences of the HVR-3 was created using the CLUSTAL W Multiple Sequence Alignment Program, version 1.83 of MegAlign module of Lasergene DNAStar software[21]. Sequences generated in the frame of this study were submitted to the GenBank database with accession numbers showed in Table 1.
Forty SPF chicks of one-day-old were obtained from the Nile SPF company, Kom-Oshim, Fayoum, Egypt. The birds were housed in separate bio-safety level-3 chicken isolators in RLQP under strict hygienic conditions. Chickens were tagged with wing bands for identification and randomly divided into 4 groups 10 birds each including the negative control group. The first three groups were assigned for determination of pathogenicity. Birds were inoculated at one day by intranasal instillation of 105EID50/100 μL of IBV viruses (IBV-EG/1212B-2012, IBV/EG/IBV1-2011 and IBV-EG/11539F-2011) according to Purcell et al[22], while the remaining negative control group was kept uninfected.
The birds were observed for clinical signs, gross lesions and virus pathogenicity. The clinical signs were recorded daily for up to 14 d post-infection, according to clinical scoring formula presented by Wang et al[1]. Gross lesions in the trachea and kidney as well as pathogenicity index were calculated based on the criteria described in Wang et al[1]. The presence of IBV was checked in samples obtained from the inoculated groups at 14 d post-infection. The real-time rRT-PCR test was performed for the detection of virus in the tissues. Histopathological examinations were carried out on both trachea and kidneys, according to Bancroft et al[23].
Samples representing the twenty flocks in this study showed positive results for detection of IBV using rRT-PCR. Virus isolation was obtained from the homogenate pool of the trachea and kidney from each flock. The allantoic fluid from the 3rd passage of each sample further confirmed positive using rRT-PCR.
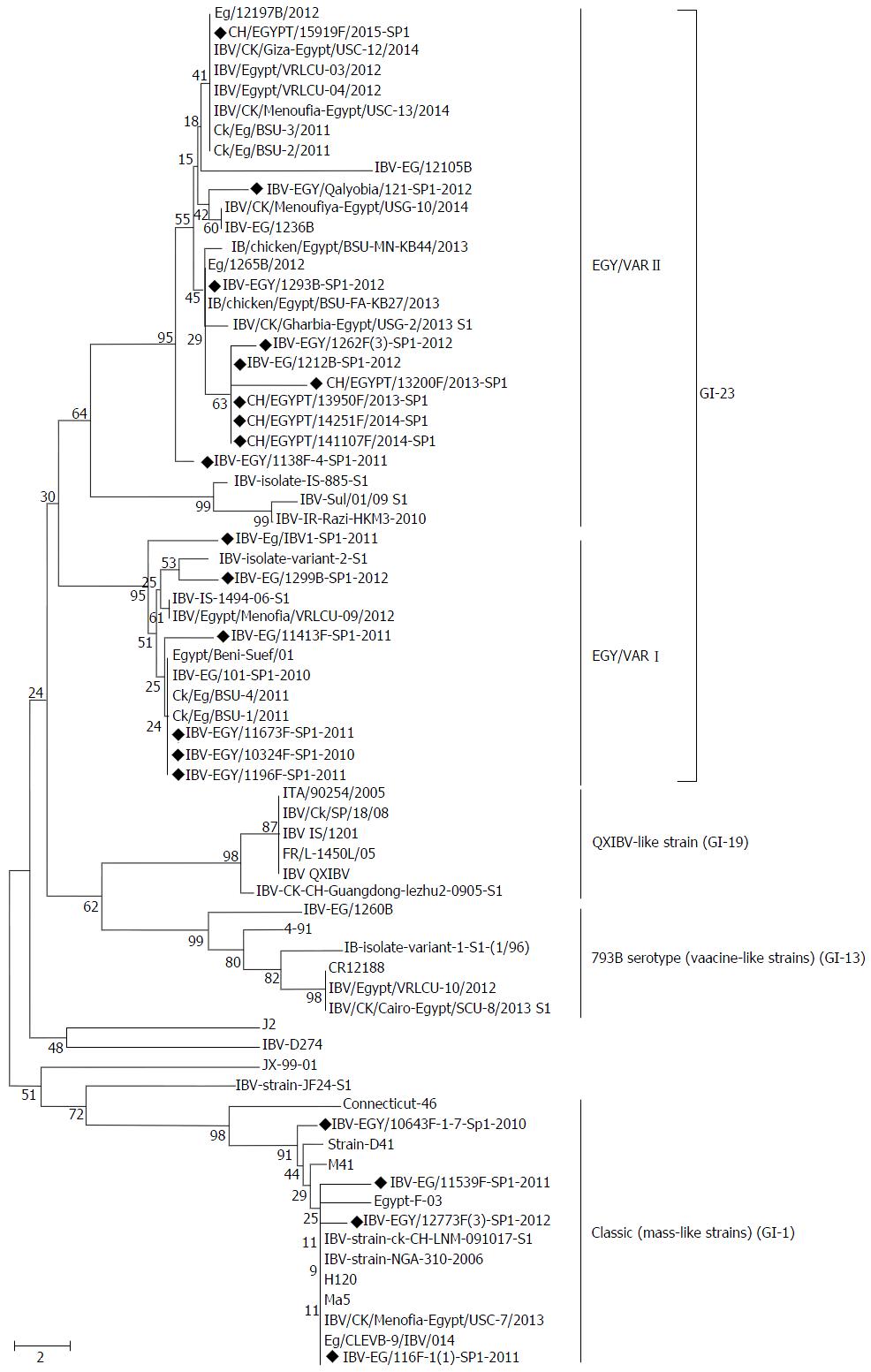
The 400 bp amplified PCR products of the HVR-3 of S1 gene were obtained from the 20 positive samples, then the partial sequencing of HVR-3 and sequence analysis was conducted. Phylogenetic tree was constructed from the amino acid sequences of HVR-3 of the S1 protein (Figure 1). The results indicated that Egyptian IBV viruses in this study were divided into two distinct groups (classic and variant). Sixteen isolates, isolate numbers 5 to 20 (Table 1), were found to be closely related to the variant group and 4 isolates, numbers 1 to 4 (Table 1), were genetically related to the classic genotype of Massachusetts strain.
The sixteen variant isolates were further subdivided into two subgroups: IBV Egy/Var-I and II. Where, virus isolates, numbers from 5-10 (Table 1), were found to be related to Egy/Var-I and they were very close to both the ancestral Egyptian virus (Egypt/Beni-Suef/01, Genbank accession number JX174183.1) and other viruses of Egy/Var-I available in the GenBank, also to IBV-IS-1494-06. They share amino acid identities from 96% to 100% with each other (Table 2). Virus isolates, numbers from 11 to 20, were clustered within the Egy/Var-IIsubgroup (Ck/Eg/BSU-2/2011, Ck/Eg/BSU-3/2011 and IBV/IS/885-00) and other Egyptian related strains in the GenBank (Table 2, Figure 1).
| Strain namenucleotide identity (%) (upper right)amino acid identity (%) (lower left) | Egypt/Beni-Suef/01 | IB-isolate-variant-2-S1 | IBV-IS-1494-06-S1 | IBV-isolate-IS-885-S1 | QXIBV | IBV-H120 | IBV-Ma5 | IBV-M41 | IBV-variant-1-S1-(1/96) | IBV-4-91 | IBV-CR12188 | IBV-D274 | IBV-Eg/11539F-2011 (Classic) | IBV-Eg/IBV1-2011 (EGY-Var-1) | IBV-Eg/1212B-2012 (EGY-Var-2) |
| Egypt/Beni-Suef/01 | 72 | 73 | 70 | 86 | 65 | 65 | 64 | 81 | 68 | 80 | 82 | 68 | 98 | 89 | |
| IB-isolate-variant-2-S1 | 97 | 99 | 92 | 91 | 87 | 87 | 87 | 63 | 90 | 63 | 66 | 84 | 70 | 67 | |
| IBV-IS-1494-06-S1 | 99 | 98 | 94 | 91 | 87 | 87 | 87 | 63 | 90 | 63 | 66 | 84 | 71 | 68 | |
| IBV-isolate-IS-885-S1 | 90 | 88 | 90 | 90 | 86 | 86 | 86 | 63 | 89 | 62 | 65 | 83 | 69 | 69 | |
| QXIBV | 86 | 85 | 86 | 84 | 85 | 85 | 85 | 63 | 92 | 63 | 65 | 82 | 67 | 66 | |
| IBV-H120 | 79 | 79 | 79 | 76 | 76 | 100 | 100 | 61 | 83 | 60 | 65 | 95 | 65 | 64 | |
| IBV-Ma5 | 79 | 79 | 79 | 76 | 76 | 100 | 100 | 61 | 83 | 60 | 65 | 95 | 65 | 64 | |
| IBV-M41 | 79 | 79 | 79 | 76 | 76 | 99 | 99 | 61 | 83 | 60 | 65 | 95 | 65 | 64 | |
| IBV-variant-1-S1 (1/96) | 80 | 80 | 80 | 79 | 85 | 72 | 72 | 71 | 69 | 98 | 82 | 63 | 80 | 81 | |
| IBV-4-91 | 83 | 83 | 83 | 82 | 87 | 74 | 74 | 73 | 95 | 68 | 66 | 80 | 66 | 65 | |
| IBV-CR12188 | 82 | 81 | 82 | 80 | 86 | 72 | 72 | 71 | 96 | 95 | 83 | 62 | 79 | 80 | |
| IBV-D274 | 82 | 82 | 83 | 80 | 83 | 78 | 78 | 77 | 80 | 83 | 83 | 68 | 82 | 81 | |
| IBV-Eg/11539F-2011 (Classic) | 77 | 77 | 77 | 74 | 74 | 98 | 98 | 97 | 71 | 73 | 71 | 76 | 81 | 78 | |
| IBV-Eg/IBV1-2011 (EGY-Var-1) | 97 | 94 | 96 | 88 | 84 | 81 | 81 | 81 | 78 | 81 | 80 | 80 | 79 | 88 | |
| IBV-Eg/1212B-2012 (EGY-Var-2) | 89 | 87 | 89 | 90 | 83 | 80 | 80 | 80 | 81 | 83 | 81 | 82 | 78 | 87 |
Alignment analysis of the S1-HVR3 for both nucleotide and deduced amino acid were performed and compared with the previously published reference and vaccine IBV strains commonly used in the field (H120, Ma5, D274, 4/91, CR88121 and 1/96) as well as original Egyptian viruses Egy/Var-I and Egy/Var-II. One virus represents the consensus of each group was selected for the comparative analysis (Table 2). Classic Egyptian IBV isolates (1 to 4) showed amino acid identities reached up to 98% with the Mass-like strains (H120, Ma5 and M41), while they showed only about 78% amino acid identities with the variant group. In comparison to vaccine strains used in Egypt, the viruses isolated in this study share different amino acid identities: For Egy/Var-I strains, including strain number from 5 to 10 they showed identity from 78% to 81% for strains H120, Ma5, 4/91, CR88, D274 and 1/96 (Table 2). In the meantime, Egy/Var-II strains shared 80% to 83% amino acid with them (Table 2). The strains included in the classic group (1 to 4) showed only about 71% to 76% with 4/91, CR88, D274 and 1/96 serotypes (Table 2).
The pathogenicity of the IBV strains was evaluated by a standard pathogenicity assay using SPF chicken. For each group, the clinical and pathogenicity scores were recorded (Tables 3 and 4). In group 1 and 2 (inoculated with variant strains) sick chicks showed varying degrees of coughing, sneezing, tracheal rales, and watery feces. The severity of the signs increases in group 1 (Egy/Var-II) than group 2 (Egy/Var-I); however, for group 3 (classic strain) only mild respiratory signs were observed. The clinical scores were recorded in Table 4. The mortalities were 50%, 40%, and 10% and the recorded clinical scores were 3, 2 and 1 in the three groups 1, 2 and 3, respectively (Table 4).
| Group | IBV isolates code | Necropsy | |||||||
| Trachea | Kidney | ||||||||
| No Lesion | Slight mucin | Excessive mucin | Mucosal congestion | No Lesion | Swelling | Ureate | Congestion | ||
| 1 | IBV-EG/1212B-2012 | 0 | 2 | 5 | 3 | 0 | 6 | 2 | 2 |
| 2 | IBV-EG/IBV1-2011 | 0 | 5 | 3 | 2 | 0 | 5 | 2 | 3 |
| 3 | IBV-EG/11539F-2011 | 3 | 6 | 0 | 1 | 10 | 0 | 0 | 0 |
| 4 | Negative control | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
Pathogenicity indices were 25, 24 and 8 for the isolates in groups 1 (Egy/Var-II), 2 (Egy/Var-I) and 3 (classic), respectively (Table 4). Based on the lesion in both kidney and trachea, isolates could be classified according to the pathogenicity index to high virulent (Egy/Var-II and Egy/Var-I) and low virulent (classic) (Table 4). The main commonly reported macroscopic lesions included: Congestion with casious plug in the trachea, congested lung, swollen and congested kidneys with ureters distended with ureate. Grossly, the kidney lesions were more severe in Egy/Var-II group than in Egy/Var-I group; however, the lungs were only affected in Egy/Var-II group (Tables 3 and 4). While in the classic group the tracheal lesions were less severe than the other 2 variant groups.
Virus distribution in tissues following virus inoculation of one-day-old SPF-chicks with 3 IBV isolated strains: IBV-Eg/1212B-2012 (Egy/Var-II), IBV-Eg/IBV1-2011 (Egy/Var-I) and IBV-Eg/11539F-2011 (Mass-like) were described (Table 5). The virus was found in the trachea and kidney in groups infected with variant strains (Egy/Var-I, Egy/Var-II). The virus was not detected in the lungs of Egy/Var-I group also from lung and kidney of the classic group. The negative control group had no virus in tissues (Table 5).
| Group | IBV isolates | Phylogenetic group | Virus detection (number of positive/total) | ||
| Trachea | Kidney | Lung | |||
| 1 | IBV-EG/1212B-2012 | Egy Var II | 10/10 | 9/10 | 6/10 |
| 2 | IBV-EG/IBV1-2011 | Egy Var I | 8/10 | 10/10 | 0/10 |
| 3 | IBV-EG/11539F-2011 | Classic (mass-like strain) | 5/10 | 0/10 | 0/10 |
| 4 | Negative control | 0/10 | 0/10 | 0/10 | |
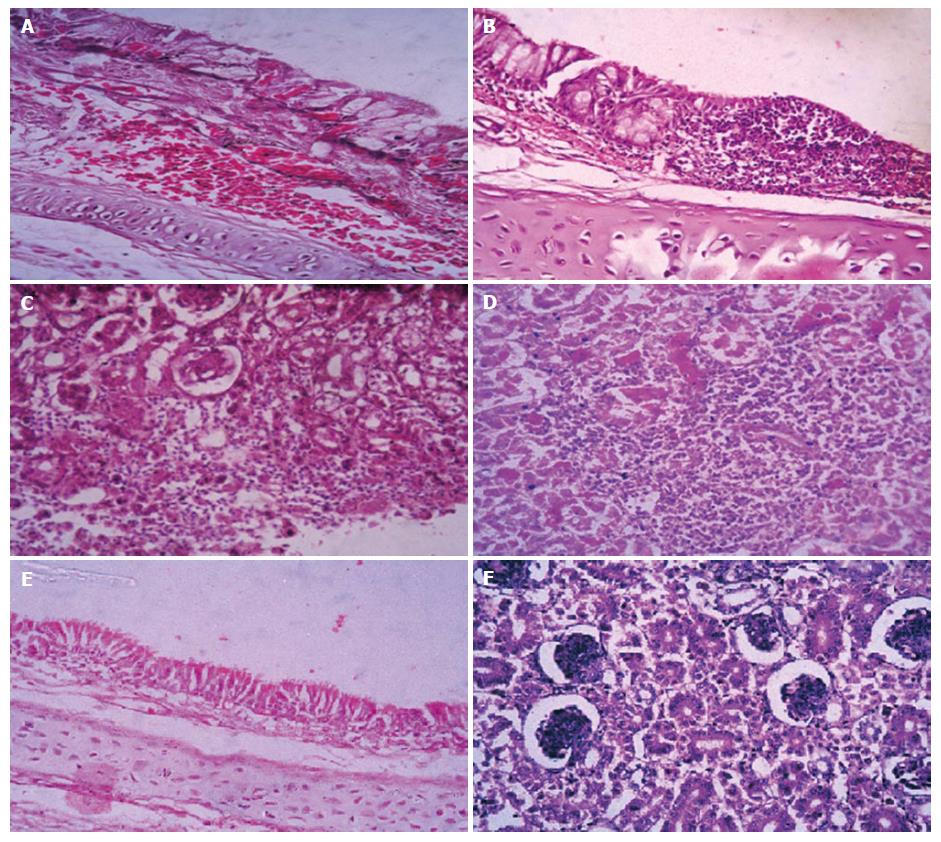
Microscopic examination of sacrificed birds at 14 d Post- infection and freshly dead birds revealed marked loss of cilia, epithelial desquamation, lymphocytic cell infiltration, epithelial hyperplasia and congested trachea in both 2 subgroups of variant viruses (Egy/Var-I, Egy/Var-II) (Figure 2A and B). Kidneys showed severe changes, including hemorrhages, degeneration in renal tubules and hyper cellularity of the renal glomeruli as well as focal infiltration of inflammatory lymphocytes in group 1 (Egy/Var-II) of variant viruses and to lesser extent in group 2 (Egy/Var-I,) (Figure 2C and D). The lesion for the classic group 3 of Eg/11539F-2011 (Mass-like) showed the lowest severity in trachea and kidneys in comparison to variant groups (data not shown).
One of the main problems of IBV is the frequent emergence of several IBV serotypes or antigenic variants due to high rates of S1 gene mutation[2,24]. Therefore, it is important to detect these new emerging viruses and to choose an appropriate vaccine against IBV infection. In the S1 gene, there are three HVRs located within amino acids 38-67 (HVR-1), 91-141 (HVR-2) and 274-387 (HVR-3)[25]. Genotyping of IBV based on S1 gene sequencing, especially the HVRs, is the most reliable way to classify IBV isolates. Usually IBV serotypes have a wide range of genetic variations in the S1 gene ranged from 2% to 25%[6,26]. In Egypt, many strains of live attenuated and inactivated vaccines used to control IBV. However, the outbreaks of the disease have continued to cause severe infections[13].
In this work, 20 IBV isolates from commercial broiler chicken flocks from 12 governorates were genotyped by sequencing of the HVR-3 of the S1 gene. The molecular data indicated that the IBVs isolated in Egypt during the last five years evolved into two groups; variant (GI-23) and classic (GI-1). The strains of the variant group (GI-23) are indigenous and predominate in the Middle East[9], and in Egypt were subdivided into 2 subgroups according to the sequence of the HVR-3[14,16]. The first subgroup of Egy/Var-I, represented by 6 viruses (Table 1), is very close to the original Egyptian strain Egypt/Beni-Suef/01 and to Ck/EG/BSU-1,4,5/2011 isolated in 2011[16]. In this work, viruses of Egy/Var-I subgroup were also found to be closely related to IS/1494/2006 with 96%-97% amino acid identity. The second subgroup Egy/Var-II included 10 viruses and they were mostly related to IS/885/00 strain with 90% amino acid identities (Tables 1 and 2, Figure 1). Accordingly, Egy/Var-II subgroup was shown to be widely spread in Egypt during the last 5 years[15,16]. However, full genome sequencing will provide more accurate information about different subgroups[9]. In the meantime, the 4 isolates in the classic group (Mass-like strain of GI-1) were genetically related to Massachusetts strains and had the same phylogenetic origin. It showed high amino acid similarity (98%) to H120 and Ma5 vaccine strains with 95% nucleotide identities (Table 2, Figure 1). The four isolates of Mass-like strain were obtained from vaccinated farms with the same strain. Furthermore, previous studies have confirmed that live IBV vaccines persist in chicks for many weeks after administration with virus isolation[27-29].
Phylogenetic analysis revealed that the variant IBV isolates (Egy/Var-I and Egy/Var-II) had a distant relation to vaccine strains commonly used in Egypt, including Ma5, H120, M41, 4/91, CR88, D274 and 1/96 (Table 2, Figure 1). The new IBV variants frequently emerge as a result of a few changes in the amino acid structure along the S1 protein[6]. These changes may be due to immunological pressure caused by the wide use of live vaccines of different strain types along with field virus infection. This can lead to genetic alterations and recombinations allowing new field strains to evolve[3].
In this work, the three IBV strains (IBV-Eg/1212B-2012 of Egy/Var-II, IBV-EG/IBV1-2011 of Egy/Var-I and IBV-EG/11539F-2011 of classic Mass-like genotype) were able to induce respiratory signs post-inoculation with clinical scores of 3, 2 and 1; respectively (Table 4). In addition, respiratory and renal lesions were recorded (Tables 3 and 4). The virulence of the three strains (Egy/Var-II, Egy/Var-I and classic Mass-like) was assessed in one-day-old SPF chicks for comparison. The three IBV strains were able to produce 50%, 40% and 10% deaths; respectively. It is well known that the most severe clinical symptoms of IBV appear in very young chicks and the severity decreased in older chickens[30]. This fact explains the high mortality rate observed in infected chicks with IBV-Eg/1212B-2012 (50%) and IBV/EG/IBV1-2011 (40%) (Table 4). These findings matched with Wang et al[31], who reported mortality rates ranged from 10% to 60% in experimentally infected chicks with QX strain of IBV. Furthermore, these findings agreed with Ignjatović et al[32], who found that strains of IBV differed in their virulence for the respiratory tract, kidney or oviduct. The majority of IBV strains, including those of the Mass-like serotype produce prominent respiratory disease[33]. The possibility of re-isolations of H120 vaccine strain cannot be excluded. Although vaccine strains induced lesions in chickens, but the mortality did not exceed 10%[31].
Trachea, lung and kidney were collected from infected birds after intra-nasal inoculation and the virus tropism was detected by rRT-PCR, the results were shown in Table 5. IBV nucleic acids were detected more frequently in the tracheal tissues than in the lungs, and kidney (Table 5). Terregino et al[34] isolated IBV from kidney, trachea, ovary, and oviduct following infection with the QX-IBV strain.
The presence of acute interstitial nephritis and gross renal ureates deposition and histological lesions in the experimental chicks at day 14 post infection indicated that IBV-Eg/1212B-2012 and IBV-EG/IBV1-2011 were nephrogenic viruses. Severe renal hemorrhages were observed grossly and histopathologically in dead birds for the two viruses denoting that the deaths might be resulted from acute renal failure rather than the respiratory distress (Figure 2). Similarly, variants of IBV were reported as nephrogenic strains in Egypt: D274, D3896, D1559, Egypt/Beni-Suef/01, 720/99 Israel, 4/91, IS/1494[13,29]. The microscopic picture of the renal tubules matched with the general findings recorded with nephrogenic IBV strains[35].
Regarding microscopic lesions in trachea associated with IBV infection in day old SPF chicks and examined at 14 d pi, the findings appeared similar to those previously recorded by Cavanagh et al[33], including: Deciliation, degenerative changes and edema of the tracheal mucosa, irregular loss of epithelium, desquamation of the epithelium in the tracheal lumen, goblet cell activation and focal aggregation to diffuse massive lymphocytic infiltration (Figure 2).
In conclusion, our results provide evidence of evolving the recent Egyptian IBV strains and showed two groups of variants are co-circulating in Egypt with high mortality in SPF chicks. The distinctive dissimilarity between these variants and the widely used IBV vaccine reveal that the antigenic drift is likely to occur under the long-term immune pressure. Further epidemiological surveillance studies are needed in order to explain the mechanism of emergence of variants and their biological properties, including pathogenicity and vaccine trails to help in disease control.
We would like to thank all the staff of the Gene analysis unit and Virology Unit, Reference laboratory for veterinary quality control on poultry production, animal health research institute, Egypt for their help throughout this work.
Infectious bronchitis virus (IBV), a major pathogen of commercial poultry flocks, circulates in the form of different genotypes. Three IBV genotypes were isolated from broiler chickens showing severe respiratory and renal lesions in vaccinated and non-vaccinated flocks.
Different research articles discuss the current situation of IBV depending on the sequence of either hypervariable region (HVR)-1, 2 or HVR-3 of the S1 gene. While other characterize IBV depending on the full S1 gene sequence.
In this study three genotypes were characterized depending on the sequence of the HVR 3 of the S1 gene. The virulence of the three genotypes (Egy/Var-II, Egy/Var-I and classic) was assessed in one-day-old SPF chicks for comparison.
This work includes detection of the currently circulating strains of IBV. The high genetic variation between these variants and the widely used IBV vaccine reveal that the antigenic drift is likely to occur under the long-term immune pressure. Also, this work focus on the high mortality caused by the variant strains of IBV in the poultry flocks.
HVR-3, a part of the S1 gene used intensively to characterize IBV genotype.
In this study, Ali Zanaty et al isolated 20 IBV isolates from 20 chicken flocks in Egypt and characterized the genotypes of the isolates to be two groups (classic and variant, and the latter was further classified into two subgroups) and tested the pathogenicity of three selected isolates to SPF chicks. The work contributed to the epidemiology and biology of IBV. The experiments were well designed and the manuscript is well organized.
Manuscript source: Invited manuscript
Specialty type: Virology
Country of origin: Egypt
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen YD, Ferreira HL, Qiu HJ S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Wang CH, Huang YC. Relationship between serotypes and genotypes based on the hypervariable region of the S1 gene of infectious bronchitis virus. Arch Virol. 2000;145:291-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Lee CW, Hilt DA, Jackwood MW. Typing of field isolates of infectious bronchitis virus based on the sequence of the hypervariable region in the S1 gene. J Vet Diagn Invest. 2003;15:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Liu S, Kong X. A new genotype of nephropathogenic infectious bronchitis virus circulating in vaccinated and non-vaccinated flocks in China. Avian Pathol. 2004;33:321-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Gelb J, Wolff JB, Moran CA. Variant serotypes of infectious bronchitis virus isolated from commercial layer and broiler chickens. Avian Dis. 1991;35:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Carstens E. Report from the 40th meeting of the Executive Committee of the International Committee of Taxonomy of Viruses, 2009: 1571–1574. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Cavanagh D, Davis PJ, Cook JK, Li D, Kant A, Koch G. Location of the amino acid differences in the S1 spike glycoprotein subunit of closely related serotypes of infectious bronchitis virus. Avian Pathol. 1992;21:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Casais R, Dove B, Cavanagh D, Britton P. Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. J Virol. 2003;77:9084-9089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 225] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Boursnell ME, Brown TD, Foulds IJ, Green PF, Tomley FM, Binns MM. Completion of the sequence of the genome of the coronavirus avian infectious bronchitis virus. J Gen Virol. 1987;68:57-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 329] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Valastro V. Holmes EC. Britton P. Fusaro A. Jackwood MW, Cattoli G, Monne I. S1 gene-based phylogeny of infectious bronchitis virus: An attempt to harmonize virus classification. Infect Genet Evol. 2016;39:349-364. |
| 10. | Sjaak de Wit JJ, Cook JK, van der Heijden HM. Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathol. 2011;40:223-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Alvarado IR, Villegas P, Mossos N, Jackwood MW. Molecular characterization of avian infectious bronchitis virus strains isolated in Colombia during 2003. Avian Dis. 2005;49:494-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32:567-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 238] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Abd El Rahman S, Hoffmann M, Lueschow D, Eladl A, Hafez HM. Isolation and characterization of new variant strains of infectious bronchitis virus in Northern Egypt. Adv Anim Vet Sci. 2015;3:362-371. |
| 14. | Ganapathy K, Ball C, Forrester A. Genotypes of infectious bronchitis viruses circulating in the Middle East between 2009 and 2014. Virus Res. 2015;210:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Sultan H, Abdel-Razik AG, Shehata AA, Ibrahim M, Talaat S, Abo-Elkhair M, Bazid AE, Moharam IM and Vahlenkamp T. Characterization of Infectious Bronchitis Viruses Circulating in Egyptian chickens during 2012 and 2013. J Vet Sci Med Diagn. 2015;4:5. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Abdel-Moneim AS, Afifi MA, El-Kady MF. Emergence of a novel genotype of avian infectious bronchitis virus in Egypt. Arch Virol. 2012;157:2453-2457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Callison SA, Jackwood MW, Hilt DA. Molecular characterization of infectious bronchitis virus isolates foreign to the United States and comparison with United States isolates. Avian Dis. 2001;45:492-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Momayez R, Pourbakhsh SA, Khodashenas M, Banani M. Isolation and Identification of Infectious Bronchitis Virus from Commercial Chickens. Arch Razi Ins. 2002;53:1. |
| 19. | Adzhar A, Gough RE, Haydon D, Shaw K, Britton P, Cavanagh D. Molecular analysis of the 793/B serotype of infectious bronchitis virus in Great Britain. Avian Pathol. 1997;26:625-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30102] [Cited by in RCA: 27948] [Article Influence: 2329.0] [Reference Citation Analysis (0)] |
| 21. | Ziegler AF, Ladman BS, Dunn PA, Schneider A, Davison S, Miller PG, Lu H, Weinstock D, Salem M, Eckroade RJ. Nephropathogenic infectious bronchitis in Pennsylvania chickens 1997-2000. Avian Dis. 2002;46:847-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Purcell DA, Tham VL, Surman PG. The histopathology of infectious bronchitis in fowls infected with a nephrotropic “T” strain of virus. Aust Vet J. 1976;52:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Bancroft JD, Stevens A. Theory and practices of histologic techniques 2nd Eds. Churchill, Living Stone Edingburgh, London Melborne and New York, 1977. . |
| 24. | Li M, Mo ML, Huang BC, Fan WS, Wei ZJ, Wei TC, Li KR, Wei P. Continuous evolution of avian infectious bronchitis virus resulting in different variants co-circulating in Southern China. Arch Virol. 2013;158:1783-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Moore KM, Jackwood MW, Hilt DA. Identification of amino acids involved in a serotype and neutralization specific epitope within the s1 subunit of avian infectious bronchitis virus. Arch Virol. 1997;142:2249-2256. [PubMed] |
| 26. | Kingham BF, Keeler CL, Nix WA, Ladman BS, Gelb J. Identification of avian infectious bronchitis virus by direct automated cycle sequencing of the S-1 gene. Avian Dis. 2000;44:325-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Alvarado IR, Villegas P, El-Attrache J, Jackwood MW. Detection of Massachusetts and Arkansas serotypes of infectious bronchitis virus in broilers. Avian Dis. 2006;50:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Worthington KJ, Currie RJ, Jones RC. A reverse transcriptase-polymerase chain reaction survey of infectious bronchitis virus genotypes in Western Europe from 2002 to 2006. Avian Pathol. 2008;37:247-257. [PubMed] |
| 29. | Abdel-Moneim AS, El-Kady MF, Ladman BS, Gelb J. S1 gene sequence analysis of a nephropathogenic strain of avian infectious bronchitis virus in Egypt. Virol J. 2006;3:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Dhinakar Raj G, Jones RC. Protectotypic differentiation of avian infectious bronchitis viruses using an in vitro challenge model. Vet Microbiol. 1996;53:239-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Wang CH, Hsieh MC, Chang PC. Isolation, pathogenicity, and H120 protection efficacy of infectious bronchitis viruses isolated in Taiwan. Avian Dis. 1996;40:620-625. [PubMed] |
| 32. | Ignjatović J, Sapats S. Avian infectious bronchitis virus. Rev Sci Tech. 2000;19:493-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Cavanagh D, Naqi SA. Infectious bronchitis. In B.W. Calnek, H.J. Barnes, C.W. Bearol, L.R. Mc Daugald, and Y.M. Saif (eds). Disease of Poultry 10th Ed. Lawa University Press: Ames, 1997: 511-526. . |
| 34. | Terregino C, Toffan A, Beato MS, De Nardi R, Vascellari M, Meini A, Ortali G, Mancin M, Capua I. Pathogenicity of a QX strain of infectious bronchitis virus in specific pathogen free and commercial broiler chickens, and evaluation of protection induced by a vaccination programme based on the Ma5 and 4/91 serotypes. Avian Pathol. 2008;37:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Albassam MA, Winterfield RW, Thacker HL. Comparison of the nephropathogenicity of four strains of infectious bronchitis virus. Avian Dis. 1986;30:468-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 1.2] [Reference Citation Analysis (0)] |