Published online Aug 12, 2015. doi: 10.5501/wjv.v4.i3.178
Peer-review started: April 28, 2015
First decision: June 18, 2015
Revised: June 25, 2015
Accepted: July 21, 2015
Article in press: July 23, 2015
Published online: August 12, 2015
Processing time: 109 Days and 12.8 Hours
In 2009, several groups reported that interleukin-28B (IL28B) genotypes are associated with the response to peginterferon plus ribavirin therapy for chronic hepatitis C virus (HCV) infection in a genome-wide association study, although the mechanism of this association is not yet well understood. However, in recent years, tremendous progress has been made in the treatment of HCV infection. In Japan, some patients infected with HCV have the IL28B major genotype, which may indicate a favorable response to interferon-including regimens; however, certain patients within this group are also interferon-intolerant or ineligible. In Japan, interferon-free 24-wk regimens of asunaprevir and daclatasvir are now available for HCV genotype 1b-infected patients who are interferon-intolerant or ineligible or previous treatment null-responders. The treatment response to interferon-free regimens appears better, regardless of IL28B genotype. Maybe other interferon-free regimens will widely be available soon. In conclusion, although some HCV-infected individuals have IL28B favorable alleles, importance of IL28B will be reduced with availability of oral interferon free regimen.
Core tip: Genome-wide association studies have revealed that interleukin-28B (IL28B) genotypes are associated with the response to interferon therapy for chronic hepatitis C. The mechanism of this association is not yet clear. Although many hepatitis C virus (HCV)-infected individuals have IL28B favorable alleles, in the near future, HCV-infected patients in Japan may be treated with interferon-free regimens, which avoid the adverse events caused by interferon plus ribavirin therapy.
- Citation: Kanda T, Nakamoto S, Yokosuka O. Is the use of IL28B genotype justified in the era of interferon-free treatments for hepatitis C? World J Virology 2015; 4(3): 178-184
- URL: https://www.wjgnet.com/2220-3249/full/v4/i3/178.htm
- DOI: https://dx.doi.org/10.5501/wjv.v4.i3.178
Chronic hepatitis C virus (HCV) infection is a major cause of end-stage liver diseases and hepatocellular carcinoma (HCC) in Japan and the United States[1-4]. Chronic hepatitis C is an important health problem worldwide[5]. The eradication of HCV by interferon-including treatment could lead to the following benefits[6]: (1) fibrotic regression[7-9]; (2) reduction of HCC occurrence and recurrence[10-12]; (3) reduction of other complications, including liver failure, liver-related death[13,14] and liver-unrelated death[15]; and (4) improved quality of life[15]. A sustained virologic response (SVR), which is defined as HCV RNA negativity 24 wk after completion of antiviral therapy, could have beneficial effects in HCV-infected patients. In the era of direct-acting antivirals (DAA) against HCV, regimens including interferon remain important treatments for HCV eradication[5,16-33], although interferon-free regimens should be available worldwide soon[34]. In this review, we focused the distribution of interleukin-28B (IL28B) status in Japanese patients currently infected with HCV, and their treatment.
In 2009, several groups reported that a genetic polymorphism near the IL28B gene, which encodes interferon-lambda-3 (IL28B genotypes), was associated with the response to peginterferon plus ribavirin therapy for chronic hepatitis C in a genome-wide association study[35-37]. The IL28B minor genotype plays a crucial role in interferon resistance[38]. The host genetic polymorphism may be useful for predicting drug response[37,39]. IL28B major or minor genotype, respectively, could predict better or poor response to interferon therapy in patients infected with HCV. An association between inosine triphosphatase (ITPA) genetic variants and treatment-induced anemia has been reported in HCV-infected patients treated with peginterferon plus ribavirin[40-42]. ITPA major genotype could predict profound anemia induced by peginterferon plus ribavirin treatment in HCV-infected patients. A genetic polymorphism of interferon-lambda-4 has also been associated with the treatment response to interferon-including regimens for chronic hepatitis C infection[43-45]. Similar to IL28B genotypes, interferon-lambda-4 major or minor genotype, respectively, could predict better or poor response to interferon therapy in HCV-infected patients.
Recently, Aoki et al[46] reported that serum IL28B levels are increased in patients with chronic hepatitis C, regardless of the IL28B genotype. They also suggested that serum IL28B is a biomarker of the activity and fibrosis of liver disease; however serum IL28B is not correlated with the responsiveness to peginterferon plus ribavirin therapy[46]. The same group reported that IL28B genotype affects IL28B production but that the outcome of peginterferon plus ribavirin treatment depends on the amount of IL28B protein[47].
Hepatic interferon-stimulated genes (ISGs) have been significantly associated with the IL28B polymorphism, and expression level of hepatic ISG was significantly higher in patients with the minor genotype than those with the major genotype[48,49]. Lagging et al[50] found that the favorable IL28B variants were associated with lower baseline plasma interferon-gamma-inducible protein-10 (IP-10), although high baseline levels of IP-10 predicted a slower first phase decline in HCV RNA and poor outcome following interferon plus ribavirin therapy in patients with chronic hepatitis C[51-53]. We also reported that IL28B genotypes and hepatic STAT1-nuclear translocation are independent predictors of treatment response[54]. IL28B overexpression in HepG2 cells induces ISGs that have been associated with the progression of HCV-related pathogenesis and antiviral activities against HCV[55]. Sugiyama et al[56] reported that the A (TA) dinucleotide repeat rs72258881 is associated with the transcriptional activity of IL28B. A functional polymorphism (rs4803217) in the 3’ untranslated region (UTR) of IL28B has been shown to influence the AU-rich element (ARE)-mediated decay (AMD) of IL28B mRNA and binding of HCV-induced microRNAs during infection[57]. At the present, we do not know the precise mechanisms between IL28B variants and treatment response to interferon. Additional studies investigating these mechanisms are needed.
Kobayashi et al[58] analyzed IL28B genotypes in 1518 Japanese patients infected with HCV and reported that TT at rs8099917 and CC at rs12979860 as IL28B major genotypes were detected in 77.7% and 76.8% of patients, respectively, and that TG/GG at rs8099917 and CT/TT at rs12979860 as IL28B minor genotypes were detected in 22.3% and 23.2% of patients, respectively. Although there are some discrepancies between these two sets of genotypes, the linkage disequilibrium between two IL28B polymorphisms at rs8099917 and rs12979860 is strong in Japanese HCV patients[58]. In 2010, Akkarathamrongsin et al[59] found that genotyping by both rs8103142 and rs11881222 indicated that 77.9% and 22.1% of the patients had the major and minor genotypes, respectively. In 2011, we also reported that TT and TG/GG at rs8099917 as IL28B major and minor genotypes, respectively, were detected in 65.6% and 34.4% of HCV-infected patients, respectively[38]. Kurosaki et al[60] reported that TT and TG/GG at rs8099917 as IL28B major and minor genotypes, respectively, were detected in 69.6% and 30.4% of HCV genotype 1-infected patients, respectively.
Thomas et al[61] reported that HCV clearance was observed much more frequently than expected (53%) in the CC IL28B genotypes at rs12979860, although the proportion of individuals with CT/TT IL28B genotypes at rs12979860 who cleared the virus (28%) was similar to a general population expectation, because HCV clearance occurs in approximately 30% of HCV-infected patients. Approximately 65%-70% of Japanese patients infected with HCV had the IL28B major genotype. In 2011, telaprevir, a first-generation HCV NS3/4A protease inhibitor with peginterferon plus ribavirin was introduced as treatment for HCV genotype 1 infection in Japan[22,45], and in 2013, simeprevir, a second-generation HCV NS3/4A protease inhibitor with peginterferon plus ribavirin was also made available in Japan[27,62]. We next examined the current status of IL28B genotypes in Japanese patients infected with HCV.
The IL28B genotype is a strong predictor of treatment response in HCV-infected patients treated with interferon-including regimens. We examined the current status of the IL28B genotype rs8099917 distribution of the outpatients infected with HCV. Blood samples were obtained from 432 HCV-infected outpatients (mean age: 59.9 years, male/female: 224/208, HCV genotypes 1/2/3/unknown: 314/102/1/15) in our hospital. The IL28B genotype at rs8099917 was determined by TaqMan SNP genotyping assay using the Step One real-time PCR system (Applied Biosystems, Foster City, CA, United States). Clinical backgrounds, including the present status of HCV RNA positivity, were also examined. Written informed consent was obtained from all patients, and the study protocol was approved by the Ethics Committee of Chiba University, School of Medicine (number 508). Some patients had been included in previous studies[38,42,54,63-66].
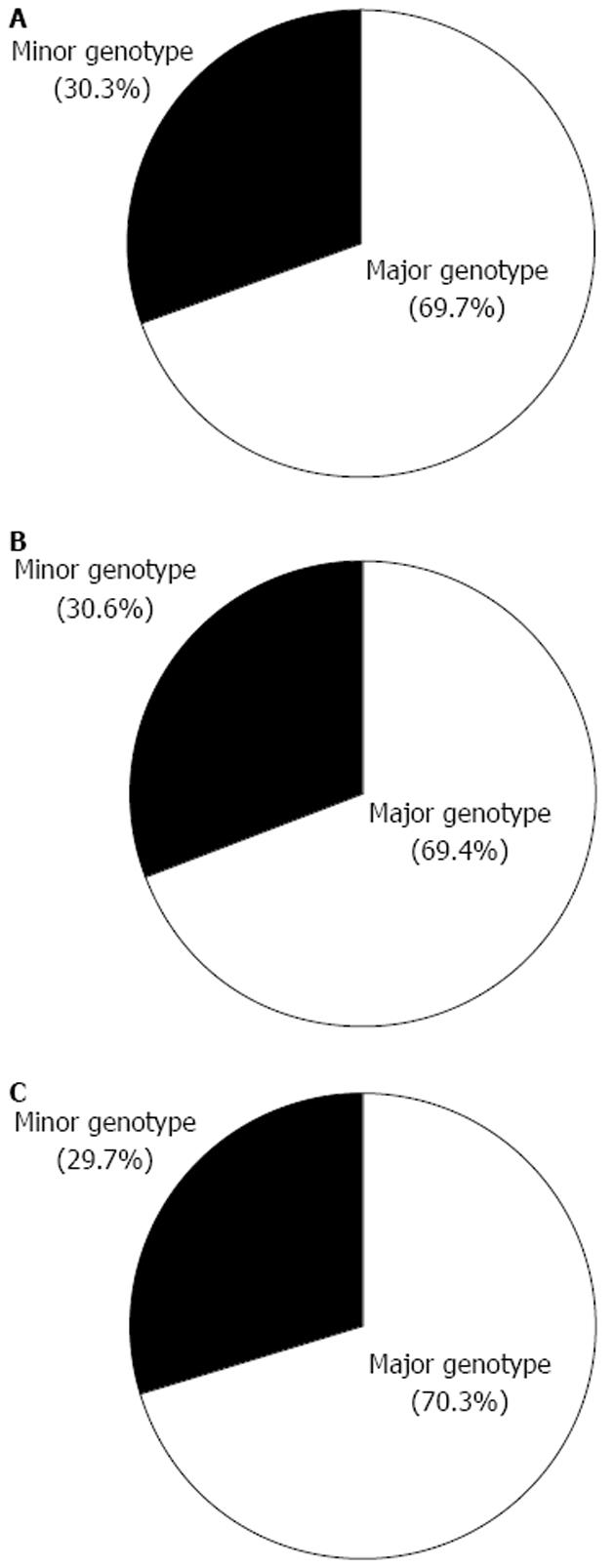
Of the 432 patients, 301 and 131 had the IL28B major and minor genotypes, respectively (Figure 1A), and 87.7% were treated at least once with an interferon-including regimen, resulting in 184 SVR, 184 non-SVR, and 64 untreated/others, respectively. Of the 314 patients with HCV genotype 1, 218 and 96 had the IL28B major and minor genotypes, respectively (Figure 1B), and 122, 143, and 49 patients had SVR, non-SVR/untreated, or other, respectively. Of the 143 patients with HCV genotype 1 with non-SVR or untreated, 85 and 58 had the IL28B major and minor genotypes, respectively, and 22 (25.9%) of the 85 patients with HCV genotype 1 and the IL28B major type are now interferon-intolerant or ineligible. Of the 118 patients with HCV genotype non-1, 83 and 35 had the IL28B major and minor genotypes, respectively, and 62, 41, and 14 patients had SVR, non-SVR/untreated, or other, respectively. In the 41 patients with HCV genotype non-1 with non-SVR or untreated, 27 and 14 had the IL28B major and minor genotypes, respectively (Figure 1C), and 10 (37%) of the 27 patients with HCV genotype 1 and the IL28B major type are now interferon-intolerant or ineligible. The distribution of IL28B genotypes is not significantly different between HCV genotype 1 and non-1 (P = 0.947; Figure 1B and C).
Thus, the patients infected with HCV genotypes 1 and non-1, who had IL28B minor genotypes in 40.6% (58/143) and 34.1% (14/41), respectively, should be treated. Further, some patients who had the IL28B major genotype are interferon-intolerant or ineligible. Regarding the current status of IL28B genotype rs8099917 distribution, we re-confirmed that the HCV-infected population in Japan should be treated with interferon-free regimens, although interferon-including regimens may be effective in certain patients. The rs8099917 TT genotype may be significantly independently predictive of rapid virologic response, which is the single best predictor of SVR, in Asian HCV genotype patients[67].
In Japan, interferon-free 24-wk regimens of asunaprevir, a HCV NS3/4A inhibitor, and daclatasvir, a HCV NS5A inhibitor, can now be used for HCV genotype 1b-infected patients who are interferon-intolerant or ineligible, or previous-treatment null-responders[68-70]. In the near future, interferon-free 12-wk regimens of sofosbuvir plus ribavirin for HCV genotype 2-infected patients will be available[71]. Interferon-free 12-wk regimens of sofosbuvir, a HCV NS5B nucleotide polymerase inhibitor, and ledipasvir, a HCV NS5A inhibitor, for HCV genotype 1-infected patients will also be available[72]. The response to the treatment with interferon-free regimens appears to have no association with IL28B genotypes. In conclusion, although some HCV-infected individuals have IL28B favorable alleles, importance of IL28B will be reduced with availability of oral interferon free regimen.
P- Reviewer: Frider B, Quarleri J, Tetsuya T S- Editor: Ji FF L- Editor: A E- Editor: Yan JL
| 1. | Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, Kikuchi S, Watanabe Y, Koi S, Onji M, Ohta Y. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:6547-6549. [PubMed] |
| 2. | Akamatsu N, Sugawara Y, Kokudo N, Eguchi S, Fujiwara T, Ohdan H, Nagano H, Taketomi A, Kitagawa Y, Shimada M. Outcomes of living donor liver transplantation for hepatitis C virus-positive recipients in Japan: results of a nationwide survey. Transpl Int. 2014;27:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Di Bisceglie AM. Hepatitis C and hepatocellular carcinoma. Hepatology. 1997;26:34S-38S. [PubMed] |
| 4. | Kim WR, Terrault NA, Pedersen RA, Therneau TM, Edwards E, Hindman AA, Brosgart CL. Trends in waiting list registration for liver transplantation for viral hepatitis in the United States. Gastroenterology. 2009;137:1680-1686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Kanda T, Imazeki F, Yokosuka O. New antiviral therapies for chronic hepatitis C. Hepatol Int. 2010;4:548-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Omata M, Kanda T, Yu ML, Yokosuka O, Lim SG, Jafri W, Tateishi R, S Hamid S, Chuang WL, Chutaputti A. APASL consensus statements and management algorithms for hepatitis C virus infection. Hepatol Int. 2012;6:409-435. [RCA] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 7. | Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Huang JF, Yu ML, Lee CM, Dai CY, Hou NJ, Hsieh MY, Wang JH, Lu SN, Sheen IS, Lin SM. Sustained virological response to interferon reduces cirrhosis in chronic hepatitis C: a 1,386-patient study from Taiwan. Aliment Pharmacol Ther. 2007;25:1029-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Maruoka D, Imazeki F, Arai M, Kanda T, Fujiwara K, Yokosuka O. Longitudinal changes of the laboratory data of chronic hepatitis C patients with sustained virological response on long-term follow-up. J Viral Hepat. 2012;19:e97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 780] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 11. | Shiratori Y, Shiina S, Teratani T, Imamura M, Obi S, Sato S, Koike Y, Yoshida H, Omata M. Interferon therapy after tumor ablation improves prognosis in patients with hepatocellular carcinoma associated with hepatitis C virus. Ann Intern Med. 2003;138:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 218] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Yu ML, Lin SM, Chuang WL, Dai CY, Wang JH, Lu SN, Sheen IS, Chang WY, Lee CM, Liaw YF. A sustained virological response to interferon or interferon/ribavirin reduces hepatocellular carcinoma and improves survival in chronic hepatitis C: a nationwide, multicentre study in Taiwan. Antivir Ther. 2006;11:985-994. [PubMed] |
| 13. | Shiratori Y, Ito Y, Yokosuka O, Imazeki F, Nakata R, Tanaka N, Arakawa Y, Hashimoto E, Hirota K, Yoshida H. Antiviral therapy for cirrhotic hepatitis C: association with reduced hepatocellular carcinoma development and improved survival. Ann Intern Med. 2005;142:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Deuffic-Burban S, Deltenre P, Louvet A, Canva V, Dharancy S, Hollebecque A, Boitard J, Henrion J, Yazdanpanah Y, Mathurin P. Impact of viral eradication on mortality related to hepatitis C: a modeling approach in France. J Hepatol. 2008;49:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Yoshida H, Arakawa Y, Sata M, Nishiguchi S, Yano M, Fujiyama S, Yamada G, Yokosuka O, Shiratori Y, Omata M. Interferon therapy prolonged life expectancy among chronic hepatitis C patients. Gastroenterology. 2002;123:483-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 164] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, McNair L, Alam J, Muir AJ. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 809] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 17. | Hézode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, Bronowicki JP, Bourlière M, Gharakhanian S, Bengtsson L. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 794] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 18. | McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, Heathcote EJ, Zeuzem S, Reesink HW, Garg J. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 535] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 19. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1862] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 20. | Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1214] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 21. | Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, Adler M, Reesink HW, Martin M. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 602] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 22. | Kumada H, Toyota J, Okanoue T, Chayama K, Tsubouchi H, Hayashi N. Telaprevir with peginterferon and ribavirin for treatment-naive patients chronically infected with HCV of genotype 1 in Japan. J Hepatol. 2012;56:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 23. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1981] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 24. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1308] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 25. | Manns M, Marcellin P, Poordad F, de Araujo ES, Buti M, Horsmans Y, Janczewska E, Villamil F, Scott J, Peeters M. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 291] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 26. | Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, Moroz L, Craxi A, Peeters M, Lenz O. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 357] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 27. | Hayashi N, Izumi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Ki R, Komada Y, Seto C. Simeprevir with peginterferon/ribavirin for treatment-naïve hepatitis C genotype 1 patients in Japan: CONCERTO-1, a phase III trial. J Hepatol. 2014;61:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 28. | Reddy KR, Zeuzem S, Zoulim F, Weiland O, Horban A, Stanciu C, Villamil FG, Andreone P, George J, Dammers E. Simeprevir versus telaprevir with peginterferon and ribavirin in previous null or partial responders with chronic hepatitis C virus genotype 1 infection (ATTAIN): a randomised, double-blind, non-inferiority phase 3 trial. Lancet Infect Dis. 2015;15:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | McPhee F, Hernandez D, Zhou N, Yu F, Ueland J, Monikowski A, Chayama K, Toyota J, Izumi N, Yokosuka O. Virological escape in HCV genotype-1-infected patients receiving daclatasvir plus ribavirin and peginterferon alfa-2a or alfa-2b. Antivir Ther. 2014;19:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Izumi N, Yokosuka O, Kawada N, Osaki Y, Yamamoto K, Sata M, Ishikawa H, Ueki T, Hu W, McPhee F. Daclatasvir combined with peginterferon alfa-2a and ribavirin in Japanese patients infected with hepatitis C genotype 1. Antivir Ther. 2014;19:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Zeuzem S, Soriano V, Asselah T, Bronowicki JP, Lohse AW, Müllhaupt B, Schuchmann M, Bourlière M, Buti M, Roberts SK. Faldaprevir and deleobuvir for HCV genotype 1 infection. N Engl J Med. 2013;369:630-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 32. | Ferenci P, Asselah T, Foster GR, Zeuzem S, Sarrazin C, Moreno C, Ouzan D, Maevskaya M, Calinas F, Morano LE. STARTVerso1: A randomized trial of faldaprevir plus pegylated interferon/ribavirin for chronic HCV genotype-1 infection. J Hepatol. 2015;62:1246-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Rodriguez-Torres M, Stoehr A, Gane EJ, Serfaty L, Lawitz E, Zhou A, Bourque M, Bhanja S, Strizki J, Barnard RJ. Combination of vaniprevir with peginterferon and ribavirin significantly increases the rate of SVR in treatment-experienced patients with chronic HCV genotype 1 infection and cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1029-37.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Kanda T, Yokosuka O, Omata M. Treatment of hepatitis C virus infection in the future. Clin Transl Med. 2013;2:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1775] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 36. | Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2776] [Cited by in RCA: 2723] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 37. | Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1505] [Cited by in RCA: 1504] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 38. | Nakamoto S, Kanda T, Imazeki F, Wu S, Arai M, Fujiwara K, Yokosuka O. Simple assay based on restriction fragment length polymorphism associated with IL28B in chronic hepatitis C patients. Scand J Gastroenterol. 2011;46:955-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286:487-491. [PubMed] |
| 40. | Fellay J, Thompson AJ, Ge D, Gumbs CE, Urban TJ, Shianna KV, Little LD, Qiu P, Bertelsen AH, Watson M. ITPA gene variants protect against anaemia in patients treated for chronic hepatitis C. Nature. 2010;464:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 371] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 41. | Thompson AJ, Fellay J, Patel K, Tillmann HL, Naggie S, Ge D, Urban TJ, Shianna KV, Muir AJ, Fried MW. Variants in the ITPA gene protect against ribavirin-induced hemolytic anemia and decrease the need for ribavirin dose reduction. Gastroenterology. 2010;139:1181-1189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 42. | Miyamura T, Kanda T, Nakamoto S, Wu S, Jiang X, Arai M, Fujiwara K, Imazeki F, Yokosuka O. Roles of ITPA and IL28B genotypes in chronic hepatitis C patients treated with peginterferon plus ribavirin. Viruses. 2012;4:1264-1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Prokunina-Olsson L, Muchmore B, Tang W, Pfeiffer RM, Park H, Dickensheets H, Hergott D, Porter-Gill P, Mumy A, Kohaar I. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet. 2013;45:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 759] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 44. | Bibert S, Roger T, Calandra T, Bochud M, Cerny A, Semmo N, Duong FH, Gerlach T, Malinverni R, Moradpour D. IL28B expression depends on a novel TT/-G polymorphism which improves HCV clearance prediction. J Exp Med. 2013;210:1109-1116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 45. | Miyamura T, Kanda T, Nakamoto S, Arai M, Nakamura M, Wu S, Jiang X, Sasaki R, Haga Y, Yasui S. IFNL4 ss469415590 Variant Is Associated with Treatment Response in Japanese HCV Genotype 1 Infected Individuals Treated with IFN-Including Regimens. Int J Hepatol. 2014;2014:723868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Aoki Y, Sugiyama M, Murata K, Yoshio S, Kurosaki M, Hashimoto S, Yatsuhashi H, Nomura H, Kang JH, Takeda T. Association of serum IFN-λ3 with inflammatory and fibrosis markers in patients with chronic hepatitis C virus infection. J Gastroenterol. 2014;Epub ahead of print. [PubMed] |
| 47. | Murata K, Sugiyama M, Kimura T, Yoshio S, Kanto T, Kirikae I, Saito H, Aoki Y, Hiramine S, Matsui T. Ex vivo induction of IFN-λ3 by a TLR7 agonist determines response to Peg-IFN/ribavirin therapy in chronic hepatitis C patients. J Gastroenterol. 2014;49:126-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Honda M, Sakai A, Yamashita T, Nakamoto Y, Mizukoshi E, Sakai Y, Yamashita T, Nakamura M, Shirasaki T, Horimoto K. Hepatic ISG expression is associated with genetic variation in interleukin 28B and the outcome of IFN therapy for chronic hepatitis C. Gastroenterology. 2010;139:499-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 344] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 49. | Urban TJ, Thompson AJ, Bradrick SS, Fellay J, Schuppan D, Cronin KD, Hong L, McKenzie A, Patel K, Shianna KV. IL28B genotype is associated with differential expression of intrahepatic interferon-stimulated genes in patients with chronic hepatitis C. Hepatology. 2010;52:1888-1896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 314] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 50. | Lagging M, Askarieh G, Negro F, Bibert S, Söderholm J, Westin J, Lindh M, Romero A, Missale G, Ferrari C. Response prediction in chronic hepatitis C by assessment of IP-10 and IL28B-related single nucleotide polymorphisms. PLoS One. 2011;6:e17232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 51. | Romero AI, Lagging M, Westin J, Dhillon AP, Dustin LB, Pawlotsky JM, Neumann AU, Ferrari C, Missale G, Haagmans BL. Interferon (IFN)-gamma-inducible protein-10: association with histological results, viral kinetics, and outcome during treatment with pegylated IFN-alpha 2a and ribavirin for chronic hepatitis C virus infection. J Infect Dis. 2006;194:895-903. [PubMed] |
| 52. | Lagging M, Romero AI, Westin J, Norkrans G, Dhillon AP, Pawlotsky JM, Zeuzem S, von Wagner M, Negro F, Schalm SW. IP-10 predicts viral response and therapeutic outcome in difficult-to-treat patients with HCV genotype 1 infection. Hepatology. 2006;44:1617-1625. [PubMed] |
| 53. | Askarieh G, Alsiö A, Pugnale P, Negro F, Ferrari C, Neumann AU, Pawlotsky JM, Schalm SW, Zeuzem S, Norkrans G. Systemic and intrahepatic interferon-gamma-inducible protein 10 kDa predicts the first-phase decline in hepatitis C virus RNA and overall viral response to therapy in chronic hepatitis C. Hepatology. 2010;51:1523-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 54. | Miyamura T, Kanda T, Nakamoto S, Wu S, Fujiwara K, Imazeki F, Yokosuka O. Hepatic STAT1-nuclear translocation and interleukin 28B polymorphisms predict treatment outcomes in hepatitis C virus genotype 1-infected patients. PLoS One. 2011;6:e28617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Kanda T, Jiang X, Nakamoto S, Nakamura M, Miyamura T, Wu S, Yokosuka O. Different effects of three interferons L on Toll-like receptor-related gene expression in HepG2 cells. Cytokine. 2013;64:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Sugiyama M, Tanaka Y, Wakita T, Nakanishi M, Mizokami M. Genetic variation of the IL-28B promoter affecting gene expression. PLoS One. 2011;6:e26620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | McFarland AP, Horner SM, Jarret A, Joslyn RC, Bindewald E, Shapiro BA, Delker DA, Hagedorn CH, Carrington M, Gale M. The favorable IFNL3 genotype escapes mRNA decay mediated by AU-rich elements and hepatitis C virus-induced microRNAs. Nat Immunol. 2014;15:72-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 58. | Kobayashi M, Suzuki F, Akuta N, Sezaki H, Suzuki Y, Hosaka T, Kawamura Y, Kobayashi M, Saitoh S, Arase Y. Association of two polymorphisms of the IL28B gene with viral factors and treatment response in 1,518 patients infected with hepatitis C virus. J Gastroenterol. 2012;47:596-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Akkarathamrongsin S, Sugiyama M, Matsuura K, Kurbanov F, Poovorawan Y, Tanaka Y, Mizokami M. High sensitivity assay using serum sample for IL28B genotyping to predict treatment response in chronic hepatitis C patients. Hepatol Res. 2010;40:956-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Kurosaki M, Tanaka Y, Nishida N, Sakamoto N, Enomoto N, Honda M, Sugiyama M, Matsuura K, Sugauchi F, Asahina Y. Pre-treatment prediction of response to pegylated-interferon plus ribavirin for chronic hepatitis C using genetic polymorphism in IL28B and viral factors. J Hepatol. 2011;54:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 61. | Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1706] [Cited by in RCA: 1687] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 62. | Kanda T, Nakamoto S, Wu S, Yokosuka O. New treatments for genotype 1 chronic hepatitis C - focus on simeprevir. Ther Clin Risk Manag. 2014;10:387-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Nakamura M, Kanda T, Miyamura T, Wu S, Nakamoto S, Yokosuka O. Alanine aminotransferase elevation during peginterferon alpha-2a or alpha-2b plus ribavirin treatment. Int J Med Sci. 2013;10:1015-1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 64. | Miyamura T, Kanda T, Nakamura M, Jiang X, Wu S, Nakamoto S, Mikami S, Takada N, Imazeki F, Yokosuka O. IL-28B polymorphisms and treatment response in hepatitis C virus patients with persistently normal alanine aminotransferase. World J Hepatol. 2013;5:635-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 65. | Kanda T, Nakamoto S, Nishino T, Takada N, Tsubota A, Kato K, Miyamura T, Maruoka D, Wu S, Tanaka T. Peginterferon Alfa-2a plus ribavirin in Japanese patients infected with hepatitis C virus genotype 2 who failed previous interferon therapy. Int J Med Sci. 2013;10:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 66. | Kanda T, Nakamoto S, Wu S, Yokosuka O. Role of IL28B genotype in older hepatitis C virus-infected patients. World J Immunol. 2013;3:54-61. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 67. | Yu ML, Huang CF, Huang JF, Chang NC, Yang JF, Lin ZY, Chen SC, Hsieh MY, Wang LY, Chang WY. Role of interleukin-28B polymorphisms in the treatment of hepatitis C virus genotype 2 infection in Asian patients. Hepatology. 2011;53:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 68. | Chayama K, Takahashi S, Toyota J, Karino Y, Ikeda K, Ishikawa H, Watanabe H, McPhee F, Hughes E, Kumada H. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology. 2012;55:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 69. | Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, Reindollar R, Rustgi V, McPhee F, Wind-Rotolo M. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 476] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 70. | Kumada H, Suzuki Y, Ikeda K, Toyota J, Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 453] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 71. | Omata M, Nishiguchi S, Ueno Y, Mochizuki H, Izumi N, Ikeda F, Toyoda H, Yokosuka O, Nirei K, Genda T. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat. 2014;21:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 72. | Mizokami M, Yokosuka O, Takehara T, Sakamoto N, Korenaga M, Mochizuki H, Nakane K, Enomoto H, Ikeda F, Yanase M. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis. 2015;15:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 301] [Article Influence: 30.1] [Reference Citation Analysis (0)] |