Revised: May 4, 2014
Accepted: May 9, 2014
Published online: May 12, 2014
Processing time: 190 Days and 17.8 Hours
AIM: To study the effect of seminal plasma on Chemokine (C-C motif) ligand 20 (CCL20) production by epithelial cells and its relationship with lactoferrin.
METHODS: HEC-1A cells, a cell line derived from a monostratified endocervical epithelium, were incubated with samples of seminal plasma (diluted 1:10 in culture medium) recovered from human immunodeficiency virus (HIV) seronegative (HIV-) or HIV seropositive (HIV+) subjects. Recombinant human interleukin 1 beta (IL-1β) was used as positive control, and culture medium only as negative control. The measurement of CCL20 production in the supernatants of HEC-1A cells and of lactoferrin in seminal plasma was determined by enzyme-linked immunosorbent assay techniques. A fractionation of seminal plasma proteins was performed by ion exchange chromatography on a pool of seminal plasma specimens from HIV- subjects. Each fraction was tested for its ability to stimulate the production of CCL20 by HEC-1A cells and for its lactoferrin concentration. The HIV viral load in seminal plasma samples from HIV+ patients was measured using the HIV-Monitor kit (Roche Diagnostic Systems, Branchburg, NJ, United States).
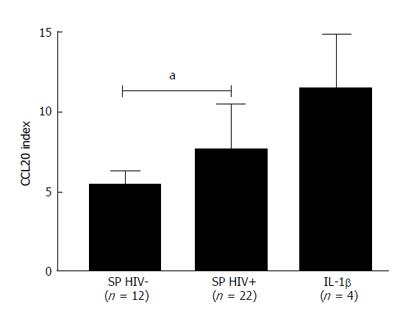
RESULTS: The positive control IL-1β was responsible for an increase of 11.36 ± 3.36 times in the production of CCL20. Stimulation of HEC-1A cells was performed in 34 seminal plasma samples (22 from HIV+ subjects and 12 from HIV- subjects). The mean production of CCL20 by HEC-1A in presence of seminal plasma from HIV- and HIV+ subjects was respectively 5.38 ± 0.91 and 7.57 ± 3.26 times higher than that obtained with the untreated cells (P < 0.05 between the two groups). Using the same 34 specimens of seminal plasma, no correlation was observed between the concentration of total proteins in seminal plasma and their ability to stimulate the secretion of CCL20 by HEC-1 cells. In contrast, the ability to produce CCL20 by HEC-1A cells correlated to the concentration of lactoferrin in the seminal plasma samples (r coefficient = 0.56; CI: 0.26-0.76; P < 0.001). After fractionation by ion exchange chromatography, the seminal plasma fractions exhibiting the highest concentrations of lactoferrin were responsible for the greatest stimulation of CCL20 production by HEC-1A cells (r coefficient = 0.89; CI: 0.78-0.95; P < 0.0001).
CONCLUSION: Lactoferrin present in seminal plasma correlated with an increased production of CCL20 by HEC-1A cells and therefore could facilitate HIV entry through the genital mucosa.
- Citation: Lourenço AG, Komesu MC, Machado AA, Quintana SM, Bourlet T, Pozzetto B, Delézay O. Semen lactoferrin promotes CCL20 production by epithelial cells: Involvement in HIV transmission. World J Virol 2014; 3(2): 11-17
- URL: https://www.wjgnet.com/2220-3249/full/v3/i2/11.htm
- DOI: https://dx.doi.org/10.5501/wjv.v3.i2.11
Sexual transmission of human immunodeficiency virus type 1 (HIV-1) accounts for 60% to 90% of new infections, especially in developing countries. During male-to-female transmission, the virus is typically deposited in the vagina as cell-free or cell associated virions carried by semen[1]. In the absence of breaches in the genital mucosa, the epithelium crossing by HIV could occur through the recruitment of immune cells with migratory properties, such as macrophages, lymphocytes or Langerhans cells (LCs), the latter cells being considered as one of the first target for this virus[2]. HIV entry may be observed at different levels of the female genital tract including the vagina, the ectocervix and the endocervix. The epithelial architecture is variable in these regions. The epithelium of vagina and ectocervix is composed by multi-layered, pluristratified epithelial cells that do not form a polarized epithelium. In contrast, the epithelium of the endocervix is a single layer of polarized, columnar epithelial cells with tight junctions, dividing the epithelium into apical and basolateral domains[3]. These simple mono-layered epithelia provide a lower degree of protection.
The Chemokine (C-C motif) ligand 20 (CCL20) is liberated by epithelial cells from different tissues including skin[4,5], oral mucosa[6] and vaginal epithelium[7]. CCL20 is an important immune effector molecule that is chemotactic for immature dendritic cells (DCs) and lymphocytes[8,9]. DCs also likely contribute to the array of cells potentially involved in HIV entry into the vaginal and ectocervical mucosae. DCs efficiently capture, disseminate, and transmit viruses to mononuclear target cells; however, HIV does not productively infect the DCs themselves[3]. CCL20 secretion by human vaginal epithelial cells has been shown to be enhanced in the presence of semen resulting in chemoattraction of LCs that are permissive to HIV infection[10], but the compound(s) involved in this stimulation is (are) not yet characterized.
This study was performed for analyzing the ability of seminal plasma from HIV seronegative (HIV-) and HIV seropositive (HIV+) subjects to promote the production of CCL20 by monolayers of endocervical epithelium cells (HEC-1A cell line). This secretion correlated to the amount of lactoferrin present in the seminal plasma specimen.
Semen samples were collected from 22 HIV+ and 12 HIV- subjects. The patients gave their fully-informed written consent. The study was reviewed and approved by the Ethics Committee of the School of Medicine of Ribeirão Preto, University of São Paulo, Brazil (CH-SMRP-USP No. 4926/2009). The inclusion criteria were as follows: being over 18 years old, having never undergone radiotherapy or chemotherapy treatment, and not having used antimicrobials or anti-inflammatory drugs during the last 6 mo. HIV- men were tested for the absence of common sexually transmitted diseases including syphilis, Chlamydia trachomatis and Neisseria gonorrhoeae infection, herpes simplex virus infection and hepatitis B virus infection.
The participants were instructed to auto-perform an aseptic collection of semen by masturbation into a universal collector, with the use of neither lubricants nor water. Within 4 h after ejaculation, the semen specimens were submitted to the following protocol: they were diluted 1:2 in PBS and then centrifuged at 800 g for 30 min. The supernatant constituted the seminal plasma that was stored at -80 °C. The frozen samples were sent to the GIMAP team, Saint Etienne, France, for analysis.
The HEC-1A cell line was used for mimicking the female genital tract. It was cultured in Dulbecco’s minimal essential medium (DMEM-F12 medium, Cambrex BioScience, Verviers, Belgium) to which were added 2% fetal bovine serum (FBS) and 1% solution containing penicillin, streptomycin and amphotericin B (Sigma-Aldrich, St. Louis, MO, United States). The experiments of stimulation were performed on 96-well culture plates (BD Falcon, Franklin Lakes, NJ, United States) seeded with cells cultured for 2 d with a final density of 100000 cells/well.
HIV- or HIV+ seminal plasmas diluted 1:10 in culture medium were added to the HEC-1A cells. Recombinant human interleukin 1 beta (IL-1β) (Peprotech, Neuilly-Sur-Seine, France) at the concentration 25 ng/mL was used as positive control, as previously reported[7,10]. Culture medium DMEM-F12 served as negative control. After an overnight incubation, the CCL20 production was measured in the supernatants of HEC-1A cells by using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Quantikine, R and D Systems, Abingdon, United Kingdom) as recommended by the manufacturer. Each assay was performed in duplicate. The results were expressed as relative CCL20 index corresponding to the ratio between the amount of CCL20 produced by the specimen and the negative control (culture medium only).
The HIV-Monitor kit (Roche Diagnostic Systems, Branchburg, NJ, United States) was used to quantify the HIV RNA in seminal plasma samples from HIV+ subjects. The detection lower limit was of 50 copies/mL. The RNA was extracted from the specimens using a modified silica protocol (QIAmp RNA viral kit; Qiagen, Chatsworth, CA, United States).
Measurement of lactoferrin in seminal plasma specimens was performed by ELISA technique. A standard curve was prepared by using different concentrations of lactoferrin from human milk (Sigma-Aldrich). Seminal plasma samples (diluted 1:10 in PBS) were distributed into wells at the concentration of 100 μL per well and incubated at 37 °C for 1 h. Albumin from chicken egg whites (Sigma-Aldrich) was used for blocking. Lactoferrin was detected with rabbit anti-human lactoferrin antibodies (L3262; Sigma-Aldrich) incubated for 1 h at room temperature followed by peroxidase conjugated anti-rabbit antibodies (Sigma-Aldrich). After several washes, o-phenylenediamine was used as substrate and optical densities at 492 nm were measured. Each assay was performed in duplicate.
The Bradford technique was used for the measurement of the total protein content[11]. Seminal plasma was diluted 1:75 in PBS and distributed in triplicate in microplate wells under a volume of 150 μL per well in addition to the same volume of Bradford reagent (Sigma-Aldrich). Bovine serum albumin was used to perform the standard curve. The reading was performed by spectrophotometry at 590 nm.
Specimens of seminal plasma from HIV- subjects were diluted 1:10 in 50 mmol/L NaCl pH 7.4 (buffer A) and applied onto a column of affinity (Hitrap Q FF, GE Healthcare Life sciences, Velizy-Villacoublay, France) equilibrated at room temperature with the same buffer. A discontinuous gradient was used for the elution of seminal plasma proteins by using six different concentrations (5%, 10%, 20%, 30%, 40%, 50%) of a buffer containing 500 mmol/L NaCl (buffer B). Buffer B was passed through the column at a flow rate of 0.5 mL/min using the HPLC AKTA purifier system (GE Healthcare Life sciences). The successive fractions were tested for their capability to induce the secretion of CCL20 by HEC-1A cells as well as for lactoferrin and total protein content as described above.
The data expressed in experimental units are presented as mean ± SD. Statistical analyses were performed using the GraphPad Prism software (San Diego, CA, United States). The Mann-Whitney test was used to compare two means. Correlations were analyzed using the Spearman’s r test. P values < 0.05 were considered as statistically significant.
The secretion of CCL20 was measured by ELISA in the supernatants of HEC-1A cells incubated for 17 h with culture medium DMEM-F12 only (negative control), IL-1β (25 ng/mL, positive control) or each of 34 seminal plasma specimens (22 from HIV+ subjects and 12 from HIV- subjects) diluted 1:10 in DMEM-F12. The CCL20 stimulation was expressed in number of times its production increased in comparison to untreated cells (CCL20 index). IL-1β used as positive control was responsible for an increase of 11.36 ± 3.36 times in the production of CCL20. The mean production of CCL20 by HEC-1A in presence of seminal plasma from HIV- and HIV+ subjects was increased by respectively 5.38 ± 0.91 and 7.57 ± 3.26 times with comparison to untreated cells (negative control). The difference between the two groups was statistically significant (P < 0.05 by Mann-Whitney test) (Figure 1).
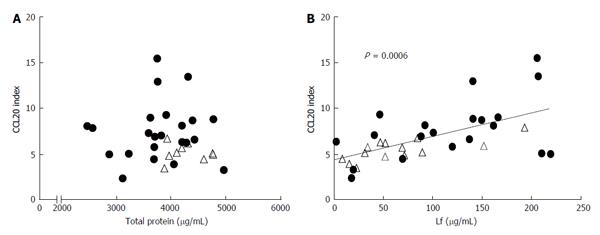
Using the same 34 specimens of seminal plasma (12 from HIV- and 22 from HIV+ subjects), no correlation was observed between the concentration of total proteins in seminal plasma and their ability to stimulate the secretion of CCL20 by HEC-1 cells (Figure 2A). In contrast, the ability to produce CCL20 by HEC-1A cells positively correlated to the concentration of lactoferrin in the seminal plasma samples (r coefficient = 0.56; CI: 0.26-0.76; P < 0.001 by the Spearman’s r test) (Figure 2B).
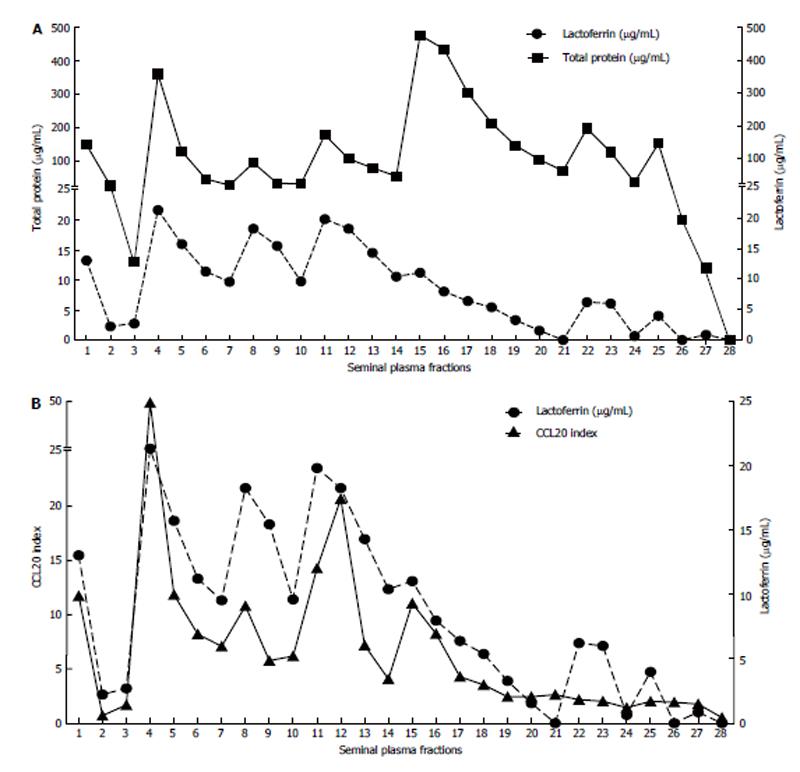
In order to verify whether lactoferrin present in seminal plasma was responsible for the production of CCL20 by HEC-1A cells, the proteins from a pool of seminal plasma specimens from 12 HIV- subjects were fractionated by ion exchange chromatography. Each fraction was then tested for its ability to stimulate the production of CCL20 and for its concentration of lactoferrin and total proteins (Figure 3).
As shown in Figure 3B, the amount of CCL20 produced by HEC-1A cells was closely related to the concentration of lactoferrin present in the plasma fraction (r = 0.8942, CI: 0.7773-0.9514, P < 0.0001 by the Spearman’s r test). Fractions with the greatest concentration of lactoferrin (fractions 1, 3-5, 7-9, 10-13 in Figure 3B) corresponded to those exhibiting the highest capacity for inducing the production of CCL20 by HEC-1A cells.
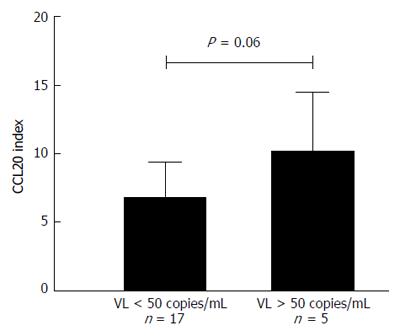
From the 22 seminal plasma specimens from subjects tested seropositive for HIV, the 5 samples exhibiting a detectable viral load (> 50 copies/mL) increased the production of CCL20 by a factor of 10.3 ± 4.2 times as compared to the negative control whereas the 17 samples with undetectable viral load (< 50 copies/mL) stimulated the production of CCL20 by a factor of 6.7 ± 2.6 times with reference to the negative control. A trend was observed between the 2 groups but the difference was not statistically significant due to the small size of effectives (Figure 4).
Heterosexual route is the most common way for HIV transmission resulting in a significant increase of HIV-infected women in recent years[12,13]. Women are more susceptible to HIV transmission, notably because of the large size of genital mucosa that is exposed to semen and also because semen contains higher concentrations of virus than vaginal fluid[14].
Seminal plasma confers a survival advantage to spermatozoids within the relative hostile environment of the female genital tract[15]. However, more recent studies have shown that seminal plasma is able to provide to vaginal mucosa a set of signaling molecules that are capable of interacting with epithelial cells of the female reproductive tract, these interactions triggering molecular and cellular changes that resemble an inflammatory response[16].
Signaling molecules present in seminal plasma may increase the secretion of chemokines and cause vascular changes that lead to the recruitment and activation of macrophages, granulocytes and DCs[17] including LCs[10]. LCs present in the vaginal mucosa are known as “Trojan horse” that facilitate the passage of HIV through the vaginal mucosa and present them to the CD4+ cells[18]. CCL20 is the main chemokine involved in the recruitment of LCs and its production by epithelial cells could be related to an increased risk of HIV infection. In this way, Li et al[19] demonstrated that the reduction of CCL20 secretion by epithelial cells treated with glycerol monolaurate, a vaginal microbicide, prevented the mucosal transmission of Simian Immunodeficiency Virus. These data are an additional argument for the determining role of CCL20 in the contamination process by HIV.
In this study, we found that seminal plasma was able to stimulate the production of CCL20 by HEC-1A, with a statistically significant advantage for that originated from HIV+ patients as compared to HIV- subjects. These results confirm those previously published by our team[10] that showed a higher increase in the production of CCL20 by the SiHa cell line derived from vaginal epithelium when stimulated with seminal plasma from HIV+ subjects, however without statistical significance. Sharkey et al[17] also showed that human seminal plasma is capable of interacting with cervical and vaginal tissues for inducing the production of proinflammatory cytokines.
Cremel et al[7] demonstrated that vaginal epithelial cells increased the secretion of CCL20 in response to stimulation by proinflammatory cytokine IL-1β. The present study shows that seminal plasma from HIV+ and HIV- subjects produces similar effects on the cells lining the endocervical monostratified (HEC-1A), suggesting that seminal plasma contains components able to generate a response, even if not specific, in the female genital mucosa, mediated by CCL20 secretion.
One of the potential candidates that could stimulate CCL20 secretion by female genital epithelial cells is lactoferrin, a globular glycoprotein of the transferrin family with a molecular mass of 80 kDa and present in large amounts in various secretions. Lactoferrin is considered as an important element of nonspecific humoral immunity and was shown to exhibit a protective effect, particularly against HIV because of its interference with the viral gp 120 and Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin receptor receptor[20,21]. In contrast, other studies have reported that some peptides from the cleavage of this protein by elastase or proteinase type III enzymes exhibit a strong pro-inflammatory activity in different mucosae[22,23]. In this study, we found a positive correlation between the concentration of lactoferrin in seminal plasma and the production of CCL20 stimulated by HEC-1A, even if it cannot be excluded that an additional factor could contribute to this activation. Another finding of our study that suggests the participation of lactoferrin or its cleavage products as activating factors of increased secretion of CCL20 by genital mucosal cells is the result obtained after chromatography fractionation performed on a pool of seminal plasma samples from HIV- subjects (the volume of seminal plasma was not enough to perform the same experiments with seminal plasma samples from HIV+ subjects). Indeed, the fractions that were the most efficient for CCL20 secretion stimulation were those containing the highest concentration of lactoferrin. The fact that the lactoferrin activity was distributed in discontinuous pattern along the chromatogram (Figure 3B) could be explained by the tetrameric conformation of the protein that can correspond to associations of different molecular masses, and also by the contribution of degradation products of lactoferrin after enzymatic digestion, which were shown to exhibit a strong pro-inflammatory effect[22,23].
Interestingly, as shown in Figure 4 for the subgroup of HIV+ subjects, the specimens exhibiting high viral loads were shown to stimulate more efficiently the production of CCL20 (although the difference did not reach statistical significance due to the small size of effectives); this finding is an additional evidence for the existence of a correlation between the HIV load of seminal fractions and their ability to promote CCL20 stimulation. Viral shedding in seminal plasma was recently shown to be closely related to the presence of high levels of pro-inflammatory cytokines, including granulocyte colony stimulating factor, tumor necrosis factor-alpha, interferon-gamma and IL-10[24]. In the light of the above discussion regarding lactoferrin, it can be hypothesized that the amount of pro-inflammatory components derived from this protein may be higher in HIV+ than in HIV- subjects, and notably in those with high seminal HIV load.
All these data argue in favor of a significant role of lactoferrin or its degradation products on CCL20 secretion by female genital mucosa. Despite the need of complementary studies for confirming these findings, our results are indicative of the role of some of these proteins in HIV transmission through the female epithelium tract and suggest that they must be taken into consideration for the prevention of HIV heterosexual contamination process. From a clinical point of view, it would be useful to identify the molecules implicated in this facilitation in order to develop intra-vaginal products capable of neutralizing their activity.
Sexual transmission of human immunodeficiency virus type 1 (HIV-1) accounts for 60% to 90% of new infections, especially in developing countries. During male-to-female transmission, in the absence of breaches in the genital mucosa, the epithelium crossing by HIV could occur through the recruitment of immune cells with migratory properties, such as macrophages, lymphocytes or Langerhans cells.
The Chemokine (C-C motif) ligand 20 (CCL20) secretion by human vaginal epithelial cells has been shown to be enhanced in the presence of semen resulting in chemoattraction of Langerhans cells that are permissive to HIV infection, but the compound(s) involved in this stimulation is (are) not yet characterized.
In the present study, seminal plasma was shown to promote the induction of secretion of CCL20 by monolayers of endocervical epithelium cells (HEC-1A cell line). The effect was significantly higher with seminal plasma from HIV seropositive than HIV seropositive subjects. CCL20 production by HEC-1A cells correlated with the concentration of lactoferrin in seminal plasma. After fractionation of seminal plasma, those with the highest concentrations of lactoferrin were responsible for the greatest stimulation of CCL20 by HEC-1A cells. In conclusion, lactoferrin present in seminal plasma correlated with an increased production of CCL20 by HEC-1A cells and therefore could facilitate HIV entry through the genital mucosa.
Lactoferrin itself or, more likely, some of its degradation products could facilitate HIV entry through the recruitment of immune cells. It would be interesting to characterize precisely the molecules involved in this phenomenon in order to evaluate if they may constitute a target for antiviral protection.
The CCL20 is an important immune effector molecule that is able to attract immature immune cells. Lactoferrin is a globular glycoprotein of the transferrin family that is present in large amounts in various secretions, including seminal plasma; it is considered as an important element of nonspecific humoral immunity.
The current article described the seminal plasma/ lactoferrin affects the CCL20 production by HEC-1 cells. It is an interesting and important topic.
P- Reviewers: Gokul S, Louboutin JP, Shih WL, Said ZNA S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | Doncel GF, Joseph T, Thurman AR. Role of semen in HIV-1 transmission: inhibitor or facilitator? Am J Reprod Immunol. 2011;65:292-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Lore K, Larsson M. The role of dendritic cells in the pathogenesis of HIV-1 infection. APMIS. 2003;111:776-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Shen R, Richter HE, Smith PD. Early HIV-1 target cells in human vaginal and ectocervical mucosa. Am J Reprod Immunol. 2011;65:261-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Sperling T, Ołdak M, Walch-Rückheim B, Wickenhauser C, Doorbar J, Pfister H, Malejczyk M, Majewski S, Keates AC, Smola S. Human papillomavirus type 8 interferes with a novel C/EBPβ-mediated mechanism of keratinocyte CCL20 chemokine expression and Langerhans cell migration. PLoS Pathog. 2012;8:e1002833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Dieu-Nosjean MC, Massacrier C, Homey B, Vanbervliet B, Pin JJ, Vicari A, Lebecque S, Dezutter-Dambuyant C, Schmitt D, Zlotnik A. Macrophage inflammatory protein 3alpha is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J Exp Med. 2000;192:705-718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 285] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 6. | Abiko Y, Nishimura M, Kusano K, Nakashima K, Okumura K, Arakawa T, Takuma T, Mizoguchi I, Kaku T. Expression of MIP-3alpha/CCL20, a macrophage inflammatory protein in oral squamous cell carcinoma. Arch Oral Biol. 2003;48:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Cremel M, Berlier W, Hamzeh H, Cognasse F, Lawrence P, Genin C, Bernengo JC, Lambert C, Dieu-Nosjean MC, Delézay O. Characterization of CCL20 secretion by human epithelial vaginal cells: involvement in Langerhans cell precursor attraction. J Leukoc Biol. 2005;78:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Blengio F, Raggi F, Pierobon D, Cappello P, Eva A, Giovarelli M, Varesio L, Bosco MC. The hypoxic environment reprograms the cytokine/chemokine expression profile of human mature dendritic cells. Immunobiology. 2013;218:76-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 618] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 10. | Berlier W, Cremel M, Hamzeh H, Lévy R, Lucht F, Bourlet T, Pozzetto B, Delézay O. Seminal plasma promotes the attraction of Langerhans cells via the secretion of CCL20 by vaginal epithelial cells: involvement in the sexual transmission of HIV. Hum Reprod. 2006;21:1135-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [PubMed] |
| 12. | Cohn SE, Clark RA. Sexually transmitted diseases, HIV, and AIDS in women. Med Clin North Am. 2003;87:971-995. [PubMed] |
| 13. | Higgins JA, Hoffman S, Dworkin SL. Rethinking gender, heterosexual men, and women’s vulnerability to HIV/AIDS. Am J Public Health. 2010;100:435-445. [PubMed] |
| 14. | Yi TJ, Shannon B, Prodger J, McKinnon L, Kaul R. Genital immunology and HIV susceptibility in young women. Am J Reprod Immunol. 2013;69 Suppl 1:74-79. [PubMed] |
| 15. | Denison FC, Grant VE, Calder AA, Kelly RW. Seminal plasma components stimulate interleukin-8 and interleukin-10 release. Mol Hum Reprod. 1999;5:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005;322:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 320] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 17. | Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod. 2007;13:491-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 18. | Piguet V, Blauvelt A. Essential roles for dendritic cells in the pathogenesis and potential treatment of HIV disease. J Invest Dermatol. 2002;119:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 515] [Cited by in RCA: 503] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 20. | Groot F, Geijtenbeek TB, Sanders RW, Baldwin CE, Sanchez-Hernandez M, Floris R, van Kooyk Y, de Jong EC, Berkhout B. Lactoferrin prevents dendritic cell-mediated human immunodeficiency virus type 1 transmission by blocking the DC-SIGN--gp120 interaction. J Virol. 2005;79:3009-3015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Kazmi SH, Naglik JR, Sweet SP, Evans RW, O’Shea S, Banatvala JE, Challacombe SJ. Comparison of human immunodeficiency virus type 1-specific inhibitory activities in saliva and other human mucosal fluids. Clin Vaccine Immunol. 2006;13:1111-1118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Komine K, Komine Y, Kuroishi T, Kobayashi J, Obara Y, Kumagai K. Small molecule lactoferrin with an inflammatory effect but no apparent antibacterial activity in mastitic mammary gland secretion. J Vet Med Sci. 2005;67:667-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Komine K, Kuroishi T, Ozawa A, Komine Y, Minami T, Shimauchi H, Sugawara S. Cleaved inflammatory lactoferrin peptides in parotid saliva of periodontitis patients. Mol Immunol. 2007;44:1498-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Olivier AJ, Masson L, Ronacher K, Walzl G, Coetzee D, Lewis DA, Williamson AL, Passmore JA, Burgers WA. Distinct cytokine patterns in semen influence local HIV shedding and HIV target cell activation. J Infect Dis. 2014;209:1174-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |