Published online Jun 25, 2024. doi: 10.5501/wjv.v13.i2.92944
Revised: May 21, 2024
Accepted: June 3, 2024
Published online: June 25, 2024
Processing time: 132 Days and 13.7 Hours
The advent of coronavirus disease 2019 (COVID-19) unveiled the worst national blood crisis that the United States had witnessed in over a decade. With the pandemic influencing the different stages of the acquisition of blood products outside the hospital setting, we aimed to explore the possible barriers contributing to the shortage of blood products within the medical community.
To assess the adherence to restrictive blood transfusion practices for patients in the COVID era and pre-COVID era.
We conducted a retrospective cross-sectional study on hospitalized patients distinguishing the pattern of blood transfusion during the COVID and pre-COVID era in a community hospital. Data was tabulated to include the number of red blood cell (RBC) transfusions and if transfusions met restrictive blood transfusion criteria as per institutional guidelines. Chi-square was applied to test the statistical association between qualitative variables. Unpaired t test and Mann Whitney U test were applied respectively to test the mean difference of quantitative variables.
A total of 208 patients were included in the study, of which 108 were during COVID era and 100 were during pre-COVID era. The leading reason for admission in both the COVID era and pre-COVID era transfused patients was shortness of breath (53.7% and 36% P = 0.001), followed by gastrointestinal bleeding (25.9% and 21% P = 0.001). There was a higher percentage of RBC transfusions in the intensive care unit in the COVID-era group than in the pre-COVID era group (38.9% vs 22%, P = 0.008). The restrictive transfusion criteria were met in 62% vs 79% in the COVID and pre-COVID eras, respectively (P = 0.008).
The COVID-era group received RBC transfusions with less stringent adherence to restrictive blood transfusion practices in comparison to pre-COVID era group.
Core Tip: Our study showed the percentage of liberal transfusions in a community teaching hospital almost doubled during the coronavirus disease (COVID) era compared to the pre-COVID era, shedding light on a possible change in physician mindset in adhering to restrictive transfusion guidelines during the early COVID-19 pandemic. It reiterates the importance for timely physician education on restrictive red blood cell transfusion guidelines.
- Citation: Arun Kumar S, Prabhu S, Sanghvi A, Gogtay M, Suresh MG, Khosla H, Singh Y, Mishra AK, George S. Paradigm shift in transfusion practices during early COVID-19 pandemic: A single center retrospective study. World J Virol 2024; 13(2): 92944
- URL: https://www.wjgnet.com/2220-3249/full/v13/i2/92944.htm
- DOI: https://dx.doi.org/10.5501/wjv.v13.i2.92944
Coronavirus disease 2019 (COVID-19) began in the city of Wuhan, China, with the first reported case in December 2019 while the United States recorded the first two cases of the severe acute respiratory syndrome coronavirus 2 in Illinois on January 24, 2020 leading to declaration of national emergency on March 13, 2020. With supply of many commodities across the globe facing challenges, the U.S. Food and Drug Administration released a list of medication shortages in the country which included cardiovascular medications like furosemide, labetalol, and anticoagulants such as heparin[1].
On January 1, 2022, the first day of 'National blood donor month, the American Red Cross, which supplies 40% of the nation's blood, declared the worst national blood crisis in over a decade. The pandemic of COVID-19 impacted the practices of blood product procurement and transfusion in multiple ways[2-5]. The pre-transfusion process was impacted more severely than the clinical transfusion phase[6]. One of the main reasons causing reduction in blood products was social distancing precautions to avoid public gatherings issued during the pandemic by various national governments[7]. A systematic review and meta-analysis showed significantly decreased blood donation rates across the globe during the pandemic[8]. Low donor turnouts limiting the supply of short-lived blood products, compounded the predicament. Resource-limited community medical centers nationwide faced challenges in securing products for blood transfusion[9].
The Association for the Advancement of Blood and Biotherapies (AABB) published the restrictive transfusion guidelines in 2016 (Table 1). The recommendations stemmed from summarizing several trials that demonstrated restrictive red blood cell (RBC) transfusion thresholds to not be harmful compared to the liberal transfusion threshold (transfusing RBCs at higher hemoglobin thresholds- 9 g/dL to 10 g/dL). The restrictive transfusion threshold approach of 7 g/dL or 8 g/dL was synchronous with decreased blood product use and associated cost of hospitalization coupled with no greater impact on rates of adverse clinical outcomes, including 30-day mortality, myocardial infarction, cerebrovascular accident, re-bleeding, pneumonia, or thromboembolism. Though there are definite guidelines for admitted hemodynamically stable patients, the threshold for RBC transfusion is not well defined in a situation of active blood loss or rapidly down trending hemoglobin[10]. This often leads to heterogeneity in guidelines for RBC transfusion in different institutions.
| Transfusion threshold | Patient population |
| Hemoglobin ≤ 7 g/dL | Hospitalized adult patients who are hemodynamically stable, including critically ill patients |
| Hemoglobin ≤ 8 g/dL | Patients undergoing orthopedic surgery, cardiac surgery, and those with preexisting cardiovascular disease |
With the pandemic influencing the different stages of the acquisition of blood products outside the hospital setting, we aimed to explore the possible barriers contributing to the shortage of blood products within the medical community. We found a lack of evidence in the literature looking into internal factors contributing to the national blood shortage, specifically exploring the physician component. Hence, we hypothesized that one such factor could be the adherence to restrictive transfusion practice in the COVID era compared to the pre-COVID era. We conducted a retrospective cross-sectional study observing the pattern of RBC transfusions during the COVID and pre-COVID era in a community hospital.
We performed a retrospective cross-sectional study to compare the adherence to restrictive transfusion practice in patients admitted to at a 329 - bed community teaching hospital in central Massachusetts. The patients who received RBC transfusions from April 1st 2020 to August 31st 2020 were deemed to be in the COVID era and those who received RBC transfusions from July 1st 2019 to November 30th 2019 were in the pre-COVID era, irrespective of their COVID-19 diagnosis. The above duration was chosen to be representative of the time period around the declaration of COVID-19 related national emergency. The study was approved by the institutional review board. The study inclusion criteria consisted of: (1) Age > 18 years; and (2) Received packed RBC transfusion during the hospital course. Patients with hemoglobinopathies, patients undergoing elective or emergent surgeries, active acute coronary syndrome, transfusion-dependent anemias and pregnant patients were excluded. We excluded these patients due to lack of definite RBC transfusion guidelines by AABB. A detailed chart review was conducted for each patient by two different investigators. The data was tabulated to include the number of RBC transfusions and other blood products received in patients admitted to both the medical floors and intensive care unit (ICU). Demographic data included age, gender, ethnicity, admission diagnosis, pre-existing cardiovascular disease (CVD) or prior gastrointestinal bleeding (GIB), use of chronic antiplatelet or anticoagulation such as aspirin, clopidogrel, direct oral anticoagulants (DOACs), vitamin K antagonists, and use of venous thromboembolism (VTE) prophylaxis during hospitalization. The institutional blood transfusion guidelines remained unchanged during the pandemic.
The documented indication for transfusion was noted, which included: hemoglobin ≤ 7 g/ dL, hemoglobin ≤ 8 g/ dL with pre-existing CVD, hemoglobin ≤ 10 g/dL with active bleeding and hemoglobin ≤ 10 g/dL with down-trending hemoglobin. These documented indications for RBC transfusion were derived from the pre-available options of the transfusion order set for physicians at our institution. We analyzed whether the documented indication met the restrictive blood transfusion criteria as per institutional guidelines (Table 2).
The data was collected in Microsoft excel and was analyzed using SPSS. The baseline demographic characteristics between the two study populations were assessed using Chi-square to test the statistical association between qualitative variables. Unpaired t test and Mann Whitney U test were applied respectively to test the mean difference of quantitative variables following normal and non-normal distribution. The level of significance was set at < 5%. The modalities of Medline, PubMed and Embase were utilized to analyze high impact articles relevant to the current field of study and were incorporated in the discussion.
(1) COVID era is defined from April 1st 2020 to August 31st 2020; (2) Pre-COVID era is defined from July 1st 2019 to November 30th 2019; (3) Pre-existing CVD is defined as past history of at least one episode of angina irrespective of percutaneous coronary intervention; (4) Active bleed is defined as documented witnessed bleed by nurse/physician or hemodynamic instability attributed to bleeding; and (5) Down-trending hemoglobin is defined as fall in hemoglobin more than or equal to 2 g/dL.
Two hundred fifteen patients were screened, and 208 met the inclusion criteria. There were 108 patients who received RBC transfusions in the COVID era group and 100 patients who received RBC transfusions in the pre-COVID era group. The mean age of patients in the COVID and pre-COVID eras were 68.01 and 70.22 years (P = 0.29), respectively. Gender and race were equally distributed between both groups. Pre-existing CVD and prior GIB were equally distributed between both groups (Table 3).
| Variable | COVID era (n = 108) | Pre COVID era (n = 100) | P value |
| Demographics | |||
| Mean age, years | 68 | 70 | 0.295 |
| Sex | |||
| Male | 51 (47.2) | 49 (49) | 0.798 |
| Female | 57 (52.8) | 51 (51) | 0.798 |
| Race1 | |||
| Caucasian | 86 (79.6) | 84 (84) | 0.758 |
| Hispanics | 1 (0.9) | 1 (1) | 0.758 |
| African American | 8 (7.4) | 3 (3) | 0.758 |
| Asian | 2 (1.9) | 2 (2) | 0.758 |
| Others | 11 (10.2) | 10 (10) | 0.758 |
| Clinical characteristics | |||
| Preexisting CVD | 15 (13.9) | 29 (29) | 0.008 |
| Prior GIB | 11 (10.2) | 8 (8) | 0.585 |
| Active antiplatelet/anticoagulant use | 20 (18.5) | 46 (46) | 0.001 |
| Inpatient VTE prophylaxis2 | 15 (13.9) | 29 (29) | 0.001 |
| RBC transfusions in ICU | 42 (38.9) | 22 (22) | 0.008 |
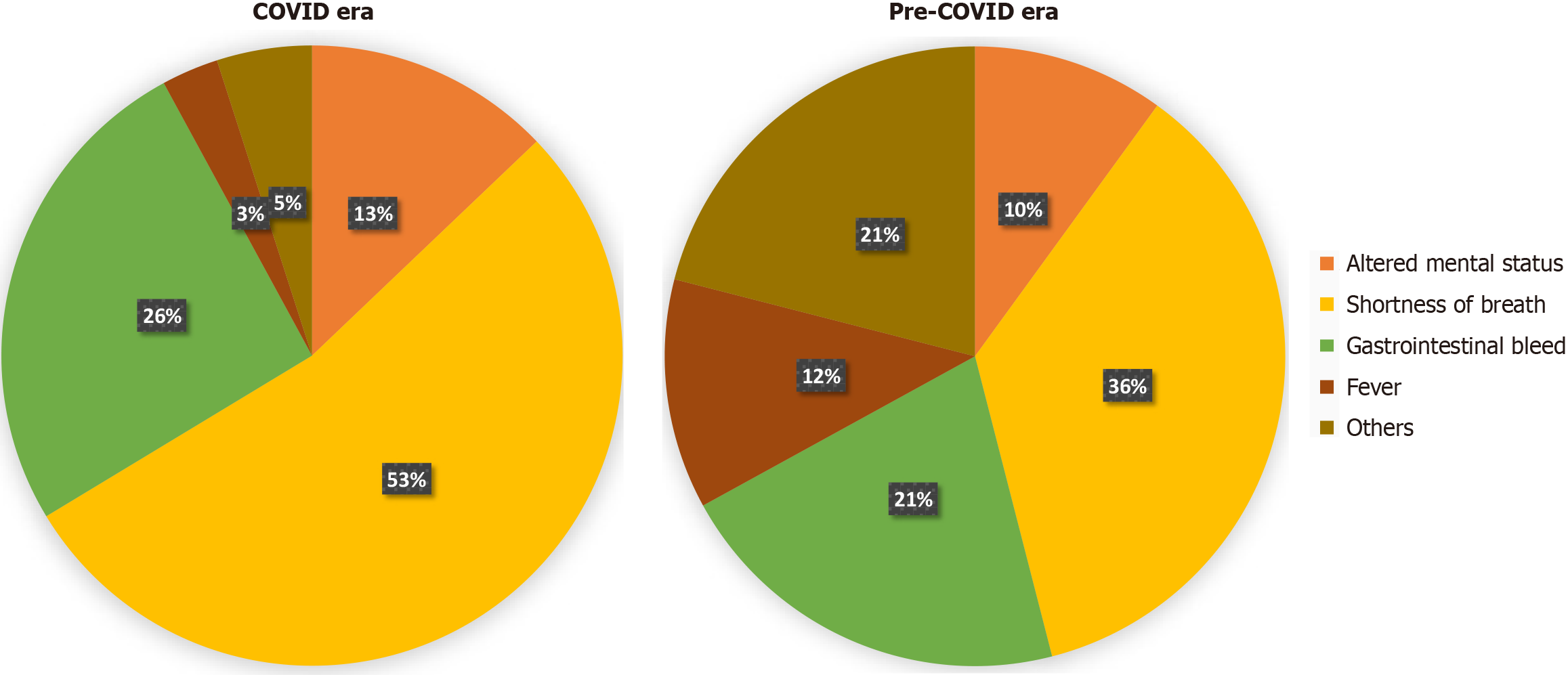
The leading reason for admission in patients who received RBC transfusions in both the COVID era and pre-COVID era was shortness of breath (53.7% and 36% P = 0.001), followed by GIB (25.9% and 21% P = 0.001) (Figure 1).
In COVID era group, 10 patients were found to have COVID-19 infection confirmed by positive real time polymerase chain reaction. These patients did not qualify for convalescent plasma treatment due to their immunocompetent status. The COVID era group as compared to pre-COVID era group had a lower proportion of patients on chronic anticoagulant or antiplatelet therapy (18.5% and 46% P = 0.001). The distribution of patients based on chronic anticoagulant or antiplatelet therapy in COVID and pre-COVID era groups are as follows: aspirin 4.6% vs 11%, aspirin and clopidogrel 1.9% vs 8%, DOACs 6.5% vs 7% and vitamin K antagonists 5.6% vs 20% respectively. Similarly, the COVID era group had a lower proportion of patients on pharmacological prophylaxis for VTE compared to the pre-COVID era patients (13.9% and 29% P = 0.001). The distribution of patients based on the types of VTE prophylaxis received in COVID and pre-COVID era groups are as follows: unfractionated heparin 6.5% vs 22%, low molecular weight heparin 7.4% vs 7% and mechanical prophylaxis 84.3% vs 63% respectively. There was a higher percentage of RBC transfusions in the ICU in the COVID-19 era group than in the pre-COVID-era group (38.9% vs 22%, P = 0.008).
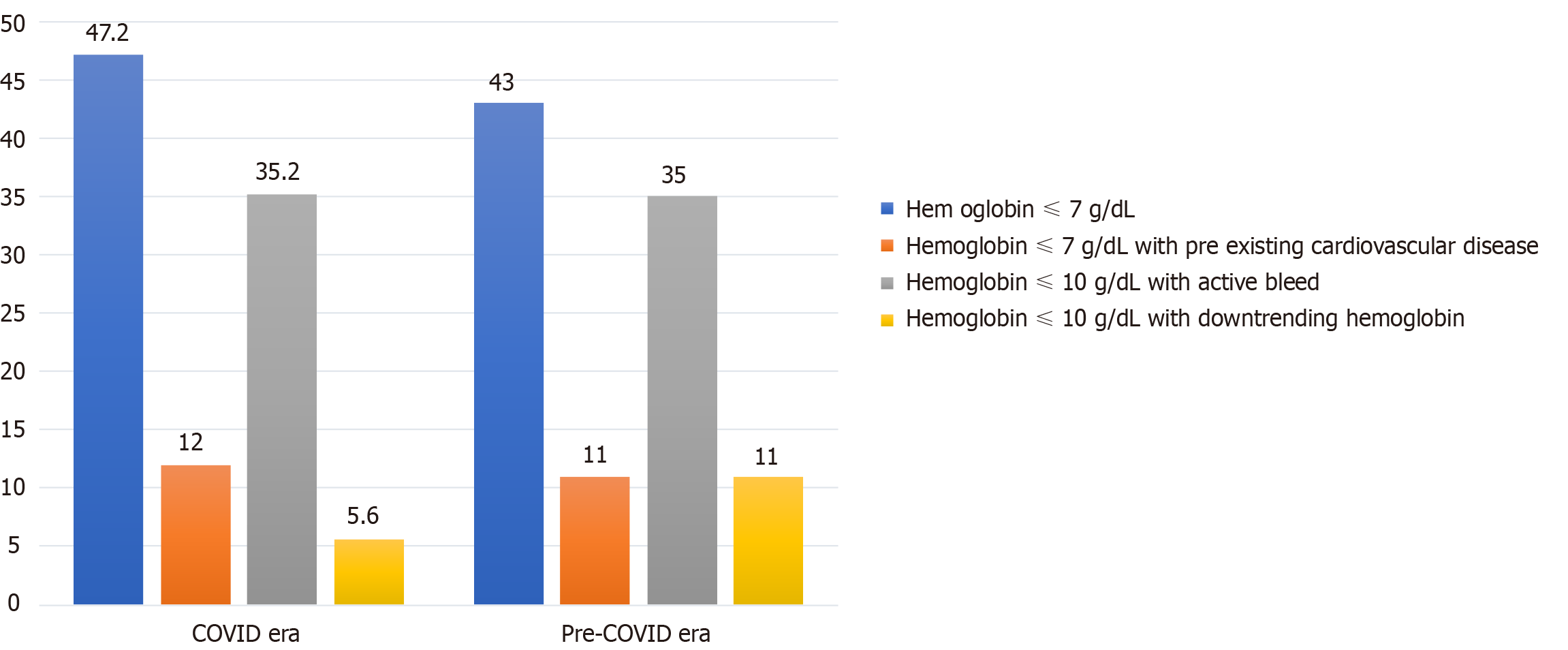
Documented indications for RBC transfusions in COVID and pre-COVID era groups were hemoglobin ≤ 7 g/ dL (47.2% vs 43%), hemoglobin ≤ 8 g/ dL with pre-existing CVD (12% vs 11%), hemoglobin ≤ 10 g/dL with active bleeding (35.2% vs 35%) and hemoglobin ≤ 10 g/dL with down-trending hemoglobin (5.6% vs 11%) respectively (Figure 2).
Restrictive transfusion criteria were met in 62% of total RBC transfusions in the COVID era vs 79% in the pre-COVID era group (P = 0.008) (Table 4). The clinical characteristics of patients who did meet the restrictive transfusion criteria in the COVID and pre-COVID eras are noted in Supplementary Table 1.
| Restrictive transfusion criteria met | Number and percentage of red blood cell transfusions | |
| COVID era | Pre-COVID era | |
| Yes | 67 (62) | 79 (79) |
| No | 41 (38) | 21 (29) |
| P value | 0.008 | |
The majority of patients in both groups received 1-unit PRBC transfusion. The distribution based on the number of units of RBC transfused in COVID-19 and pre-COVID-19 era groups respectively include: one-unit RBC 74.1% vs 83%, two units RBC 25.9% vs 11%, three units of RBC 0 vs 3% and four units of RBC 0 vs 3%. Other blood products received during the COVID and pre-COVID eras included platelets 1.9% vs 1% and fresh frozen plasma 11.1% vs 8%, respectively.
Blood transfusions are the most overused in-hospital procedure, and various observational studies show a definite lack of restrictive transfusion practices[11]. Ours is the first retrospective study in a teaching community hospital to evaluate the pattern of blood transfusions during the COVID and pre-COVID era, specifically highlighting the adherence to restrictive blood transfusion practices. Our study showed 21% of total RBC transfusions in the pre-COVID era did not meet our restrictive transfusion guidelines and that the percentage of liberal transfusions almost doubled (37.9%) during the COVID era. Although different hospitals practice restrictive transfusion strategies differently, the transfusion guidelines at our institution were a projection of the restrictive transfusion guidelines from AABB. By comparing two different timelines in the same institution, we aimed to remove biases from heterogeneous institutional practices.
The time period of 4 months for the COVID era was chosen from April 1st 2020 to August 31st 2020 after the declaration of the national emergency in March 2020 and the pre-COVID era from July 1st 2019 to November 30th 2019 was prior to the first case of COVID 19 in December 2019. From prior studies, blood product utilization in a tertiary care hospital did not show statistically significant variation across different time periods in a year[12]. Many hospitals worldwide saw significant reduction in hospitalizations and postponed elective surgeries during the early pandemic which may have contributed to increased blood product inventory[13]. However, our study saw fairly equal number of RBC transfusions in both the COVID (n = 108) and pre-COVID era (n = 100). The primary presenting clinical complaint in both groups was shortness of breath, with a more significant number in the COVID era group (54% vs 36%), due to suspected COVID-19 infection from a sick contact. In our study, fewer patients receiving RBC transfusions in the COVID era group were on chronic antiplatelet/anticoagulant treatment at the time of presentation (18.5% vs 46%) and pharmacologic VTE prophylaxis (13.9% vs 29%), as compared to the pre-COVID era group. This could be secondary to a lower number of patients with pre-existing CVD and a higher number of patients with prior GIB in the COVID era group compared to the pre-COVID era group. There is evidence in the literature to support increased use of anticoagulants during the pandemic, however this does not apply to our study since the study period was prior to the widespread use of therapeutic anticoagulation for admitted COVID-19 positive patients[14,15]. Irrespective of the differences in the patient characteristics between the COVID and the pre-COVID era (Table 3), the transfusion guidelines defined by AABB support adherence to restrictive transfusion criteria.
It is well established that there was a rise in admissions to the ICU during the pandemic and in our study, we observed an increased number of RBC transfusions in the ICU in the COVID era group[16]. Nevertheless, there is no evidence to show if COVID-19 patients had an increased need for blood transfusion; however, studies indicate that critically ill patients with COVID-19 may have required more blood transfusions compared to non-critically ill patients[17]. The Transfusion Requirements in Critical Care (TRICC) trial that the restrictive transfusion strategy is as effective as the liberal transfusion strategy with improved mortality in critically ill patients[18]. The AABB guidelines remain firm on restrictive transfusion practices for hemodynamically stable patients, irrespective of ICU level of care[10].
The adherence to a restrictive transfusion strategy of 7 g/dL to 8 g/dL can help decrease the number of RBC transfusions[19]. Not only does it impact blood product availability logistically, but it also has clinical consequences. Evidence suggests that the liberal transfusion strategy is associated with worsening disease burden by causing circulatory overload and increased thrombogenicity[20,21]. These adverse effects are in addition to the increased risk of hospital-acquired infections in liberal transfusion policy as compared to restrictive transfusion policy[22]. The guidelines published by AABB summarize various trials highlighting no greater rate of adverse outcomes in the restrictive transfusion strategy than in the liberal transfusion strategy. Even though RBC transfusions are relatively safe, unnecessary transfusions should be assessed for risk vs benefit and associated cost. Given the evidence for the similar safety profile of the restrictive transfusion approach, it necessitates a change in the mindset of the medical community to incorporate the same in clinical practice.
Worldwide, the COVID-19 pandemic brought an era of uncertainty and paranoia. The number of critically ill hospitalized patients and ICU admissions increased dramatically[15,23]. We question whether the pandemic's mortality and paranoia obliged physicians to have a more liberal mindset toward blood transfusions? Did it coerce physicians to practice defensive medicine? As evident from our results, the COVID era group, despite having comparable, if not fewer, risk factors associated with blood loss, received a blood transfusion with less stringent adherence to restrictive blood transfusion guidelines. The results of our study indicate that there could have been more liberal use of blood products while caring for patients during COVID-19 since physicians were still searching for various modalities to approach COVID-19 infection and its related complications[24].
Our study was a retrospective cross-sectional study, and given its observational nature, there was a lack of data regarding the clinical judgment made by the physician that preceded the decision to transfuse liberally. Our data is primarily from one healthcare center.
Further studies are needed to pool data from larger geographical locations to comment if liberal transfusion practices during the pandemic was a universal finding. It would also be imperative to see if this trend continued beyond the early pandemic era. Streamlining transfusion practices by being cognizant of the internal factors within the medical community will help prevent a future blood crisis.
The role of several external factors that contributed to blood shortages worldwide during the COVID-19 pandemic is well established. However, to our knowledge, ours is the first retrospective study in a tertiary care community teaching hospital to explore the possible role of internal factors within the medical community. Our study sheds light on a possible change in physician mindset in adhering to restrictive transfusion guidelines during the early COVID-19 pandemic. It begs the question of physicians’ possible practice of defensive medicine being a preventable cause, though minor, for the National Blood Crisis in 2022. Hence it is pivotal for timely physician education on restrictive RBC transfusion guidelines. There is also a dire need to educate ancillary staff and blood bank associates on these practices, as they would function as critical checkpoints against liberal transfusion policies.
| 1. |
|
| 2. | Raturi M, Kusum A. The blood supply management amid the COVID-19 outbreak. Transfus Clin Biol. 2020;27:147-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Silva-Malta MCF, Rodrigues DOW, Chaves DG, Magalhães NNS, Ribeiro MA, Cioffi JGM, Martins ML. Impact of COVID-19 in the attendance of blood donors and production on a Brazilian Blood Centres. Transfus Med. 2021;31:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Covington ML, Voma C, Stowell SR. The impact of the COVID-19 pandemic on source plasma donations. J Clin Apher. 2023;38:644-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Basavaraju SV, Free RJ, Chavez Ortiz JL, Stewart P, Berger J, Sapiano MRP. Impact of the COVID-19 pandemic on blood donation and transfusions in the United States in 2020. Transfusion. 2023;63 Suppl 4:S1-S7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Yu SC, Yao YT; Evidence in Cardiovascular Anesthesia (EICA) Group. The influence of the COVID-19 pandemic on blood donation and supply in China. Transfus Med. 2024;34:124-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Cai X, Ren M, Chen F, Li L, Lei H, Wang X. Blood transfusion during the COVID-19 outbreak. Blood Transfus. 2020;18:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 8. | Chiem C, Alghamdi K, Nguyen T, Han JH, Huo H, Jackson D. The Impact of COVID-19 on Blood Transfusion Services: A Systematic Review and Meta-Analysis. Transfus Med Hemother. 2021;30:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Ngo A, Masel D, Cahill C, Blumberg N, Refaai MA. Blood Banking and Transfusion Medicine Challenges During the COVID-19 Pandemic. Clin Lab Med. 2020;40:587-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 10. | Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, Gernsheimer T, Holcomb JB, Kaplan LJ, Katz LM, Peterson N, Ramsey G, Rao SV, Roback JD, Shander A, Tobian AA. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316:2025-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 758] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 11. | Sadana D, Kummangal B, Moghekar A, Banerjee K, Kaur S, Balasubramanian S, Tolich D, Han X, Wang X, Hanane T, Mireles-Cabodevila E, Quraishy N, Duggal A, Krishnan S. Adherence to blood product transfusion guidelines-An observational study of the current transfusion practice in a medical intensive care unit. Transfus Med. 2021;31:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Gaur DS, Negi G, Chauhan N, Kusum A, Khan S, Pathak VP. Utilization of blood and components in a tertiary care hospital. Indian J Hematol Blood Transfus. 2009;25:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Pál S, Réger B, Alizadeh H, Szomor Á, Vereczkei A, Kiss T, Miseta A, Solymár M, Faust Z. Use of blood products during the first months of COVID-19 pandemic period: A single center report. Heliyon. 2023;9:e14391. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Sanz C, Faundez A, García Carulla A, Rodriguez Aliberas M, Coromoto A, Pereira A. Hemorrhage Is a Major Cause of Blood Transfusion in COVID-19 Patients. Blood. 2020;136:21-22. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators, Lawler PR, Goligher EC, Berger JS, Neal MD, McVerry BJ, Nicolau JC, Gong MN, Carrier M, Rosenson RS, Reynolds HR, Turgeon AF, Escobedo J, Huang DT, Bradbury CA, Houston BL, Kornblith LZ, Kumar A, Kahn SR, Cushman M, McQuilten Z, Slutsky AS, Kim KS, Gordon AC, Kirwan BA, Brooks MM, Higgins AM, Lewis RJ, Lorenzi E, Berry SM, Berry LR, Aday AW, Al-Beidh F, Annane D, Arabi YM, Aryal D, Baumann Kreuziger L, Beane A, Bhimani Z, Bihari S, Billett HH, Bond L, Bonten M, Brunkhorst F, Buxton M, Buzgau A, Castellucci LA, Chekuri S, Chen JT, Cheng AC, Chkhikvadze T, Coiffard B, Costantini TW, de Brouwer S, Derde LPG, Detry MA, Duggal A, Džavík V, Effron MB, Estcourt LJ, Everett BM, Fergusson DA, Fitzgerald M, Fowler RA, Galanaud JP, Galen BT, Gandotra S, García-Madrona S, Girard TD, Godoy LC, Goodman AL, Goossens H, Green C, Greenstein YY, Gross PL, Hamburg NM, Haniffa R, Hanna G, Hanna N, Hegde SM, Hendrickson CM, Hite RD, Hindenburg AA, Hope AA, Horowitz JM, Horvat CM, Hudock K, Hunt BJ, Husain M, Hyzy RC, Iyer VN, Jacobson JR, Jayakumar D, Keller NM, Khan A, Kim Y, Kindzelski AL, King AJ, Knudson MM, Kornblith AE, Krishnan V, Kutcher ME, Laffan MA, Lamontagne F, Le Gal G, Leeper CM, Leifer ES, Lim G, Lima FG, Linstrum K, Litton E, Lopez-Sendon J, Lopez-Sendon Moreno JL, Lother SA, Malhotra S, Marcos M, Saud Marinez A, Marshall JC, Marten N, Matthay MA, McAuley DF, McDonald EG, McGlothlin A, McGuinness SP, Middeldorp S, Montgomery SK, Moore SC, Morillo Guerrero R, Mouncey PR, Murthy S, Nair GB, Nair R, Nichol AD, Nunez-Garcia B, Pandey A, Park PK, Parke RL, Parker JC, Parnia S, Paul JD, Pérez González YS, Pompilio M, Prekker ME, Quigley JG, Rost NS, Rowan K, Santos FO, Santos M, Olombrada Santos M, Satterwhite L, Saunders CT, Schutgens REG, Seymour CW, Siegal DM, Silva DG Jr, Shankar-Hari M, Sheehan JP, Singhal AB, Solvason D, Stanworth SJ, Tritschler T, Turner AM, van Bentum-Puijk W, van de Veerdonk FL, van Diepen S, Vazquez-Grande G, Wahid L, Wareham V, Wells BJ, Widmer RJ, Wilson JG, Yuriditsky E, Zampieri FG, Angus DC, McArthur CJ, Webb SA, Farkouh ME, Hochman JS, Zarychanski R. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N Engl J Med. 2021;385:790-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 574] [Cited by in RCA: 676] [Article Influence: 169.0] [Reference Citation Analysis (0)] |
| 16. | Abate SM, Ahmed Ali S, Mantfardo B, Basu B. Rate of Intensive Care Unit admission and outcomes among patients with coronavirus: A systematic review and Meta-analysis. PLoS One. 2020;15:e0235653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 17. | Al Mahmasani L, Hodroj MH, Finianos A, Taher A. COVID-19 pandemic and transfusion medicine: the worldwide challenge and its implications. Ann Hematol. 2021;100:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Zhang W, Zheng Y, Yu K, Gu J. Liberal Transfusion vs Restrictive Transfusion and Outcomes in Critically Ill Adults: A Meta-Analysis. Transfus Med Hemother. 2021;48:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Carson JL, Stanworth SJ, Roubinian N, Fergusson DA, Triulzi D, Doree C, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2016;10:CD002042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 20. | Gili S, D'Ascenzo F, Lococo MF, Moretti C, Gaita F, Raposeiras-Roubín S, Abu-Assi E, Henriques JP, Saucedo J, González-Juanatey JR, Wilton SB, Kikkert WJ, Nuñez-Gil I, Ariza-Sole A, Song X, Alexopoulos D, Liebetrau C, Kawaji T, Huczek Z, Nie SP, Fujii T, Correia L, Kawashiri MA, García-Acuña JM, Southern D, Alfonso E, Terol B, Garay A, Zhang D, Chen Y, Xanthopoulou I, Osman N, Möllmann H, Shiomi H, Scarano S, Kowara M, Filipiak K, Wang X, Yan Y, Fan JY, Ikari Y, Nakahashi T, Sakata K, Yamagishi M, Kalpak O, Kedev S. Impact of blood transfusion on in-hospital myocardial infarctions according to patterns of acute coronary syndrome: Insights from the BleeMACS registry. Int J Cardiol. 2016;221:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Silvain J, Abtan J, Kerneis M, Martin R, Finzi J, Vignalou JB, Barthélémy O, O'Connor SA, Luyt CE, Brechot N, Mercadier A, Brugier D, Galier S, Collet JP, Chastre J, Montalescot G. Impact of red blood cell transfusion on platelet aggregation and inflammatory response in anemic coronary and noncoronary patients: the TRANSFUSION-2 study (impact of transfusion of red blood cell on platelet activation and aggregation studied with flow cytometry use and light transmission aggregometry). J Am Coll Cardiol. 2014;63:1289-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Rohde JM, Dimcheff DE, Blumberg N, Saint S, Langa KM, Kuhn L, Hickner A, Rogers MA. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA. 2014;311:1317-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 508] [Cited by in RCA: 484] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 23. | Shanmugavel Geetha H, Prabhu S, Sekar A, Gogtay M, Singh Y, Mishra AK, Abraham GM, Martin S. Use of inflammatory markers as predictor for mechanical ventilation in COVID-19 patients with stages IIIb-V chronic kidney disease? World J Virol. 2023;12:286-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | John KJ, Mishra AK, Ramasamy C, George AA, Selvaraj V, Lal A. Heart failure in COVID-19 patients: Critical care experience. World J Virol. 2022;11:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |