Published online Dec 25, 2024. doi: 10.5501/wjv.v13.i4.95986
Revised: August 14, 2024
Accepted: August 27, 2024
Published online: December 25, 2024
Processing time: 177 Days and 11.7 Hours
The diagnosis of West Nile virus (WNV) is challenging due to short-term and low-level viremia, flavivirus cross-reactivity, and long immunoglobulin M (IgM) persistence.
To evaluate different methods for WNV detection [reverse transcription-polymerase chain reaction (RT-PCR), IgM/IgG antibodies, IgG avidity] in serum, cerebrospinal fluid (CSF), and urine samples of patients with confirmed WNV infection.
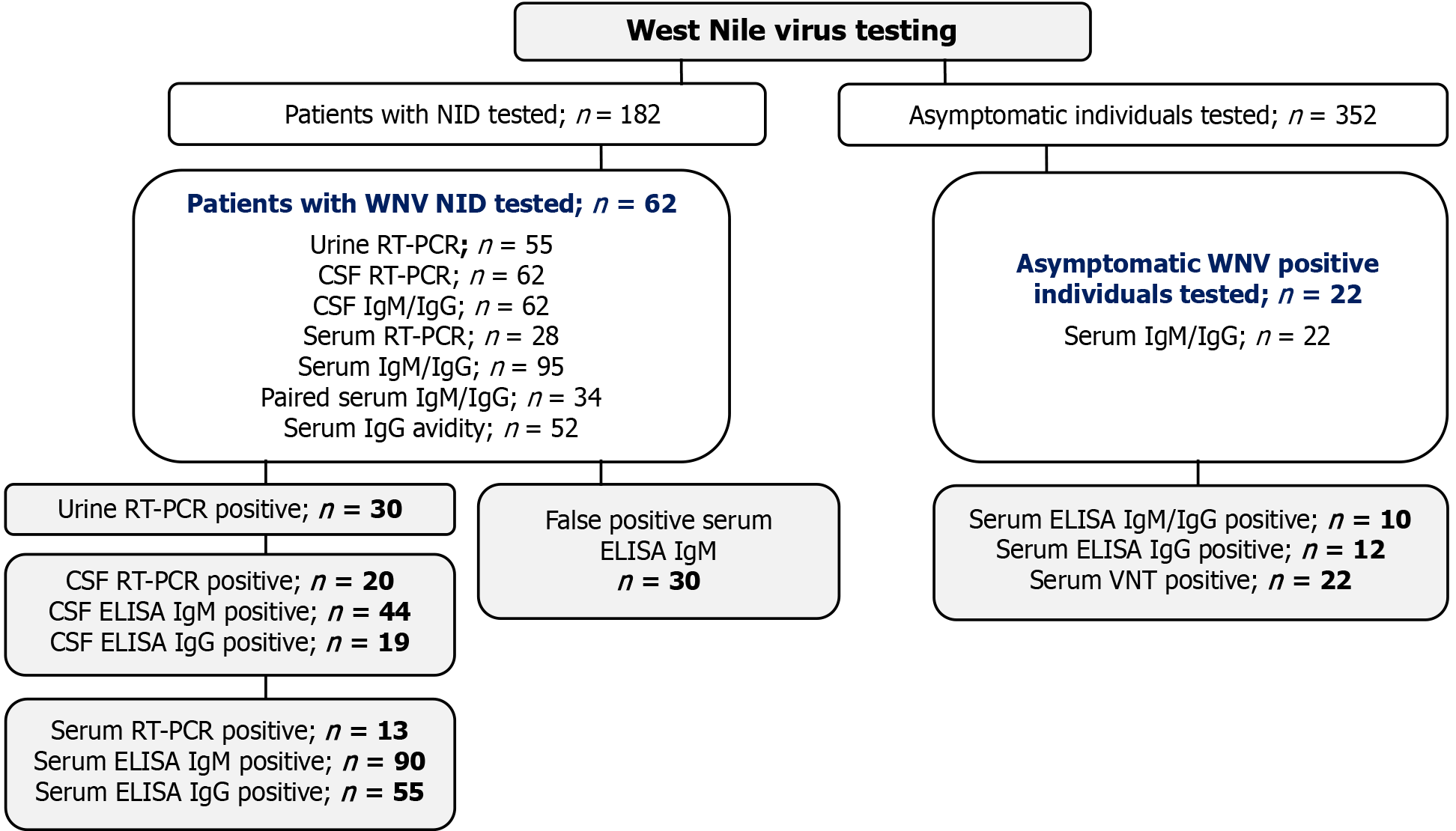
The study included patients with confirmed WNV neuroinvasive infection (n = 62), asymptomatic WNV seropositive individuals (n = 22), and individuals with false-positive WNV IgM antibodies (n = 30). WNV RNA was detected using RT-PCR. A commercial ELISA was used to detect WNV IgM/IgG antibodies with confirmation of cross-reactive samples using a virus neutralization test (VNT). IgG-positive samples were tested for IgG avidity.
The WNV-RNA detection rates were significantly higher in the urine (54.5%)/serum (46.4%) than in CSF (32.2%). According to the sampling time, the WNV-RNA detection rates in urine collected within 7 days/8-14/≥ 15 days were 29.4/66.6/62.5% (P = 0.042). However, these differences were not observed in the CSF. The median RT-PCR cycle threshold values were significantly lower in urine (32.5, IQR = 28-34) than in CSF (34.5, IQR = 33-36). The frequency of positive WNV IgM and IgG significantly differed according to the sampling time in serum but not in CSF. Positive IgM/IgG antibodies were detected in 84.3/9.3% of serum samples collected within 7 days, 100/71.1% of samples collected 8-14, and 100% samples collected after ≥ 15 days. Recent WNV infection was confirmed by low/borderline avidity index (AI) in 13.6% of asymptomatic individuals. A correlation between ELISA and AI was strong negative for IgM and strong positive for IgG. No significant correlation between ELISA IgG and VNT was found.
The frequency of WNV RNA and antibody detection depends on the sampling time and type of clinical samples. IgG avidity could differentiate recent WNV infections from long-persisting IgM antibodies.
Core Tip: We analyzed different diagnostic methods in patients with West Nile virus (WNV) neuroinvasive disease and asymptomatic seropositive individuals. The WNV RNA detection rate was significantly higher in the urine/serum than in cerebrospinal fluid (CSF). The RT-PCR cycle threshold (Ct) values were significantly lower in urine than in CSF and serum samples. The frequency of WNV RNA and IgM/IgG antibody detection rates depends on the sampling time and type of clinical samples (CSF or serum). The correlation between ELISA and IgG avidity was negative for IgM and positive for IgG. No correlation was observed between ELISA IgG and virus neutralization test.
- Citation: Vilibic-Cavlek T, Bogdanic M, Savic V, Hruskar Z, Barbic L, Stevanovic V, Antolasic L, Milasincic L, Sabadi D, Miletic G, Coric I, Mrzljak A, Listes E, Savini G. Diagnosis of West Nile virus infections: Evaluation of different laboratory methods. World J Virol 2024; 13(4): 95986
- URL: https://www.wjgnet.com/2220-3249/full/v13/i4/95986.htm
- DOI: https://dx.doi.org/10.5501/wjv.v13.i4.95986
West Nile virus (WNV) is an emerging flavivirus of public health importance. In nature, the WNV transmission cycle includes birds as virus reservoirs and mosquitoes, mainly of the genus Culex as vectors. WNV is endemic in many parts of the world (Europe, the United States, the Middle East, Asia, and Africa) causing outbreaks and sporadic infections in both endemic and non-endemic areas[1]. Although the majority of WNV infections (80%) are asymptomatic or present as a non-specific febrile disease (WNV fever), some patients develop neuroinvasive disease (meningitis, encephalitis, myelitis). The mortality rate can reach 10% in severe neuroinvasive forms of the disease[2].
WNV shares many features with some other neuroinvasive arboviruses, especially flaviviruses such as tick-borne encephalitis virus (TBEV), and Usutu virus (USUV). Due to overlapping geographic distributions and clinical symptoms with these infections, the WNV diagnosis should be confirmed using virological methods[3]. The Centers for Disease Control and Prevention/European Center for Disease Control and Prevention (ECDC) laboratory criteria for WNV confirmation include: (1) Direct detection of virus by culture, antigen, or viral RNA; (2) A four-fold rise in antibody titers between acute- and convalescent-phase serum samples; or (3) Virus-specific immunoglobulin M (IgM) confirmed by detection of neutralizing antibodies. The detection of specific IgM antibodies in the cerebrospinal fluid (CSF) and the lack of IgM to other endemic arboviruses are also criteria for confirmation of WNV neuroinvasive disease (NID)[4,5].
Because the majority of arboviruses, including WNV, have a short period of replication (short-term and low-level viremia), limiting the utility of molecular tests, serology is commonly used for disease confirmation. However, antibody cross-reactivity between viruses within the same serocomplex and persistent antibodies from previous arboviral infections complicate the interpretation of serology results[6].
ELISA and indirect immunofluorescence assay are the most commonly used screening tests for WNV serology. Although next-generation tests have improved performance by selecting the best epitopes and enhancing antigen purity, challenges in serological diagnosis still exist. In samples with cross-reactive antibodies, neutralization tests such as virus neutralization test (VNT) and plaque reduction neutralization test (PRNT), which still represent gold standard serology tests, are necessary for confirmation of the first-line serology[7].
Long IgM persistence is frequently observed in WNV infections. In a study conducted in Greece, WNV IgM antibodies were detectable for more than 3 years in 12% of patients with WNV infection[8]. Moreover, a study from Huston has found WNV IgM persistence (positive or equivocal results) in 42%, 34%, and 23% of WNV-infected patients approximately 1 year, 6 years, and 8 years post-infection, respectively[9]. Long-term persistence of WNV IgM antibodies in the CSF was also observed. In a study among patients with WNV NID from Michigan, IgM antibodies were detectable in the CSF of three patients for 110, 141, and 199 days post-acute phase[10]. In cases with long IgM persistence, IgG avidity determination could be useful for the differentiation of acute vs previous WNV infection[11].
Molecular methods such as RT-PCR in blood/serum and CSF samples are limited to the minority of patients who present with ongoing viremia or central nervous system replication[7]. Recent investigations indicated that WNV is excreted in urine longer and at higher levels compared to blood[12].
WNV grows and plaques efficiently on Vero cell culture, usually inducing a cytopathic effect[13]. Virus isolation is time-consuming and laborious[14]. Because WNV is classified as a biosafety level 3 (BSL-3) agent, virus cultivation is restricted to reference laboratories[15].
In Croatia, human WNV infections (WNV NID and WNV fever) have been reported continuously since 2012 in continental counties. In addition, some other flaviviruses are endemic in the same geographic areas, such as TBEV or occur sporadically (USUV)[16].
Because there are still many challenges in flavivirus diagnostics, this study aimed to analyze the characteristics of different methods for the diagnosis of WNV infection.
This retrospective study included patients with NID (n = 182) and asymptomatic individuals (n = 352) tested for WNV during the two Croatian outbreaks (2017-2018). Patients with confirmed WNV NID (IgM/IgG positive and/or RT-PCR positive; n = 62), asymptomatic WNV IgM and/or IgG seropositive individuals (n = 22), and individuals with false positive WNV IgM antibodies (n = 30) were analyzed (Figure 1). Serum and CSF samples were collected in all patients, while urine samples were available for 55 patients. The samples were collected in the period from 4-19 days after disease onset (median 9, IQR = 7-13). In addition, paired serum samples taken 2-3 weeks after the first one were collected in 34 patients. Characteristics of patients with WNV NID are presented in Table 1.
| Characteristic | Subcategory | n (%)1 |
| Sex | Male | 35 (56.4) |
| Female | 27 (43.6) | |
| Age in median years (IQR) | 68 (58-76) | |
| Clinical diagnosis | Febrile headache | 2 (3.2) |
| Meningitis | 36 (58.1) | |
| Meningoencephalitis | 20 (32.3) | |
| Meningoencephalomyelitis | 2 (3.2) | |
| Polyradiculoneuritis | 2 (3.2) |
Serum, CSF, and urine samples were tested for the presence of WNV RNA using real-time RT-PCR as described previously[17]. Initial serological screening (WNV IgM and IgG antibodies) was performed using a commercial ELISA. Samples were also screened for potential cross-reactivity with other flaviviruses endemic in Croatia (TBEV/USUV). WNV cross-reactive samples were confirmed using a VNT[18]. In addition, WNV IgM/IgG positive and IgM negative/IgG positive samples were further tested for IgG avidity to confirm or exclude recent infection[11]. Characteristics of laboratory methods used for the diagnosis of WNV infection are presented in Table 2.
| Method | Manufacturer/Protocol | Reference values |
| RT-PCR | FP: AAG TTG AGT AGA CGG TGC TG; RP: AGA CGG TTC TGA GGG CTT AC; Probe: FAM-CAA CCC CAG GAG GAC TGG-TAMRA | |
| IgM ELISA | Euroimmun, Lübeck, Germany | Ratio < 0.8 negative; 0.8-1.1 borderline; > 0.1 positive |
| IgG ELISA | Euroimmun, Lübeck, Germany | RU/mL < 16 negative, 16-22 borderline; > 22 positive |
| IgG avidity | Euroimmun, Lübeck, Germany | AI < 40% low; 40%-60% borderline; > 60% high |
| VNT | In house | Titer ≥ 10 positive |
WNV positive detection rates were presented as numbers and percentages with 95% confidence intervals (CI). The differences in the positive WNV RNA and antibody detection rates according to clinical sample and sampling time were compared using a χ2 test. A Kruskal-Wallis test was used to compare the differences in RT-PCR cycle threshold (Ct) values. The correlation between ELISA IgM/IgG levels and avidity indices (AI) and IgG/VNT titer was calculated using Spearman’s rank correlation coefficient. P value < 0.05 was considered statistically significant. For statistical analysis, a Social Science Statistics program was used (https://www.socscistatistics.com/tests/).
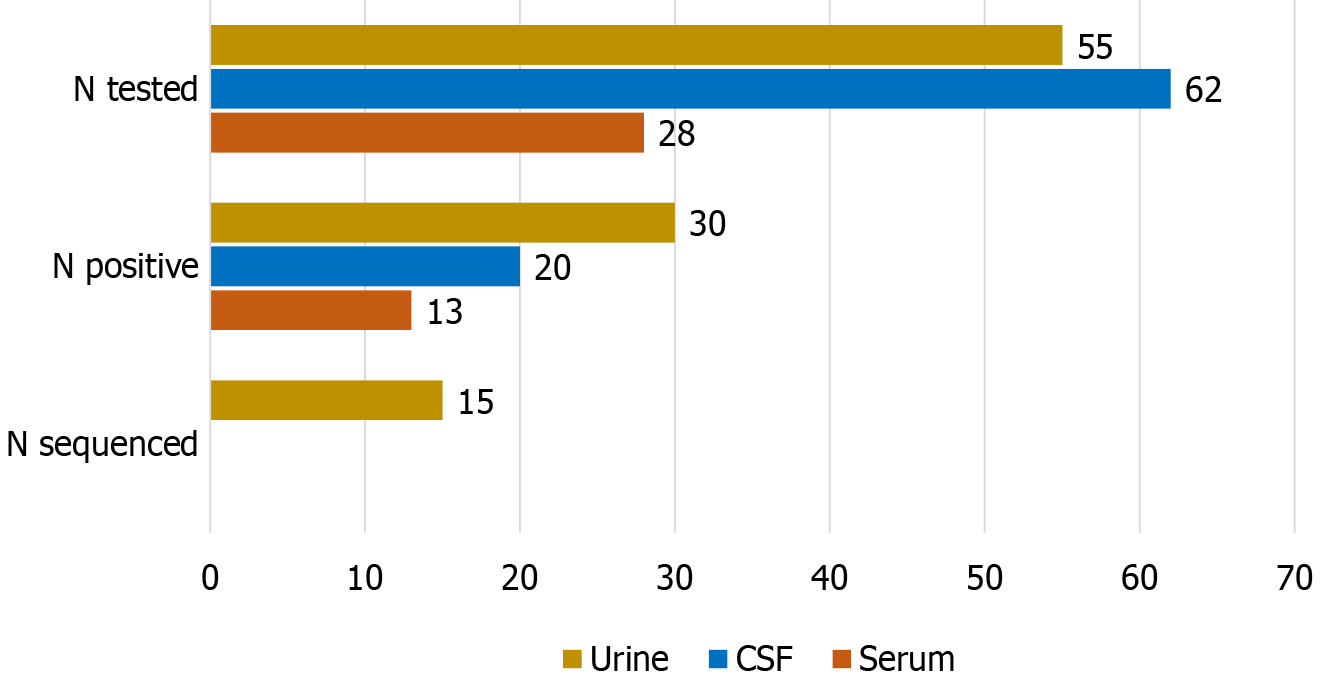
The WNV-positive detection rate was significantly higher (χ2 test P = 0.049) in the urine (30/55; 54.5%) and serum (13/28; 46.4%) than in CSF samples (20/62; 32.2%). Nucleotide sequencing was successful in 15 (27.3%) urine samples (Figure 2).
The RT-PCR positive detection rate depended on the sampling time. In the CSF, the positive detection rate was higher in samples collected within 7 days after disease onset (36.4%) compared to samples collected 8-14 days (31.2%) and ≥ 15 days (25.0%). In contrast, the frequency of WNV RNA positive detection was lower in in urine samples collected within 7 days (29.5%) than in urine samples collected within 8-14 days (66.6%) and ≥ 15 days (62.5%). The observed differences were statistically significant for urine (χ2 test P = 0.042) but not for CSF (χ2 test P = 0.828) (Table 3).
| Days after symptoms onset | WNV RT-PCR CSF | WNV RT-PCR Urine | ||
| Tested | Positive | Tested | Positive | |
| ≤ 7 | 22 (35.5) | 8 (36.4) | 17 (30.9) | 5 (29.4) |
| 8–14 | 32 (51.6) | 10 (31.2) | 30 (54.6) | 20 (66.6) |
| ≥ 15 | 8 (12.9) | 2 (25.0) | 8 (14.5) | 5 (62.5) |
| Total | 62 (100) | 20 (32.2) | 55 (100) | 30 (54.4) |
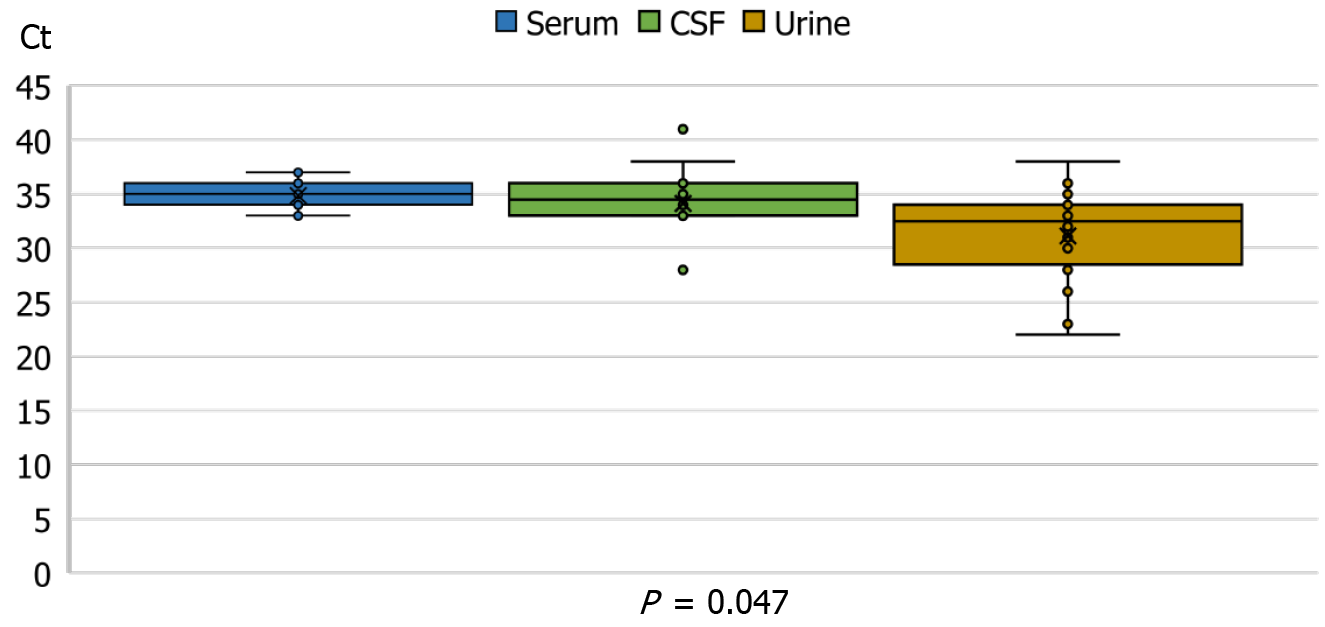
The RT-PCR Ct values were significantly lower in urine samples (median 32.5, IQR = 28-34) than in CSF (median 34.5, IQR = 33-36) or serum samples (median 35, IQR = 34-36), (Kruskal-Wallis test P = 0.047, Figure 3).
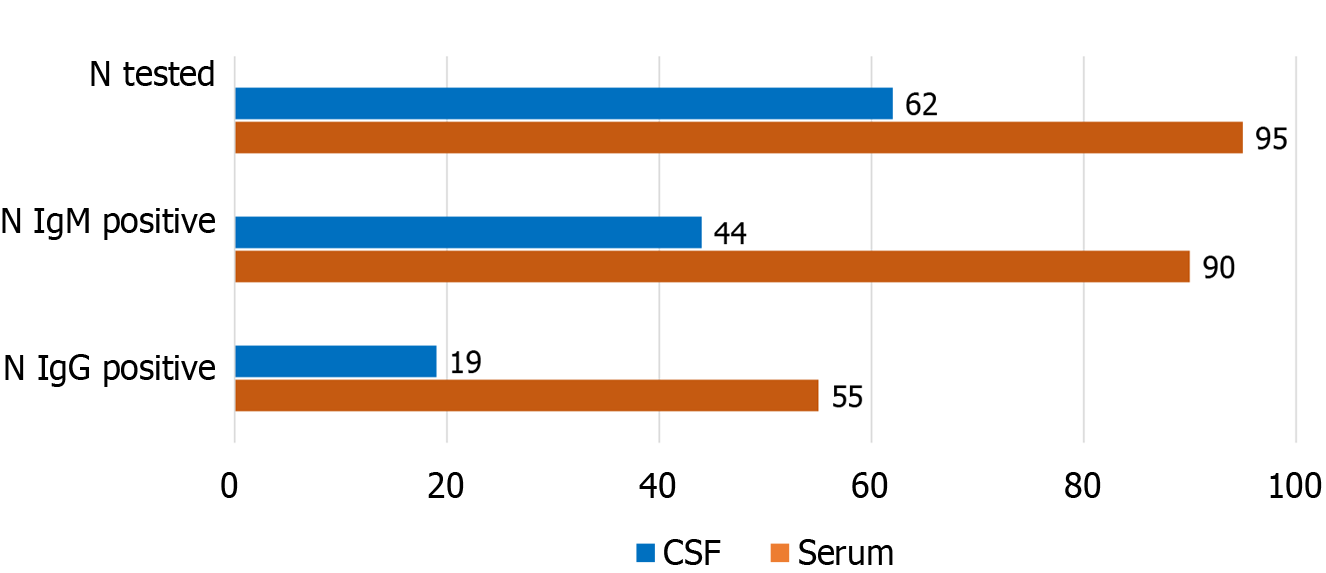
WNV antibody detection rates in serum and CSF samples are presented in Figure 4 and Table 4. Using ELISA, IgM antibodies were detected in 90/95 (94.7%) of serum and 44/62 (70.9%) of CSF samples. IgG antibodies were detected in 55 (57.8%) of serum and 19/62 (30.6%) of CSF samples (Figure 4).
| Days after symptoms onset | Tested | WNV IgM antibodies | WNV IgG antibodies | ||
| Positive | Borderline | Positive | Borderline | ||
| ≤ 7 | 32 (33.7) | 27 (84.3) | 0 (0) | 3 (9.3) | 2 (6.2) |
| 8 – 14 | 38 (40.0) | 38 (100) | 0 (0) | 27 (71.1) | 5 (13.1) |
| ≥ 15 | 25 (26.3) | 25 (100) | 0 (0) | 25 (100) | 0 (0) |
| Total | 95 (100) | 90 (94.7) | 0 (0) | 55 (57.8) | 7 (7.3) |
The frequency of positive results significantly differed according to sampling time in serum, but not in CSF samples. In serum samples collected within the 7 days after disease onset, the IgM-positive detection rate was 84.3%, compared to 100% in samples collected after more than 8 days (χ2 test P = 0.005). Similarly, the IgG-positive detection rate was lowest in samples collected in the first 7 days (9.3% positive and 6.2% borderline). In samples collected 8-14 days after disease onset, IgG antibodies were positive in 71.1% and borderline in 13.1% of samples. All samples collected ≥ 15 days showed WNV IgG antibodies (χ2 test P < 0.001) (Table 4).
The positive IgM detection rates in the CSF samples collected within 7 days, 8-14 days, and ≥ 15 days were 62.5, 73.9, and 100%, respectively (P = 0.145). The frequency of IgG detection was similar in the period ≤ 7 days and 8-14 days (28.1 and 26.1%, respectively), while it was higher in samples collected ≥ 15 days (57.1%; χ2 test P = 0.001) (Table 5).
| WNV IgM antibodies | WNV IgG antibodies | ||||
| Days after symptoms onset | Tested | Positive | Borderline | Positive | Borderline |
| ≤ 7 | 32 (51.6) | 20 (62.5) | 1 (3.1) | 9 (28.1) | 2 (6.2) |
| 8–14 | 23 (37.1) | 17 (73.9) | 0 (0) | 6 (26.1) | 1 (4.3) |
| ≥ 15 | 7 (11.3) | 7 (100) | 0 (0) | 4 (57.1) | 0 (0) |
| Total | 62 (100) | 44 (70.9) | 1 (1.6) | 19 (30.6) | 3 (4.8) |
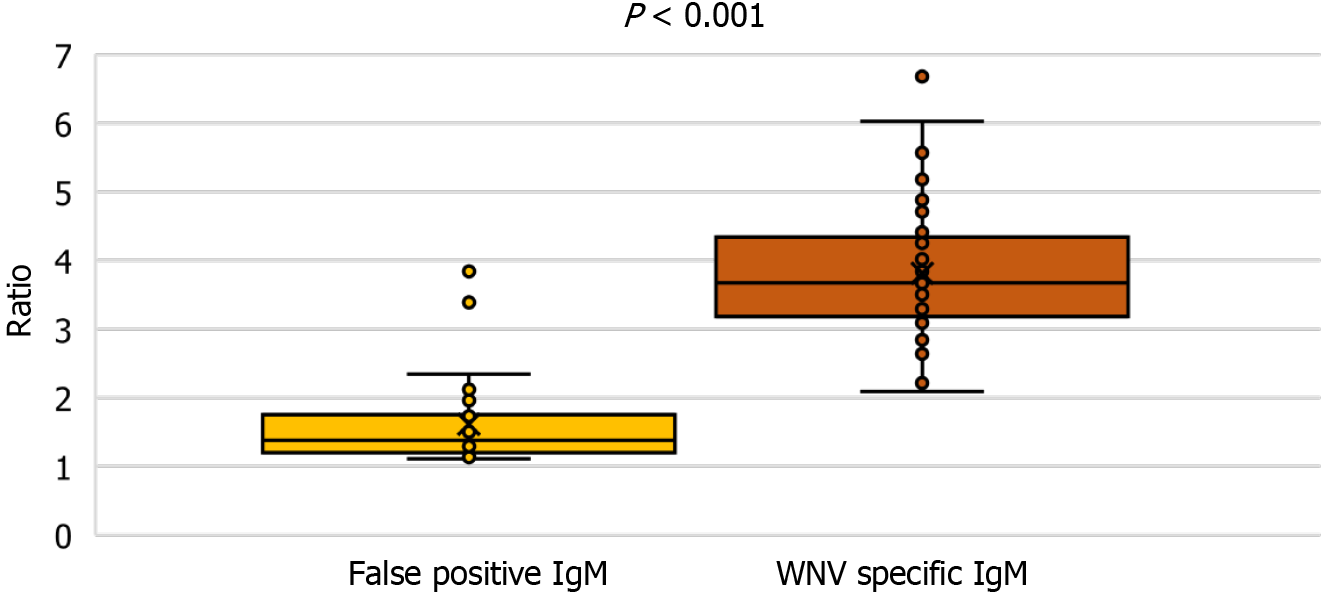
Samples with false positive WNV IgM antibodies (n = 30) were also included in the study. Analyzing the IgM antibody levels, patients with confirmed WNV infection showed a significantly higher IgM (median ratio 3.8, IQR = 3.1-4.3), than patients with false-positive IgM antibodies (median ratio 1.4, IQR = 1.1-1.8) (Kruskall-Wallis test P < 0.001) (Figure 5).
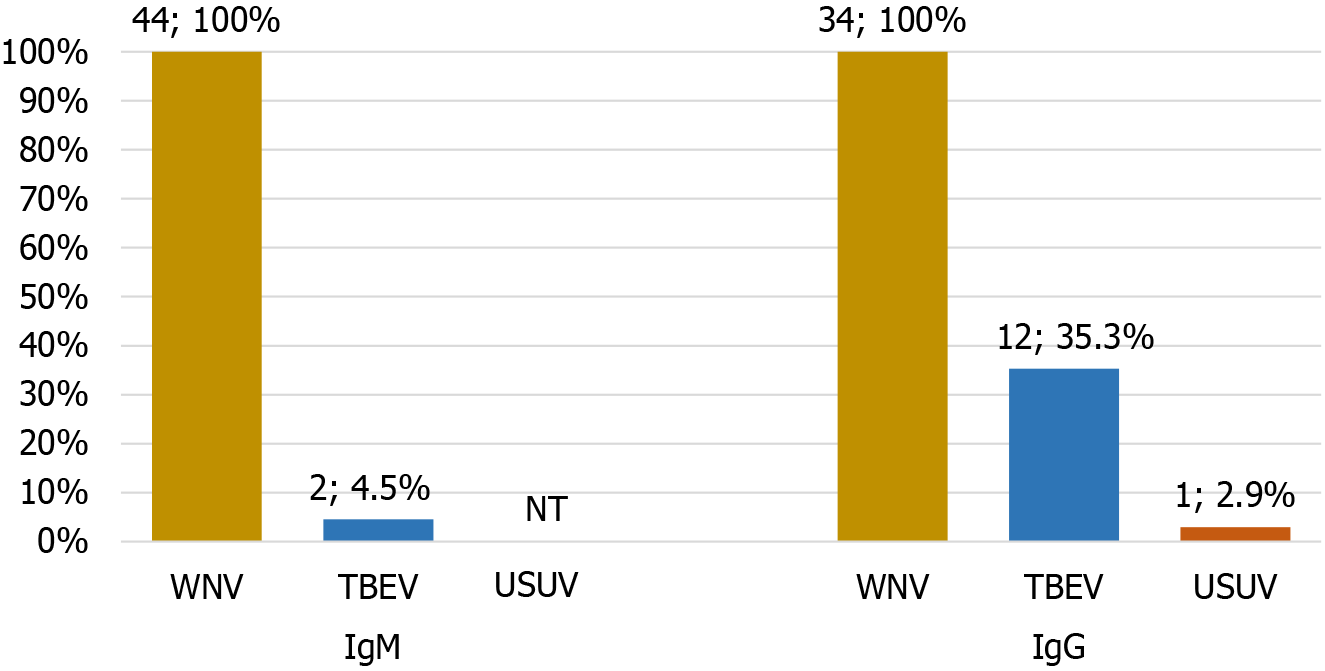
Cross-reactive WNV IgM and IgG antibodies with TBEV/USUV were detected in 4.5% and 38.2% serum samples (Figure 6). The frequency of cross-reactive IgM antibodies was significantly lower than the frequency of cross-reactive IgG antibodies (χ2 test P = 0.001).
IgG avidity was performed in IgM/IgG positive patients with WNV NID (n = 52) and asymptomatic WNV seropositive individuals (n = 22). In patients with NID, 50 samples showed low AI (median 13%, IQR = 7-21), and two samples showed borderline AI (42 and 40%), respectively.
In asymptomatic WNV seropositive individuals, IgG avidity was performed in IgM-positive/IgG-positive samples (n = 10) and IgM-negative/IgG-positive samples (n = 12) (Table 6). Three samples (13.6%) showed low/borderline AI indicating acute/recent WNV infection. Using IgG avidity determination, recent WNV infections were confirmed in two IgM-positive patients and one IgM-negative individual.
| Serology result | IgG avidity index | ||
| Low | Borderline | High | |
| IgM/IgG positive, n = 10 | 1 (10.0) | 1 (10.0) | 8 (80.0) |
| IgM negative/IgG positive, n = 12 | 1 (8.3) | 0 (0) | 11 (91.7) |
| Total, n = 22 | 2 (9.1) | 1 (4.5) | 19 (84.6) |
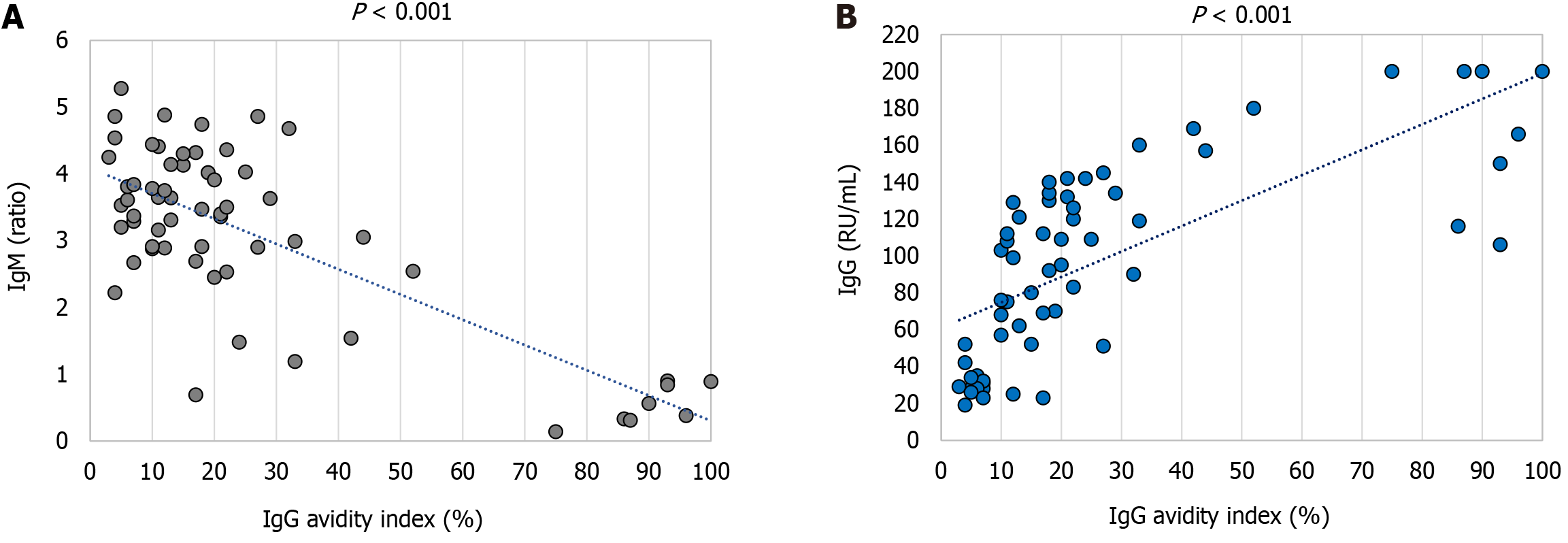
A strong negative correlation (Spearman's rho coefficient -0.512, P < 0.001) between the ELISA IgM levels (ratio) and AI (%) was observed. Samples with the highest IgM levels were associated with the lowest AI values. For IgG antibodies, a correlation between the IgG levels (RU/mL) and AI (%) was a strong positive (Spearman's rho coefficient 0.802, P < 0.001) (Figure 7).
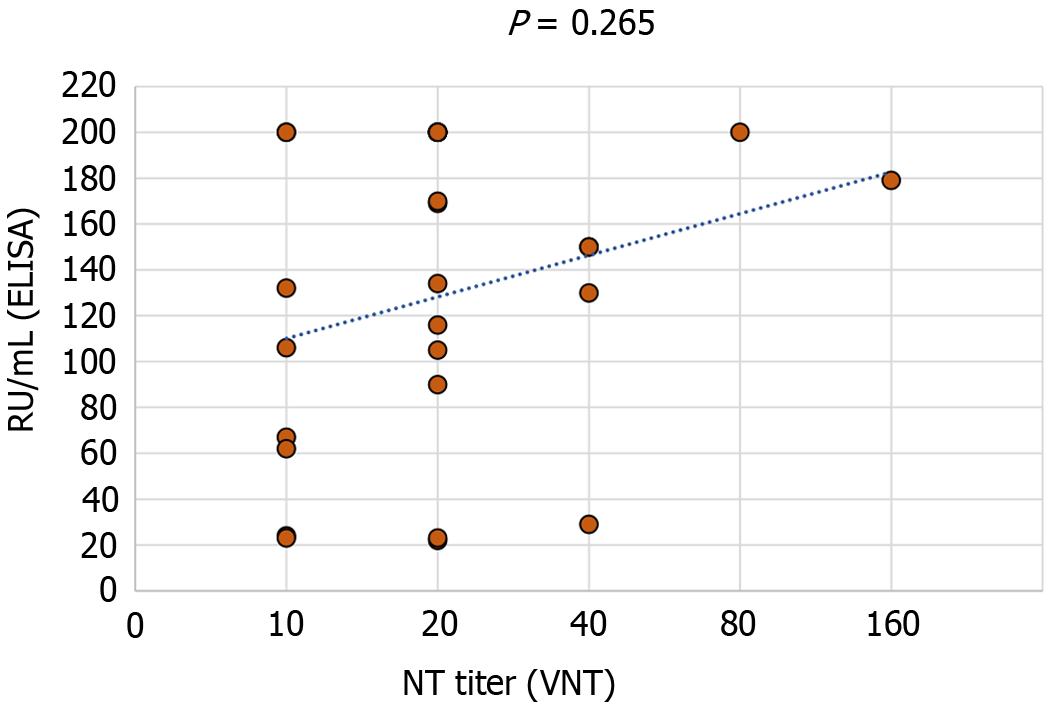
A total of 26 IgM and/or IgG-positive samples were confirmed using VNT. No significant correlation between ELISA IgG levels (RU/mL) and VNT titer was found (Spearman's rho coefficient 0.221, P = 0.265) (Figure 8).
The diagnosis of WNV in humans is challenging due to the short arbovirus viremic phase, low-level viremia (humans are dead-end hosts), the cross-reactivity between flaviviruses, and long-term IgM persistence[6,8,11].
In the present study, WNV RNA positive detection rates depend on the sample type (46.4% in serum, 32.2% in CSF, and 54.4% in urine samples). Similarly, in a study from Italy, patients with WNV NID and WNV fever had detectable WNV RNA in urine at a higher rate (43.8%) than WNV RNA in plasma (37.5%) and CSF (7.1%)[19]. Furthermore, in a study conducted in Israel, whole blood samples were most frequently positive using RT-PCR (86.8%), followed by urine samples (58.3%). The positivity of serum and plasma was 26% and 20%, respectively, while CSF was RT-PCR positive only in 16.5% of patients. All samples were collected on average 11 days after symptom onset. These results suggested that in patients with acute WNV fever, WNV RNA is present in whole blood significantly more frequently than any other sample type tested[20].
Although the period in which WNV RNA is detectable in the CSF varies, it can last several weeks in some patients[1]. In our study, the frequency of WNV RNA detection differs according to the sampling time. In the period within 7 days after symptom onset, 36.4% of CSF samples were RT-PCR positive compared to 31.2% 8-14 days and 25.0% ≥ 15 days after symptom onset. In contrast, the frequency of positive WNV RNA detection rate in urine was lowest in samples collected within 7 days (29.4%) compared to 66.6% and 62.5% for samples collected afterward. In a recently published study from Serbia, WNV infection was confirmed by positive WNV RT-PCR in serum and/or CSF samples in 46.3% of patients. Thirty-one percent more cases were confirmed using urine WNV RNA testing. In contrast to our results, there was no association between the sampling time and WNV RNA urine positivity[12].
Consistent with our findings (the lowest median RT-PCR Ct in urine 32.5, compared to serum 35, and CSF 34.5), in a study from Israel, the WNV viral load in urine was higher than that of whole blood, CSF, serum, and plasma, even though the urine sensitivity was lower than that of whole blood[20].
WNV-specific antibody testing is currently the most widely used approach for the diagnosis of WNV infection. WNV IgM and IgG antibodies are typically detectable by days 4 and 8 after the onset of clinical symptoms[21]. Serological testing of patients included in the present study showed IgM and IgG antibodies in 94.7% and 57.8% of serum samples, respectively. In addition, 70.9% of CSF samples were IgM positive and 30.6% were IgG positive. In the early acute phase of the illness, there is a negative serological window period of detection, as the antibodies have not yet been developed[14]. Our study showed a negative IgM serology in 15.7% of serum and 37.5% of CSF samples collected within 7 days after disease onset. No false negative serum samples were detected after day 8. However, analyzing the CSF serology, 26.1% of CSF samples tested IgM negative in the period 8-14 days, whereas all samples were positive after 14 days. The IgG positivity in serum was 9.3, 71.1, and 100% in samples collected ≤ 7 days, 8-14 days, and ≥ 15 days. In the CSF samples, IgG antibody detection rates were 28.1 and 26.1% in samples collected by day 14 and 57.1% in samples collected after 14 days.
Serological cross-reactivity between flaviviruses is common due to antigenic similarities, especially between the viruses that belong to the same serocomplex. Both species-specific and cross-reactive antibodies are produced during flavivirus infections[22]. People who live in areas where different arboviruses are endemic gradually accumulate cross-reactive antibodies from previous exposures with increasing age[6]. Therefore, it can be challenging to identify the most recent infection in patients with multiple exposures to different flaviviruses[23]. Cross-reacting antibodies are frequently observed in ELISA, while some degree of cross-reactivity was also found in a more specific VNT[24]. Given that WNV and USUV are antigenically closer by genomic phylogeny than TBEV, cross-reactions between WNV and USUV in VNT are usually more common[25]. In our study, ELISA revealed WNV cross-reactivity with TBEV and USUV. IgM cross-reactivity was significantly lower (4.5% of serum samples) than IgG cross-reactivity (38.2% of serum samples). WNV IgG antibodies cross-reacted with TBEV in 35.3% of samples and USUV in 2.9% of samples.
Despite their obvious clinical relevance, IgM antibodies experience false-positive results, which can result in WNV misdiagnosis[26]. Thirty samples with false positive WNV IgM antibodies were also analyzed in the present study. Comparing the levels of false positive IgM antibodies and WNV-specific antibodies, significant differences were observed. WNV-specific antibody levels were significantly higher (median ratio 3.8) than false positive IgM levels (median ratio 1.4).
The diagnostic implications of serum WNV IgM persistence are noteworthy since the presence of IgM antibodies is generally considered evidence of an acute or recent WNV infection. Since the long-term persistence of IgM antibodies is frequently observed in patients with WNV infection[8-10], this should be taken into account when interpreting serology results. IgG avidity was useful to differentiate between recent and previous WNV infection in both patients with NID and WNV fever[11]. In the present study, using avidity determination, 80.0% of tested IgM/IgG-positive asymptomatic individuals were classified as having recent WNV infection, while 20.0% were classified as having previous WNV exposure (long-persisting IgM antibodies). Furthermore, 8.3% of IgM negative/IgG positive individuals had low IgG avidity, suggestive of recent infection.
Our study found a strong negative correlation between IgM levels and IgG avidity, consistent with previous findings in Croatian patients with confirmed WNV infection. The samples with the highest WNV IgM levels had the lowest AI values, indicating that determination of IgG avidity is not required in cases with very high IgM results[11].
Because of its high specificity, neutralization tests are the most reliable serological assays. A correlation between neutralizing flavivirus antibody detection and the presence of specific IgG in blood specimens was observed in some studies[27]. In this study, VNT was used for confirmation of cross-reactive serum samples. However, we found no correlation between WNV-neutralizing antibody titers with binding antibody levels (ELISA). Similarly, some studies have found that the ELISA titers in convalescent WNV patients did not correlate with neutralization nor did neutralization titers increase over time[28].
A limitation of this study that needs to be addressed is the small number of patients tested. Therefore, further studies on a large cohort of WNV patients are needed to confirm our observations.
The frequency of WNV RNA and antibody detection depend on the sampling time and type of clinical samples, which should be considered when interpreting the results. Testing should include flaviviruses that circulate in a specific area to exclude cross-reactive antibodies. IgG avidity determination is a useful diagnostic method for differentiating IgM positivity in recent infections from long-persisting WNV IgM antibodies.
| 1. | Habarugira G, Suen WW, Hobson-Peters J, Hall RA, Bielefeldt-Ohmann H. West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and "One Health" Implications. Pathogens. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 2. | Roberts JA, Kim CY, Dean A, Kulas KE, St George K, Hoang HE, Thakur KT. Clinical and Diagnostic Features of West Nile Virus Neuroinvasive Disease in New York City. Pathogens. 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Simonin Y. Usutu, West Nile, and Tick-Borne Encephalitis Viruses. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | McDonald E, Mathis S, Martin SW, Erin Staples J, Fischer M, Lindsey NP. Surveillance for West Nile virus disease - United States, 2009-2018. Am J Transplant. 2021;21:1959-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Riccetti N, Ferraccioli F, Fasano A, Stilianakis NI. Demographic characteristics associated with West Nile virus neuroinvasive disease - A retrospective study on the wider European area 2006-2021. PLoS One. 2023;18:e0292187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 6. | Kasbergen LMR, Nieuwenhuijse DF, de Bruin E, Sikkema RS, Koopmans MPG. The increasing complexity of arbovirus serology: An in-depth systematic review on cross-reactivity. PLoS Negl Trop Dis. 2023;17:e0011651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 7. | Piantadosi A, Kanjilal S. Diagnostic Approach for Arboviral Infections in the United States. J Clin Microbiol. 2020;58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Papa A, Anastasiadou A, Delianidou M. West Nile virus IgM and IgG antibodies three years post- infection. Hippokratia. 2015;19:34-36. [PubMed] |
| 9. | Murray KO, Garcia MN, Yan C, Gorchakov R. Persistence of detectable immunoglobulin M antibodies up to 8 years after infection with West Nile virus. Am J Trop Med Hyg. 2013;89:996-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Kapoor H, Signs K, Somsel P, Downes FP, Clark PA, Massey JP. Persistence of West Nile Virus (WNV) IgM antibodies in cerebrospinal fluid from patients with CNS disease. J Clin Virol. 2004;31:289-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Vilibic-Cavlek T, Kristofic B, Savic V, Kolaric B, Barbic L, Tabain I, Peric L, Sabadi D, Miklausic B, Potocnik-Hunjadi T, Zember S, Stevanovic V, Listes E, Savini G. Diagnostic significance of immunoglobulin G avidity in symptomatic and asymptomatic West Nile virus infection. Rev Soc Bras Med Trop. 2018;51:591-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Cvjetković IH, Radovanov J, Kovačević G, Turkulov V, Patić A. Diagnostic value of urine qRT-PCR for the diagnosis of West Nile virus neuroinvasive disease. Diagn Microbiol Infect Dis. 2023;107:115920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | McAuley AJ, Beasley DW. Propagation and Titration of West Nile Virus on Vero Cells. Methods Mol Biol. 2016;1435:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Chan KR, Ismail AA, Thergarajan G, Raju CS, Yam HC, Rishya M, Sekaran SD. Serological cross-reactivity among common flaviviruses. Front Cell Infect Microbiol. 2022;12:975398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 15. | Mayo DR, Beckwith WH 3rd. Inactivation of West Nile virus during serologic testing and transport. J Clin Microbiol. 2002;40:3044-3046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Vilibic-Cavlek T, Janev-Holcer N, Bogdanic M, Ferenc T, Vujica Ferenc M, Krcmar S, Savic V, Stevanovic V, Ilic M, Barbic L. Current Status of Vector-Borne Diseases in Croatia: Challenges and Future Prospects. Life (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 17. | Tang Y, Anne Hapip C, Liu B, Fang CT. Highly sensitive TaqMan RT-PCR assay for detection and quantification of both lineages of West Nile virus RNA. J Clin Virol. 2006;36:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Di Gennaro A, Lorusso A, Casaccia C, Conte A, Monaco F, Savini G. Serum neutralization assay can efficiently replace plaque reduction neutralization test for detection and quantitation of West Nile virus antibodies in human and animal serum samples. Clin Vaccine Immunol. 2014;21:1460-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Barzon L, Pacenti M, Franchin E, Pagni S, Martello T, Cattai M, Cusinato R, Palù G. Excretion of West Nile virus in urine during acute infection. J Infect Dis. 2013;208:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Lustig Y, Mannasse B, Koren R, Katz-Likvornik S, Hindiyeh M, Mandelboim M, Dovrat S, Sofer D, Mendelson E. Superiority of West Nile Virus RNA Detection in Whole Blood for Diagnosis of Acute Infection. J Clin Microbiol. 2016;54:2294-2297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Lustig Y, Sofer D, Bucris ED, Mendelson E. Surveillance and Diagnosis of West Nile Virus in the Face of Flavivirus Cross-Reactivity. Front Microbiol. 2018;9:2421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Rathore APS, St John AL. Cross-Reactive Immunity Among Flaviviruses. Front Immunol. 2020;11:334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 23. | Mansfield KL, Horton DL, Johnson N, Li L, Barrett ADT, Smith DJ, Galbraith SE, Solomon T, Fooks AR. Flavivirus-induced antibody cross-reactivity. J Gen Virol. 2011;92:2821-2829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 24. | Oliveira RA, de Oliveira-Filho EF, Fernandes AI, Brito CA, Marques ET, Tenório MC, Gil LH. Previous dengue or Zika virus exposure can drive to infection enhancement or neutralisation of other flaviviruses. Mem Inst Oswaldo Cruz. 2019;114:e190098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Lorusso A, Marini V, Di Gennaro A, Ronchi GF, Casaccia C, Carelli G, Passantino G, D'Alterio N, D'Innocenzo V, Savini G, Monaco F, Horton DL. Antigenic relationship among zoonotic flaviviruses from Italy. Infect Genet Evol. 2019;68:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Landry ML. Immunoglobulin M for Acute Infection: True or False? Clin Vaccine Immunol. 2016;23:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Musso D, Despres P. Serological Diagnosis of Flavivirus-Associated Human Infections. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Throsby M, Goudsmit J, Kruif JD. The Human Antibody Response Against WNV. In: West Nile Encephalitis Virus Infection. Emerging Infectious Diseases of the 21st Century. New York: Springer New York, 2009: 401-416. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |