Published online Mar 25, 2024. doi: 10.5501/wjv.v13.i1.88660
Peer-review started: October 4, 2023
First decision: October 9, 2023
Revised: November 9, 2023
Accepted: December 29, 2023
Article in press: December 29, 2023
Published online: March 25, 2024
Processing time: 159 Days and 8.1 Hours
Monoclonal antibodies (mAbs) have shown clinical benefits against coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Several studies have reported the use of bamlani
To synthesize the latest evidence for the efficacy and safety of bamlanivimab alone in the treatment of adult patients with COVID-19.
A literature search was conducted in PubMed, Cochrane Library, Web of Science, medRxiv, and Google Scholar using “SARS-CoV-2”, “COVID-19”, “LY-CoV555”, and “Bamlanivimab” keywords up to January 25, 2023. The quality of included studies was assessed using the Cochrane bias tools. The Comprehensive Meta-Analysis software version 3.0 was used to analyze the data.
A total of 30 studies involving 47368 patients were included. A significant difference was observed between the bamlanivimab and standard of care/placebo groups in terms of mortality rate [risk ratio (RR) = 50, 95% confidence interval (CI): 0.36-0.70], hospitalization rate (RR = 0.51; 95%CI: 0.39-0.68), and emergency department (ED) visits (RR = 0.69; 95%CI: 0.47-0.99); while the two groups exhibited no significant difference in terms of intensive care unit (ICU) admission (P > 0.05). Compared to other mAbs, bamlanivimab was associated with a higher rate of hospitalization (RR = 1.44; 95%CI: 1.07-1.94). However, no significant difference was detected between the bamlanivimab and other mAbs groups in terms of mortality rate, ICU admission, and ED (P > 0.05). The incidence of any adverse events was similar between the bamlanivimab and control groups (P > 0.05).
Although the results suggest the efficacy and safety of bamlanivimab in COVID-19 patients, further research is required to confirm the efficacy of this drug for the current circulating SARS-CoV-2 variants.
Core Tip: The present study is the most comprehensive systematic review and meta-analysis on the efficacy and safety of bamlanivimab in the treatment of coronavirus disease 2019 (COVID-19). A significant difference was observed between the bamlanivimab and standard of care/placebo groups in terms of mortality rate, hospitalization rate, and emergency department visits. While the two groups exhibited no significant difference in terms of intensive care unit admission. The present results suggested that bamlanivimab might be effective and safe for the treatment of COVID-19.
- Citation: Amani B, Khodavirdilou L, Rajabkhah K, Kardan Moghaddam V, Akbarzadeh A, Amani B. Efficacy and safety of bamlanivimab in patients with COVID-19: A systematic review and meta-analysis. World J Virol 2024; 13(1): 88660
- URL: https://www.wjgnet.com/2220-3249/full/v13/i1/88660.htm
- DOI: https://dx.doi.org/10.5501/wjv.v13.i1.88660
Despite a high rate of vaccination, cases of breakthrough coronavirus disease 2019 (COVID-19) have been reported worldwide[1]. Consequently, numerous pharmaceutical interventions have been proposed to prevent and manage severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the primary cause of COVID-19[2]. Numerous clinical studies have demonstrated that various monoclonal antibodies (mAbs), including sotrovimab[3], casirivimab/imdevimab[3,4], cilgavimab/tixagevimab[5], regdanvimab[6], bamlanivimab/etesevimab[7], and bamlanivimab[8] could be potentially effective in reducing mortality and morbidity in patients with mild to moderate COVID-19. These interventions specifically target the spike protein of the SARS-CoV-2 virus, thereby, inhibiting its activity[9]. In particular, bamlani
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses was utilized as a reporting guideline for conducting this systematic review and meta-analysis of primary studies[16].
An extensive literature search was conducted to gather relevant evidence. The search was performed in PubMed, Cochrane Library, Web of Science, medRxiv, and Google Scholar, up to January 25, 2023. Apart from the database search, the reference lists of the included studies were also examined to identify any additional relevant records. No language restrictions were applied. The search strategy in PubMed included keywords such as “Coronavirus”, “COVID-19”, “SARS-CoV-2”, “Bamlanivimab”, and “LY-CoV555”. The specific search strategy in PubMed was as follows: ((((((((Coronavirus[Title/Abstract]) OR (Coronavirus[MeSH Terms])) OR (COVID-19 [Title/Abstract])) OR (SARS-CoV-2 [Title/Abstract])) OR (COVID-19[MeSH Terms])) OR (SARS-CoV-2 [MeSH Terms])) OR (2019 novel coronavirus infection[Title/Abstract])) OR (2019-nCoV infection[Title/Abstract])) AND ((Bamlanivimab [Title/Abstract] OR (LY-CoV555 [Title/Abstract]).
To be included in the study, the selected studies had to meet the following criteria: (1) Addressing adult patients who with positive COVID-19 results based on the polymerase chain reaction test; (2) Bamlanivimab alone as treatment; (3) Using placebo (PBO), standard of care (SOC), and other therapeutic interventions as the control group; and (4) Addressing mortality rate, hospitalization rate, emergency department (ED) visits, ICU admission rate, and incidence of adverse events as the measures of efficacy and safety. Animal studies, case reports, letters to the editor, and studies that did not report relevant outcomes were excluded from the analysis.
Two authors independently assessed the bias risk in observational studies and randomized controlled trials (RCTs) using Nonrandomized Studies of Interventions (ROBINS-I) tool and Cochrane Risk of Bias (ROB) tool, respectively[17,18].
Two authors independently extracted the following data from the included studies: (1) Study characteristics including information such as the name of the first author, year of publication, location of the study, and study design; (2) Participant characteristics such as sample size, sex distribution, and the mean age of the participants; (3) Intervention and control including details on the sample size of both intervention and control groups, and the treatment dosage and duration; and (4) Efficacy and safety outcomes consisted of the reported efficacy (i.e., mortality rate, hospitalization rate, ED visits, ICU admission rate, and the incidence of adverse events). By independently extracting this data, the authors ensured a thorough and accurate collection of information for analysis.
The Comprehensive Meta-Analysis software was employed to compare the efficacy and safety between bamlanivimab and the control groups. The risk ratio (RR), along with a 95% confidence interval (CI), was employed to analyze the dichotomous variables. The level of heterogeneity was assessed using the I2 statistic, with a value greater than 50% or a P-value less than 0.1, indicating high heterogeneity (I2 > 50% or P < 0.1). A random-effects model was employed in highly heterogeneous studies, while a fixed-effects model was used for studies with low heterogeneity. Both RCTs and observational studies were analyzed together to estimate the effect size. Subgroup analyses were conducted based on the age of patients (less than 65 years or 65 and over), sample size, and study design. Moreover, a sensitivity analysis was performed by excluding studies with remarkable risk of bias for outcomes of mortality rate and hospitalization rate. Publication bias was assessed by Begg’s test and Egger’s test.
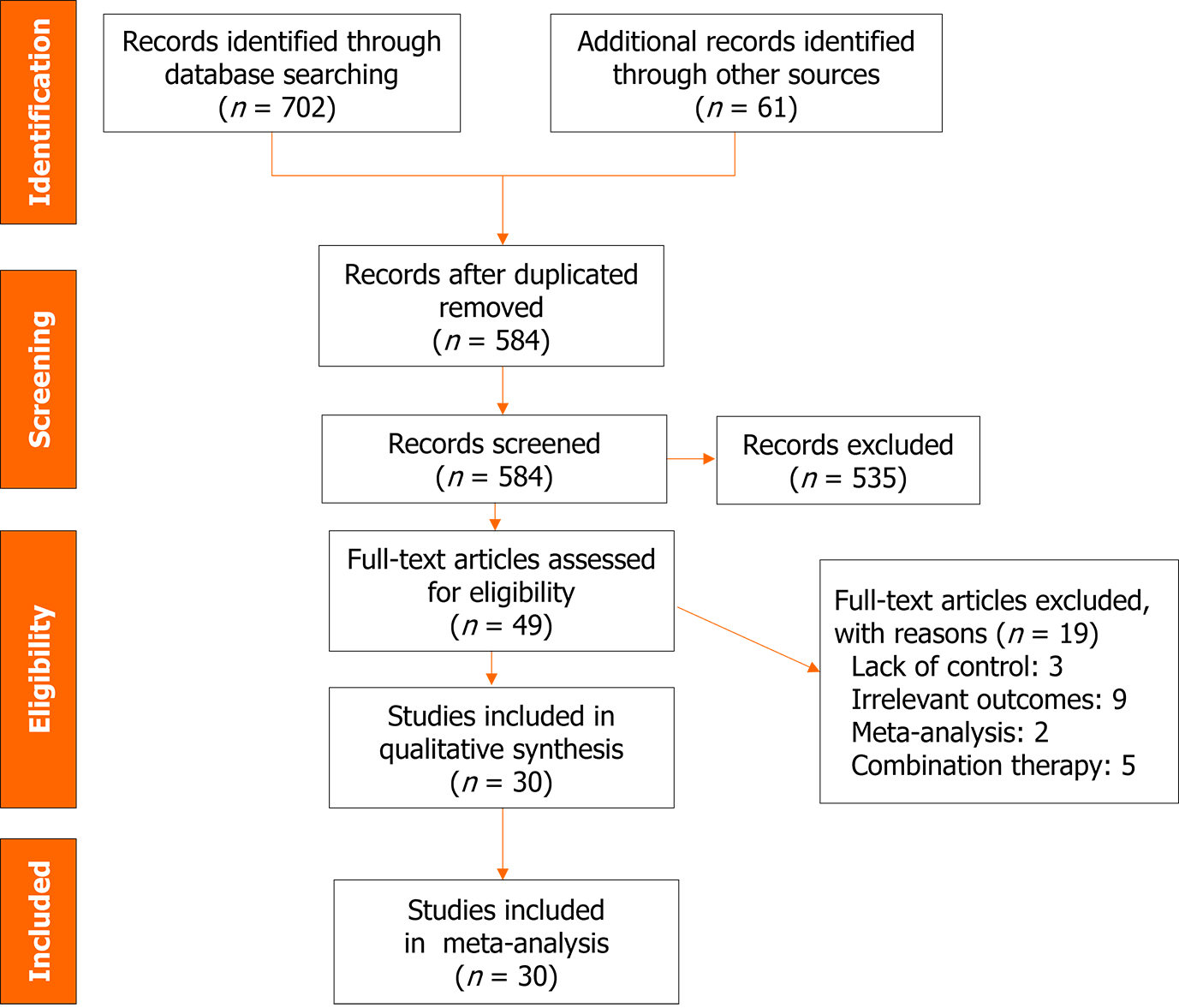
Figure 1 depicts the study selection process, starting from the initial literature search, removal of duplicates, and screening based on title, abstract, and full-text. Out of the initial 584 studies identified after removing duplicates, 49 full-text studies were considered for eligibility assessment. Ultimately, a total of 30 studies with 47368 patients were included in the meta-analysis[8,10,11,13,14,19-43]. Excluded studies are presented in Figure 1 along with their corresponding reason. The majority of the included studies were of retrospective nature and conducted in the United States. Furthermore, most of the studies were published in 2021, coinciding with the SARS-CoV-2 Delta wave. COVID-19 vaccination status was reported in a few number of studies. Studies mainly evaluated the efficacy of bamlanivimab in patients with mild-to-moderate COVID-19 infection. In most studies, bamlanivimab was administered at a dose of 700 mg. In some studies, however, patients received doses of 2800 and 7000 mg. More detailed information on the characteristics of the included studies is listed in Table 1.
| Ref. | Country | Design | Sample size | Male % | Severity of COVID-19 | Bamlanivimab | Comparison(s) | |||||
| n | Mean age | Comorbidity1 | Name | n | Mean age | Comorbidity1 | ||||||
| Alam et al[19], 2021 | United States | RS | 264 | 44 | MM | 160 | 81 | 58.1 | SOC | 86 | 84 | 51.2 |
| Bariola et al[13], 2021 | United States | RS | 1392 | 44.39 | MM | 232 | 67.3 | 75.9 | SOC | 1160 | 67.1 | 73.6 |
| Brock et al[20], 2021 | United States | RS | 108 | NA | MM | 58 | NA | 100 | SOC | 58 | NA | 100 |
| Chen et al[21], 2021 | United States | RCT | 24 | 54 | MC | 18 | NA | NA | PBO | 6 | 43.2 | NA |
| Chen et al[21], 2021 | United States | RCT | 452 | 44.9 | MM | 309 | 45 | 69.6 | PBO | 143 | 46 | 66.4 |
| Chew et al[23], 2022 | United States | RCT | 317 | 51.1 | MS | 159 | NA | NA | PBO | 158 | NA | NA |
| Cooper et al[24], 2021 | United States | RS | 5758 | 45.13 | NA | 1718 | 60 | 56.3 | SOC, B/E, C/I | 4040 | NA | > 50 |
| Corwin et al[25], 2021 | United States | RS | 6117 | 42.7 | MM | 780 | 62.6 | 68.1 | SOC | 5337 | 56.7 | 47.1 |
| Destache et al[14], 2021 | United States | RS | 234 | 47 | MM | 117 | 72 | 69.2 | SOC | 117 | 72 | 63.3 |
| Djuric et al[26], 2022 | Serbia | RS | 31 | 67.74 | MS | 13 | 62.2 | 30.8 | SOC | 18 | 65.9 | 38.9 |
| Farcy et al[11], 2022 | United States | PS | 321 | 60.12 | MM | 201 | 64.2 | 56.2 | C/I | 120 | 66.3 | 58.3 |
| San Filippo et al[39], 2022 | United States | RS | 453 | 47.01 | MM | 183 | 66.9 | 44.8 | C/I | 270 | 63.4 | 51.9 |
| Ganesh et al[27], 2021 | United States | RS | 4670 | 50.62 | MM | 2335 | 63 | 54.2 | SOC | 2335 | 63 | 55.1 |
| Ganesh et al[28], 2021 | United States | RS | 3596 | 50.02 | MM | 2747 | NA | 53.3 | C/I | 849 | NA | 48.3 |
| Gottlieb et al[29], 2021 | United States | RCT | 577 | 45.40 | MM | 309 | NA | NR | PBO, B/E | 156 | NA | NA |
| Heller et al[31], 2023 | Germany | RS | 26 | 45 | MM | 10 | 81 | NR | SOC, C/I | 23 | NA | NA |
| Iqbal et al[8], 2021 | United States | RS | 284 | NA | MM | 144 | NR | 10.3 | SOC | 140 | NA | 63.60 |
| Karr et al[10], 2022 | United States | RS | 46 | 63.04 | MM | 40 | 69 | 65 | SOC | 6 | 69 | 50 |
| Kumar et al[32], 2022 | United States | RS | 403 | 52.10 | MM | 218 | 66 | 50.5 | SOC | 185 | 62 | 43.8 |
| ACTIV-3/TICO LY-CoV555 Study Group et al[30], 2021 | United States | RCT | 314 | 57.32 | NA | 163 | 63 | 72 | PBO | 151 | 59 | 65 |
| McCreary et al[33], 2021 | United States | RCT | 1935 | 46.20 | MM | 128 | 57 | 47 | B/E, C/I | 1807 | NA | NA |
| Monday et al[34], 2022 | United States | RCT | 643 | 42.76 | MM | 294 | 61 | 72.8 | B/E | 349 | 55 | 72.4 |
| Murillo et al[35], 2022 | United States | RS | 107 | 42.99 | MM | 39 | NA | NA | SOC | 63 | NA | NA |
| Priest et al[36], 2022 | United States | RS | 758 | 49 | MM | 379 | NA | 88 | SOC | 379 | NA | 88 |
| Quenzer et al[37], 2022 | United States | RS | 270 | 51.85 | MM | 134 | 60.3 | 92.5 | SOC | 136 | 63.3 | 69.1 |
| Rubin et al[38], 2021 | United States | RS | 1257 | 43.75 | NA | 191 | 64 | NR | SOC | 1066 | 64.6 | NA |
| Savoldi et al[40], 2022 | Italy | PS | 635 | 61.57 | MM | 161 | 63 | 72.7 | B/E, C/I | 474 | NR | NA |
| Sridhara et al[41], 2023 | United States | RS | 2182 | 42.98 | NA | 1099 | 64 | 52.8 | SOC | 1091 | 46 | 20.9 |
| Voelker and Jerath[42], 2022 | United States | PS | 678 | 43.65 | NA | 380 | NA | NA | C/I | 298 | NA | NA |
| Webb et al[43], 2021 | United States | QES | 13534 | 55.19 | NA | 479 | 65 | 90.8 | SOC, C/I | 5651 | NA | NA |
Supplementary Tables 1 and 2 respectively show the risk of bias assessment determined by ROB and ROBINS-I tools. Accordingly, the included studies had acceptable quality.
Mortality rate: The pooled estimate revealed a significant difference in mortality rate of the bamlanivimab compared to the SOC/PBO groups (RR = 0.50; 95%CI: 0.36-0.70, P < 0.05, I2 = 15%) (Figure 2A). However, no significant difference was observed between bamlanivimab and other mAbs in terms of mortality rate (RR = 1.71; 95%CI: 0.85-3.44, P = 0.12, I2 = 0%) (Supplementary Figure 1).
Hospitalization rate: A significant difference was observed in the hospitalization rate of bamlanivimab-receiving patients compared to those treated with SOC/PBO (RR = 0.51; 95%CI: 0.39-0.68, P < 0.05, I2 = 80%) (Figure 2B). Moreover, a significant difference was detected between the hospitalization rate of the bamlanivimab group compared to mAbs one (RR = 1.44; 95%CI: 1.07-1.94, P = 01, I2 = 53%) (Supplementary Figure 2).
ED visits: The combined analysis of these studies revealed a significant difference in the frequency of ED visits between bamlanivimab-treated patients and those receiving SOC (RR = 0.69; 95%CI: 0.47-0.99, P = 0.04, I2 = 58%). No significant difference was observed between bamlanivimab and other mAbs in terms of ED visits (RR = 0.96; 95%CI: 0.76-1.20, P = 0.74, I2 = 0%) (Figure 2C and Supplementary Figure 3).
ICU admission: The result of meta-analysis showed no significant difference in the ICU admission rate of the bamlanivimab-treated patients and those receiving SOC (RR = 0.82; 95%CI: 0.57-1.18, P = 0.29, I2 = 42%) (Figure 2D). No significant difference was observed between bamlanivimab and other mAbs in terms of ICU admission (RR = 1.60; 95%CI: 0.86-2.98, P = 0.13, I2 = 0%) (Supplementary Figure 4).
Any adverse events: The pooled estimate of included studies showed no significant difference in adverse events between the bamlanivimab and SOC/PBO groups (RR = 1.01; 95%CI: 0.81-1.26, P = 0.88, I2 = 0%) (Figure 2E). Moreover, no significant difference was observed in adverse events between the bamlanivimab and other mAb groups (RR = 6.13; 95%CI: 0.71-52.72, P = 0.09, I2 = 1%) (Supplementary Figure 5).
Publication bias: No evidence of publication bias was detected for pooled estimate of mortality rate (P = 0.24) and hospitalization rate (P = 0.11) based on Begg test. However, Egger’s test indicated a publication bias for pooled estimates of mortality rate (P = 0.01) and hospitalization rate (P = 0.004) (Supplementary Figures 6 and 7).
Subgroup and sensitivity analyses: The subgroup analysis showed no significance difference in mortality rate and hospitalization rate by mean age of patients treated with bamlanivimab compared to SOC/PBO and by sample size (Table 2). Sensitivity analysis also exhibited no significant change compared to the excluded studies (Table 2).
| Analysis | Studies, n | Sample size, n | Point estimate (95%CI) | P value | Heterogeneity | ||
| Q value | P value | I2 | |||||
| Sensitivity analysis | |||||||
| Mortality rate soc (excluding Brock 2021and Djuric 2021) | 16 | 29091 | 0.52 (0.37-0.73) | < 0.001 | 18.45 | 0.24 | 18.71 |
| Hospitalization rate (excluding Brock 2021) | 17 | 26565 | 0.55 (0.42-0.71) | < 0.001 | 69.65 | 0.21 | 77.02 |
| Hospitalization rate (excluding Voelker 2022) | 7 | 8177 | 1.38 (1.00-1.92) | 0.04 | 14.25 | 0.02 | 57.91 |
| ICU admission (excluding Brock 2021) | 6 | 15759 | 0.88 (0.60-1.29) | 0.52 | 7.79 | 0.16 | 35.81 |
| Subgroup analysis | |||||||
| Hospitalization rate by design, BAM vs SOC/PBO | |||||||
| OS | 16 | 25904 | 0.67 (0.60-0.75) | < 0.001 | 82.27 | < 0.001 | 81.76 |
| RCT | 2 | 769 | 0.44 (0.21-0.94) | 0.03 | 1.95 | 0.16 | 48.80 |
| Hospitalization rate by sample size, BAM vs SOC/PBO | |||||||
| < 1000 | 11 | 23453 | 0.51 (0.43-0.61) | < 0.001 | 50.51 | < 0.001 | 80.20 |
| ≥ 1000 | 7 | 3220 | 0.77 (0.67-0.89) | < 0.001 | 22.22 | 0.001 | 73.00 |
| Hospitalization rate by mean age, BAM vs SOC/PBO | |||||||
| < 65 | 8 | 22783 | 0.77 (0.67-0.88) | < 0.001 | 26.91 | < 0.001 | 73.99 |
| ≥ 65 | 4 | 1918 | 0.46 (0.32-0.66) | < 0.001 | 0.30 | 0.96 | < 0.001 |
| Mortality rate by design, BAM vs SOC/PBO | |||||||
| OS | 17 | 28916 | 0.44 (0.31-0.62) | < 0.001 | 14.67 | 0.54 | 0.00 |
| RCT | 1 | 314 | 1.67 (0.57-4.86) | 0.34 | 0.00 | 1.00 | 0.00 |
| Mortality rate by sample size, BAM vs SOC/PBO | |||||||
| < 1000 | 12 | 4030 | 0.48 (0.31-0.76) | 0.002 | 15.55 | 0.15 | 29.29 |
| ≥ 1000 | 6 | 25200 | 0.51 (0.31-0.84) | 0.009 | 4.43 | 0.48 | 0.00 |
| Mortality rate by mean age, BAM vs SOC/PBO | |||||||
| < 65 | 8 | 25639 | 0.60 (0.37-0.96) | 0.037 | 9.89 | 0.19 | 29.26 |
| ≥ 65 | 5 | 2189 | 0.40 (0.20-0.79) | 0.008 | 5.16 | 0.27 | 22.53 |
The objective of this study was to analyze and synthesize the most recent evidence on the effectiveness and safety of bamlanivimab, a mAb intervention, during the prevalence of the SARS-CoV-2 Omicron variant. Despite the protective role of vaccines against SARS-CoV-2 infection, effective treatments are still required to manage COVID-19 disease, particularly with the emergence of new variants[44]. The results demonstrated the efficacy of bamlanivimab in achieving positive clinical outcomes among patients diagnosed with COVID-19.
The results of the meta-analysis revealed a significantly lower mortality rate in the bamlanivimab-receiving individuals compared to those treated with SOC/PBO. However, this difference was not significant between the bamlanivimab and other mAb groups. Clinical studies showed the similar efficacy of mAb treatments in reducing COVID-19-induced death[11,40]. Consistent to our findings, meta-analyses conducted on the efficacy of bamlanivimab revealed that treatment with bamlanivimab is significantly associated with a lower mortality rate compared to the control group[45,46]. In general, the clinical evidence suggests that mAb treatments may contribute to a reduction in the mortality rate among patients with COVID-19[4,30,34,42,47]. Targeting the spike protein of the SARS-CoV-2 virus with anti-SARS-CoV-2 mAbs may serve as a potential mechanism for reducing the mortality rate of COVID-19 patients[48].
According to results of the present meta-analysis, bamlanivimab-treated patients had a lower likelihood of being admitted to the hospital compared to those receiving SOC/PBO. However, hospitalization rate was higher in the bamlanivimab group than the other mAbs group. Bamlanivimab treatment may contribute to a reduced rate of hospitalization among COVID-19 patients. Consistent with the mentioned finding, other meta-analyses[45,46] on the efficacy of this drug also demonstrated that treatment with bamlanivimab is associated with a lower rate of hospitalization in patients with mild to moderate COVID-19 compared to control groups. This further supports the potential effectiveness of bamlanivimab in reducing the hospital admission in individuals with COVID-19. Indeed, real-world studies demonstrated that therapeutic mAbs, including bamlanivimab[38,40], sotrovimab, casirivimab/imdevimab[4,40], and bamlanivimab/etesevimab[40] can significantly reduce the rate of COVID-19-related hospitalization. According to these studies, the use of these mAbs can effectively lower the severity of the disease and decrease the need for hospitalization in individuals affected by COVID-19.
The results of the present study demonstrate a significant positive effect of bamlanivimab on reducing the need for ED visits in patients with COVID-19 compared to SOC/PBO. However, this difference was not significant between the bamlanivimab and other mAb groups. A meta-analysis of RCTs comparing mAbs-receiving patients with PBO group indicated a significant association of mAbs with a lower rate of ED visits[49]. A possible explanation for this difference could be due to differences in the type of mAb treatments as intervention or included in the study design.
According to the present meta-analysis, treatment with bamlanivimab was not significantly associated with a lower rate of admission to ICU compared to SOC/PBO or mAbs. On the contrary, a meta-analysis by Xiang et al[45] showed a significant association of bamlanivimab with reduced ICU admission rate compared to the controls. This difference can be due to the number of studies included in the quantitative analysis. Compared to Xiang et al[45], the present research identified and included more studies in the meta-analysis of data on ICU admission rate.
Consistent with previously published meta-analyses[45] on the safety profile of bamlanivimab, the present study found similar incidence of adverse events in both the bamlanivimab and control groups. In general, the bamlanivimab-related incidence of adverse events in COVID-19 patients was mild and well-tolerated[11,19,39]. The most frequent adverse events in studies included nausea, diarrhea, headache, and respiratory distress[21,29]. In terms of severe adverse events, no significant difference was observed between the bamlanivimab and control groups. Chen et al[21] found no cases of discontinuations due to adverse events in bamlanivimab-treated patients at different doses (700, 2800, and 7000 mg). Gottlieb et al[29] also found similar results in COVID-19 patients receiving bamlanivimab doses of 700, 2800, and 7000 mg.
Two important points should be considered in the interpretation of the present results. First, several studies have documented evidence of post-COVID-19 condition among individuals after the initial SARS-CoV-2 infection which is a serious problem for many recovered COVID-19 patients[50-52]. Given the importance of post-COVID-19 conditions in designing effective treatments for COVID-19, and considering the lack of validated treatment for these conditions, it is crucial to conduct longitudinal monitoring of COVID-19 patients. This monitoring is vital for the development of effective therapeutic agents[52]. Second, studies have shown the resistance of some SARS-COV-2 variants to bamlanivimab. Hoffmann et al[53] reported the resistance of SARS-CoV-2 variant B.1.1.7 to bamlanivimab. A study conducted by Peiffer-Smadja et al[54] showed the emergence of resistance mutants in bamlanivimab-receiving COVID-19 patients. A RCT conducted by Choudhary et al[55] reported the emergence of SARS-CoV-2 escape mutations in COVID-19 patients during treatment with bamlanivimab (700 mg). However, no resistance mutations were identified in patients treated with 7000 mg bamlanivimab. These findings highlight the importance of viral resistance during the development of treatments for COVID-19 patients. SARS-CoV-2 mutations may also lower the effectiveness of current preventive therapies in individuals, including vaccines. The SARS-CoV-2 variant B.1.351 could significantly reduce the efficacy of Novavax COVID-19 vaccine[56].
The present study has some remarkable limitations. Firstly, the included studies did not report the type of SARS-CoV-2 variant. Therefore, the present findings may not be applicable to some SARS-COV-2 variants of interest. Secondly, the majority of studies included in the meta-analysis were retrospective, causing an inherent risk of bias. Moreover, many of these retrospective studies did not utilize propensity score matching to minimize selection bias and confounding variables. Thirdly, we could not perform subgroup analyses based on these variables as the information on the com-orbidity percentage and COVID-19 vaccine status of the studies was not complete. Therefore, the present results cannot be generalized to patients with unknown COVID-19 vaccine status. Finally, the present results should be interpreted with caution due to the presence of potential publication bias in several outcomes.
The present meta-analysis demonstrated the association of bamlanivimab treatment with a reduction in the mortality rate, hospitalization rate, and ED visits in patients with COVID-19 compared to SOC-receiving group. However, it did not show a significant efficacy in improving clinical outcomes compared to other mAb treatments. In terms of safety, bamlanivimab was safe and well-tolerated in patients with COVID-19. However, studies did not report the specific type of SARS-CoV-2 variants. Therefore, the findings may not be directly applicable to patients with current SARS-CoV-2 variants. Future research should be focused on the efficacy of bamlanivimab against the current SARS-CoV-2 variants, especially in immunocompromised patients who are more susceptible to the new SARS-CoV-2 variants in terms of mutations and resistance to treatment with mAbs. Moreover, the comorbidity percentage and COVID-19 vaccination rate should be considered in evaluating the efficacy of bamlanivimab in COVID-19 patients.
Bamlanivimab, a monoclonal antibody (mAb), has been used as a therapeutic agent for patients with coronavirus disease 2019 (COVID-19). Previous studies have shown that bamlanivimab may be effective in treating COVID-19 patients.
Despite several studies evaluating the clinical benefit of bamlanivimab in COVID-19 patients, there is currently no comprehensive systematic review and meta-analysis assessing its efficacy and safety as a treatment.
This study aims to evaluate the use of bamlanivimab in improving efficacy outcomes compared to other treatments in COVID-19 patients. Additionally, the safety profile of bamlanivimab is compared to control groups.
A thorough search was conducted in PubMed, Cochrane Library, Web of Science, medRxiv, and Google Scholar up to January 25, 2023. Cochrane bias tools were utilized to assess the risk of bias in the included studies. Data analysis was performed using Comprehensive Meta-Analysis software (version 3).
A total of 30 studies were identified and included in the meta-analysis. The meta-analysis revealed a significant difference between the bamlanivimab and standard of care/placebo groups in terms of mortality rate, hospitalization rate, and emergency department (ED) visits. However, there was no significant difference between the two groups regarding intensive care unit (ICU) admission. When compared to other mAbs, bamlanivimab did not demonstrate superior efficacy in terms of hospitalization rate, mortality rate, ICU admission, and ED visits. No significant difference was observed between the treatment groups in terms of adverse events.
Although the present results demonstrate the efficacy and safety of bamlanivimab in treating COVID-19, further research is necessary to confirm its effectiveness against novel circulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants.
In the future, studies should be focused on the efficacy of bamlanivimab against the current SARS-CoV-2 variants, especially in immunocompromised patients who are more susceptible to the new SARS-CoV-2 variants in terms of mutations and resistance to treatment with mAbs. Moreover, the comorbidity percentage and COVID-19 vaccination rate should be considered in evaluating the efficacy of bamlanivimab in COVID-19 patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Virology
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao S, China; Sukocheva OA, Australia; Wang K, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Zhao S
| 1. | Lanzavecchia S, Beyer KJ, Evina Bolo S. Vaccination Is Not Enough: Understanding the Increase in Cases of COVID-19 in Chile despite a High Vaccination Rate. Epidemiologia (Basel). 2021;2:377-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Ceramella J, Iacopetta D, Sinicropi MS, Andreu I, Mariconda A, Saturnino C, Giuzio F, Longo P, Aquaro S, Catalano A. Drugs for COVID-19: An Update. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Huang DT, McCreary EK, Bariola JR, Minnier TE, Wadas RJ, Shovel JA, Albin D, Marroquin OC, Kip KE, Collins K, Schmidhofer M, Wisniewski MK, Nace DA, Sullivan C, Axe M, Meyers R, Weissman A, Garrard W, Peck-Palmer OM, Wells A, Bart RD, Yang A, Berry LR, Berry S, Crawford AM, McGlothlin A, Khadem T, Linstrum K, Montgomery SK, Ricketts D, Kennedy JN, Pidro CJ, Nakayama A, Zapf RL, Kip PL, Haidar G, Snyder GM, McVerry BJ, Yealy DM, Angus DC, Seymour CW. Effectiveness of Casirivimab-Imdevimab and Sotrovimab During a SARS-CoV-2 Delta Variant Surge: A Cohort Study and Randomized Comparative Effectiveness Trial. JAMA Netw Open. 2022;5:e2220957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 4. | Razonable RR, Pawlowski C, O'Horo JC, Arndt LL, Arndt R, Bierle DM, Borgen MD, Hanson SN, Hedin MC, Lenehan P, Puranik A, Seville MT, Speicher LL, Tulledge-Scheitel SM, Venkatakrishnan AJ, Wilker CG, Badley AD, Ganesh R. Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. EClinicalMedicine. 2021;40:101102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 5. | Kertes J, Shapiro Ben David S, Engel-Zohar N, Rosen K, Hemo B, Kantor A, Adler L, Shamir Stein N, Mizrahi Reuveni M, Shahar A. Association Between AZD7442 (Tixagevimab-Cilgavimab) Administration and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection, Hospitalization, and Mortality. Clin Infect Dis. 2023;76:e126-e132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 6. | Lee S, Lee SO, Lee JE, Kim KH, Lee SH, Hwang S, Kim SW, Chang HH, Kim Y, Bae S, Kim AS, Kwon KT. Regdanvimab in patients with mild-to-moderate SARS-CoV-2 infection: A propensity score-matched retrospective cohort study. Int Immunopharmacol. 2022;106:108570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Vena A, Cenderello G, Balletto E, Mezzogori L, Santagostino Barbone A, Berruti M, Ball L, Battaglini D, Bonsignore A, Dentone C, Giacobbe DR, Eldin TK, Mikulska M, Rebesco B, Robba C, Scintu A, Stimamiglio A, Taramasso L, Pelosi P, Artioli S, Bassetti M. Early Administration of Bamlanivimab in Combination with Etesevimab Increases the Benefits of COVID-19 Treatment: Real-World Experience from the Liguria Region. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Iqbal L, Terlau TJ, Hernandez A, Woods K. Efficacy of Bamlanivimab in Reducing Hospitalization and Mortality Rates in COVID-19 Patients in a Rural Community. Cureus. 2021;13:e16477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Taylor PC, Adams AC, Hufford MM, de la Torre I, Winthrop K, Gottlieb RL. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol. 2021;21:382-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 485] [Cited by in RCA: 520] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 10. | Karr E, Chung T, Burtson K, Markert R, Kelly D. Bamlanivimab Use in a Military Treatment Facility. Mil Med. 2022;187:e1261-e1264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Farcy DA, Dalley MT, Miro G, Swalley P, Sherman D, Nash J, Jodoin K, Cubeddu LX, Zitek T, Goldszer R. A Comparison of SARS-COV-2 Neutralizing Antibody Therapies in High-Risk Patients with Mild to Moderate COVID-19 Disease at a Single Academic Hospital. J Emerg Med. 2022;62:83-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Dougan M, Nirula A, Azizad M, Mocherla B, Gottlieb RL, Chen P, Hebert C, Perry R, Boscia J, Heller B, Morris J, Crystal C, Igbinadolor A, Huhn G, Cardona J, Shawa I, Kumar P, Adams AC, Van Naarden J, Custer KL, Durante M, Oakley G, Schade AE, Holzer TR, Ebert PJ, Higgs RE, Kallewaard NL, Sabo J, Patel DR, Dabora MC, Klekotka P, Shen L, Skovronsky DM; BLAZE-1 Investigators. Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19. N Engl J Med. 2021;385:1382-1392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 550] [Cited by in RCA: 510] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 13. | Bariola JR, McCreary EK, Wadas RJ, Kip KE, Marroquin OC, Minnier T, Koscumb S, Collins K, Schmidhofer M, Shovel JA, Wisniewski MK, Sullivan C, Yealy DM, Nace DA, Huang DT, Haidar G, Khadem T, Linstrum K, Seymour CW, Montgomery SK, Angus DC, Snyder GM. Impact of Bamlanivimab Monoclonal Antibody Treatment on Hospitalization and Mortality Among Nonhospitalized Adults With Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Open Forum Infect Dis. 2021;8:ofab254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 14. | Destache CJ, Aurit SJ, Schmidt D, Peet Erkes L, Tierney M, Vivekanandan R. Bamlanivimab use in mild-to-moderate COVID-19 disease: A matched cohort design. Pharmacotherapy. 2021;41:743-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Tao K, Tzou PL, Kosakovsky Pond SL, Ioannidis JPA, Shafer RW. Susceptibility of SARS-CoV-2 Omicron Variants to Therapeutic Monoclonal Antibodies: Systematic Review and Meta-analysis. Microbiol Spectr. 2022;10:e0092622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 16. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47118] [Article Influence: 2944.9] [Reference Citation Analysis (0)] |
| 17. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 10807] [Article Influence: 1200.8] [Reference Citation Analysis (2)] |
| 18. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24780] [Article Influence: 1770.0] [Reference Citation Analysis (3)] |
| 19. | Alam MM, Mahmud S, Aggarwal S, Fathma S, Al Mahi N, Shibli MS, Haque SM, Ahmed Z. Clinical Impact of the Early Use of Monoclonal Antibody LY-CoV555 (Bamlanivimab) on Mortality and Hospitalization Among Elderly Nursing Home Patients: A Multicenter Retrospective Study. Cureus. 2021;13:e14933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Brock P, Dagher H, Wechsler AH, Lipe DN, Chaftari P, Chaftari AM, Gaeta MS, Johnson TN, Coussirat DJ, Aitken SL, Jiang Y, Malek A, Hachem RY, Raad II. 542. Use of Bamlanivimab in Cancer Patients with Mild-to-Moderate COVID-19. Open Forum Infect Dis. 2021;8:S372-S373. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Chen P, Datta G, Grace Li Y, Chien J, Price K, Chigutsa E, Brown-Augsburger P, Poorbaugh J, Fill J, Benschop RJ, Rouphael N, Kay A, Mulligan MJ, Saxena A, Fischer WA, Dougan M, Klekotka P, Nirula A, Benson C. First-in-Human Study of Bamlanivimab in a Randomized Trial of Hospitalized Patients With COVID-19. Clin Pharmacol Ther. 2021;110:1467-1477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, Shawa I, Adams AC, Van Naarden J, Custer KL, Shen L, Durante M, Oakley G, Schade AE, Sabo J, Patel DR, Klekotka P, Skovronsky DM; BLAZE-1 Investigators. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N Engl J Med. 2021;384:229-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1009] [Cited by in RCA: 1009] [Article Influence: 252.3] [Reference Citation Analysis (0)] |
| 23. | Chew KW, Moser C, Daar ES, Wohl DA, Li JZ, Coombs RW, Ritz J, Giganti M, Javan AC, Li Y, Choudhary MC, Deo R, Malvestutto C, Klekotka P, Price K, Nirula A, Fischer W, Bala V, Ribeiro RM, Perelson AS, Fletcher CV, Eron JJ, Currier JS; ACTIV-2/A5401 Study Team, Hughes MD, Smith DM. Antiviral and clinical activity of bamlanivimab in a randomized trial of non-hospitalized adults with COVID-19. Nat Commun. 2022;13:4931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 24. | Cooper MH, Christensen PA, Salazar E, Perez KK, Graviss EA, Nguyen D, Musser JM, Huang HJ, Liebl MG. Real-world Assessment of 2879 COVID-19 Patients Treated With Monoclonal Antibody Therapy: A Propensity Score-Matched Cohort Study. Open Forum Infect Dis. 2021;8:ofab512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Corwin DS, Ender PT, Sahu N, Durgham RA, McGorry DM Jr, Rahman A, Stoltzfus J, Jahre JA. The Efficacy of Bamlanivimab in Reducing Emergency Department Visits and Hospitalizations in a Real-world Setting. Open Forum Infect Dis. 2021;8:ofab305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Djuric P, Bogicevic J, Pešić S, Davidovic Z, Naumovic R. MO659: First Experience of Bamlanivimab for Covid-19 Positive Haemodialysis Patients: A Case-Control Study. Nephrol Dial Transplant. 2022;37:gfac077.019. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 27. | Ganesh R, Pawlowski CF, O'Horo JC, Arndt LL, Arndt RF, Bell SJ, Bierle DM, Borgen MD, Hanson SN, Heyliger A, Larsen JJ, Lenehan PJ, Orenstein R, Puranik A, Speicher LL, Tulledge-Scheitel SM, Venkatakrishnan AJ, Wilker CG, Badley AD, Razonable RR. Intravenous bamlanivimab use associates with reduced hospitalization in high-risk patients with mild to moderate COVID-19. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Ganesh R, Philpot LM, Bierle DM, Anderson RJ, Arndt LL, Arndt RF, Culbertson TL, Destro Borgen MJ, Hanson SN, Kennedy BD, Kottke BB, Larsen JJ, Ramar P, Rosedahl JK, Seville MT, Speicher LL, Tulledge-Scheitel SM, Wilker CG, Razonable RR. Real-World Clinical Outcomes of Bamlanivimab and Casirivimab-Imdevimab Among High-Risk Patients With Mild to Moderate Coronavirus Disease 2019. J Infect Dis. 2021;224:1278-1286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 29. | Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, Shawa I, Kumar P, Adams AC, Van Naarden J, Custer KL, Durante M, Oakley G, Schade AE, Holzer TR, Ebert PJ, Higgs RE, Kallewaard NL, Sabo J, Patel DR, Klekotka P, Shen L, Skovronsky DM. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA. 2021;325:632-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 724] [Article Influence: 181.0] [Reference Citation Analysis (0)] |
| 30. | ACTIV-3/TICO LY-CoV555 Study Group; Lundgren JD, Grund B, Barkauskas CE, Holland TL, Gottlieb RL, Sandkovsky U, Brown SM, Knowlton KU, Self WH, Files DC, Jain MK, Benfield T, Bowdish ME, Leshnower BG, Baker JV, Jensen JU, Gardner EM, Ginde AA, Harris ES, Johansen IS, Markowitz N, Matthay MA, Østergaard L, Chang CC, Davey VJ, Goodman A, Higgs ES, Murray DD, Murray TA, Paredes R, Parmar MKB, Phillips AN, Reilly C, Sharma S, Dewar RL, Teitelbaum M, Wentworth D, Cao H, Klekotka P, Babiker AG, Gelijns AC, Kan VL, Polizzotto MN, Thompson BT, Lane HC, Neaton JD. A Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:905-914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 328] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 31. | Heller M, Henrici C, Büttner J, Leube S, Treske I, Pospischil P, Doll M, Schanz I, Hallier A, Herrmann E, Schmidt M, Sarrazin C. SARS-CoV-2 neutralizing antibody therapies: an early retrospective cohort study of 26 hospitalized patients treated with bamlanivimab or casirivimab/imdevimab. Int J Infect Dis. 2023;129:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 32. | Kumar RN, Wu EL, Stosor V, Moore WJ, Achenbach C, Ison MG, Angarone MP. Real-World Experience of Bamlanivimab for Coronavirus Disease 2019 (COVID-19): A Case-Control Study. Clin Infect Dis. 2022;74:24-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 33. | McCreary EK, Bariola JR, Minnier T, Wadas RJ, Shovel JA, Albin D, Marroquin OC, Kip KE, Collins K, Schmidhofer M, Wisniewski MK, Nace DA, Sullivan C, Axe M, Meyer R, Weissman A, Garrard W, Peck-Palmer OM, Wells A, Bart RD, Yang A, Berry L, Berry S, Crawford A, McGlothin A, Khadem T, Linstrum K, Montgomery SK, Ricketts D, Kennedy JN, Pidro CJ, Haidar G, Snyder GM, McVerry BJ, Angus DC, Kip PL, Seymour CW, Huang DT. A learning health system randomized trial of monoclonal antibodies for COVID-19. 2021 Preprint. Available from: medRxiv: 2021:2021.09.03.21262551. [DOI] [Full Text] |
| 34. | Monday LM, Brar I, Alangaden G, Ramesh MS. SARS-CoV-2 neutralizing antibodies for COVID-19: Outcomes for bamlanivimab versus bamlanivimab-etesevimab combination in a racially diverse cohort of patients with significant comorbidities. J Clin Pharm Ther. 2022;47:1438-1443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 35. | Murillo M, Lomiguen C, Terrell M, King A, Lin J, Ferretti S. Effect of SARS CoV2-Neutralizing Monoclonal Antibody on Hospitalization and Mortality in Long-Term Care Facility Residents. Aging Dis. 2022;13:1523-1531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Priest DH, Blanchette LM, Hekman AL, Maddikunta R, Burleson PE. Bamlanivimab for the Prevention of Hospitalizations and Emergency Department Visits in SARS-CoV-2-Positive Patients in a Regional Health Care System. Infect Dis Clin Pract. 2022;30:1-4. |
| 37. | Quenzer FC, Lafree AT, Grey L, Singh S, Smyers C, Balog B, Guedez HM, McIntyre K, Wulfovich S, Ramirez J, Saikhon T, Tomaszewski C. Bamlanivimab Reduces ED Returns and Hospitalizations and May Reduce COVID-19 Burden on Low-resource Border Hospitals. West J Emerg Med. 2022;23:302-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 38. | Rubin EB, Boiarsky JA, Canha LA, Giobbie-Hurder A, Liu M, Townsend MJ, Dougan M. Bamlanivimab Efficacy in Older and High-BMI Outpatients With COVID-19 Selected for Treatment in a Lottery-Based Allocation Process. Open Forum Infect Dis. 2021;8:ofab546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | San Filippo S, Crovetto B, Bucek J, Nahass RG, Milano M, Brunetti L. Comparative Efficacy of Early COVID-19 Monoclonal Antibody Therapies: A Retrospective Analysis. Open Forum Infect Dis. 2022;9:ofac080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Savoldi A, Morra M, De Nardo P, Cattelan AM, Mirandola M, Manfrin V, Scotton P, Giordani MT, Brollo L, Panese S, Lanzafame M, Scroccaro G, Berkell M, Lippi G, Konnova A, Smet M, Malhotra-Kumar S, Kumar-Singh S, Tacconelli E; mAb Working Group. Clinical efficacy of different monoclonal antibody regimens among non-hospitalised patients with mild to moderate COVID-19 at high risk for disease progression: a prospective cohort study. Eur J Clin Microbiol Infect Dis. 2022;41:1065-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Sridhara S, Gungor AB, Erol HK, Al-Obaidi M, Zangeneh TT, Bedrick EJ, Ariyamuthu VK, Shetty A, Qannus AA, Mendoza K, Murugapandian S, Gupta G, Tanriover B. Lack of effectiveness of Bebtelovimab monoclonal antibody among high-risk patients with SARS-Cov-2 Omicron during BA.2, BA.2.12.1 and BA.5 subvariants dominated era. PLoS One. 2023;18:e0279326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 42. | Voelker D, Jerath M. Monoclonal antibody infusion for COVID-19 infection: results from a tertiary referral center. J Allergy Clin Immunol. 2022;149:AB194. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 43. | Webb BJ, Buckel W, Vento T, Butler AM, Grisel N, Brown SM, Peltan ID, Spivak ES, Shah M, Sakata T, Wallin A, Stenehjem E, Poulsen G, Bledsoe J. Real-world Effectiveness and Tolerability of Monoclonal Antibody Therapy for Ambulatory Patients With Early COVID-19. Open Forum Infect Dis. 2021;8:ofab331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 44. | Liu Y, Arase H. Neutralizing and enhancing antibodies against SARS-CoV-2. Inflamm Regen. 2022;42:58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 45. | Xiang HR, He B, Li Y, Cheng X, Zhang QZ, Peng WX. Bamlanivimab plus etesevimab treatment have a better outcome against COVID-19: A meta-analysis. J Med Virol. 2022;94:1893-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Zuo L, Ao G, Wang Y, Gao M, Qi X. Bamlanivimab improves hospitalization and mortality rates in patients with COVID-19: A systematic review and meta-analysis. J Infect. 2022;84:248-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Cheng MM, Reyes C, Satram S, Birch H, Gibbons DC, Drysdale M, Bell CF, Suyundikov A, Ding X, Maher MC, Yeh W, Telenti A, Corey L. Real-World Effectiveness of Sotrovimab for the Early Treatment of COVID-19 During SARS-CoV-2 Delta and Omicron Waves in the USA. Infect Dis Ther. 2023;12:607-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 48. | Cox M, Peacock TP, Harvey WT, Hughes J, Wright DW; COVID-19 Genomics UK (COG-UK) Consortium, Willett BJ, Thomson E, Gupta RK, Peacock SJ, Robertson DL, Carabelli AM. SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies. Nat Rev Microbiol. 2023;21:112-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 198] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 49. | Lin WT, Hung SH, Lai CC, Wang CY, Chen CH. The impact of neutralizing monoclonal antibodies on the outcomes of COVID-19 outpatients: A systematic review and meta-analysis of randomized controlled trials. J Med Virol. 2022;94:2222-2229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 50. | Boaventura P, Macedo S, Ribeiro F, Jaconiano S, Soares P. Post-COVID-19 Condition: Where Are We Now? Life (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 51. | Bull-Otterson L, Baca S, Saydah S, Boehmer TK, Adjei S, Gray S, Harris AM. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and≥ 65 years-United States, March 2020-November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:713-717. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 209] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 52. | Sukocheva OA, Maksoud R, Beeraka NM, Madhunapantula SV, Sinelnikov M, Nikolenko VN, Neganova ME, Klochkov SG, Amjad Kamal M, Staines DR, Marshall-Gradisnik S. Analysis of post COVID-19 condition and its overlap with myalgic encephalomyelitis/chronic fatigue syndrome. J Adv Res. 2022;40:179-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 91] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 53. | Hoffmann M, Hofmann-Winkler H, Krüger N, Kempf A, Nehlmeier I, Graichen L, Arora P, Sidarovich A, Moldenhauer AS, Winkler MS, Schulz S, Jäck HM, Stankov MV, Behrens GMN, Pöhlmann S. SARS-CoV-2 variant B.1.617 is resistant to bamlanivimab and evades antibodies induced by infection and vaccination. Cell Rep. 2021;36:109415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 173] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 54. | Peiffer-Smadja N, Bridier-Nahmias A, Ferré VM, Charpentier C, Garé M, Rioux C, Allemand A, Lavallée P, Ghosn J, Kramer L, Descamps D, Yazdanpanah Y, Visseaux B. Emergence of E484K Mutation Following Bamlanivimab Monotherapy among High-Risk Patients Infected with the Alpha Variant of SARS-CoV-2. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 55. | Choudhary MC, Chew KW, Deo R, Flynn JP, Regan J, Crain CR, Moser C, Hughes MD, Ritz J, Ribeiro RM, Ke R, Dragavon JA, Javan AC, Nirula A, Klekotka P, Greninger AL, Fletcher CV, Daar ES, Wohl DA, Eron JJ, Currier JS, Parikh UM, Sieg SF, Perelson AS, Coombs RW, Smith DM, Li JZ; ACTIV-2/A5401 Study Team. Emergence of SARS-CoV-2 escape mutations during Bamlanivimab therapy in a phase II randomized clinical trial. Nat Microbiol. 2022;7:1906-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 56. | Beeraka NM, Sukocheva OA, Lukina E, Liu J, Fan R. Development of antibody resistance in emerging mutant strains of SARS CoV-2: Impediment for COVID-19 vaccines. Rev Med Virol. 2022;32:e2346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |