Published online Jun 25, 2024. doi: 10.5501/wjv.v13.i2.95273
Revised: May 8, 2024
Accepted: June 5, 2024
Published online: June 25, 2024
Processing time: 78 Days and 13.4 Hours
Kidney transplant recipients (KTR) are at risk of severe coronavirus disease 2019 (COVID-19) disease and mortality after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. We predicted that hospitalization for COVID-19 and subsequent admission to the intensive care unit (ICU) would yield worse outcomes in KTRs.
To investigate outcomes among KTRs hospitalized at our high-volume transplant center either on the general hospital floor or the ICU.
We retrospectively describe all adult KTRs who were hospitalized at our center with their first SARS-CoV-2 infection between 04/2020 and 04/2022 and had at least 12 months follow-up (unless they experienced graft failure or death). The cohort was stratified by ICU admission. Outcomes of interest included risk factors for ICU admission and mortality, length of stay (LOS), respiratory symptoms at admission, all-cause graft failure at the last follow-up, and death related to COVID-19.
96 KTRs were hospitalized for SARS-COV-2 infection. 21 (22%) required ICU admission. The ICU group had longer hospital LOS (21.8 vs 8.6 days, P < 0.001) and were more likely to experience graft failure (81% vs 31%, P < 0.001). Of those admitted to the ICU, 76% had death at last-follow up, and 71% had death related to COVID-19. Risk factors for ICU admission included male sex (aHR: 3.11, 95%CI: 1.04-9.34; P = 0.04). Risk factors for all-cause mortality and COVID-19-related mortality included ICU admission and advanced age at SARS-CoV-2 diagnosis. Mortality was highest within a month of COVID-19 diagnosis, with the ICU group having increased risk of all-cause (aHR: 11.2, 95%CI: 5.11-24.5; P < 0.001) and COVID-19-related mortality (aHR: 27.2, 95%CI: 8.69-84.9; P < 0.001).
ICU admission conferred an increased risk of mortality, graft failure, and longer LOS. One-fifth of those hospitalized died of COVID-19, reflecting the impact of COVID-19-related morbidity and mortality among KTRs.
Core Tip: This retrospective study investigated risk factors and outcomes among kidney-only transplant recipients who were diagnosed with and hospitalized for severe acute respiratory syndrome coronavirus 2 infection at a large volume transplant center within the first two years of the coronavirus disease 2019 (COVID-19) pandemic. Recipients were divided into two groups based on whether they were admitted and/or transferred to the intensive care unit (ICU) or the general care floors. Recipients admitted to the ICU had longer hospital length of stays, higher risk of graft failure, and higher all-cause and COVID-19-related mortality rates compared to the general care group. Male sex was a risk factor for ICU admission.
- Citation: Zona EE, Gibes ML, Jain AS, Smith JA, Garonzik-Wang JM, Mandelbrot DA, Parajuli S. Long-term follow-up of kidney transplant recipients admitted to a tertiary care transplant center with SARS-CoV-2. World J Virol 2024; 13(2): 95273
- URL: https://www.wjgnet.com/2220-3249/full/v13/i2/95273.htm
- DOI: https://dx.doi.org/10.5501/wjv.v13.i2.95273
According to the World Health Organization, as of first week of February 2024, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) resulted in 774593066 confirmed cases and claimed the lives of 7028881 individuals worldwide[1]. SARS-CoV-2 has had a disproportionate and devastating impact on immunocompromised populations who are at increased risk of severe coronavirus disease 2019 (COVID-19) disease and adverse outcomes[2]. Early studies among solid organ transplant recipients (SOTRs) reported a mortality rate of approximately 20%, which was higher than the general population[3]. Some studies even report (KTRs) have the highest mortality among SOTRs[4].
Although the current literature has identified many risk factors associated with negative outcomes among KTRs with COVID-19, few have studied this among those hospitalized with the disease. A single-center cohort study of 2500 KTRs found that vaccinated patients had improved outcomes concerning mortality, hospitalization, and intensive care unit (ICU) admission[5]. In another single-center study of 400 KTRs that compared unvaccinated and vaccinated recipients, there were no significant differences in the rates of ICU admission, length of hospital stay, death, or graft failure, although the incidence of SARS-CoV-2 infection was higher in the unvaccinated group[6]. Another study found that the severity of COVID-19 disease necessitating the hospitalization of KTRs was predominantly influenced by comorbid conditions and baseline kidney function [baseline estimated glomerular filtration rate (eGFR)]; however, they found that when adjusted for comorbidity and renal function, they found no statistically significant differences in mortality, ICU admission, and length of stay between adults hospitalized for COVID-19 who had undergone kidney transplant and non-transplanted counterparts[7]. Further study is necessary to understand risk factors and outcomes for ICU admission in this vulnerable population.
To explore this further, we sought to investigate outcomes among KTRs hospitalized at our high-volume transplant center either on the general hospital floor or the ICU. Outcomes of interest were risk factors for ICU admission, risk for all-cause mortality and COVID-19-related mortality.
We evaluated all adult (> 18 years of age) kidney-only transplant recipients at the University of Wisconsin who were subsequently diagnosed with and hospitalized for SARS-CoV-2 infection between April 2020 and April 2022. We excluded individuals who were followed at our center but did not have a functioning allograft. Given that in April 2020 the first case of the SARS-CoV-2 was isolated in our kidney transplant recipient population, April 2020 was selected as the start date for this review. All recipients had at least one year of follow-up unless they had allograft failure or death. Multiorgan transplant recipients or recipients less than 18 years of age at the time of diagnosis of SARS-CoV-2 infection were excluded.
Recipients were divided into two groups based on whether they were admitted/transferred to the ICU or not. Risk factors for ICU admission, all-cause mortality, and COVID-19-related mortality were outcomes of interest. Additionally, we evaluated for changes in allograft function with serum creatinine and eGFR at various periods post-SARS-CoV-2 infection. During hospitalization, monitoring of other non-invasive biomarkers was not routinely performed.
This study was approved by the University of Wisconsin School of Medicine and Public Health Institutional Review Board (IRB protocol number: 2014-1072). This study was in adherence to the Declaration of Helsinki. The clinical and research activities being reported were consistent with the Principles of the Declaration of Istanbul as outlined in ‘The Declaration of Istanbul on Organ Trafficking and Transplant Tourism’. Due to the nature of the study (retrospective, observational) informed consent pertinent to this study was not obtained from the recipients.
We follow our kidney transplant recipients at either the University Hospital or various regional outreach clinics at least once a year until graft failure or until the patient decides to transfer their care to a different center as previously described. All major health events were documented in our master database and were included in the study.
COVID-19-related death was defined as SARS-CoV-2 infection as the cause of mortality as documented in the electronic health record. Every death occurred in the setting of a functional graft. Death-censored graft failure was characterized by either the necessity to resume dialysis or undergo re-transplantation. All rejections were confirmed through biopsy. Vaccination was defined as receiving at least one dose of an available SARS-CoV-2 vaccine. ICU admission was defined as any admission or transfer to the ICU for greater than or equal to 1 day. Indications for ICU admission for SARS-CoV-2 paralleled with any other indications for ICU admission including severe respiratory distress needing high flow oxygen or intubation, hemodynamical instability, and many more.
As previously described, the majority of KTRs are managed with a triple immunosuppressant regimen, primarily comprising tacrolimus, mycophenolic acid, and prednisone[8,9]. A minority of KTRs underwent early steroid withdrawal or received alternative immunosuppression regimens. Once the patient is admitted to the ICU, all regular maintenance immunosuppressive are usually held and recipients are maintained on high-dose intravenous steroids.
Our KTRs are urged to inform the transplant center upon testing positive for or being suspected of having COVID-19. If they test positive, we advise them to pursue early treatment for the disease. Furthermore, patients are encouraged to seek urgent evaluation if their oxygen saturation falls below 90%, or if they encounter worsening shortness of breath, inability to hydrate due to vomiting or diarrhea, or altered mental status.
Categorical data were analyzed using Fisher’s exact test or chi-square test, while continuous data were compared with the Student’s t-test or the Wilcoxon rank-sum test. P values ≤ 0.05 were regarded as statistically significant. Risk factors linked to ICU admission, all-cause mortality, and COVID-19-related mortality were examined through univariate and multivariate stepwise Cox regression analyses. Variables showing associations with outcomes at a significance level of P ≤ 0.10 in the univariate analysis were retained for inclusion in the multivariate analyses. Kaplan-Meier analyses were used to analyze all-cause mortality and COVID-19-related mortality.
96 KTRs received a SARS-CoV-2 infection diagnosis. Of these, 21 (22%) needed ICU admission and 75 (78%) received general hospital-based care. All KTRs included in this study were admitted to our University hospital. The median interval from COVID-19 to the last follow-up in those requiring ICU admission was 0.71 (IQR: 0.49-1.10) months and those who did not requiring ICU admission was 17.04 (IQR: 12.6-28.3) months (P < 0.001). Table 1 summarizes the baseline characteristics. Baseline serum creatinine before COVID-19 diagnosis was higher in the non-ICU group (P < 0.001), but otherwise there were no differences between the two groups.
| Characteristics | ICU admission, n = 21 | Non-ICU admission, n = 75 | P value | |
| Age at transplant (years) | 54.7 ± 13.9 | 50.4 ± 15.2 | 0.68 | |
| Age at COVID-19 diagnosis (years) | 59.5 ± 14.1 | 57.1 ± 14.4 | 0.97 | |
| Male | 16 (76) | 40 (53) | 0.06 | |
| Nonwhite | 4 (19) | 20 (27) | 0.50 | |
| Cause of ESKD | Diabetes mellitus | 6 (28) | 21 (28) | 0.56 |
| Hypertension | 4 (19) | 9 (12) | ||
| Glomerular disease | 8 (38) | 23 (31) | ||
| Polycystic kidney disease | 0 | 6 (8) | ||
| Other | 3 (14) | 16 (21) | ||
| Living donor | 3 (14) | 28 (37) | 0.04 | |
| Previous transplant | 3 (14) | 23 (31) | 0.14 | |
| Maintenance immunosuppressive | Tacrolimus + Mycophenolic acid + prednisone | 18 (86) | 53 (71) | 0.17 |
| Prednisone based immunosuppression | 19 (91) | 69 (91) | 0.98 | |
| Vaccinated | 11 (52) | 44 (59) | 0.61 | |
| Rejection within six months before COVID-19 | 0 | 2 (3) | 0.45 | |
| Baseline serum creatinine before COVID-19 (mg/dL) | 1.57 ± 0.73 | 1.89 ± 1.57 | < 0.001 | |
| Baseline eGFR before COVID-19 (mL/m2) | 53.7 ± 21.1 | 42.3 ± 19.5 | 0.61 | |
| The interval from transplant to the first COVID-19 (month) | 58.5 ± 54.7 | 81.8 ± 68.4 | 0.26 | |
Table 2 compares various outcomes between recipients admitted to the ICU and those admitted to general hospital care. Mean hospital LOS was greater in the ICU group (21.8 ± 19.4) compared with the non-ICU group (8.6 ± 9.8). At last follow-up, patients admitted to the ICU were more likely to experience graft failure and death compared with the non-ICU admission group (P < 0.001). 17 recipients (81%) in the ICU group experienced graft failure compared with 23 recipients (31%) in the non-ICU group. Notably, of the 16 recipients (76%) in the ICU group who had death at last follow-up, 15 (71%) of these deaths were because of COVID-19. In the non-ICU group, 4 (5%) of the 13 (17%) deaths at last follow-up were primarily attributed to COVID-19.
| Characteristics | ICU admission | Non-ICU admission | P value |
| Respiratory symptoms for admission | 15 (71) | 49 (65) | 0.6 |
| Mean hospital length of stay (days) | 21.8 ± 19.4 | 8.6 ± 9.8 | < 0.001 |
| Use of remdesivir for management of SARS-CoV-2 | 5 (24) | 23 (31) | 0.54 |
| Serum creatinine at time of SARS-CoV-2 infection (mg/dL) | 1.91 ± 0.25 | 2.50 ± 2.17 | 0.002 |
| Serum eGFR at the time of SARS-CoV-2 infection (mL/m2) | 48.1 ± 24.6 | 37.9 ± 21.1 | 0.34 |
| Serum creatinine 1-month post SARS-CoV-2 infection (mg/dL) | 1.22 ± 0.29 (n = 6) | 1.86 ± 1.47 (n = 66) | 0.12 |
| Serum eGFR 1 month SARS-CoV-2 infection (mL/m2) | 73 ± 30.7 | 46.4 ± 21.7 | 0.18 |
| Serum creatinine 6 months post SARS-CoV-2 infection (mg/dL) | 1.34 ± 0.62 (n = 5) | 1.67 ± 0.72 (n = 62) | 0.86 |
| Serum eGFR 6 months SARS-CoV-2 infection (mL/m2) | 67.0 ± 32.8 | 47.0 ± 19.5 | 0.07 |
| Serum creatinine 1 year post SARS-CoV-2 infection (mg/dL) | 1.32 ± 0.70 (n = 4) | 1.93 ± 1.21 (n = 61) | 0.39 |
| Serum eGFR 1 year post SARS-CoV-2 infection (mL/m2) | 70.3 ± 34.9 | 44.3 ± 20.8 | 0.09 |
| Serum creatinine at last follow-up (mg/dL) | 1.29 ± 0.84 (n = 4) | 1.88 ± 1.30 (n = 53) | 0.5 |
| Serum eGFR at last follow-up (mL/m2) | 76.8 ± 37.0 | 48.1 ± 21.9 | 0.1 |
| Uncensored graft failure at last follow-up | 17 (81) | 23 (31) | < 0.001 |
| Death at last follow-up | 16 (76) | 13 (17) | < 0.001 |
| Death related to COVID-19 | 15 (71) | 4 (5) | < 0.001 |
Assessing the risk factors for ICU admission (Table 3), male sex was the only factor significantly associated with increased risk for ICU admission in the univariate (HR: 3.29; 95%CI: 1.10-9.88; P = 0.03) and multivariate (HR: 3.29; 95%CI: 1.10-9.88; P = 0.04) analyses. Interestingly, vaccination (HR: 0.93; 95%CI: 0.38-2.25; P = 0.87) and living donor recipient (HR: 0.35; 95%CI: 0.10-1.19; P = 0.09) were equally represented in patients admitted to the ICU and to the floor.
| Covariate | Univariate analyses | Multivariate analyses | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age at SARS-CoV-2 infection diagnosis (per year) | 1.01 | 0.98-1.05 | 0.35 | |||
| Male recipient | 3.29 | 1.10-9.88 | 0.03 | 3.11 | 1.04-9.34 | 0.04 |
| Nonwhite recipient | 0.71 | 0.24-2.13 | 0.55 | |||
| Diabetes as a cause of ESKD vs other | 1.09 | 0.42-2.83 | 0.86 | |||
| Living donor recipient | 0.35 | 0.10-1.19 | 0.09 | 0.38 | 0.11-1.30 | 0.12 |
| Previous transplant | 0.46 | 0.13-1.55 | 0.2 | |||
| Tacrolimus + MPA + prednisone maintenance vs other | 2.07 | 0.61-7.08 | 0.24 | |||
| Prednisone based immunosuppression | 0.89 | 0.21-3.82 | 0.87 | |||
| Treatment of rejection before SARS-CoV-2 infection | -- | -- | -- | |||
| Vaccinated | 0.93 | 0.38-2.25 | 0.87 | |||
| Baseline eGFR pre- SARS-CoV-2 infection (per mL/m2) | 1.01 | 0.99-1.03 | 0.23 | |||
| The interval from transplant to COVID-19 (per month) | 0.99 | 0.98-1.01 | 0.4 | |||
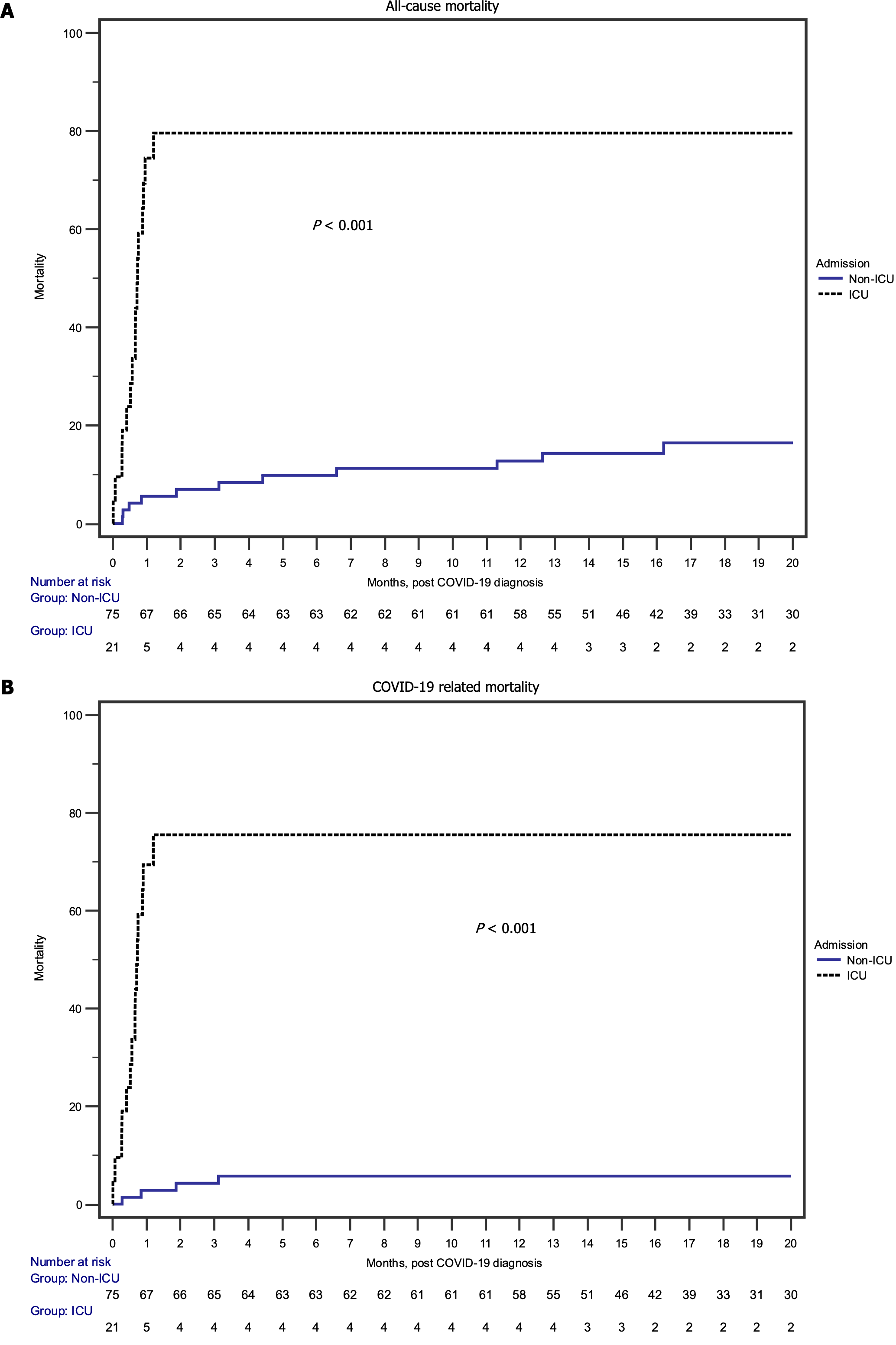
Furthermore, examining the risk of all-cause mortality (Table 4) in univariate analysis, ICU admission (HR: 10.2; 95%CI: 4.72-21.9; P < 0.001) and advanced age (HR: 1.05; 95%CI: 1.02-1.08; P = 0.002) were associated with increased risk. This finding carried over into the multivariate analyses as well. ICU admission was associated with an 11-fold increase in the risk of all-cause mortality, especially evident during the early post-SARS-CoV-2 infection period, as illustrated in Figure 1A.
| Covariate | Univariate analyses | Multivariate analyses | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| ICU admission | 10.2 | 4.72-21.9 | < 0.001 | 11.2 | 5.11-24.5 | < 0.001 |
| Age at COVID-19 diagnosis (per year) | 1.05 | 1.02-1.08 | 0.002 | 1.06 | 1.02-1.09 | 0.001 |
| Male recipient | 1.90 | 0.86-4.17 | 0.11 | |||
| Nonwhite recipient | 0.60 | 0.23-1.58 | 0.31 | |||
| Diabetes as a cause of ESKD vs other | 1.13 | 0.52-2.50 | 0.75 | |||
| Living donor recipient | 0.72 | 0.32-1.64 | 0.44 | |||
| Previous transplant | 0.66 | 0.27-1.61 | 0.36 | |||
| Tacrolimus + MPA + prednisone maintenance vs other | 1.18 | 0.51-2.78 | 0.69 | |||
| Prednisone based immunosuppression | 0.87 | 0.26-2.87 | 0.82 | |||
| Treatment of rejection before SARS-CoV-2 infection | -- | -- | -- | |||
| Vaccinated | 0.81 | 0.39-1.69 | 0.58 | |||
| Baseline eGFR (per mL/m2) | 1.0 | 0.98-1.02 | 0.94 | |||
| Interval from transplant to COVID-19 (per month) | 1.0 | 0.99-1.01 | 0.73 | |||
| Respiratory symptoms for hospital admission | 1.22 | 0.55-2.68 | 0.63 | |||
| Remdesivir for management of COVID | 0.48 | 0.18-1.26 | 0.14 | |||
COVID-19-related mortality risk showed similar outcomes (Table 5 and Figure 1B). In the univariate analysis, ICU admission (HR: 25.5; 95%CI: 8.26-78.8; P < 0.001) was associated with increased risk for COVID-19-related mortality, but this time by over 25-fold. This increased risk associated with ICU admission persisted in the multivariate analysis as well (HR: 27.2; 95%CI: 8.69-84.9; P < 0.001). Though advanced age was not quite statistically significant in the univariate analysis (P = 0.06), it was significantly associated with increased risk in the multivariate analysis (HR: 1.04; 95%CI: 1.00-1.08; P = 0.04). Vaccination, male sex, nonwhite recipients, diabetes as the cause of end-stage kidney disease (ESKD), living donor recipients, and baseline eGFR conferred neither increased nor decreased risk for COVID-19-related mortality once hospitalized with COVID-19.
| Covariate | Univariate analyses | Multivariate analyses | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| ICU admission | 25.5 | 8.26-78.8 | < 0.001 | 27.2 | 8.69-84.9 | < 0.001 |
| Age at SARS-CoV-2 infection diagnosis (per year) | 1.04 | 0.99-1.07 | 0.06 | 1.04 | 1.0-1.08 | 0.04 |
| Male recipient | 1.75 | 0.66-4.59 | 0.26 | |||
| Nonwhite recipient | 0.54 | 0.16-1.85 | 0.33 | |||
| Diabetes as a cause of ESKD vs other | 0.66 | 0.22-1.99 | 0.46 | |||
| Living donor recipient | 0.68 | 0.25-1.90 | 0.47 | |||
| Previous transplant | 0.48 | 0.14-1.66 | 0.25 | |||
| Tacrolimus + MPA + prednisone maintenance vs other | 1.33 | 0.44-4.02 | 0.61 | |||
| Prednisone based immunosuppression | 0.52 | 0.15-1.77 | 0.30 | |||
| Treatment of rejection before SARS-CoV-2 infection | -- | -- | -- | |||
| Vaccinated | 0.66 | 0.26-1.60 | 0.35 | |||
| Baseline eGFR (per mL/m2) | 1.01 | 0.99-1.03 | 0.27 | |||
| Interval from transplant to SARS-CoV-2 infection (per month) | 1.0 | 0.99-1.01 | 0.68 | |||
| Respiratory symptoms for hospital admission | 1.13 | 0.43-2.99 | 0.80 | |||
| Remdesivir for management of COVID | 0.62 | 0.21-1.88 | 0.40 | |||
Among this group of 96 KTRs admitted to a single institution during the initial two years of the COVID-19 pandemic, we presented important features associated with SARS-CoV-2 infection and its outcomes. This study distinguishes hospitalization based on whether the KTRs required ICU admission, allowing us to further characterize risk factors and outcomes according to this variable. To summarize, KTRs admitted to the ICU had a higher mean hospital LOS, higher risk of graft failure, and higher mortality rate compared to the non-ICU admission group. 81% of KTRs in the ICU group experienced graft failure, a stark contrast to the 31% rate of graft failure in the non-ICU group. Additionally, 15 out of 16 KTRs (94%) in the ICU group who had death at the last follow-up experienced death attributed to COVID-19. Again, this contrasts with the 4 out of 13 KTRs (31%) in the non-ICU group who had death at the last follow-up experienced death attributed to COVID-19. ICU admission increased risk of all-cause mortality by 11-fold and increased risk of COVID-19-related mortality by 27-fold. Surprisingly, vaccination, living donor recipients, diabetes as the cause of ESKD, and baseline eGFR were neither associated with increased nor decreased risk for ICU admission, all-cause mortality, and COVID-19-related mortality. Unsurprisingly, advanced age significantly increased risk for ICU admission, all-cause mortality, and COVID-19-related mortality in this study.
Advanced age has been widely documented in the literature as an important risk factor for severe COVID-19 disease and mortality[5,9]. While our study is consistent regarding the variable of advanced age, these data diverge from other studies regarding the impacts of vaccination status, comorbid conditions, and immunosuppressive regimens. Many studies document the protective effect of vaccination in reducing the risk of morbidity and mortality from COVID-19 disease in KTRs. One study of 2500 KTRs found that the number of SARS-CoV-2 vaccination doses was inversely correlated with mortality, hospitalization, ICU admission, and acute kidney injury (AKI)[5]. On the other hand, a single-center study of 400 KTRs found no statistically significant differences between unvaccinated and vaccinated KTRs in terms of hospitalization (outpatient, general floor, ICU), length of stay, death, and graft failure[6]. It is important to note that in the study which noted a significant influence in vaccination on outcomes that it analyzed results according to the different SARS-CoV-2 variants, namely pre and post Omicron. This same study also showed that a steroid-free immunosuppressive regimen was a protective factor against hospitalization for COVID-19 disease, COVID-19-related AKI, and ICU admission, with other immunosuppressive agents not being significantly associated with CVOID-19 mortality or morbidity[5]. In contrast to this finding, our study showed no difference in ICU admission among KTRs who received prednisone-based immunosuppression. Regarding graft function, we found that KTRs admitted to the ICU had an increased risk of graft failure. This finding is consistent with results from a study that revealed a sex-specific association between relative eGFR decline and COVID-19 severity among male KTRs.
Current guidelines for the treatment of solid organ transplant recipients with severe and critical COVID-19 recommend intravenous remdesivir with or without immunomodulation agents including dexamethasone, tocilizumab, or baricitinib[10]. Our findings in this study did not show a mortality benefit associated with remdesivir for management of COVID-19. One study of SOTRs hospitalized with SARS-CoV2 infection found that severe COVID-19 cases show high mortality despite antiviral treatment with RDV[11]. Another cohort study found that remdesivir protected KTRs from severe COVID-19 disease only in those hospitalized with early administration of the drug (within 7 days of symptom onset)[12].
As a single-center observational study, this research carries the limitations associated with such a design which include the specific clinical approach and population of our institution. This consistency of the clinical practices reinforces the strength of the study, as all participants were hospitalized at the same center, reducing the risk of confounding variables associated with hospitalization at different locations. As our center is a tertiary care university academic institution, patients admitted or transferred to our center are usually very sick.
In conclusion, our patients, especially SOTRs, continue to suffer from the adverse effects of SARS-CoV-2 infection. We remain motivated to continue rigorous efforts to better understand and characterize risk factors and protective factors related to COVID-19 such that we can prevent devastating outcomes and promote the health and safety of our patients.
| 1. |
|
| 2. | Kompaniyets L, Pennington AF, Goodman AB, Rosenblum HG, Belay B, Ko JY, Chevinsky JR, Schieber LZ, Summers AD, Lavery AM, Preston LE, Danielson ML, Cui Z, Namulanda G, Yusuf H, Mac Kenzie WR, Wong KK, Baggs J, Boehmer TK, Gundlapalli AV. Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized With COVID-19, March 2020-March 2021. Prev Chronic Dis. 2021;18:E66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 208] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 3. | Kates OS, Haydel BM, Florman SS, Rana MM, Chaudhry ZS, Ramesh MS, Safa K, Kotton CN, Blumberg EA, Besharatian BD, Tanna SD, Ison MG, Malinis M, Azar MM, Rakita RM, Morilla JA, Majeed A, Sait AS, Spaggiari M, Hemmige V, Mehta SA, Neumann H, Badami A, Goldman JD, Lala A, Hemmersbach-Miller M, McCort ME, Bajrovic V, Ortiz-Bautista C, Friedman-Moraco R, Sehgal S, Lease ED, Fisher CE, Limaye AP; UW COVID-19 SOT Study Team. Coronavirus Disease 2019 in Solid Organ Transplant: A Multicenter Cohort Study. Clin Infect Dis. 2021;73:e4090-e4099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 4. | Massie AB, Werbel WA, Avery RK, Po-Yu Chiang T, Snyder JJ, Segev DL. Quantifying excess deaths among solid organ transplant recipients in the COVID-19 era. Am J Transplant. 2022;22:2077-2082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Mikhailov M, Budde K, Halleck F, Eleftheriadis G, Naik MG, Schrezenmeier E, Bachmann F, Choi M, Duettmann W, von Hoerschelmann E, Koch N, Liefeldt L, Lücht C, Straub-Hohenbleicher H, Waiser J, Weber U, Zukunft B, Osmanodja B. COVID-19 Outcomes in Kidney Transplant Recipients in a German Transplant Center. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Singh P, Von Stein L, McGowan M, Nolan A, Ross A, Kaur M, Maxwell M, Ma J, Peng J, Pesavento T. Comparison of outcomes in vaccinated vs unvaccinated COVID-19 kidney transplant recipients, a single center retrospective study-Is the taboo justified? Clin Transplant. 2024;38:e15187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 7. | Escalante EJ, Rodríguez JG, Salas JDC, Castañeda Z, Conde MLM. Clinical Course, Nosocomial, and Opportunistic Infections Among Kidney Transplant Recipients with COVID-19: A Retrospective Single Center Study. Transplant Proc. 2023;55:1829-1842. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Zona E, Jorgenson M, Dolma S, Santos A, Garg N, Aziz F, Mohamed M, Saddler CM, Smith JA, Mandelbrot D, Parajuli S. Discordance in cytomegalovirus viremia in kidney recipients from the same donor is associated with the worst outcomes. Clin Transplant. 2023;37:e14979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 9. | Zona EE, Gibes ML, Jain AS, Danobeitia JS, Garonzik-wang J, Smith JA, Mandelbrot DA, Parajuli S. Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection among Kidney Transplant Recipients: A Large Single-Center Experience. Critical Care Research and Practice. 2024;2024:1-9. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 10. | Mendoza MA, Razonable RR. Coronavirus Disease 2019 Management Strategies in Solid Organ Transplant Recipients. Infect Dis Clin North Am. 2023;37:475-493. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Fesu D, Bohacs A, Hidvegi E, Matics Z, Polivka L, Horvath P, Czaller I, Sutto Z, Eszes N, Vincze K, Muller V. Remdesivir in Solid Organ Recipients for COVID-19 Pneumonia. Transplant Proc. 2022;54:2567-2569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Cacho J, Nicolás D, Bodro M, Cuadrado-Payán E, Torres-Jaramillo V, Gonzalez-Rojas Á, Ventura-Aguiar P, Montagud-Marrahi E, Herrera S, Rico V, Cofàn F, Oppenheimer F, Revuelta I, Diekmann F, Cucchiari D. Use of remdesivir in kidney transplant recipients with SARS-CoV-2 Omicron infection. Kidney Int. 2022;102:917-921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |