Published online Nov 12, 2015. doi: 10.5501/wjv.v4.i4.372
Peer-review started: May 9, 2015
First decision: June 3, 2015
Revised: September 9, 2015
Accepted: September 29, 2015
Article in press: September 30, 2015
Published online: November 12, 2015
Processing time: 188 Days and 9.4 Hours
AIM: To characterize the prevalence of rotavirus (RV) and adenovirus (AdV) infections in immunocompromised patients with acute gastroenteritis.
METHODS: The presence of RV and AdV (serotypes 40 and 41) was evaluated in 509 stool samples obtained between January 2009 and December 2010 from 200 immunocompromised patients (83 females and 117 males; median age 21 years old, range 0-72. The diagnosis of infection was performed as a routine procedure and the presence of RV and AdV (serotypes 40 and 41) was determined by immunochromatography using the RIDA® Quick Rota-Adeno-Kombi kit (r-Biopharm, Darmstadt, Germany). The data analysis and description of seasonal frequencies were performed using computer software IBM® SPSS® (Statistical Package for Social Sciences) Statistics version 20.0 for Mac. The frequencies of infection were compared into different age and gender groups by χ2 test.
RESULTS: The study revealed 12.4% AdV positive samples and 0.8% RV positive samples, which correspond to a prevalence of 6.5% and 1.5%, respectively. AdV was more frequent between October 2009 and April 2010, while RV was identified in April 2010 and July 2010. The stool analysis revealed that from the 509 samples, 63 (12.4%) were positive for AdV and 4 (0.8%) positive for RV, which by resuming the information of each patient, lead to an overall prevalence of AdV and RV of 6.5% (13/200 patients) and 1.5% (3/200 patients), respectively. The stratification of the analysis regarding age groups showed a tendency to an increased prevalence of infection in paediatric patients between 0-10 years old. Considering the seasonal distribution of these infections, our study revealed that AdV infection was more frequent between October 2009 and April 2010, while RV infection was characterized by two distinct peaks (April 2010 and July 2010).
CONCLUSION: The overall prevalence of AdV and RV infection in immunocompromised patients with acute gastroenteritis was 8% and AdV was the most prevalent agent.
Core tip: Acute gastroenteritis has been associated with significant rates of morbidity and mortality among immunocompromised patients. Rotavirus (RV) and adenovirus (AdV) are described as common agents of viral gastroenteritis causing acute diarrhoea. This is the first study in Portugal to characterize the prevalence and seasonal features of RV and AdV infections in immunocompromised patients with acute gastroenteritis. Results revealed 12.4% AdV positive samples and 0.8% RV positive samples, which correspond to a prevalence of 6.5% and 1.5%, respectively. Our results also demonstrate the importance of to add more screening methods for other emergent enteric viruses, in order to avoid the morbidity and mortality of the immunocompromised patients.
- Citation: Ribeiro J, Ferreira D, Arrabalde C, Almeida S, Baldaque I, Sousa H. Prevalence of adenovirus and rotavirus infection in immunocompromised patients with acute gastroenteritis in Portugal. World J Virology 2015; 4(4): 372-376
- URL: https://www.wjgnet.com/2220-3249/full/v4/i4/372.htm
- DOI: https://dx.doi.org/10.5501/wjv.v4.i4.372
Acute gastroenteritis, one of the main causes of morbidity and mortality in the world, is a consequence of microbial infections, which in industrialized countries are mainly caused by viruses, such as rotavirus (RV), enteric adenovirus (AdV), astrovirus and human calicivirus[1-3].
RV is the most common cause of severe diarrhoea in children under 5 years of age, adults in close contact with infected children, hospitalised patients and the elderly[3,4]. Data from United States reveal that RV infection is responsible for approximately 3 million cases of acute diarrhoea in children each year[5]. RV infection is associated with high rates of morbidity throughout the world and high rates of mortality in developing countries, where gastroenteritis caused by RV account for more than 800000 deaths per year due to poor nutrition and lack of health care[6]. In the majority of countries, RV infections occur dispersed throughout the year, nevertheless in temperate climates is characterized by a peak during the winter months[1-3].
AdV, especially serotypes 40 and 41 (enteric AdV), are frequently related with high morbidity and mortality in immunocompromised patients with the most susceptible groups to be children and patients submitted to transplantation[7-9]. Enteric AdV have been related with acute diarrhoea in variable frequency (ranging from 1.4% to 10%), depending on the geographic location and type of patients[10,11]. AdV is known for its large distribution worldwide and the majority of the studies refer it to be equally distributed during all the seasons of the year[8,9].
Immunocompromised patients constitute an important group for prevention of gastrointestinal infections[7,12,13]. In fact, the incidence of infection-related post-transplant diarrhoea has been reported to be up to 40%, with viruses being the most common pathogens[2,3,12]. Considering the importance of gastroenteritis prevention in immunocompromised individuals, the diagnosis of AdV and RV at early stages of the disease is extremely important, in order to reduce morbidity and mortality.
The aim of this study was to characterize the prevalence of RV and AdV infection in immunocompromised patients with acute gastroenteritis from the North region of Portugal treated at the Portuguese Institute of Oncology of Porto.
This study was performed as a cross-sectorial retrospective hospital-based case study with 509 stool samples obtained between January 2009 and December 2010 from immunocompromised patients diagnosed with acute diarrhoea at Portuguese Institute of Oncology of Porto (IPO Porto). Samples were obtained from 200 immunocompromised patients with different haematological malignancies (median age 21 years old, range 0-72): 83 female (median age 15 years old, range 0-65) and 117 male (median age 39 years old, range 0-72).
The diagnosis of infection was performed as a routine procedure at the Virology Service of IPO Porto. The stool specimens were tested as soon as possible after collection and the presence of RV and AdV (serotypes 40 and 41) was determined by immunochromatography using the RIDA® Quick Rota-Adeno-Kombi kit (r-Biopharm, Darmstadt, Germany) according to manufacturer instructions. The faecal samples were diluted in the dilution buffer supplied with the kit. This is a ready-to-use test based on a nitrocellulose membrane sensitized with antibodies directed against RV and AdV (test lines).
Data analysis and description of seasonal frequencies were performed using computer software IBM® SPSS® (Statistical Package for Social Sciences) Statistics version 20.0 for Mac. The frequencies of infection were compared into different age and gender groups by χ2 test.
In total, 509 stool samples were tested and it was possible to detect 63 (12.4%) positive samples for AdV and 4 (0.8%) positive samples for RV (Table 1). Comparing the number of cases during the period between January 2009 and December 2010, it was possible to observe a substantial increase in 2010, however the infection rates were similar.
| AdV positive | RV positive | Negative | |
| Total (n = 509) | 63 (12.4) | 4 (0.8) | 442 (86.8) |
| Year 2009 (n = 189) | 24 (12.7) | - | 165 (87.3) |
| Year 2010 (n = 320) | 39 (12.2) | 4 (1.3) | 277 (86.5) |
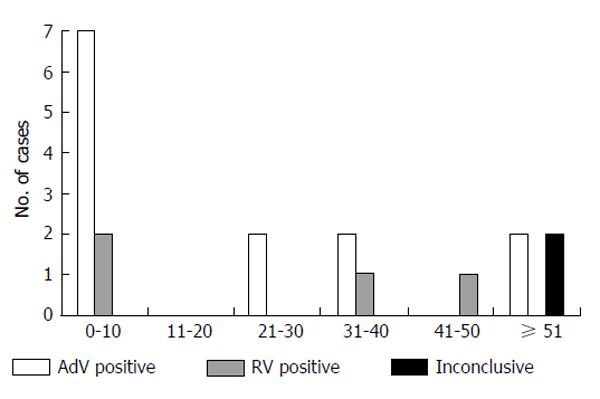
Considering only patients-related data, where we have combined the results of all samples obtained from each individual, the overall frequency of AdV and RV infection was 6.5% (13/200 patients) and 1.5% (3/200 patients), respectively. The prevalence of infection was characterized with stratification of individuals according to age groups and genre and the results showed a tendency to an increased prevalence of infection in paediatric patients between 0-10 years old (Figure 1 and Table 2).
| AdV | RV | |||||
| Positive | Negative | IC | Positive | Negative | IC | |
| Total (n = 200) | 13 (6.5) | 185 (92.5) | 2 (1.0) | 3 (1.5) | 196 (98.0) | 1 (0.5) |
| Age group | ||||||
| 0-10 (n = 60) | 7 (11.7) | 53 (88.3) | - | 2 (3.3) | 58 (96.7) | - |
| 11-20 (n = 21) | - | 21 (100.0) | - | - | 21 (100.0) | - |
| 21-30 (n = 19) | 2 (10.5) | 17 (89.5) | - | - | 19 (100.0) | - |
| 31-40 (n = 19) | 2 (10.5) | 17 (89.5) | - | 1 (5.3) | 18 (94.7) | - |
| 41-50 (n = 19) | - | 18 (94.7) | 1 (5.3) | - | 19 (100.0) | - |
| ≥ 51 (n = 62) | 2 (3.2) | 59 (95.2) | 1 (1.6) | - | 61 (98.4) | 1 (1.6) |
| Genre | ||||||
| Male (n = 117) | 9 (7.7) | 107 (91.4) | 1 (0.9) | 1 (0.9) | 115 (98.2) | 1 (0.9) |
| Female (n = 83) | 4 (4.8) | 78 (94.0) | 1 (1.2) | 2 (2.4) | 81 (97.6) | - |
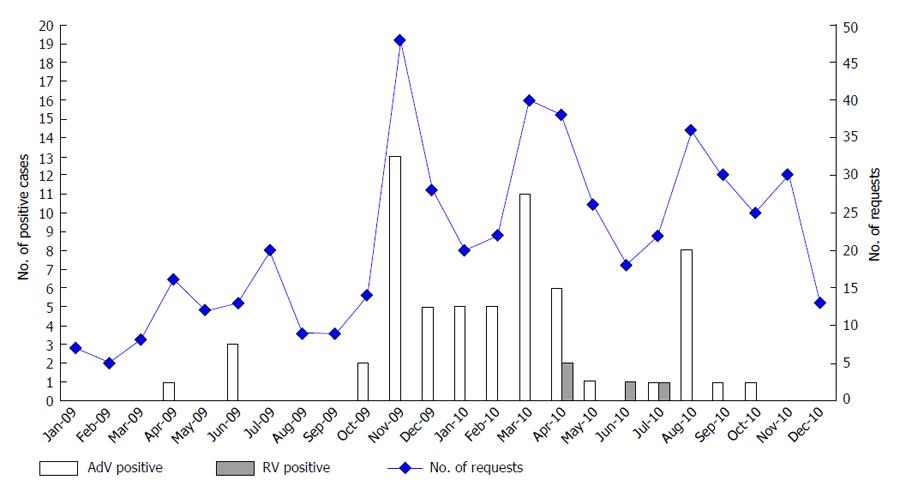
Considering the seasonal distribution of these infections (Figure 2), our study revealed that AdV infection was more frequent between October 2009 and April 2010, while RV infection was characterized by two distinct peaks (April 2010 and July 2010).
Immunosuppression treatments lead patients to be more susceptible to opportunistic infections, either bacterial or viral. Many studies have shown that RV and AdV are the most frequent pathogenic virus during acute diarrhoea in immunocompromised patients[3,7,9,13]. The overall distribution worldwide has been described in literature[5,14], although little is known about the epidemiology of these viruses in Portugal, and therefore we aimed to add epidemiological information of the prevalence of these infections in immunocompromised patients with haematological diseases treated at IPO Porto.
Firstly, it is important to refer that our results should be discussed considering the limitations of the diagnostic test. This test is used to screen only infections by RV and enteric AdV, which could limit the identification of positive cases since the range of viruses involved in acute gastroenteritis is larger. Nevertheless, the frequencies that we have obtained were considerably low when compared with the number samples. These results might be explained by the fact that other virus such as Norovirus, or even bacteria, could be the cause of the acute gastroenteritis[15-18]. Moreover, acute diarrhoea is sometimes overestimated since chemotherapeutic agents and radiotherapy may also promote it[19]. In addition, there are no reports of these viruses in Portuguese immunocompromised patients, thus the fact that results are distinct from other studies remain to be further clarified[20].
Recent data indicate that enteric AdV infection in immunocompromised patients remains constant along the year[3,8], and the RV infection is more prevalent during the winter and beginning of the spring[2-4]. These features were not observed in our population, and in fact, our study showed that enteric AdV was more prevalent between November 2009 and April 2010, with a seasonal peak in August 2010. Between November 2010 and December 2010 there were no positive cases, but the number of suspected samples was also lower than in the same period the year before.
In contrast with literature, RV incidence was very low, with only four positive cases to be identified, and therefore no seasonal prevalence was estimated[2,3]. However, it is possible to observe that positive cases were identified in April and June/July months. These data are in agreement with previous studies performed in Portugal, which describe a higher prevalence of RV infection in paediatric population[21,22]. Rodrigues et al[22] refers that the prevalence of RV presents clear differences between years and that in 2010 there were two distinct peaks with significant RV activity occurring much later and lasting into July.
To the best of our knowledge, this is the first study to characterize the prevalence of RV and enteric AdV in immunocompromised patients from Portugal. Despite the lower incidence of both viral infections, this study emphasizes the importance of vigilance and prevention of viral infections in the gastrointestinal tract. Moreover, as the results reveal a lower incidence of RV and enteric AdV, it is extremely important to add more screening methods for other emergent enteric viruses, in order to avoid the morbidity and mortality of the immunocompromised patients.
Authors are grateful to the Portuguese League Against Cancer (Liga Portuguesa Contra o Cancro - Núcleo Regional do Norte) for the grant of the first author.
Acute gastroenteritis is one of the main causes of morbidity and mortality in the world, especially for immunocompromised patients. Immunosuppression treatments lead patients to be more susceptible to opportunistic infections, either bacterial or viral. Many studies have shown that rotavirus (RV) and adenovirus (AdV) are the most frequent pathogenic virus during acute diarrhoea in immunocompromised patients.
Characterization of RV and AdV prevalence and seasonal distribution in immunocompromised patients with acute gastroenteritis.
The overall prevalence of AdV and RV infection in immunocompromised patients with acute gastroenteritis was 8%. AdV was the most prevalent with 6.5% (13/200 patients) followed by RV with a prevalence of 1.5% (3/200 patients). Results revealed a lower prevalence of RV and enteric AdV than expected.
The lower incidence of RV and enteric AdV observed in the authors’ study pointed that it is extremely important to add more screening methods for other emergent enteric viruses, in order to avoid the morbidity and mortality of the immunocompromised patients.
This study provides the first update on the prevalence of RV and AdV infection as agents of acute gastroenteritis in immunocompromised patients.
This is an interesting study on the cause of diarrhea by viral agents. The study is well-designed and the manuscript is well-written.
P- Reviewer: Kamal SA, Mattner J S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Wilhelmi I, Roman E, Sánchez-Fauquier A. Viruses causing gastroenteritis. Clin Microbiol Infect. 2003;9:247-262. [PubMed] |
| 2. | Carraturo A, Catalani V, Tega L. Microbiological and epidemiological aspects of rotavirus and enteric adenovirus infections in hospitalized children in Italy. New Microbiol. 2008;31:329-336. [PubMed] |
| 3. | Akan H, Izbırak G, Gürol Y, Sarıkaya S, Gündüz TS, Yılmaz G, Hayran O, Vitrinel A. Rotavirus and adenovirus frequency among patients with acute gastroenteritis and their relationship to clinical parameters: a retrospective study in Turkey. Asia Pac Fam Med. 2009;8:8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Podkolzin AT, Fenske EB, Abramycheva NY, Shipulin GA, Sagalova OI, Mazepa VN, Ivanova GN, Semena AV, Tagirova ZG, Alekseeva MN. Hospital-based surveillance of rotavirus and other viral agents of diarrhea in children and adults in Russia, 2005-2007. J Infect Dis. 2009;200 Suppl 1:S228-S233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Elhag WI, Saeed HA, Omer el FE, Ali AS. Prevalence of rotavirus and adenovirus associated with diarrhea among displaced communities in Khartoum, Sudan. BMC Infect Dis. 2013;13:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2395] [Cited by in RCA: 2571] [Article Influence: 214.3] [Reference Citation Analysis (0)] |
| 7. | Walls T, Shankar AG, Shingadia D. Adenovirus: an increasingly important pathogen in paediatric bone marrow transplant patients. Lancet Infect Dis. 2003;3:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Ison MG. Adenovirus infections in transplant recipients. Clin Infect Dis. 2006;43:331-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 247] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 9. | Echavarría M. Adenoviruses in immunocompromised hosts. Clin Microbiol Rev. 2008;21:704-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 338] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 10. | Ramani S, Kang G. Viruses causing childhood diarrhoea in the developing world. Curr Opin Infect Dis. 2009;22:477-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Tran A, Talmud D, Lejeune B, Jovenin N, Renois F, Payan C, Leveque N, Andreoletti L. Prevalence of rotavirus, adenovirus, norovirus, and astrovirus infections and coinfections among hospitalized children in northern France. J Clin Microbiol. 2010;48:1943-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Liakopoulou E, Mutton K, Carrington D, Robinson S, Steward CG, Goulden NJ, Cornish JM, Marks DI. Rotavirus as a significant cause of prolonged diarrhoeal illness and morbidity following allogeneic bone marrow transplantation. Bone Marrow Transplant. 2005;36:691-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Aggarwal V, Williams MD, Beath SV. Gastrointestinal problems in the immunosuppressed patient. Arch Dis Child. 1998;78:5-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Al-Thani A, Baris M, Al-Lawati N, Al-Dhahry S. Characterising the aetiology of severe acute gastroenteritis among patients visiting a hospital in Qatar using real-time polymerase chain reaction. BMC Infect Dis. 2013;13:329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Karst SM. Pathogenesis of noroviruses, emerging RNA viruses. Viruses. 2010;2:748-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Weber DJ, Rutala WA, Miller MB, Huslage K, Sickbert-Bennett E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control. 2010;38:S25-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 510] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 17. | Man SM. The clinical importance of emerging Campylobacter species. Nat Rev Gastroenterol Hepatol. 2011;8:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 313] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 18. | Rackoff LA, Bok K, Green KY, Kapikian AZ. Epidemiology and evolution of rotaviruses and noroviruses from an archival WHO Global Study in Children (1976-79) with implications for vaccine design. PLoS One. 2013;8:e59394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Donaldson SS, Lenon RA. Alterations of nutritional status: impact of chemotherapy and radiation therapy. Cancer. 1979;43:2036-2052. [PubMed] |
| 20. | Fischer Walker CL, Perin J, Aryee MJ, Boschi-Pinto C, Black RE. Diarrhea incidence in low- and middle-income countries in 1990 and 2010: a systematic review. BMC Public Health. 2012;12:220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 302] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 21. | Rodrigues F, Iturriza M, Gray J, Januário L, Lemos L. Epidemiology of rotavirus in Portugal: G9 as a major cause of diarrhoea in non-hospitalised children. J Clin Virol. 2007;40:214-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Rodrigues F, Iturriza-Gómara M, Marlow R, Gray J, Nawaz S, Januário L, Finn A. The evolving epidemiology of rotavirus gastroenteritis in central Portugal with modest vaccine coverage. J Clin Virol. 2013;56:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |