Revised: February 3, 2013
Accepted: February 8, 2013
Published online: February 12, 2013
Processing time: 34 Days and 22.4 Hours
Betanodaviruses cause viral nervous necrosis, an infectious neuropathological condition in fish that is characterized by necrosis of the central nervous system, including the brain and retina. This disease can cause mass mortality in larval and juvenile populations of several teleost species and is of global economic importance. The mechanism of brain and retina damage during betanodavirus infection is poorly understood. In this review, we will focus recent results that highlight betanodavirus infection-induced molecular death mechanisms in vitro. Betanodavirus can induce host cellular death and post-apoptotic necrosis in fish cells. Betanodavirus-induced necrotic cell death is also correlated with loss of mitochondrial membrane potential in fish cells, as this necrotic cell death is blocked by the mitochondrial membrane permeability transition pore inhibitor bongkrekic acid and the expression of the anti-apoptotic Bcl-2 family member zfBcl-xL. Moreover, this mitochondria-mediated necrotic cell death may require a caspase-independent pathway. A possible cellular death pathway involving mitochondrial function and the modulator zfBcl-xs is discussed which may provide new insights into the necrotic pathogenesis of betanodavirus.
- Citation: Hong JR. Betanodavirus: Mitochondrial disruption and necrotic cell death. World J Virol 2013; 2(1): 1-5
- URL: https://www.wjgnet.com/2220-3249/full/v2/i1/1.htm
- DOI: https://dx.doi.org/10.5501/wjv.v2.i1.1
Betanodaviruses cause viral nervous necrosis, an infectious neuropathological condition in fish that is characterized by necrosis of the central nervous system, including the brain and retina[1]. This disease can cause mass mortality in larval and juvenile populations of several teleost species and is of global economic importance[2].
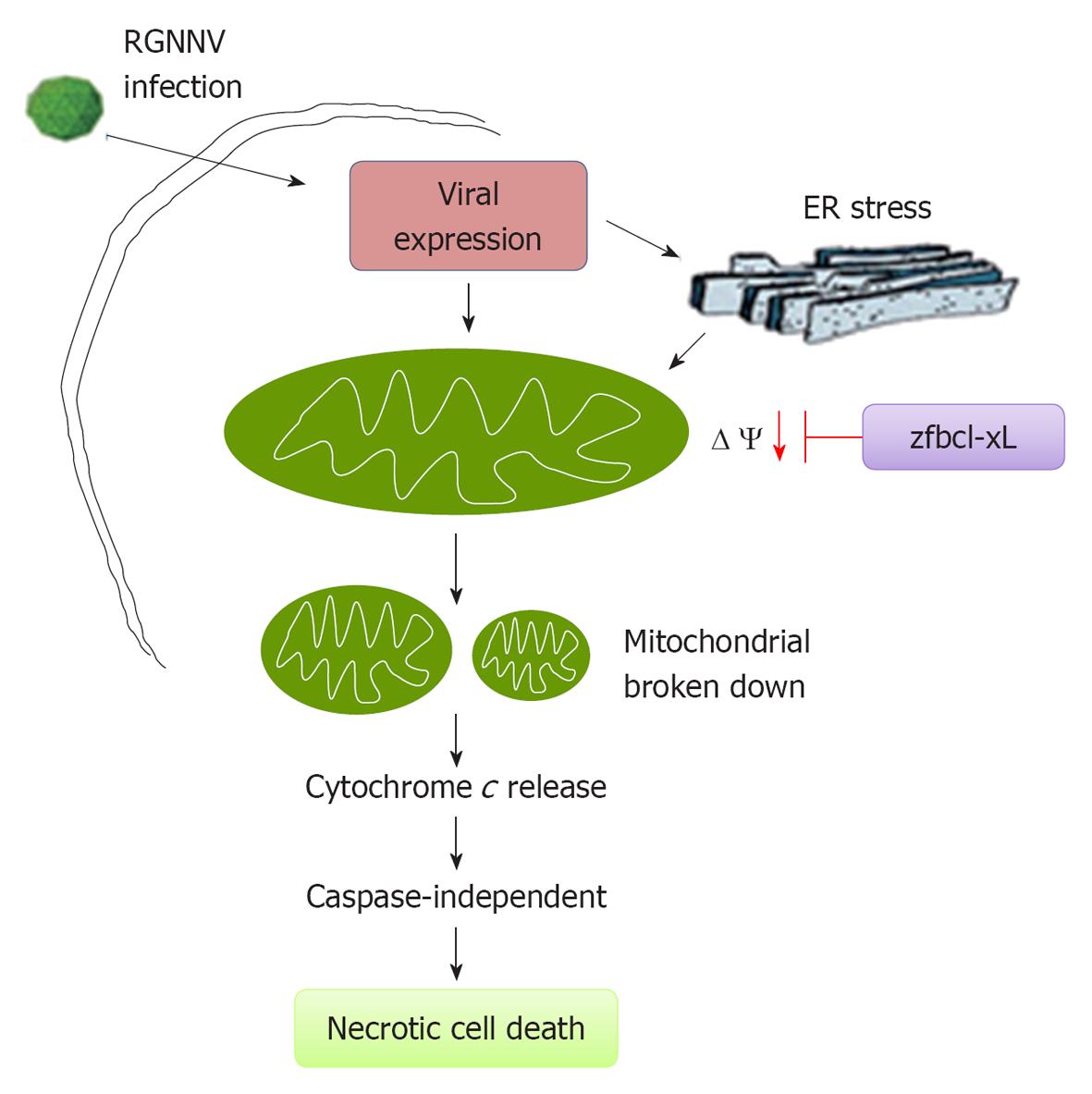
The family Nodaviridae is comprised of the genera Alphanodavirus and Betanodavirus. Alphanodavirus predominantly infects insects, while Betanodavirus predominantly infects fish[3-7]. Nodaviruses are small, nonenveloped, spherical viruses with bipartite positive-sense RNA genomes (RNA1 and RNA2) that are capped but not polyadenylated[3]. RNA1 encodes a non-structural protein of approximately 110 kDa that has been designated RNA-dependent RNA polymerase or protein A. This protein is vital for replication of the viral genome. RNA2 encodes a 42 kDa capsid protein that may also function in the induction of cell death[8,9]. Nodaviruses also synthesize RNA3, a sub-genomic RNA species from the 3’ terminus of RNA1. RNA3 contains two putative open reading frames that potentially encode a 111 amino-acid protein B1 and a 75 amino-acid protein B2[3,10,11]. Recently, the betanodavirus B1 protein has been shown to have an anti-necrotic death function during the early replication stages[10]. In contrast, the betanodavirus B2 protein appears to function as a suppressor of host siRNA silencing[12,13] or as a necrotic death factor[14,15]. In addition, red-spotted grouper nervous necrosis virus (RGNNV) infection and expression can trigger the ER stress response, which results in the upregulation of the 78 kDa glucose regulated protein at the early replication stage[16]. Very recently, RGNNV has been shown to induce the production of reactive oxygen species (ROS) during the early and middle replication stages[17].
Apoptosis and necrosis are two stereotyped mechanisms by which nucleated eukaryotic cells die[18,19]. Necrosis is considered a pathological reaction to major perturbations in the cellular environment such as anoxia[20], while apoptosis is a physiological process that preserves homeostasis by facilitating normal tissue turnover[21,22]. The mechanisms leading to apoptosis are better understood[23-26].
Tumor necrosis factor-α (TNF-α) is a crucial regulator of the innate and adaptive immune response against microbial infection via its regulation of cell death and survival[27]. TNF-α is a pro-inflammatory cytokine that plays important roles in diverse host responses, such as cell proliferation, differentiation, necrosis, apoptosis, and induction of other cytokines. Recently, TNF-α has been shown to induce either nuclear factor κB-initiated survival or apoptosis, depending on the cellular context[28]. As such, many viruses have strategies to neutralize TNF-α either by direct binding and inhibition of the ligand or receptor or by modulation of various downstream signaling events[29,30].
The death receptors (DRs), including TNF receptor-1 (TNF-R1), Fas, DR3, DR4, DR5, and TRAIL, contain an intracellular “death domain” that influences downstream signaling pathways by means of homotypic interactions with adaptor proteins, such as FADD, TRADD, and receptor-interacting protein-1 (RIP1)[31]. These DRs induce apoptosis in many cell types through activation of caspase 8. Activated caspase 8 may act indirectly to induce apoptosis through cleavage of Bid. The truncated Bid protein acts on the mitochondria to cause the release of cytochrome c, which further activates downstream caspase 9. Furthermore, TNF-R1 is also involved in the initiation of necrotic cell death (Figure 1)[32]. TNFα and other cytokines that bind to receptors of different-classes promote the generation of ROS, which functions as a second messenger in the necrotic cell death pathway[33,34].
RIP1 is an intracellular adaptor molecule with kinase activity[35]. The RIP1[36] and RIP3[37] proteins appear to be crucial for the initiation of caspase-independent cell death. RIP1 is also necessary for the generation of ROS by TNF-α[33,34].
Other research has shown that TNF-α activates RIP1 kinase-mediated signaling, promoting the induction of downstream genes influencing necrosis or apoptosis[38].
In aquatic betanodavirus systems, RGNNV induces exposure of phosphatidylserine (PS; an early apoptotic marker) at an early apoptotic stage[39], as determined by annexin-V assays. Secondary necrotic morphological changes are also evident at middle and late stages under phase-contrast microscopy in RGNNV-infected grouper cells using acridine orange (AO) and ethidium bromide (EtBr) to identify apoptotic and post-apoptotic necrotic cells; double-stained cells are often observed. Furthermore, RGNNV infection can induce ROS production in mitochondria at the early replication stage [24 h postinfection (p.i.)]. Viral expression during this stage leads to ROS production, triggering an oxidative stress response[17], which may contribute to secondary necrotic cell death. In our system, RGNNV induces necrotic cell death, but whether or not this requires RIP1 kinase-mediated signaling is still unknown.
Apoptosis is controlled at the mitochondrial level by the sequestration of apoptogenic proteins in the mitochondrial intermembrane space and the cytosolic release of these factors on exposure to proapoptotic signals[39,40]. Disruption of the mitochondrial membrane potential (MMP) initiates the caspase cascade, leading to downstream activation of apoptosis[40,41]. MMP can affect both the inner and outer mitochondrial membranes, and this precedes the signs of necrotic or apoptotic cell death, including the apoptosis-specific activation of caspases[42]. Adenine nucleotide translocase (ANT) plays a role in the exchange of ATP for ADP through the inner mitochondrial membrane, thus supplying the cytoplasm with ATP newly synthesized by oxidative phosphorylation. In a search for proapoptotic proteins, Bauer et al[43] identified the protein ANT1 as the main inducer of apoptosis. The overexpression of ANT1 produces rapid cell death, with a concomitant decrease in MMP and an increase in nucleosomal DNA degradation. Since this cell death is sensitive to caspase inhibitors and to inhibitors of the mitochondrial permeability transition pore (MPTP), such as bongkrekic acid (BKA), apoptosis and the involvement of MPTP are thus implicated[43]. Hence, the mitochondrion is appreciated as a central integrator of pro-death stimuli, streamlining various types of proapoptotic signals into a common caspase-dependent pathway[41].
In a betanodavirus system, secondary necrosis is correlated with loss of MMP in grouper liver cells[44] and mitochondrial breakdown at the middle and late apoptotic stages[11]. The loss of MMP is dramatically inhibited by the ANT specific inhibitor BKA, which enhances host-cell viability at the early and middle apoptotic stages[44]. Furthermore, RGNNV-induced mitochondrial cytochrome c release is also blocked following BKA treatment at the early (24 h p.i.) and middle (48 h p.i.) stages.
Apoptosis removes damaged, infected, and superfluous cells. In most circumstances, a cell’s decision to live or die rests largely with the Bcl-2 family of interacting proteins[45,46]. The Bcl-2 family of proteins includes both anti- and pro-apoptotic molecules that act at a critical intracellular decision point along a common death pathway[47]. The ratio of antagonists (Bcl-2, Bcl- xL, Mcl-1, Bcl-W, and A1) to agonists (Bax, Bak, Bcl-xS, Bid, Bik, Bad, PUMA, and NOXA) dictates whether a cell responds to a proximal apoptotic stimulus[46,47]. The Bcl-2 family member proteins also interact with mitochondria to regulate MMP[42]. Changes in MMP, which can include permeabilization of both the inner and outer membranes, precede necrotic or apoptotic cell death[40], highlighting the central role of the mitochondrion as a integrator of pro-death stimuli[41]. Cytochrome c release from mitochondria into the cytosol is initiated by the interaction of mitochondria with one or more members of the Bcl-2 family. Thus, Bcl-2 proteins, which critically regulate apoptosis, function prior to the irreversible damage of cellular constituents[48-50].
In our fish system, we found that RGNNV infection can induce downregulation of the anti-apoptotic Bcl-2 genes at the middle apoptotic stage (48 h p.i.)[16]. Subsequently, mitochondrial damage and RGNNV-induced necrotic cell death were assessed in stable cell lines producing the anti-apoptotic Bcl-2 proteins, zfBcl-xL or zfMcl-1a. Both zfBcl-xL and zfMcl-1a strongly inhibited RGNNV-induced necrotic cell death and reduced the percentage of necrotic cells at 36 h p.i. by up to 90% (zfBcl-xL) and 93% (zfMcl-1a), respectively, when compared with the NNV-infected control group. Cell viability was correspondingly enhanced at 36 h p.i. by 102% (zfBcl-xL) and 98% (zfMcl-1a), respectively, when compared with the NNV-infected control group[11]. Furthermore, overexpression of zfBcl-xL dramatically blocked RGNNV viral death factor protein α[9] and B2[14] induction of cell death.
The mitochondrion is seen as a central integrator of pro-death stimuli, streamlining various types of proapoptic signals into a common caspase-dependent pathway[41], although the absolute requirement for caspase activation in apoptosis is no longer considered dogma[51,52].
The molecular cornerstones of apoptosis are the family of cysteinyl aspartate-specific proteases, collectively known as caspases. At least 13 caspases have been identified[53], and members of this family can be subdivided into two groups: initiators and executioners. Initiator caspases serve to relay death signals from proapoptotic signals to executioner caspases, which then cleave key proteins involved in cellular structure and function. Known initiators include caspase 8 and caspase 9, whereas known effectors include caspase 3[54], caspase 6, and caspase 7.
Our analysis of caspase 3, caspase 8, and caspase 9 activities revealed no significant differences relative to normal control cells at 0, 24, 48, and 72 h p.i. with RGNNV (MOI = 5), and cell death was not effectively blocked by treatment with a pan-caspase inhibitor[11]. The results of these assays suggest that betanodavirus can induce caspase-dependent and caspase-independent death pathways that may be dependent on the specific cell line used. In grouper liver cells, RGNNV may preferentially induce caspase-independent death, but GGNNV induces caspase-dependent death in sea bass cells[8].
We have reviewed the cellular impact of RGNN viral infection on cell viability via modulation of mitochondrial necrotic cell death in fish cells. Over recent years, our knowledge about mitochondria-mediated apoptotic cell death has expanded, but our understanding of mitochondria-mediated necrotic cell death is still limited, especially in lower vertebrates. In addition, we are beginning to uncover the physiological roles of mitochondria-mediated caspase-independent necrotic cell death. However, despite these recent advances, many questions remain largely unanswered. What signaling occurs upstream of necrotic cell death following betanodavirus infection Does induction of autophagy affect necrotic cell death during viral replication What parameters, in addition to mitochondria-shaping proteins, control mitochondrial fusion and fission[15,55] Hopefully, future studies will increase our understanding of the mechanisms underlying mitochondria-mediated necrosis, its functions in multiple biological processes, and the regulatory signaling pathways that control its activation. This knowledge will be of great importance for validating mitochondria-mediated necrosis as an effective target for the treatment of various diseases, including RNA viral infections.
P-Reviewers Chaivisuthangkura P, Ghiringhelli PD S- Editor Song XX L- Editor Hughes D E- Editor Zheng XM
| 1. | Bovo G, Nishizawa T, Maltese C, Borghesan F, Mutinelli F, Montesi F, De Mas S. Viral encephalopathy and retinopathy of farmed marine fish species in Italy. Virus Res. 1999;63:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Tan C, Huang B, Chang SF, Ngoh GH, Munday B, Chen SC, Kwang J. Determination of the complete nucleotide sequences of RNA1 and RNA2 from greasy grouper (Epinephelus tauvina) nervous necrosis virus, Singapore strain. J Gen Virol. 2001;82:647-653. [PubMed] |
| 3. | Ball LA, Johnson KL. Reverse genetics of nodaviruses. Adv Virus Res. 1999;53:229-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Schneemann A, Reddy V, Johnson JE. The structure and function of nodavirus particles: a paradigm for understanding chemical biology. Adv Virus Res. 1998;50:381-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Toffolo V, Negrisolo E, Maltese C, Bovo G, Belvedere P, Colombo L, Valle LD. Phylogeny of betanodaviruses and molecular evolution of their RNA polymerase and coat proteins. Mol Phylogenet Evol. 2007;43:298-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Delsert C, Morin N, Comps M. A fish encephalitis virus that differs from other nodaviruses by its capsid protein processing. Arch Virol. 1997;142:2359-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Mori K, Nakai T, Muroga K, Arimoto M, Mushiake K, Furusawa I. Properties of a new virus belonging to nodaviridae found in larval striped jack (Pseudocaranx dentex) with nervous necrosis. Virology. 1992;187:368-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 256] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Guo YX, Wei T, Dallmann K, Kwang J. Induction of caspase-dependent apoptosis by betanodaviruses GGNNV and demonstration of protein alpha as an apoptosis inducer. Virology. 2003;308:74-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Wu HC, Chiu CS, Wu JL, Gong HY, Chen MC, Lu MW, Hong JR. Zebrafish anti-apoptotic protein zfBcl-xL can block betanodavirus protein alpha-induced mitochondria-mediated secondary necrosis cell death. Fish Shellfish Immunol. 2008;24:436-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Chen LJ, Su YC, Hong JR. Betanodavirus non-structural protein B1: A novel anti-necrotic death factor that modulates cell death in early replication cycle in fish cells. Virology. 2009;385:444-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Chen SP, Wu JL, Su YC, Hong JR. Anti-Bcl-2 family members, zfBcl-x(L) and zfMcl-1a, prevent cytochrome c release from cells undergoing betanodavirus-induced secondary necrotic cell death. Apoptosis. 2007;12:1043-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Fenner BJ, Goh W, Kwang J. Sequestration and protection of double-stranded RNA by the betanodavirus b2 protein. J Virol. 2006;80:6822-6833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Iwamoto T, Mise K, Takeda A, Okinaka Y, Mori K, Arimoto M, Okuno T, Nakai T. Characterization of Striped jack nervous necrosis virus subgenomic RNA3 and biological activities of its encoded protein B2. J Gen Virol. 2005;86:2807-2816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Su YC, Wu JL, Hong JR. Betanodavirus non-structural protein B2: A novel necrotic death factor that induces mitochondria-mediated cell death in fish cells. Virology. 2009;385:143-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Su YC, Hong JR. Betanodavirus B2 causes ATP depletion-induced cell death via mitochondrial targeting and complex II inhibition in vitro and in vivo. J Biol Chem. 2010;285:39801-39810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Su YC, Wu JL, Hong JR. Betanodavirus up-regulates chaperone GRP78 via ER stress: roles of GRP78 in viral replication and host mitochondria-mediated cell death. Apoptosis. 2011;16:272-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Chang CW, Su YC, Her GM, Ken CF, Hong JR. Betanodavirus induces oxidative stress-mediated cell death that prevented by anti-oxidants and zfcatalase in fish cells. PLoS One. 2011;6:e25853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4980] [Cited by in RCA: 4834] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 19. | Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3-15. [PubMed] |
| 20. | Herman B, Nieminen AL, Gores GJ, Lemasters JJ. Irreversible injury in anoxic hepatocytes precipitated by an abrupt increase in plasma membrane permeability. FASEB J. 1988;2:146-151. [PubMed] |
| 21. | Duvall E, Wyllie AH. Death and the cell. Immunol Today. 1986;7:115-119. |
| 22. | McConkey DJ, Nicotera P, Hartzell P, Bellomo G, Wyllie AH, Orrenius S. Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration. Arch Biochem Biophys. 1989;269:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 391] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | Jeurissen SH, Wagenaar F, Pol JM, van der Eb AJ, Noteborn MH. Chicken anemia virus causes apoptosis of thymocytes after in vivo infection and of cell lines after in vitro infection. J Virol. 1992;66:7383-7388. [PubMed] |
| 24. | Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3743] [Cited by in RCA: 3158] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 25. | Inoue Y, Yasukawa M, Fujita S. Induction of T-cell apoptosis by human herpesvirus 6. J Virol. 1997;71:3751-3759. [PubMed] |
| 26. | Hong JR, Wu JL. Molecular regulation of cellular apoptosis by fish infectious pancreatic necrosis virus (IPNV) infection. Curr Top Virol. 2002;2:151-160. |
| 27. | Fiers W, Beyaert R, Declercq W, Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719-7730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 643] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 28. | Ting AT, Pimentel-Muiños FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15:6189-6196. [PubMed] |
| 29. | Benedict CA. Viruses and the TNF-related cytokines, an evolving battle. Cytokine Growth Factor Rev. 2003;14:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Benedict CA, Banks TA, Ware CF. Death and survival: viral regulation of TNF signaling pathways. Curr Opin Immunol. 2003;15:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Zheng L, Bidere N, Staudt D, Cubre A, Orenstein J, Chan FK, Lenardo M. Competitive control of independent programs of tumor necrosis factor receptor-induced cell death by TRADD and RIP1. Mol Cell Biol. 2006;26:3505-3513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Morgan MJ, Kim YS, Liu ZG. TNFalpha and reactive oxygen species in necrotic cell death. Cell Res. 2008;18:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Meurer R, MacIntyre DE. Lack of effect of pertussis toxin on TNF-alpha-induced formation of reactive oxygen intermediates by human neutrophils. Biochem Biophys Res Commun. 1989;159:763-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Shakibaei M, Schulze-Tanzil G, Takada Y, Aggarwal BB. Redox regulation of apoptosis by members of the TNF superfamily. Antioxid Redox Signal. 2005;7:482-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell Death Differ. 2007;14:400-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 363] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 36. | Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1360] [Cited by in RCA: 1552] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 37. | He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1879] [Article Influence: 117.4] [Reference Citation Analysis (0)] |
| 38. | Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311-1323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 824] [Cited by in RCA: 886] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 40. | Madesh M, Antonsson B, Srinivasula SM, Alnemri ES, Hajnóczky G. Rapid kinetics of tBid-induced cytochrome c and Smac/DIABLO release and mitochondrial depolarization. J Biol Chem. 2002;277:5651-5659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 149] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 41. | Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255-E263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1110] [Cited by in RCA: 1097] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 42. | Zamzami N, Kroemer G. The mitochondrion in apoptosis: how Pandora’s box opens. Nat Rev Mol Cell Biol. 2001;2:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 750] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 43. | Bauer MK, Schubert A, Rocks O, Grimm S. Adenine nucleotide translocase-1, a component of the permeability transition pore, can dominantly induce apoptosis. J Cell Biol. 1999;147:1493-1502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 209] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 44. | Chen SP, Yang HL, Her GM, Lin HY, Jeng MF, Wu JL, Hong JR. Betanodavirus induces phosphatidylserine exposure and loss of mitochondrial membrane potential in secondary necrotic cells, both of which are blocked by bongkrekic acid. Virology. 2006;347:379-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590-8607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1107] [Cited by in RCA: 1102] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 46. | Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3451] [Cited by in RCA: 3575] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 47. | Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3154] [Cited by in RCA: 3499] [Article Influence: 205.8] [Reference Citation Analysis (0)] |
| 48. | Galluzzi L, Brenner C, Morselli E, Touat Z, Kroemer G. Viral control of mitochondrial apoptosis. PLoS Pathog. 2008;4:e1000018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 348] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 49. | Farrow SN, Brown R. New members of the Bcl-2 family and their protein partners. Curr Opin Genet Dev. 1996;6:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 194] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 50. | Shen Y, Shenk TE. Viruses and apoptosis. Curr Opin Genet Dev. 1995;5:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 265] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 51. | Chipuk JE, Green DR. Do inducers of apoptosis trigger caspase-independent cell death. Nat Rev Mol Cell Biol. 2005;6:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 52. | Kroemer G, Martin SJ. Caspase-independent cell death. Nat Med. 2005;11:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 537] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 53. | Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5088] [Article Influence: 188.4] [Reference Citation Analysis (0)] |
| 54. | Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3009] [Cited by in RCA: 3156] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 55. | Karbowski M, Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003;10:870-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 591] [Article Influence: 26.9] [Reference Citation Analysis (0)] |