Published online Jun 25, 2024. doi: 10.5501/wjv.v13.i2.92521
Revised: April 2, 2024
Accepted: April 11, 2024
Published online: June 25, 2024
Processing time: 147 Days and 16.9 Hours
An overly exuberant immune response, characterized by a cytokine storm and uncontrolled inflammation, has been identified as a significant driver of severe coronavirus disease 2019 (COVID-19) cases. Consequently, deciphering the intricacies of immune dysregulation in COVID-19 is imperative to identify specific targets for intervention and modulation. With these delicate dynamics in mind, immunomodulatory therapies have emerged as a promising avenue for miti
Core Tip: Effective management of coronavirus disease 2019 (COVID-19) requires a nuanced approach that harnesses the host’s immune response Immunomodulatory therapies play a pivotal role in fine-tuning the immune system, striking a balance between defense and avoiding excessive inflammation. In line with this, increased precision in targeting specific immune pathways, alongside personalized treatment strategies, holds promise in optimizing outcomes for COVID-19 patients. This paper explores the evolving landscape of immunomodulation, emphasizing its potential as a crucial component in the therapeutic arsenal against the virus.
- Citation: Velikova T, Valkov H, Aleksandrova A, Peshevska-Sekulovska M, Sekulovski M, Shumnalieva R. Harnessing immunity: Immunomodulatory therapies in COVID-19. World J Virol 2024; 13(2): 92521
- URL: https://www.wjgnet.com/2220-3249/full/v13/i2/92521.htm
- DOI: https://dx.doi.org/10.5501/wjv.v13.i2.92521
In late 2019, the world was overrun by a severe respiratory virus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[1]. Since the population was non-immune and the virus spread quickly from person to person, global healthcare met an immense burden. Symptoms characteristic of the coronavirus including fever, shortness of breath, fatigue, as well as major complications like pneumonia, sepsis, respiratory distress and septic shock led to the use of various types of symptomatic therapies before the development of coronavirus disease 2019 (COVID-19) vaccines[2].
Patients are treated in accordance with the degree of illness, as a specialist supervises mandatory treatment. Oxygen therapy is administered if the degree of illness is severe (such as in the case of pneumonia). Immunosuppressors are suitable for treatment, as they can disrupt the induction of interferons type I. This shows why the use of proper immunomodulators could be utilized.
Immunomodulators are medically necessary because the exact duration of the protection provided by antibodies against COVID-19, which the vaccine and infection produce, has not yet been established. Several immunomodulators have been administered to patients: immunotherapy with interferon, mAbs, anti-inflammatory cytokine, plasma, prebiotics, probiotics, stem cells, vitamins, etc.
Infection with COVID-19 induces a destructive immune hyperreaction through several pathways. One of these is Toll-like receptor (TLR) activation, which leads to a cytokine storm characterized by overproduction of inflammatory factors and cytokines[3].
Тhis cytokine storm is the underlying cause of viral sepsis and inflammation-induced lung damage, pneumonitis, acute respiratory distress syndrome (ARDS), respiratory failure, shock, organ failure and death. Therefore, it is essential to develop strategies that can counteract or neutralize this cytokine storm by modulating the immune response and thereby suppressing lung inflammation and injury, inhibiting viral replication, and promoting viral clearance, thus ultimately saving lives[4].
The global trajectory of the COVID-19 pandemic has underscored the importance of understanding and effectively modulating the intricate dynamics of the host immune response. This viral infection, caused by the novel SARS-CoV-2, manifests as a spectrum of clinical outcomes, ranging from mild respiratory symptoms to severe pneumonia and life-threatening complications. As researchers and clinicians grapple with the evolving nature of this virus, the central role of immunity has become increasingly evident. However, this recognition has brought to light a dual challenge: the need to achieve a delicate balance between an insufficient immune response that may foster viral persistence and an exaggerated response that can lead to detrimental hyperinflammation[5].
The inadequacy of the initial immune response poses a formidable obstacle in the battle against COVID-19. SARS-CoV-2, with its ability to evade immediate immune detection, gains a foothold in the respiratory system, often resulting in prolonged viral shedding and increased transmission. This delayed immune activation facilitates viral spread and allows the virus to establish a stronghold, particularly in susceptible individuals. For that reason, understanding the factors contributing to this delayed immune response is crucial for devising effective therapeutic strategies[6].
Conversely, an overly exuberant immune response, characterized by a cytokine storm and uncontrolled inflammation, has been identified as a significant driver of severe COVID-19 cases. The immune system's attempt to eliminate the virus becomes a double-edged sword, inadvertently causing collateral damage to host tissues. This hyperinflammatory state, if left unchecked, can lead to ARDS, multi-organ failure, and an increased risk of mortality. Consequently, deciphering the intricacies of immune dysregulation in COVID-19 is imperative to identify specific targets for intervention and modulation[7].
With these delicate dynamics in mind, immunomodulatory therapies have emerged as a promising avenue for mitigating the challenges posed by COVID-19. Precision in manipulating immune pathways presents an opportunity to alter the host response, optimizing antiviral defenses while curbing deleterious inflammation[8].
As the COVID-19 pandemic persists, the intricacies of the host immune response have emerged as central players in the battle against the virus. However, this nuanced interplay presents a dual challenge—on the one hand, an inadequate immune response may result in viral persistence. On the other hand, an overly exuberant reaction can lead to detrimental hyperinflammation. Navigating this delicate balance demands a comprehensive understanding of immunomodulation strategies[9].
This paper critically examines the multifaceted challenges inherent in the immunological landscape of COVID-19, emphasizing the imperative for targeted immunomodulatory interventions to navigate the fragile equilibrium between adequate viral clearance and immune-mediated pathology. By unraveling the complexities of host-virus interactions, we aim to contribute to the evolving strategies to harness immunity for optimal outcomes in the fight against COVID-19.
The infection with SARS-CoV-2 is followed by a hyperactive inflammatory response, which is crucial for the pathogenesis of COVID-19. Among the known risk factors for a more severe clinical course of COVID-19, including risk of hospitalization and death, are older age, and co-morbidities like diabetes, and cardiovascular or chronic kidney diseases, which are even more prominent risk factors in immunocompromised patients. The latter include patients with rheumatic diseases, hematological malignancies, organ transplants, HIV or other immunodeficiency disorders for which a prolonged shedding of SARS-CoV-2 RNA has been reported[5,9].
Corticosteroids (CS) have anti-inflammatory effects against a broad spectrum of cytokines and chemokines. They could suppress lung injury and multisystem organ dysfunction during hyperinflammatory stages in patients with severe or critical COVID-19 disease who require oxygen therapy. The beneficial effects of the use of CS in hospitalized patients have been proven in multiple randomized trials, which showed that the introduction of dexamethasone in the treatment protocol leads to a reduction of the mortality rate in the group of patients who required invasive mechanical ventilation or non-invasive oxygen support (RECOVERY trial, CoDEX trial, etc.)[10]. Current recommendations are against the use of dexamethasone or other systemic corticosteroid in non-hospitalized patients without other indications. Although inhaled CS suppress the inflammation in the lungs by impairing viral replication and downregulating receptor expression, currently, there is insufficient evidence for or against their use[10-13].
Studies have shown that during SARS-CoV-2 infection, the bronchial epithelial cells produce high amounts of interleukin 6 (IL-6) in a dose-dependent manner. An activated IL-6-JAK-STAT3 axis characterizes severe COVID-19 disease, and levels of IL-6 are associated with the severity of COVID-19, independent of age and sex. IL-6 inhibitors such as tocilizumab and sarilumab have been evaluated in clinical trials for treating hospitalized patients with systemic hyperinflammatory status. Currently, tocilizumab is approved for treating hypoxic patients on systemic CS[14,15].
JAK inhibitors were also discussed for COVID-19 treatment. Activation of JAK molecules by proinflammatory cytokines such as IL-6 leads to phosphorylation of STAT proteins followed by immune activation and inflammation. Thus, JAK inhibitors are studied for the treatment of COVID-19 disease. Currently, only baricitinib is approved by the US Food and Drug Administration (FDA) for the treatment of hospitalized hypoxic COVID-19 patients[16].
Early on in the pandemic, antimalarials were used as inhibitors of cytokines production and viral fusion. Systemic literature review of hydroxychloroquine (HCQ) and chloroquine (CQ) with azithromycin (AZ) did not prove any benefit. Currently, antimalarials are not recommended for treatment of hospitalized patients with COVID-19[17,18].
The impaired immune system in patients with autoimmune rheumatic diseases (AIRDs), along with the effects of CS and disease-modifying antirheumatic drugs, which further suppress the immune system, increase the risk of infections compared to the general population. Being immunosuppressed, even vaccinated, patients with AIRDs are at higher risk for COVID-19 and severe COVID-19. This risk is reported to be related also to the ongoing treatment for the underlying rheumatic condition. Thus, in using immunosuppressive agents when AIRDs, the clinician should balance the benefit-risk ratio considering the two aspects of the immunosuppressive treatment[19]. As these medications can modulate different aspects of the immune response, they could be used as monotherapy or in combination in clinical practice to reduce the severity of COVID-19 course. On the other hand, immunosuppressive agents could reduce the suppressive immune response to viral replication, thus prolonging the viral survival, infection and shedding. CS are widely used to induce remission or as bridging therapy for the long-term management of patients with AIRDs. The beneficial effect of CS in managing patients with AIRDs is related to their rapid inhibition of the immune cell response. By inhibiting the host-immune response, they potentiate a delay in viral clearance and, in case of a SARS-CoV-2 infection, could increase the risk of lung involvement. Oral CS are reported to have a negative impact on AIRD patients with COVID-19 disease, with doses over 10 mg/prednisolone equivalent daily related to increased risk of hospitalization[19]. Moreover, the dose of CS is reported to be an independent risk factor for COVID-19-related death[20]. However, glucocorticoids have side effects that include diabetes mellitus and hypertension, and should be avoid when applicable.
Data reports on clinical outcomes in patients with AIRD treated with agents suppressing T-cells (e.g., calcineurin inhibitors, mycophenolate mofetil), B-cells (anti-CD20 antibodies, anti-CD22 antibodies) or agents against type I interferon show that this treatment agents lead to more severe COVID-19 disease[9,21,22].
Treatment with rituximab has been reported to increase the risk of severe COVID-19 and to lead to the poorest outcome in different studies[23,24]. One suggested mechanism is due to the low viral clearance and persisting viremia[25,26]. Rituximab impairs B cell response to the COVID-19 vaccine in patients with AIRD, thus making the treatment with Rituximab not preferable even in vaccinated individuals[27,28].
Data on the use of anti-TNFα agents in AIRDs infected with COVID-19 show the relative safety of these agents with lower risk of hospitalization and better clinical outcomes compared to patients treated with other immunosuppressants. A possible mechanism is the blockage of TNF-α cytokine as one of the contributing factors in the "cytokine storm" related to COVID-19[29,30].
Other immunosuppressants such as azathioprine, cyclophosphamide, ciclosporin, mycophenolate or tacrolimus were reported to be associated with a higher risk of COVID-19-related death compared to patients on methotrexate monotherapy. The same was valid for patients with AIRDs not receiving any disease-modifying antirheumatic drug (DMARD)[31,32].
SARS-CoV-2 possesses many mechanisms to blunt early immune responses, allowing viral replication and worsening clinical symptoms and, in some cases, uncontrolled immune activation (i.e., cytokine storm)[33].
While the exact pathways causing cytokine release syndrome (CRS) and ARDS are still unknown, high levels of proinflammatory cytokines like IL-6, IL-1β, and TNF-α characterize the cytokine storm. There are encouraging preli
Iqbal Yatoo et al[34] focused on immunomodulatory therapies available before COVID-19 vaccines or specific treatment: convalescent plasma, immunoglobulins, monoclonal or polyclonal antibodies, immunomodulatory agents, cell-based therapies (i.e., NK cells, T cells, stem cells), cytokines and toll-like receptors based therapies.
Remdesivir (GS-5734) was the only medication for the treatment of COVID-19 with emergency use authorization issued by the FDA[35]. Incorporating into the viral RNA as a nucleotide/adenosine analog, Remdesivir results in premature termination of the viral replication[36-38]. In human airway epithelial cells, remdesivir inhibits Middle East respiratory syndrome coronavirus and SARS-CoV[39]. Nausea, vomiting, elevation of transaminases, and diarrhea are some adverse effects of remdesivir use[35]. At ten hospitals in Hubei, China, a randomized, double-blind, placebo-controlled, multicentre trial (NCT04257656) was conducted to evaluate the safety and efficacy of Remdesivir in hospitalized adult patients with severe COVID-19[40]. Two hundred thirty-seven patients were assigned to a 2:1 ratio (158 to Remdesivir and 79 to placebo), and the participants were permitted concomitant use of interferons, CS, and lopinavir-ritonavir. Despite the adequate tolerability, the study did not find statistically significant clinical benefits of Remdesivir use[40]. In a double-blind, randomized, placebo-controlled trial, Beigel et al[41] concluded that intravenous Remdesivir is superior to a placebo in shortening the time to recovery in hospitalized adult patients with COVID-19, who had evidence of lower respiratory tract infection.
Favipiravir, known as T-705, is an antiviral drug that selectively and potently inhibits the RNA-dependent RNA polymerase[42] and may serve as an emerging treatment for COVID-19[35]. Tocilizumab represents a recombinant humanized monoclonal antibody against the human IL-6 receptor[43,44]. Multicenter, randomized controlled trial enrolled 26 patients to assess the efficacy and safety of tocilizumab combined with favipiravir in patients with COVID-19. The participants were divided into three groups according to treatment: favipiravir, tocilizumab, or tocilizumab combined with tocilizumab. The study showed the beneficial effect of tocilizumab on COVID-19 patients, as tocilizumab alone or combined with favipiravir can improve pulmonary inflammation and reduce mortality and worsening of the infection[44]. Generally, Favipiravir is well tolerated, however, liver dysfunction, diarrhea, and nausea are some of the side effects related to its use[45]. Resulting in the inhibition of membrane fusion between virus particles and plasma membranes related to its intercalation into membrane lipids[46], umifenovir is another candidate drug for the treatment of COVID-19[35]. Chen et al[47], comparing favipiravir with umifenovir, did not find a significant difference in the clinical recovery rate at day 7 in adult patients with COVID-19.
CQ and its derivative, HCQ, are antimalarial drugs that showed activity against numerous RNA viruses and were considered as promising therapeutic options for COVID-19[35,48,49]. The limitation of the virus-cell fusion, the causing of alkalization of the usually acidic endosomal pH of the infected cells, and receptors modifying glycosylation are some of the multiple anti-viral mechanisms of CQ and HCQ[35,50]. The current evidence shows that HCQ is inefficient in reducing mortality and has no benefits regarding time to clinical improvement and intensive mechanical ventilation requirements[48]. Common adverse reactions related to treatment with CQ and HCQ are diarrhea, abdominal discomfort, nausea, and vomiting[51]. Cardiac toxicity and prolonged QTc interval are also described with these drugs, and retinopathy is the most severe complication of CQ/HCQ[51]. The HCQ and AZ combination showed a synergetic effect on SARS-CoV-2-infected cells in in vitro studies[52]. AZ is a macrolide associated with a higher risk of cardiac death and prolongation of the QT/QTc interval[53,54]. Based on the current evidence, HCQ, either alone or with AZ, is unsuitable for treating COVID-19[55].
Geldanamycin represents the group of ansamycins and inhibits the HSP90[56]. Anticancer and antimicrobial properties characterize Geldanamycin and demonstrate antiviral activities against viruses such as HIV-1 and Influenza[56]. Hepatotoxicity and anemia are some of the adverse effects of the use of Geldanamycin, and there is no registered trial of the impact of this medication on patients with COVID-19[35].
Thalidomide has anti-inflammatory activity, downregulates soluble levels of mediators such as TNF- α, IL-1, IL-6, and PGE2, and inhibits the COX-2[57]. The FDA approved Thalidomide for treating multiple myeloma and erythema nodosum leprosum[58]. Through its immunomodulatory and anti-inflammatory properties, Thalidomide could be tested in treating respiratory complications related to COVID-19[58]. It is a well-known fact that Thalidomide has a teratogenic effect[59].
Non-steroidal anti-inflammatory drugs (NSAIDs) are available over the counter (OTC) in most countries, and according to the available evidence, NSAIDs neither worsen outcomes of COVID-19 nor increase the likelihood of SARS-CoV-2 infection and could be used as antipyretic and analgetic drugs during COVID-19[60].
Losartan is a selective antagonist of the AT1 receptor that potentially can protect against lung damage induced by COVID-19 by inhibiting the ACE–Ang II–AT1 axis, which is implicated in fibrosis[61]. In an individual participant data meta-analysis including 325 participants, Di Stefano et al[62] did not find a benefit of losartan versus control treatment in hospitalized patients with COVID-19.
The IL-1 family consists of 11 members as IL-1α and IL-1β are proinflammatory proteins that share IL-1 receptor 1 as their common receptor[63]. Anakinra is a recombinant human IL-1 receptor antagonist[64]. Some therapeutic indications of anakinra are Still’s Disease, Rheumatoid Arthritis, and Cryopyrin-Associated Periodic Syndromes (European Medicines Agency Summary of product characteristics)[65]. In a systematic review and meta-analysis published in 2023, Dahms et al[66] concluded that compared to placebo or standard care alone, Anakinra shows no effectiveness on adult hospitalized patients with SARS-CoV-2 infection regarding mortality and clinical improvement.
Serum IL-6 is significantly elevated in patients with complicated COVID-19, and increased IL-6 predicts adverse clinical outcomes[67]. Tocilizumab represents a recombinant humanized, anti-human monoclonal antibody against membrane-bound and soluble interleukin 6 receptors (IL-6R)[42]. In a systematic review and meta-analysis, Keske et al[68] showed the effectiveness of tocilizumab in non-intubated cases with severe COVID-19. Through this systematic review and meta-analysis, they demonstrated that compared to standard-of-care (SOC) treatment tocilizumab decreased the need for invasive mechanical ventilation (OR: 0.76; 95%CI: 0.67–0.86, P < 0.001 and for the heterogeneity I2 = 6%, P = 0.39) and reduced the decreased the mortality (OR: 0.84; 95%CI: 0.73–0.96, P = 0.009, and for the heterogeneity I2 = 0%, P = 0.82)[68].
Sarilumab is a human recombinant IgG1 monoclonal antibody that inhibits IL-6-mediated signaling by binding to both soluble and membrane-bound IL-6R[69]. In the treatment of severe COVID-19, Sarilumab was considered as an alternative to tocilizumab[70]. In a national, multicenter, open-labeled, phase 3 randomized clinical trial, Mastrorosa et al[71] evaluated the clinical efficacy and safety of intravenous sarilumab in addition to the SOC in managing adults with severe COVID-19 pneumonia. The participants in the trial were randomly assigned in a 2:1 ratio to receive sarilumab in addition to SOC or SOC therapy alone[71]. Mastrorosa et al[71] did not find the efficacy of adding sarilumab in severe COVID-19. Increased risk of secondary infections, hypotension, cytopenias, and edemas are some of the side effects related to sarilumab use[35].
Via receptor-mediated endocytosis, SARS-CoV-2 enters cells after binding its spike protein to the human ACE-2 receptor[72]. AAK1 is one regulator of endocytosis whose inhibition could prevent SARS-CoV-2 entry and the intracellular assembly of virus particles[73]. Cycling GAK modulates endocytosis[36]. By binding to GAK and inhibiting the AAKI kinase, it is hypothesized that baricitinib prevented viral infection[74], and by targeting JAK1 and JAK2, baricitinib would inhibit inflammation[75]. Baricitinib is recommended as the therapeutic option for patients with severe COVID-19[70]. In a systematic review and meta-analysis, Song et al[76] showed that compared to the standard treatment, baricitinib decreases mortality and mechanical ventilation requirements in patients with severe COVID-19.
Tofacitinib is an oral, small molecule JAKi[77], which inhibits JAK1 and JAK 3 and, to a lower degree, JAK 2, modulating on this way the JAK-STAT signaling[78,79]. Tofacitinib can decrease the release of cytokines by type 1 and type 17 helper T cells by modulating the action of IL-6 and interferons[80-82]. In the STOP-COVID study, a multicenter, randomized, double-blind, placebo-controlled trial, Guimarães et al[83] evaluated the efficacy and safety of tofacitinib in hospitalized patients with COVID-19 pneumonia. The trial showed that respiratory failure or death through day 28 occurred in 29.0% of those in the placebo group in 18.1% of the patients in the tofacitinib group (risk ratio: 0.63, 95%CI: 0.41 to 0.97; P = 0.04)[83].
Glucocorticoids are among the most widely prescribed drugs with their immune-suppressive and anti-inflammatory effect[84]. The current guidelines for the treatment of COVID-19 recommend against the use of dexamethasone or other systemic CS in non-hospitalized patients in the absence of another indication[70]. The RECOVERY trial demonstrates the reduced 28-d mortality among hospitalized patients with COVID-19 using dexamethasone compared to the usual standard of care, along with other investigators, such as Ahmed and Hassan[85]. The benefit of dexamethasone was seen only among participants receiving either oxygen alone or invasive mechanical ventilation at randomization but not among those receiving no respiratory support at enrollment[85]. In a systematic review and meta-analysis, Albuquerque et al[86] showed that in comparison to tocilizumab, baricitinib, and sarilumab are associated with high probabilities of similar mortality reductions among hospitalized COVID-19 concurrently treated with CS.
As a result of the absence of SARS-CoV-2-specific antiviral medications, the effectiveness of COVID-19 treatments is reduced. Several COVID-19 therapies are now under investigation. However, the majority of them lack specificity, efficacy, and safety[87]. Immunotherapy is a ground-breaking medical treatment that manipulates the immune system to fight diseases. Translational research is rapidly progressing, recognized as a significant breakthrough in 2013[88]. Among the immunotherapeutic options for treating COVID-19 are Immunoglobulin, CP, antibodies, mAbs (mAbs), NK cells, T cells, TLR, cytokine therapies and immune modulators.
All the therapeutic options discussed above ranging from CS to targeted biologics have shown more than promising results in the battle of tackling COVID-19 infection. In patients who are hospitalized due to the severity of their condition the above-mentioned treatments are an integral part of the management plan. However, a major disadvantage in their use is that they are not readily available to the general public who are not warranted a hospital stay but are still dealing with the consequences of this viral infection.
This is where OTC drugs come into play, more specifically NSAIDs, which are a great alternative for in-home-care therapy of COVID-19[89]. NSAIDs help with pain management and fever reduction due to their analgesic and antipyretic properties respectively, which makes them suitable for symptomatic relief[90]. NSAIDs when started early enough in the disease progression have proven effective in reducing the rate of hospitalization which delivers an additional benefit for their OTC use[91].
There cannot be a critical conversation regarding the role of NSAIDs in the light of COVID-19 infection without also addressing their controversial initial speculative use at the onset of the COVID-19 pandemic when there were concerns raised about the potential for NSAIDs namely Ibuprofen to potentially heighten susceptibility for SARS-CoV-2 infection and adversely affect clinical results. These speculative claims about Ibuprofen’s potential harm originated from an article in The Lancet Respir Med[92] in which it was stated that Ibuprofen administration runs the risk of increasing the expression of ACE-2, the receptor through which the SARS-CoV-2 virus enters cells. Increased levels of ACE2 were speculated to theoretically enhance the virus's ability to infect cells.
The confusion and unfounded fear around the topic was further exacerbated by the Health Ministry of France’s endorsement of the now proven false claims[93]. Other concerns raised at the time were connected to yet another theoretical speculation of NSAIDs masking COVID-19 symptoms and thus prolonging the disease duration and therefore increasing the risk of complications[94]. All of the initial reluctance to Ibuprofen and overall NSAIDs use has been debunked and proven wrong by numerous original research articles and extensive literature reviews[95-97].
Moreover, their OTC use and lack of any harm in it is supported fully by the World Health Organization, FDA and European Medicines Agency[98].
Several characteristics, one of which is being a selective COX-2 inhibitor[99], suggest that etoricoxib could potentially suppress the cytokine storm, providing a feasible option for COVID-19 treatment. Hence, it seems justifiable to explore etoricoxib extensively to determine its suitability for repurposing as a therapeutic intervention for COVID-19[100].
While there were initial concerns about ibuprofen use in COVID-19, current evidence suggests that ibuprofen can be used for symptom management in individuals with mild to moderate illness[101]. In fact, the use of ibuprofen did not show any correlation with adverse clinical results when compared to the administration of paracetamol or abstaining from antipyretic treatment altogether[95].
Paracetamol, also known as acetaminophen, is typically not classified as an NSAID due to its limited anti-inflammatory effects. Its relieving pain and fever properties, however, make it a perfect option for OTC use in COVID-19[102]. Paracetamol is generally considered safe when used at recommended doses. It has a favorable safety profile and is well-tolerated by most individuals with fewer gastrointestinal side effects compared to NSAIDs[103].
Aspirin causes a statistical decrease in the severity of the disease expression as well as lowers the likelihood of developing SARS-CoV-2 infection altogether[104] Meta-analyses show that Aspirin usage is associated with reduced mortality rates[105,106] while also not contributing to an increased bleeding risk, which makes it a safe option for OTC use in COVID-19[107]. It is additionally associated with a reduced risk of embolism occurrence during the infection period[108].
Aspirin should not be stopped during hospitalization if the patient is already taking it as a part of their regular medication as this would cause worse outcomes, poorer clinical course and increased mortality compared to the alternative of keeping aspirin as part of the hospital treatment regimen[109].
In fact, patients who take Aspirin as their regular treatment for primary or secondary prevention of cardiovascular disease have a hypothesized already implemented protective benefit when it comes to COVID-19. Research proved that to be true for patients taking the drug with primary prevention focus[110].
For the geriatric population, however, such firm positive effects are not as easily observed. Explanation for this discrepancy provides the fact that the elderly population has a higher frequency of chronic comorbidities which are known to worsen COVID-19 outcomes[111].
More studies need to be conducted to completely assess Aspirin’s full efficacy and clinical potential in COVID-19[112]. Nevertheless, the already established advantages of its utilization make it an important part of the OCT treatment options.
There is now a large body of literature about CP in COVID-19 treatment. While initial assessments of its effectiveness may have produced inconclusive findings, a thorough examination based on biological plausibility and guided by the principles of antibody therapy demonstrates that during the early stages of the pandemic, when COVID-19 had a high mortality rate, CP significantly decreased mortality when administered promptly and in high concentrations of specific antibodies[113]. In COVID-19 disease, CP may have several advantageous effects. The primary mechanism is mostly related to the ability of antibodies derived from CP to inhibit the presence of viruses in the bloodstream. Theoretically, administering CP early in the disease course would be more beneficial, much like the strategies implemented during the SARS pandemic. The majority of viral infections have a peak in viremia within the first week of infection. Typically, the host's primary immune response is established by days 10-14 of infection, indicating the elimination of the viruses[114]. Some researchers suggest that this immune response may begin slightly sooner. Additional possible processes involve antibody-dependent cellular cytotoxicity, complement activation, and phagocytosis (ADCP). Moreover, the existence of non-neutralizing antibodies that attach to the pathogens could potentially be advantageous[115].
The utility of CP in immunocompromised individuals is particularly intriguing[116]. The utilization of CP therapy may be seen as a viable alternative for immunocompromised patients, particularly in regions with restricted availability of monoclonal antibody treatments. Aside from the study by Lang-Meli et al[117], there is insufficient data to support this medication's safety and efficacy in patients with reduced antibody levels. In their study, the authors presented the details of 16 COVID-19 patients who had primary antibody deficiency and were treated with CP. They found that plasma administration was linked to a decrease in viral load and improvement of clinical symptoms, even when implemented seven days after the infection. Aside from a transient fever reaction in one patient, there were no other significant side effects[117]. However, since a large portion of the population has gained immunity to SARS-CoV-2 due to vaccinations and spontaneous infection, nowadays CP is no longer as helpful for immunocompetent individuals. Nevertheless, CP continues to have a crucial role in managing COVID-19 in individuals with weakened immune systems, who frequently exhibit suboptimal responses to both vaccinations and infection.
Lessons learned from past epidemics (SARS, Ebola) paved the way for using mAb-based therapeutics in COVID-19 pandemics. Various mAbs, including bevacizumab, sarilumab, adalimumab, camrelizumab, eculizumab, mepolizumab, PD-1 monoclonal antibody, and tocilizumab, are now being studied as potential treatments for COVID-19[118]. These therapies are being examined in ongoing research. The literature data showed that focusing on the S1 subunit of the spike glycoprotein makes it possible to develop mAbs that are highly targeting, efficient, and can be easily manufactured for the treatment of COVID-19. This approach aims to specifically target the structures of SARS-CoV-2, the virus responsible for COVID-19.
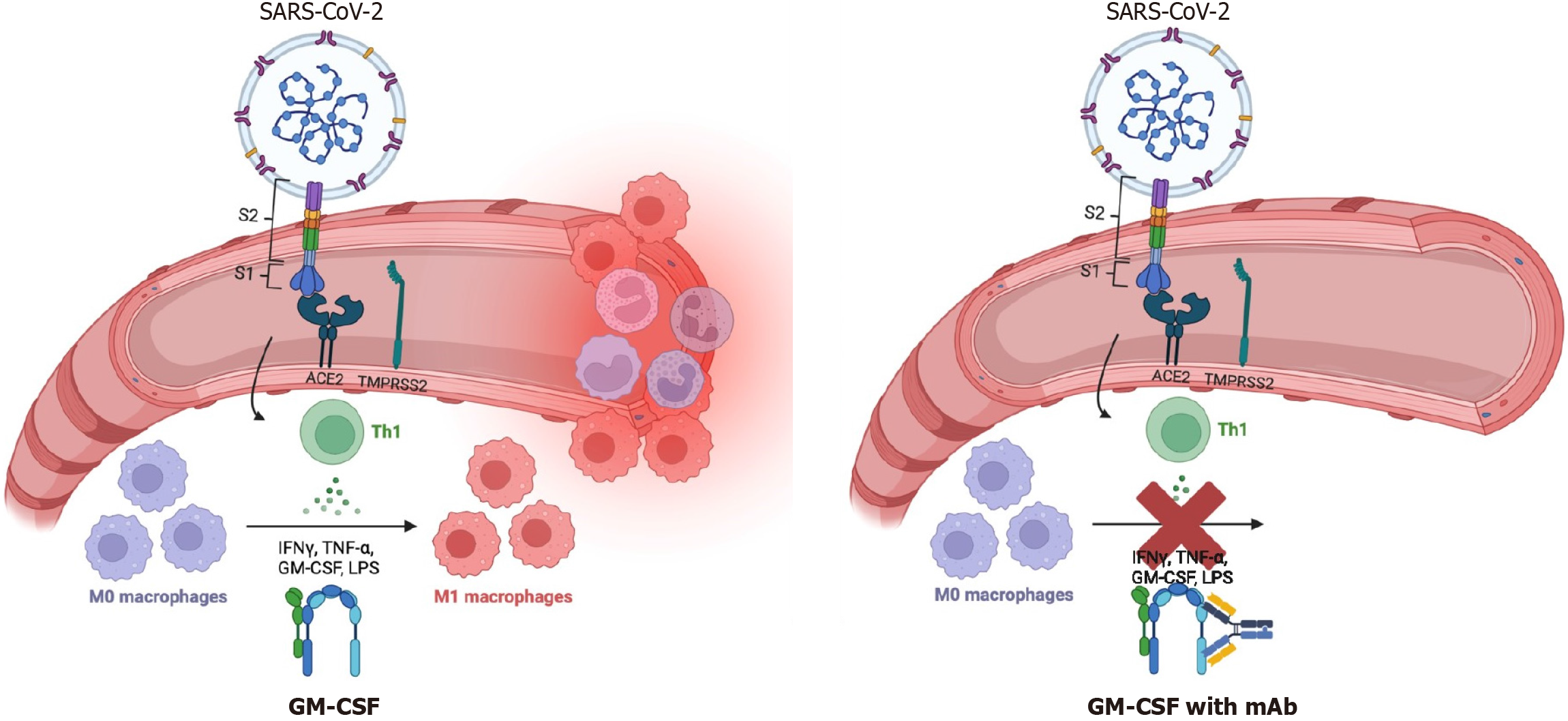
Researchers are currently assessing the potential therapeutic benefit of targeting granulocyte macrophage-colony stimulating factor with mAb to mitigate lung injury or ARDS, believed to be primarily caused by lung hyper-inflammation[119]. Effects of this therapy are shown in Figure 1.
Another interesting therapeutic option, which has the potential to be beneficial, is bevacizumab, a recombinant-humanized monoclonal antibody that specifically targets vascular endothelial growth factor (VEGF). The drug can potentially decrease the levels of VEGF induced by severe inflammation, thus inhibiting the development of edema in patients suffering from COVID-19[120]. Several other mAbs, such as adalimumab, camrelizumab, eculizumab, mepo
Some cell-based therapies discussed for COVID-19 are NK cells, T cells, CAR-T cell therapy, and stem cell therapy. It was shown that NK cells can act precisecly during immune dysfunction and cytokine storm due to their antiviral and regulatory functions[122].
A clinical trial with NK cells for COVID-19 treatment demonstrated safety and efficacy along with standard therapy. Improvements in respiratory distress and immunological parameters and decreased mortality were noted[123].
Additionally, imiquimode can enhance NK cells, further contributing to COVID-19 improvement[124].
T cell-based therapies, i.e., CD4+ CD25+ FoxP3+ regulatory T-cell, anti-CD19 CAR T-cell axicabtagene ciloleucel (Brand name: Yescarta) and tisagenlecleucel (Brand name: Kymriah) were also explored for treatment in COVID-19 patients[125,126].
To achieve the desired outcomes during the COVID-19 pandemic, Bishop stressed the importance of optimizing CAR T-cell therapy. It is possible to generate CAR T cells specific for the viral surface antigens, which can be used as therapeutic vaccines or for the targeted destruction of virally infected cells to stop the infection from spreading further within the body[127].
Hu et al[128] noted that CAR T-cell therapy was complicated during the COVID-19 pandemic and emphasized the importance of considering several medical and technical issues before, during, and following CAR-T therapy.
COVID-19 has shown promise for the use of stem cell therapy. Several clinical trials utilizing stem cells alone or with other treatment modalities are being researched[129,130].
The seven enrolled patients showed improvement in lung function and symptoms within two days of MSC transplantation. Clinical outcomes, inflammatory factors, immune function changes, and adverse effects were measured for 14 d after mesenchymal stem cell injection. The laboratory evaluation results showed that after 3–6 d, there was a significant decrease in TNF-α levels but an increase in IL-10 levels in the MSC treatment group compared to the placebo control group. Additionally, there was an absence of highly activated cytokine-secreting immune cells, such as CXCR3+ CD4 + T cells, CXCR3+ CD8 + T cells, and CXCR3+ NK cells[131].
We can also mention TLR therapies used to modulate innate immunity. TLR5 aids in activating innate immunity and stimulates TLR5 through bacterial flagellin, which can help in early modulation of immune response against COVID-19 and thus have therapeutic or prophylactic applications. Imiquimod aids in TLR7 activation, stimulation of specific and nonspecific immune response, and cytokine production, thus potentially being useful in COVID-19 therapy[88,124,132].
Immunomodulatory techniques may reduce lung inflammation and lower the risk of pneumonia or ARDS in an infected individual by modulating the immune response. However, as Verma et al[133] discussed, we must keep in mind that immunomodulatory approaches have the potential to be a double-edged sword (2023).
Therefore, it is vital to conduct extensive research on identifying new and specific prospective biological targets that may help minimize inflammation and cytokine storm. Antiviral and anti-inflammatory therapies or drugs should be administered early during COVID-19 infection due to pulmonary inflammation that can become uncontrollable and may result in immunosuppression, pneumonia, and ARDS that causes severe lung injury[134].
A common theme in regulating the detrimental effects of respiratory viruses like SARS-CoV-2 is finding ways to lessen the exacerbated inflammatory effects of the virus, which otherwise lead to respiratory failure and severe sepsis and/or shock. An astounding number of randomized trials investigating agents for COVID-19 treatment are underway, focusing on many treatment modalities. Many of the agents, as mentioned above, target the innate immune system to block proinflammatory cytokine production. These therapies are urgently needed and should be further investigated to combat a pandemic caused by a novel virus. Glucocorticoids, however, have many effects on the host immune system, including non-specific immunosuppression. Moreover, because of the glucocorticoids side effects, steroid-sparing therapy is required for COVID-19. The best agents selectively reduce an unwanted host inflammatory response without changing T-cell and monocyte-mediated antiviral activity. As a result, new therapies, like CD24Fc, have a higher safety profile than steroids and may protect better against severe COVID-19 disease by reducing the host inflammatory response without causing widespread immunosuppression.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: Bulgaria
Peer-review report’s classification
Scientific Quality: Grade A, Grade A, Grade B
Novelty: Grade A, Grade A, Grade B
Creativity or Innovation: Grade A, Grade A, Grade C
Scientific Significance: Grade A, Grade A, Grade B
P-Reviewer: Su C, China; Wang Z, China S-Editor: Lin C L-Editor: A P-Editor: Zhang L
| 1. | Li H, Liu SM, Yu XH, Tang SL, Tang CK. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents. 2020;55:105951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 692] [Cited by in RCA: 582] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 2. | Verma G, Dhawan M, Saied AA, Kumar R, Mishra RPN. Variability of severe acute respiratory syndrome coronavirus 2 infection in children and adults. Int J Surg. 2023;109:2148-2150. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30097] [Article Influence: 6019.4] [Reference Citation Analysis (3)] |
| 4. | Toldo S, Bussani R, Nuzzi V, Bonaventura A, Mauro AG, Cannatà A, Pillappa R, Sinagra G, Nana-Sinkam P, Sime P, Abbate A. Inflammasome formation in the lungs of patients with fatal COVID-19. Inflamm Res. 2021;70:7-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 5. | Hall MW, Joshi I, Leal L, Ooi EE. Immune Immunomodulation in Coronavirus Disease 2019 (COVID-19): Strategic Considerations for Personalized Therapeutic Intervention. Clin Infect Dis. 2022;74:144-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Hertanto DM, Wiratama BS, Sutanto H, Wungu CDK. Immunomodulation as a Potent COVID-19 Pharmacotherapy: Past, Present and Future. J Inflamm Res. 2021;14:3419-3428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Rommasi F, Nasiri MJ, Mirsaeidi M. Immunomodulatory agents for COVID-19 treatment: possible mechanism of action and immunopathology features. Mol Cell Biochem. 2022;477:711-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Ingraham NE, Lotfi-Emran S, Thielen BK, Techar K, Morris RS, Holtan SG, Dudley RA, Tignanelli CJ. Immunomodulation in COVID-19. Lancet Respir Med. 2020;8:544-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 9. | van de Veerdonk FL, Giamarellos-Bourboulis E, Pickkers P, Derde L, Leavis H, van Crevel R, Engel JJ, Wiersinga WJ, Vlaar APJ, Shankar-Hari M, van der Poll T, Bonten M, Angus DC, van der Meer JWM, Netea MG. A guide to immunotherapy for COVID-19. Nat Med. 2022;28:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 227] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 10. | RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6762] [Cited by in RCA: 7376] [Article Influence: 1844.0] [Reference Citation Analysis (1)] |
| 11. | Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, Silva MVAO, Baldassare FP, Costa ELV, Moura RAB, Honorato MO, Costa AN, Damiani LP, Lisboa T, Kawano-Dourado L, Zampieri FG, Olivato GB, Righy C, Amendola CP, Roepke RML, Freitas DHM, Forte DN, Freitas FGR, Fernandes CCF, Melro LMG, Junior GFS, Morais DC, Zung S, Machado FR, Azevedo LCP; COALITION COVID-19 Brazil III Investigators. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020;324:1307-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 921] [Cited by in RCA: 938] [Article Influence: 187.6] [Reference Citation Analysis (0)] |
| 12. | Bouadma L, Mekontso-Dessap A, Burdet C, Merdji H, Poissy J, Dupuis C, Guitton C, Schwebel C, Cohen Y, Bruel C, Marzouk M, Geri G, Cerf C, Mégarbane B, Garçon P, Kipnis E, Visseaux B, Beldjoudi N, Chevret S, Timsit JF; COVIDICUS Study Group. High-Dose Dexamethasone and Oxygen Support Strategies in Intensive Care Unit Patients With Severe COVID-19 Acute Hypoxemic Respiratory Failure: The COVIDICUS Randomized Clinical Trial. JAMA Intern Med. 2022;182:906-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 13. | RECOVERY Collaborative Group. Higher dose corticosteroids in patients admitted to hospital with COVID-19 who are hypoxic but not requiring ventilatory support (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2023;401:1499-1507. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Csobonyeiova M, Smolinska V, Harsanyi S, Ivantysyn M, Klein M. The Immunomodulatory Role of Cell-Free Approaches in SARS-CoV-2-Induced Cytokine Storm-A Powerful Therapeutic Tool for COVID-19 Patients. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 15. | Nasonov E, Samsonov M. The role of Interleukin 6 inhibitors in therapy of severe COVID-19. Biomed Pharmacother. 2020;131:110698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 16. | Luo W, Li YX, Jiang LJ, Chen Q, Wang T, Ye DW. Targeting JAK-STAT Signaling to Control Cytokine Release Syndrome in COVID-19. Trends Pharmacol Sci. 2020;41:531-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 17. | Das S, Bhowmick S, Tiwari S, Sen S. An Updated Systematic Review of the Therapeutic Role of Hydroxychloroquine in Coronavirus Disease-19 (COVID-19). Clin Drug Investig. 2020;40:591-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 663] [Cited by in RCA: 657] [Article Influence: 131.4] [Reference Citation Analysis (0)] |
| 19. | Haberman RH, Castillo R, Chen A, Yan D, Ramirez D, Sekar V, Lesser R, Solomon G, Neimann AL, Blank RB, Izmirly P, Webster DE, Ogdie A, Troxel AB, Adhikari S, Scher JU; NYU WARCOV Investigators. COVID-19 in Patients With Inflammatory Arthritis: A Prospective Study on the Effects of Comorbidities and Disease-Modifying Antirheumatic Drugs on Clinical Outcomes. Arthritis Rheumatol. 2020;72:1981-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 20. | Strangfeld A, Schäfer M, Gianfrancesco MA, Lawson-Tovey S, Liew JW, Ljung L, Mateus EF, Richez C, Santos MJ, Schmajuk G, Scirè CA, Sirotich E, Sparks JA, Sufka P, Thomas T, Trupin L, Wallace ZS, Al-Adely S, Bachiller-Corral J, Bhana S, Cacoub P, Carmona L, Costello R, Costello W, Gossec L, Grainger R, Hachulla E, Hasseli R, Hausmann JS, Hyrich KL, Izadi Z, Jacobsohn L, Katz P, Kearsley-Fleet L, Robinson PC, Yazdany J, Machado PM; COVID-19 Global Rheumatology Alliance. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 501] [Cited by in RCA: 497] [Article Influence: 124.3] [Reference Citation Analysis (0)] |
| 21. | Agrati C, Bartolini B, Bordoni V, Locatelli F, Capobianchi MR, Di Caro A, Castilletti C, Ippolito G. Emerging viral infections in immunocompromised patients: A great challenge to better define the role of immune response. Front Immunol. 2023;14:1147871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 22. | D'Silva KM, Serling-Boyd N, Wallwork R, Hsu T, Fu X, Gravallese EM, Choi HK, Sparks JA, Wallace ZS. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US 'hot spot'. Ann Rheum Dis. 2020;79:1156-1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 189] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 23. | Schulze-Koops H, Krueger K, Vallbracht I, Hasseli R, Skapenko A. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis. 2021;80:e67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 24. | Kow CS, Hasan SS. Use of rituximab and the risk of adverse clinical outcomes in COVID-19 patients with systemic rheumatic disease. Rheumatol Int. 2020;40:2117-2118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Tepasse PR, Hafezi W, Lutz M, Kühn J, Wilms C, Wiewrodt R, Sackarnd J, Keller M, Schmidt HH, Vollenberg R. Persisting SARS-CoV-2 viraemia after rituximab therapy: two cases with fatal outcome and a review of the literature. Br J Haematol. 2020;190:185-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 26. | Kos I, Balensiefer B, Roth S, Ahlgrimm M, Sester M, Schmidt T, Thurner L, Bewarder M, Bals R, Lammert F, Stilgenbauer S, Kaddu-Mulindwa D. Prolonged Course of COVID-19-Associated Pneumonia in a B-Cell Depleted Patient After Rituximab. Front Oncol. 2020;10:1578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Spiera R, Jinich S, Jannat-Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann Rheum Dis. 2021;80:1357-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 28. | Bitoun S, Henry J, Desjardins D, Vauloup-Fellous C, Dib N, Belkhir R, Mouna L, Joly C, Bitu M, Ly B, Pascaud J, Seror R, Roque Afonso AM, Le Grand R, Mariette X. Rituximab Impairs B Cell Response But Not T Cell Response to COVID-19 Vaccine in Autoimmune Diseases. Arthritis Rheumatol. 2022;74:927-933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 29. | Brito CA, Paiva JG, Pimentel FN, Guimarães RS, Moreira MR. COVID-19 in patients with rheumatological diseases treated with anti-TNF. Ann Rheum Dis. 2021;80:e62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 30. | Baslılar S, Pehlivan O. Evaluation of factors affecting the frequency and clinical course of COVID-19 in patients using anti-TNF-alpha agents. Rev Assoc Med Bras (1992). 2021;67:1286-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, Izadi Z, Jacobsohn L, Katz P, Lawson-Tovey S, Mateus EF, Rush S, Schmajuk G, Simard J, Strangfeld A, Trupin L, Wysham KD, Bhana S, Costello W, Grainger R, Hausmann JS, Liew JW, Sirotich E, Sufka P, Wallace ZS, Yazdany J, Machado PM, Robinson PC; COVID-19 Global Rheumatology Alliance. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 895] [Cited by in RCA: 870] [Article Influence: 174.0] [Reference Citation Analysis (0)] |
| 32. | Widhani A, Koesnoe S, Maria S, Widjanarko AL, Karjadi TH, Hasibuan AS, Yunihastuti E, Rengganis I, Djauzi S. Factors Related to Severity, Hospitalization, and Mortality of COVID-19 Infection among Patients with Autoimmune Diseases. Trop Med Infect Dis. 2023;8:227. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Khadke S, Ahmed N, Ratts R, Raju S, Gallogly M, de Lima M, Sohail MR. Harnessing the immune system to overcome cytokine storm and reduce viral load in COVID-19: a review of the phases of illness and therapeutic agents. Virol J. 2020;17:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 34. | Iqbal Yatoo M, Hamid Z, Rather I, Nazir QUA, Bhat RA, Ul Haq A, Magray SN, Haq Z, Sah R, Tiwari R, Natesan S, Bilal M, Harapan H, Dhama K. Immunotherapies and immunomodulatory approaches in clinical trials - a mini review. Hum Vaccin Immunother. 2021;17:1897-1909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Thng ZX, De Smet MD, Lee CS, Gupta V, Smith JR, McCluskey PJ, Thorne JE, Kempen JH, Zierhut M, Nguyen QD, Pavesio C, Agrawal R. COVID-19 and immunosuppression: a review of current clinical experiences and implications for ophthalmology patients taking immunosuppressive drugs. Br J Ophthalmol. 2021;105:306-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 36. | Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4289] [Cited by in RCA: 4564] [Article Influence: 912.8] [Reference Citation Analysis (0)] |
| 37. | Shiraki K, Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther. 2020;209:107512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 320] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 38. | Al-Tawfiq JA, Al-Homoud AH, Memish ZA. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med Infect Dis. 2020;34:101615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 39. | Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I, Bannister R, Park Y, Babusis D, Clarke MO, Mackman RL, Spahn JE, Palmiotti CA, Siegel D, Ray AS, Cihlar T, Jordan R, Denison MR, Baric RS. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1011] [Cited by in RCA: 1126] [Article Influence: 160.9] [Reference Citation Analysis (0)] |
| 40. | Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2337] [Cited by in RCA: 2485] [Article Influence: 497.0] [Reference Citation Analysis (0)] |
| 41. | Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5711] [Cited by in RCA: 5110] [Article Influence: 1022.0] [Reference Citation Analysis (0)] |
| 42. | Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 734] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 43. | European Medicines Agency. RoActemra. [cited 9 April 2024]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/roactemra. |
| 44. | Zhao H, Zhu Q, Zhang C, Li J, Wei M, Qin Y, Chen G, Wang K, Yu J, Wu Z, Chen X, Wang G. Tocilizumab combined with favipiravir in the treatment of COVID-19: A multicenter trial in a small sample size. Biomed Pharmacother. 2021;133:110825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 45. | Ergür FÖ, Yıldız M, Şener MU, Kavurgacı S, Ozturk A. Adverse effects associated with favipiravir in patients with COVID-19 pneumonia: a retrospective study. Sao Paulo Med J. 2022;140:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Villalaín J. Membranotropic effects of arbidol, a broad anti-viral molecule, on phospholipid model membranes. J Phys Chem B. 2010;114:8544-8554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Chen C, Zhang Y, Huang J, Yin P, Cheng Z, Wu J, Chen S, Chen B, Lu M, Luo Y, Ju L, Zhang J, Wang X. Favipiravir Versus Arbidol for Clinical Recovery Rate in Moderate and Severe Adult COVID-19 Patients: A Prospective, Multicenter, Open-Label, Randomized Controlled Clinical Trial. Front Pharmacol. 2021;12:683296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 48. | Andaluz-Ojeda D, Vidal-Cortes P, Aparisi Sanz Á, Suberviola B, Del Río Carbajo L, Nogales Martín L, Prol Silva E, Nieto Del Olmo J, Barberán J, Cusacovich I. Immunomodulatory therapy for the management of critically ill patients with COVID-19: A narrative review. World J Crit Care Med. 2022;11:269-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;55:105938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 704] [Cited by in RCA: 673] [Article Influence: 134.6] [Reference Citation Analysis (0)] |
| 50. | Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Seidah NG, Nichol ST. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1198] [Cited by in RCA: 1225] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 51. | Khuroo MS. Chloroquine and hydroxychloroquine in coronavirus disease 2019 (COVID-19). Facts, fiction and the hype: a critical appraisal. Int J Antimicrob Agents. 2020;56:106101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 52. | Nabirotchkin S, Peluffo AE, Bouaziz J, Cohen D. Focusing on the Unfolded Protein Response and Autophagy Related Pathways to Reposition Common Approved Drugs against COVID-19. 2020 Preprints. Available from: 2020030302. [DOI] [Full Text] |
| 53. | Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 663] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 54. | Lu ZK, Yuan J, Li M, Sutton SS, Rao GA, Jacob S, Bennett CL. Cardiac risks associated with antibiotics: azithromycin and levofloxacin. Expert Opin Drug Saf. 2015;14:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Bajpai J, Pradhan A, Verma AK, Kant S. Use of hydroxychloroquine and azithromycin combination to treat the COVID-19 infection. World J Exp Med. 2022;12:44-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 56. | Stalin A, Saravana Kumar P, Senthamarai Kannan B, Saravanan R, Ignacimuthu S, Zou Q. Potential inhibition of SARS-CoV-2 infection and its mutation with the novel geldanamycin analogue: Ignaciomycin. Arab J Chem. 2024;17:105493. [DOI] [Full Text] |
| 57. | Paravar T, Lee DJ. Thalidomide: mechanisms of action. Int Rev Immunol. 2008;27:111-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 58. | Khalil A, Kamar A, Nemer G. Thalidomide-Revisited: Are COVID-19 Patients Going to Be the Latest Victims of Yet Another Theoretical Drug-Repurposing? Front Immunol. 2020;11:1248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 59. | Ito T, Ando H, Handa H. Teratogenic effects of thalidomide: molecular mechanisms. Cell Mol Life Sci. 2011;68:1569-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 60. | Kushner P, McCarberg BH, Grange L, Kolosov A, Haveric AL, Zucal V, Petruschke R, Bissonnette S. The use of non-steroidal anti-inflammatory drugs (NSAIDs) in COVID-19. NPJ Prim Care Respir Med. 2022;32:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 61. | Zeinalian M, Salari-Jazi A, Jannesari A, Khanahmad H. A potential protective role of losartan against coronavirus-induced lung damage. Infect Control Hosp Epidemiol. 2020;41:752-753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Di Stefano L, Ram M, Scharfstein DO, Li T, Khanal P, Baksh SN, McBee N, Bengtson CD, Gadomski A, Geriak M, Puskarich MA, Salathe MA, Schutte AE, Tignanelli CJ, Victory J, Bierer BE, Hanley DF, Freilich DA; Pandemic Response COVID-19 Research Collaboration Platform for ACEi/ARB Pooled Analyses. Losartan in hospitalized patients with COVID-19 in North America: An individual participant data meta-analysis. Medicine (Baltimore). 2023;102:e33904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 63. | Yazdi AS, Ghoreschi K. The Interleukin-1 Family. Adv Exp Med Biol. 2016;941:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 64. | So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9:R28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 535] [Cited by in RCA: 544] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 65. | European Medicines Agency. Kineret. [cited 2 April 2024]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/kineret. |
| 66. | Dahms K, Mikolajewska A, Ansems K, Metzendorf MI, Benstoem C, Stegemann M. Anakinra for the treatment of COVID-19 patients: a systematic review and meta-analysis. Eur J Med Res. 2023;28:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 67. | Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev Med Virol. 2020;30:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 493] [Cited by in RCA: 513] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 68. | Keske Ş, Akyol M, Tanrıöver C, Özlüşen B, Akcan RE, Güler U, Sait B, Kaçmaz B, Gönen M, Ergönül Ö. Effectiveness of tocilizumab in non-intubated cases with COVID-19: a systematic review and meta-analysis. Infection. 2023;51:1619-1628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 69. | US Food and Drug Administration. KEVZARA® (sarilumab) injection, for subcutaneous use Initial U.S. Approval: 2017. [cited 2 April 2024]. Available from: https://products.sanofi.us/kevzara/kevzara.pdf. |
| 70. | National Institutes of Health. Final Coronavirus Disease (COVID-19) Treatment Guidelines (February 29, 2024). [cited 9 April 2024]. Available from: https://www.covid19treatmentguidelines.nih.gov. |
| 71. | Mastrorosa I, Gagliardini R, Segala FV, Mondi A, Lorenzini P, Cerva C, Taddei E, Bai F, Vergori A, Marcantonio N, Pinnetti C, Cicalini S, Murri R, Mazzotta V, Camici M, Mosti S, Bini T, Maffongelli G, Beccacece A, Milozzi E, Iannetta M, Lamonica S, Fusto M, Plazzi MM, Ottou S, Lichtner M, Fantoni M, Andreoni M, Sarmati L, Cauda R, Girardi E, Nicastri E, D'Arminio Monforte A, Palmieri F, Cingolani A, Vaia F, Antinori A; ESCAPE study group. Sarilumab plus standard of care vs standard of care for the treatment of severe COVID-19: a phase 3, randomized, open-labeled, multi-center study (ESCAPE study). EClinicalMedicine. 2023;57:101895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Knyazev E, Nersisyan S, Tonevitsky A. Endocytosis and Transcytosis of SARS-CoV-2 Across the Intestinal Epithelium and Other Tissue Barriers. Front Immunol. 2021;12:636966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 73. | Levy G, Guglielmelli P, Langmuir P, Constantinescu SN. JAK inhibitors and COVID-19. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 74. | Naik RR, Shakya AK, Aladwan SM, El-Tanani M. Kinase Inhibitors as Potential Therapeutic Agents in the Treatment of COVID-19. Front Pharmacol. 2022;13:806568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 75. | Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, Rawling M, Savory E, Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30-e31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 960] [Cited by in RCA: 983] [Article Influence: 196.6] [Reference Citation Analysis (0)] |
| 76. | Song W, Sun S, Feng Y, Liu L, Gao T, Xian S, Chen J. Efficacy and safety of baricitinib in patients with severe COVID-19: A systematic review and meta-analysis. Medicine (Baltimore). 2023;102:e36313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 77. | Sandborn WJ, D'Haens GR, Sands BE, Panaccione R, Ng SC, Lawendy N, Kulisek N, Modesto I, Guo X, Mundayat R, Su C, Vranic I, Panés J. Tofacitinib for the Treatment of Ulcerative Colitis: An Integrated Summary of up to 7.8 Years of Safety Data from the Global Clinical Programme. J Crohns Colitis. 2023;17:338-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 78. | López-Sanromán A, Esplugues JV, Domènech E. Pharmacology and safety of tofacitinib in ulcerative colitis. Gastroenterol Hepatol. 2021;44:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 79. | Chimenti MS, Conigliaro P, Biancone L, Perricone R. Update on the therapeutic management of patients with either psoriatic arthritis or ulcerative colitis: focus on the JAK inhibitor tofacitinib. Ther Adv Musculoskelet Dis. 2021;13:1759720X20977777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Maeshima K, Yamaoka K, Kubo S, Nakano K, Iwata S, Saito K, Ohishi M, Miyahara H, Tanaka S, Ishii K, Yoshimatsu H, Tanaka Y. The JAK inhibitor tofacitinib regulates synovitis through inhibition of interferon-γ and interleukin-17 production by human CD4+ T cells. Arthritis Rheum. 2012;64:1790-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 81. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6746] [Article Influence: 1349.2] [Reference Citation Analysis (0)] |
| 82. | Boor PPC, de Ruiter PE, Asmawidjaja PS, Lubberts E, van der Laan LJW, Kwekkeboom J. JAK-inhibitor tofacitinib suppresses interferon alfa production by plasmacytoid dendritic cells and inhibits arthrogenic and antiviral effects of interferon alfa. Transl Res. 2017;188:67-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 83. | Guimarães PO, Quirk D, Furtado RH, Maia LN, Saraiva JF, Antunes MO, Kalil Filho R, Junior VM, Soeiro AM, Tognon AP, Veiga VC, Martins PA, Moia DDF, Sampaio BS, Assis SRL, Soares RVP, Piano LPA, Castilho K, Momesso RGRAP, Monfardini F, Guimarães HP, Ponce de Leon D, Dulcine M, Pinheiro MRT, Gunay LM, Deuring JJ, Rizzo LV, Koncz T, Berwanger O; STOP-COVID Trial Investigators. Tofacitinib in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med. 2021;385:406-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 351] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 84. | Vandewalle J, Luypaert A, De Bosscher K, Libert C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol Metab. 2018;29:42-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 354] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 85. | Ahmed MH, Hassan A. Dexamethasone for the Treatment of Coronavirus Disease (COVID-19): a Review. SN Compr Clin Med. 2020;2:2637-2646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 86. | Albuquerque AM, Eckert I, Tramujas L, Butler-Laporte G, McDonald EG, Brophy JM, Lee TC. Effect of tocilizumab, sarilumab, and baricitinib on mortality among patients hospitalized for COVID-19 treated with corticosteroids: a systematic review and meta-analysis. Clin Microbiol Infect. 2023;29:13-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 87. | Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5202] [Cited by in RCA: 4631] [Article Influence: 926.2] [Reference Citation Analysis (0)] |
| 88. | Dhama K, Karthik K, Khandia R, Chakraborty S, Munjal A, Latheef SK, Kumar D, Ramakrishnan MA, Malik YS, Singh R, Malik SVS, Singh RK, Chaicumpa W. Advances in Designing and Developing Vaccines, Drugs, and Therapies to Counter Ebola Virus. Front Immunol. 2018;9:1803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 89. | Perico N, Cortinovis M, Suter F, Remuzzi G. Home as the new frontier for the treatment of COVID-19: the case for anti-inflammatory agents. Lancet Infect Dis. 2023;23:e22-e33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 90. | Aboul-Fotouh S, Mahmoud AN, Elnahas EM, Habib MZ, Abdelraouf SM. What are the current anti-COVID-19 drugs? From traditional to smart molecular mechanisms. Virol J. 2023;20:241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 91. | Consolaro E, Suter F, Rubis N, Pedroni S, Moroni C, Pastò E, Paganini MV, Pravettoni G, Cantarelli U, Perico N, Perna A, Peracchi T, Ruggenenti P, Remuzzi G. A Home-Treatment Algorithm Based on Anti-inflammatory Drugs to Prevent Hospitalization of Patients With Early COVID-19: A Matched-Cohort Study (COVER 2). Front Med (Lausanne). 2022;9:785785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 92. | Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1758] [Cited by in RCA: 1951] [Article Influence: 390.2] [Reference Citation Analysis (0)] |
| 93. | Day M. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 94. | Little P. Non-steroidal anti-inflammatory drugs and covid-19. BMJ. 2020;368:m1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 95. | Rinott E, Kozer E, Shapira Y, Bar-Haim A, Youngster I. Ibuprofen use and clinical outcomes in COVID-19 patients. Clin Microbiol Infect. 2020;26:1259.e5-1259.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 96. | Kragholm K, Torp-Pedersen C, Fosbol E. Non-steroidal anti-inflammatory drug use in COVID-19. Lancet Rheumatol. 2021;3:e465-e466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 97. | de Bruin N, Schneider AK, Reus P, Talmon S, Ciesek S, Bojkova D, Cinatl J, Lodhi I, Charlesworth B, Sinclair S, Pennick G, Laughey WF, Gribbon P, Kannt A, Schiffmann S. Ibuprofen, Flurbiprofen, Etoricoxib or Paracetamol Do Not Influence ACE2 Expression and Activity In Vitro or in Mice and Do Not Exacerbate In-Vitro SARS-CoV-2 Infection. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 98. | Reese JT, Coleman B, Chan L, Blau H, Callahan TJ, Cappelletti L, Fontana T, Bradwell KR, Harris NL, Casiraghi E, Valentini G, Karlebach G, Deer R, McMurry JA, Haendel MA, Chute CG, Pfaff E, Moffitt R, Spratt H, Singh JA, Mungall CJ, Williams AE, Robinson PN. NSAID use and clinical outcomes in COVID-19 patients: a 38-center retrospective cohort study. Virol J. 2022;19:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 99. | Cochrane DJ, Jarvis B, Keating GM. Etoricoxib. Drugs. 2002;62:2637-51; discussion 2652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 100. | Wang R. Etoricoxib may inhibit cytokine storm to treat COVID-19. Med Hypotheses. 2021;150:110557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 101. | Laughey W, Lodhi I, Pennick G, Smart L, Sanni O, Sandhu S, Charlesworth B. Ibuprofen, other NSAIDs and COVID-19: a narrative review. Inflammopharmacology. 2023;31:2147-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 102. | Ayoub SS. Paracetamol (acetaminophen): A familiar drug with an unexplained mechanism of action. Temperature (Austin). 2021;8:351-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 103. | Freo U, Ruocco C, Valerio A, Scagnol I, Nisoli E. Paracetamol: A Review of Guideline Recommendations. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 104. | Prieto-Campo Á, Zapata-Cachafeiro M, Portela-Romero M, Piñeiro-Lamas M, Figueiras A, Salgado-Barreira Á. Impact of prior use of antiplatelets on COVID-19 susceptibility, progression, and severity: a population-based study. Rev Esp Cardiol (Engl Ed). 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 105. | Wijaya I, Andhika R, Huang I, Purwiga A, Budiman KY. The effects of aspirin on the outcome of COVID-19: A systematic review and meta-analysis. Clin Epidemiol Glob Health. 2021;12:100883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 106. | Baral N, Mitchell JD, Savarapu PK, Akanbi M, Acharya B, Kambalapalli S, Seri A, Bashyal KP, Kunadi A, Ojha N, Volgman AS, Gupta T, Paul TK. All-cause and in-hospital mortality after aspirin use in patients hospitalized with COVID-19: a systematic review and meta-analysis. Biol Methods Protoc. 2022;7:bpac027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 107. | Ma S, Su W, Sun C, Lowe S, Zhou Z, Liu H, Qu G, Xia W, Xie P, Wu B, Gao J, Feng L, Sun Y. Does aspirin have an effect on risk of death in patients with COVID-19? A meta-analysis. Eur J Clin Pharmacol. 2022;78:1403-1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 108. | Chow JH, Rahnavard A, Gomberg-Maitland M, Chatterjee R, Patodi P, Yamane DP, Levine AR, Davison D, Hawkins K, Jackson AM, Quintana MT, Lankford AS, Keneally RJ, Al-Mashat M, Fisher D, Williams J, Berger JS, Mazzeffi MA, Crandall KA; N3C Consortium and ANCHOR Investigators. Association of Early Aspirin Use With In-Hospital Mortality in Patients With Moderate COVID-19. JAMA Netw Open. 2022;5:e223890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 109. | Sokolski M, Reszka K, Adamik B, Kilis-Pstrusinska K, Lis W, Pomorski M, Sokolowski J, Doroszko A, Madziarska K, Jankowska EA, Protasiewicz M. Antiplatelet therapy prior to COVID-19 infection impacts on patients mortality: a propensity score-matched cohort study. Sci Rep. 2024;14:4832. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 110. | Merzon E, Green I, Vinker S, Golan-Cohen A, Gorohovski A, Avramovich E, Frenkel-Morgenstern M, Magen E. The use of aspirin for primary prevention of cardiovascular disease is associated with a lower likelihood of COVID-19 infection. FEBS J. 2021;288:5179-5189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 111. | Zekri-Nechar K, Barberán J, Zamorano-León JJ, Durbán M, Andrés-Castillo A, Navarro-Cuellar C, López-Farré A, López-de-Andrés A, Jiménez-García R, Martínez-Martínez CH. Analysis of Prior Aspirin Treatment on in-Hospital Outcome of Geriatric COVID-19 Infected Patients. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 112. | Zareef R, Diab M, Al Saleh T, Makarem A, Younis NK, Bitar F, Arabi M. Aspirin in COVID-19: Pros and Cons. Front Pharmacol. 2022;13:849628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 113. | Yuan Y, Jiao B, Qu L, Yang D, Liu R. The development of COVID-19 treatment. Front Immunol. 2023;14:1125246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 135] [Reference Citation Analysis (0)] |
| 114. | Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3386] [Cited by in RCA: 3626] [Article Influence: 725.2] [Reference Citation Analysis (0)] |
| 115. | Gavriatopoulou M, Ntanasis-Stathopoulos I, Korompoki E, Fotiou D, Migkou M, Tzanninis IG, Psaltopoulou T, Kastritis E, Terpos E, Dimopoulos MA. Emerging treatment strategies for COVID-19 infection. Clin Exp Med. 2021;21:167-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 198] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 116. | Casadevall A, Joyner MJ, Pirofski LA, Senefeld JW, Shoham S, Sullivan D, Paneth N, Focosi D. Convalescent plasma therapy in COVID-19: Unravelling the data using the principles of antibody therapy. Expert Rev Respir Med. 2023;17:381-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 117. | Lang-Meli J, Fuchs J, Mathé P, Ho HE, Kern L, Jaki L, Rusignuolo G, Mertins S, Somogyi V, Neumann-Haefelin C, Trinkmann F, Müller M, Thimme R, Umhau M, Quinti I, Wagner D, Panning M, Cunningham-Rundles C, Laubner K, Warnatz K. Case Series: Convalescent Plasma Therapy for Patients with COVID-19 and Primary Antibody Deficiency. J Clin Immunol. 2022;42:253-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 118. | Cowan J, Amson A, Christofides A, Chagla Z. Monoclonal antibodies as COVID-19 prophylaxis therapy in immunocompromised patient populations. Int J Infect Dis. 2023;134:228-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 119. | US begins clinical trial of an artificial antibody for Covid-19 treatment. [cited 9 April 2024]. Available from: https://theprint.in/health/us-begins-clinical-trial-of-an-artificial-antibody-for-covid-19-treatment/402978/. |
| 120. | Qilu Hospital of Shandong University. Bevacizumab in Patients With Severe Covid-19 (BEST). [accessed 2023 Jun 27]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/study/NCT04305106 ClinicalTrials.gov Identifier: NCT04305106. |
| 121. |
|
| 122. | Phase 3 randomized, double-blind, placebo-controlled multi-center study to assess the efficacy and safety of ruxolitinib in patients with COVID-19 associated cytokine storm (RUXCOVID) (RUXCOVID). [accessed 2021 Oct 11]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04362137 ClinicalTrials.gov Identifier: NCT04362137. |
| 123. | Xinxiang Medical University. NK Cells Treatment for COVID-19. [accessed 2024 Feb 08]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04280224 ClinicalTrials.gov Identifier: NCT04280224. |
| 124. | Poulas K, Farsalinos K, Zanidis C. Activation of TLR7 and Innate Immunity as an Efficient Method Against COVID-19 Pandemic: Imiquimod as a Potential Therapy. Front Immunol. 2020;11:1373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 125. | Bachanova V, Bishop MR, Dahi P, Dholaria B, Grupp SA, Hayes-Lattin B, Janakiram M, Maziarz RT, McGuirk JP, Nastoupil LJ, Oluwole OO, Perales MA, Porter DL, Riedell PA; CAR T-cell Consortium. Chimeric Antigen Receptor T Cell Therapy During the COVID-19 Pandemic. Biol Blood Marrow Transplant. 2020;26:1239-1246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 126. | Stephen-Victor E, Das M, Karnam A, Pitard B, Gautier JF, Bayry J. Potential of regulatory T-cell-based therapies in the management of severe COVID-19. Eur Respir J. 2020;56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 127. | Bishop MR. Optimizing Administration of CAR T-Cell Therapy During the COVID-19 Pandemic. Clin Adv Hematol Oncol. 2020;18:400-403. [PubMed] |
| 128. | Hu Y, Tan Su Yin E, Yang Y, Wu H, Wei G, Su J, Cui Q, Jin A, Yang L, Fu S, Zhou J, Qiu L, Zhang X, Liang A, Jing H, Li Y, Blaise D, Mohty M, Nagler A, Huang H. CAR T-cell treatment during the COVID-19 pandemic: Management strategies and challenges. Curr Res Transl Med. 2020;68:111-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 129. | Khoury M, Cuenca J, Cruz FF, Figueroa FE, Rocco PRM, Weiss DJ. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J. 2020;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 182] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 130. | Moll G, Drzeniek N, Kamhieh-Milz J, Geissler S, Volk HD, Reinke P. MSC Therapies for COVID-19: Importance of Patient Coagulopathy, Thromboprophylaxis, Cell Product Quality and Mode of Delivery for Treatment Safety and Efficacy. Front Immunol. 2020;11:1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 131. | Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11:216-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 730] [Cited by in RCA: 852] [Article Influence: 170.4] [Reference Citation Analysis (0)] |
| 132. | Angelopoulou A, Alexandris N, Konstantinou E, Mesiakaris K, Zanidis C, Farsalinos K, Poulas K. Imiquimod - A toll like receptor 7 agonist - Is an ideal option for management of COVID 19. Environ Res. 2020;188:109858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 133. | Verma G, Dhawan M, Saied AA, Kaur G, Kumar R, Emran TB. Immunomodulatory approaches in managing lung inflammation in COVID-19: A double-edge sword. Immun Inflamm Dis. 2023;11:e1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 134. | Gallelli L, Zhang L, Wang T, Fu F. Severe Acute Lung Injury Related to COVID-19 Infection: A Review and the Possible Role for Escin. J Clin Pharmacol. 2020;60:815-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |