Published online Dec 25, 2023. doi: 10.5501/wjv.v12.i5.286
Peer-review started: September 4, 2023
First decision: October 17, 2023
Revised: October 26, 2023
Accepted: November 24, 2023
Article in press: November 24, 2023
Published online: December 25, 2023
Processing time: 111 Days and 13.9 Hours
Studies have shown elevated C-reactive protein (CRP) to predict mechanical ventilation (MV) in patients with coronavirus disease 2019 (COVID-19). Its utility is unknown in patients with chronic kidney disease (CKD), who have elevated baseline CRP levels due to chronic inflammation and reduced renal clearance.
To assess whether an association exists between elevated inflammatory markers and MV rate in patients with stages IIIb-V CKD and COVID-19.
We conducted a retrospective cohort study on patients with COVID-19 and stages IIIb-V CKD. The primary outcome was the rate of invasive MV, the rate of nonin
290 were screened, and 118 met the inclusion criteria. CRP, D-dimer, and ferritin were significantly different among the three groups. On univariate analysis for invasive MV (IMV), CRP had an odds ratio (OR)-5.44; ferritin, OR-2.8; LDH, OR-7.7; D-dimer, OR-3.9, (P < 0.05). The admission CRP level had an area under curve-receiver operator characteristic (AUROC): 0.747 for the IMV group (sensitivity-80.8%, specificity-50%) and 0.663 for the non-IMV (NIMV) group (area under the curve, sensitivity-69.2%, specificity-53%).
Our results demonstrate a positive correlation between CRP, ferritin, and D-dimer levels and MV and NIMV rates in CKD patients. The AUROC demonstrates a good sensitivity for CRP levels in detecting the need for MV in patients with stages IIIb-V CKD. This may be because of the greater magnitude of increased inflammation due to COVID-19 itself compared with increased inflammation and reduced clearance due to CKD alone.
Core Tip: Our study demonstrates a positive correlation between the levels of inflammatory markers, including C-reactive protein, ferritin, and D-dimer, and the rate of invasive and non-invasive mechanical ventilation (MV) among coronavirus disease 2019 patients with chronic kidney disease (CKD), suggesting that these biomarkers are clinically useful to predict the need for MV in the CKD population.
- Citation: Shanmugavel Geetha H, Prabhu S, Sekar A, Gogtay M, Singh Y, Mishra AK, Abraham GM, Martin S. Use of inflammatory markers as predictor for mechanical ventilation in COVID-19 patients with stages IIIb-V chronic kidney disease? World J Virol 2023; 12(5): 286-295
- URL: https://www.wjgnet.com/2220-3249/full/v12/i5/286.htm
- DOI: https://dx.doi.org/10.5501/wjv.v12.i5.286
A new variant of coronavirus lead to the pandemic of 2019 and was described as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Initially assumed to be a pathogen affecting the respiratory system, its effects have now been shown to be widespread affecting multiorgan infection and disease manifestation. With more than six million ad
We conducted a retrospective single-center cross-sectional study of hospitalized patients between Dec 1, 2019, to Jan 1, 2022, at a 329-bed community teaching hospital in central Massachusetts. In order to be recruited into the study participants had to meet the inclusion criteria. Inclusion criteria: (1) Inpatients admitted with clinical symptomatology and subsequently diagnosed with SARS-CoV-2 infection with polymerase chain reaction test; (2) Age > 18 years; (3) Patients with history of stages IIIb-V CKD (estimated glomerular filtration < 45 cc/min as per National Kidney Foundation guidelines); and (4) Patients with documented inflammatory markers within 24 h of admission to the hospital. Exclusion criteria included: (1) Pregnant patients; (2) Patients who had a history of renal transplantation; (3) Patients who required renal replacement therapy; and (4) Patients who failed to meet the inclusion criteria or if the required information could not be collected. The data was obtained by reviewing medical records, including demo
The primary endpoints measured included the rate of IMV, the rate of NIMV, and the rate of no requirement of mechanical ventilatory support (no-MV). As per American Thoracic Society guidelines, IMV was defined as intubation and provision of mechanical ventilatory support for respiratory failure. NIMV included bi-level positive airway pressure, high-flow oxygen, and continuous positive airway pressure support. No MV was defined as requiring oxygen via nasal cannula, oxymizer support, or those who did not require any oxygen supplementation. We assessed the levels of inflammatory markers among the three groups, including CRP, ferritin, lactate dehydrogenase (LDH), and D-dimer levels using certain cutoffs above which the levels were considered elevated. These cutoffs were designated as per institution protocol and was ≥ 100 mg/L for CRP, ≥ 530 ng/mL for ferritin, ≥ 590 U/L for LDH, and ≥ 0.5 mg/L for D-dimer respectively. We collected the baseline demographic data of the study population. Relevant clinical data associated with increased risk of MV, including a history of hypertension, diabetes mellitus, chronic liver disease, chronic pulmonary disease, coronary artery disease, and congestive heart failure, were collected. We also collected data regarding the different treatment modalities that each patient population received.
Ethical considerations: Institutional review board statement: The study was reviewed and approved by Saint Vincent-MetroWest Medical Center Institutional Review Board (approval No. 2020-035). Informed consent statement: The requirement of informed consent was waived by Saint Vincent- MetroWest Medical Center Institutional Review Board (approval No. 2020-035).
The data was collected in Microsoft excel and was analyzed using SPSS. Non-parametric tests were employed since the data showed a non-normalcy distribution when we assessed it using the Shapiro-Wilk test. Chi-square analysis was employed for analyzing categorical variables and the Mann-Whitney U test was employed for analyzing continuous variables. Univariate logistic regression was utilized to assess the association between covariates and outcomes. We also calculated the area under the curve for invasive and NIMV for the different covariates, including CRP, ferritin, and LDH. The modalities of Medline, Pubmed and RCA were utilized to analyze high impact articles relevant to the current field of study and were incorporated in the discussion
Of the 290 patients screened, 118 met the inclusion criteria, among which 26 (22%) required IMV, 26 (22%) required NIMV, and 66 (56%) patients did not require any form of mechanical ventilatory support. There was an increased number of males in the group requiring IMV compared to those requiring NIMV (P = 0.01) (Table 1). Baseline demographics, including age > 60 years, vaccination status, and history of hypertension, diabetes mellitus, chronic liver disease, chronic pulmonary disease, coronary artery disease, and congestive heart failure, was similar among the three groups. In terms of medication administration, a significant difference was observed only in steroid use between patients on NIMV compared to those without (84.6% vs 66.7%, P = 0.01) (Table 1).
| Variables | Invasive mechanical ventilation | Non-invasive mechanical ventilation | No mechanical ventilation | Total | P valuea | P valueb |
| Age > 60 yr | 24 | 25 | 62 | 111 | 1.00 | 1.00 |
| 92.3 | 96.2 | 93.9 | 94.1 | |||
| Male sex | 18 | 12 | 26 | 56 | 0.01 | 0.55 |
| 69.2 | 46.2 | 39.4 | 47.5 | |||
| Vaccinated against COVID-19 | 2 | 7 | 16 | 25 | 0.13 | 0.16 |
| 7.7 | 26.9 | 24.2 | 21.2 | |||
| Hypertension | 25 | 21 | 59 | 105 | 0.43 | 0.31 |
| 96.2 | 80.8 | 89.4 | 89.0 | |||
| Diabetes mellitus | 15 | 13 | 33 | 61 | 0.51 | 1.00 |
| 57.7 | 50.0 | 50.0 | 51.7 | |||
| Chronic liver disease | 0 | 1 | 1 | 2 | 1.00 | 0.49 |
| 0.0 | 3.8 | 1.5 | 1.7 | |||
| Chronic obstructive pulmonary disease | 8 | 11 | 13 | 32 | 0.26 | 0.26 |
| 30.8 | 42.3 | 19.7 | 27.1 | |||
| Coronary artery disease | 11 | 8 | 21 | 40 | 0.34 | 0.92 |
| 42.3 | 30.8 | 31.8 | 33.9 | |||
| Congestive heart failure | 10 | 11 | 17 | 38 | 0.23 | 0.12 |
| 38.5 | 42.3 | 25.8 | 32.2 | |||
| Remdesivir | 12 | 16 | 32 | 60 | 0.84 | 0.26 |
| 46.2 | 61.5 | 48.5 | 50.8 | |||
| Steroids | 24 | 22 | 44 | 90 | 0.01 | 0.12 |
| 92.3 | 84.6 | 66.7 | 76.3 |
The association between the levels of inflammatory markers and the use of invasive, non-invasive, and no mechanical ventilatory support was evaluated.
IMV: We observed a significant difference in the levels of inflammatory markers, including CRP (65.4% vs 25.8%, P = 0.01), ferritin (61.5% vs 36.4%, P = 0.01), troponin (42.3% vs 22.7%, P = 0.03), D-dimer (80.8% vs 51.5%, P = 0.01), and LDH (26.9% vs 4.5%, P = 0.04) between patients who required IMV and those who did not require MV (Table 2). This correlated with the significantly different mean levels of inflammatory markers observed between the two groups as well [CRP (160.2 vs 67, P = 0.001), ferritin (811 vs 295, P = 0.019), LDH (452 vs 321, P = 0.001) and D-dimer (2 vs 1, P = 0.001)]. Further univariate analysis between the inflammatory markers showed greater odds of having high inflammatory marker levels in patients who required IMV [CRP odds ratio (OR) 5.44, 95% confidence interval (CI): 2.04-14.48, ferritin (OR 2.8, 95%CI: 1.98-7.13), D-dimer (OR 3.95, 95%CI: 1.33-11.74), LDH (OR 7.73, 95%CI: 1.821-32.87), but troponin levels were not statistically significant (OR 2 .49, 95%CI: 0.947-6.56] (Table 3).
| Variables | Invasive mechanical ventilation | No mechanical ventilation | Total | P valuea |
| CRP level (mg/L) | 17 | 17 | 48 | 0.01 |
| 65.4 | 25.8 | 40.7 | ||
| Ferritin level (ng/mL) | 16 | 24 | 57 | 0.01 |
| 61.5 | 36.4 | 48.3 | ||
| LDH level (U/L) | 7 | 3 | 12 | 0.04 |
| 26.9 | 4.5 | 10.2 | ||
| Troponin (ng/mL) | 11 | 15 | 38 | 0.03 |
| 42.3 | 22.7 | 32.2 | ||
| D-dimer (mg/L) | 21 | 34 | 76 | 0.01 |
| 80.8 | 51.5 | 64.4 |
| Variables | OR | 95%CI |
| CRP level | 5.444 | 2.047-14.483 |
| Ferritin level | 2.8 | 1.098-7.138 |
| LDH level | 7.737 | 1.821-32.87 |
| Troponin level | 2.493 | 0.947-6.56 |
| D-dimer level | 3.953 | 1.331-11.74 |
NIMV: A similar phenomenon of significantly different levels of inflammatory markers was observed in patients who required NIMV in comparison to those without mechanical ventilatory support requirements [CRP (53.8% vs 25.8%, P = 0.001), ferritin (65.4% vs 36.4%, P = 0.03), D-dimer (80.8% vs 51.5%, P = 0.01), and LDH (7.7% vs 4.5%, P = 0.001), but no significant difference was demonstrated in troponin levels (46.2% vs 22.7%, P = 0.06)] (Table 4). On assessing the mean levels of inflammatory markers between the two groups, we observed a significant difference in CRP (115.9 vs 67, P = 0.002), ferritin (628 vs 295, P = 0.013), and D-dimer (2 vs 1, P = 0.001) but no significant difference in LDH (357 vs 321, P = 0.29). We subjected these inflammatory biomarkers to univariate analysis, which showed increased odds of higher levels of all biomarkers except LDH among patients who required NIMV [CRP (OR 3.63, 95%CI: 1.30-8.67), ferritin (OR 3.306, 95%CI: 1.27-8.55), D-dimer (OR 3.95, 95%CI: 1.33-11.73 ), troponin (OR 2.94, 95%CI: 1.11-7.62) but no significant difference was demonstrated in LDH (OR 1.75, 95%CI: 0.27-11.12) (Table 5).
| Variables | Non-invasive mechanical ventilation | No mechanical ventilation | Total | P valuea |
| CRP level (mg/L) | 14 | 17 | 48 | 0.001 |
| 53.8 | 25.8 | 40.7 | ||
| Ferritin level (ng/mL) | 17 | 24 | 57 | 0.03 |
| 65.4 | 36.4 | 48.3 | ||
| LDH level (U/L) | 2 | 3 | 12 | 0.001 |
| 7.7 | 4.5 | 10.2 | ||
| Troponin (ng/mL) | 12 | 15 | 38 | 0.06 |
| 46.2 | 22.7 | 32.2 | ||
| D-dimer (mg/L) | 21 | 34 | 76 | 0.01 |
| 80.8 | 51.5 | 64.4 |
| Variables | OR | 95%CI |
| CRP level | 3.363 | 1.303-8.679 |
| Ferritin level | 3.306 | 1.277-8.55 |
| LDH level | 1.750 | 0.275-11.129 |
| Troponin level | 2.914 | 1.113-7.628 |
| D-dimer level | 3.953 | 1.331-11.736 |
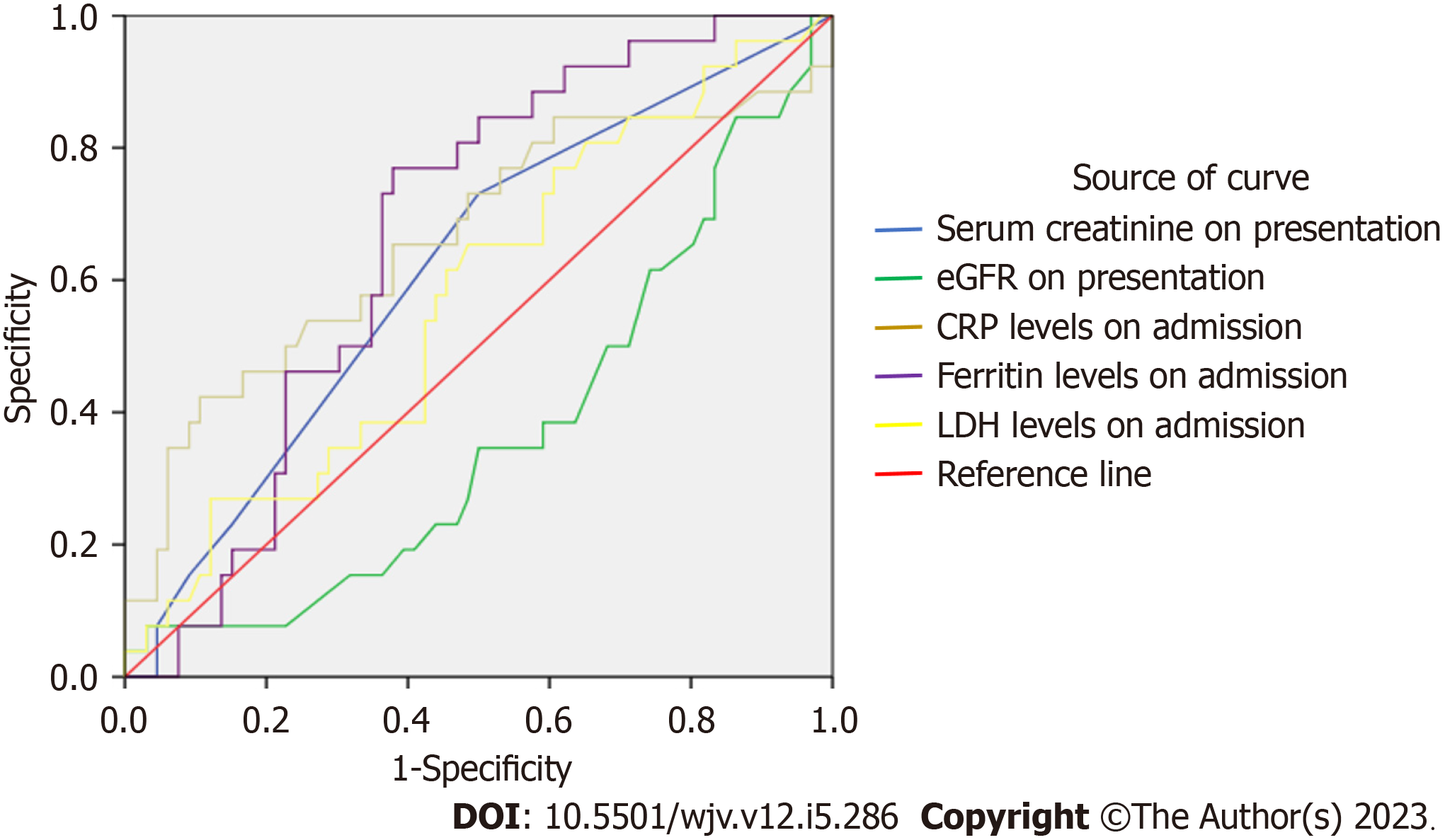
Area under curve-receiver operator characteristic (ROC) (AUROC): In order to further confirm the role of the inflammatory biomarkers in predicting the need for MV, ROC analysis was carried out. The AUROC for IMV was the following: for CRP, AUROC 0.747 (95%CI: 0.617-0.878, P = 0.001) that yielded a sensitivity of 80.8% and specificity of 50%; for ferritin, AUROC 0.658 (CI: 0.528-0.788, P = 0.019) with a sensitivity of 73% and specificity of 50%; for LDH, AUROC 0.699 (CI: 0.579-0.820, P = 0.003) with a sensitivity of 80.8% and specificity of 50%; and for D-dimer, AUC 0.751 (CI: 0.625-0.876, P = 0.001) with a sensitivity of 76.9% and specificity of 50% (Figure 1, Table 6).
| Variables on admission | AUC | P value | 95% confidence interval | Sensitivity (%) | Specificity (%) | |
| Lower limit | Upper limit | |||||
| CRP level | 0.747 | 0.001 | 0.617 | 0.878 | 80.8 | 51 |
| Ferritin level | 0.658 | 0.019 | 0.528 | 0.788 | 73 | 53 |
| LDH level | 0.699 | 0.003 | 0.579 | 0.820 | 80.8 | 51 |
| D-dimer level | 0.751 | 0.001 | 0.625 | 0.876 | 76.9 | 52 |
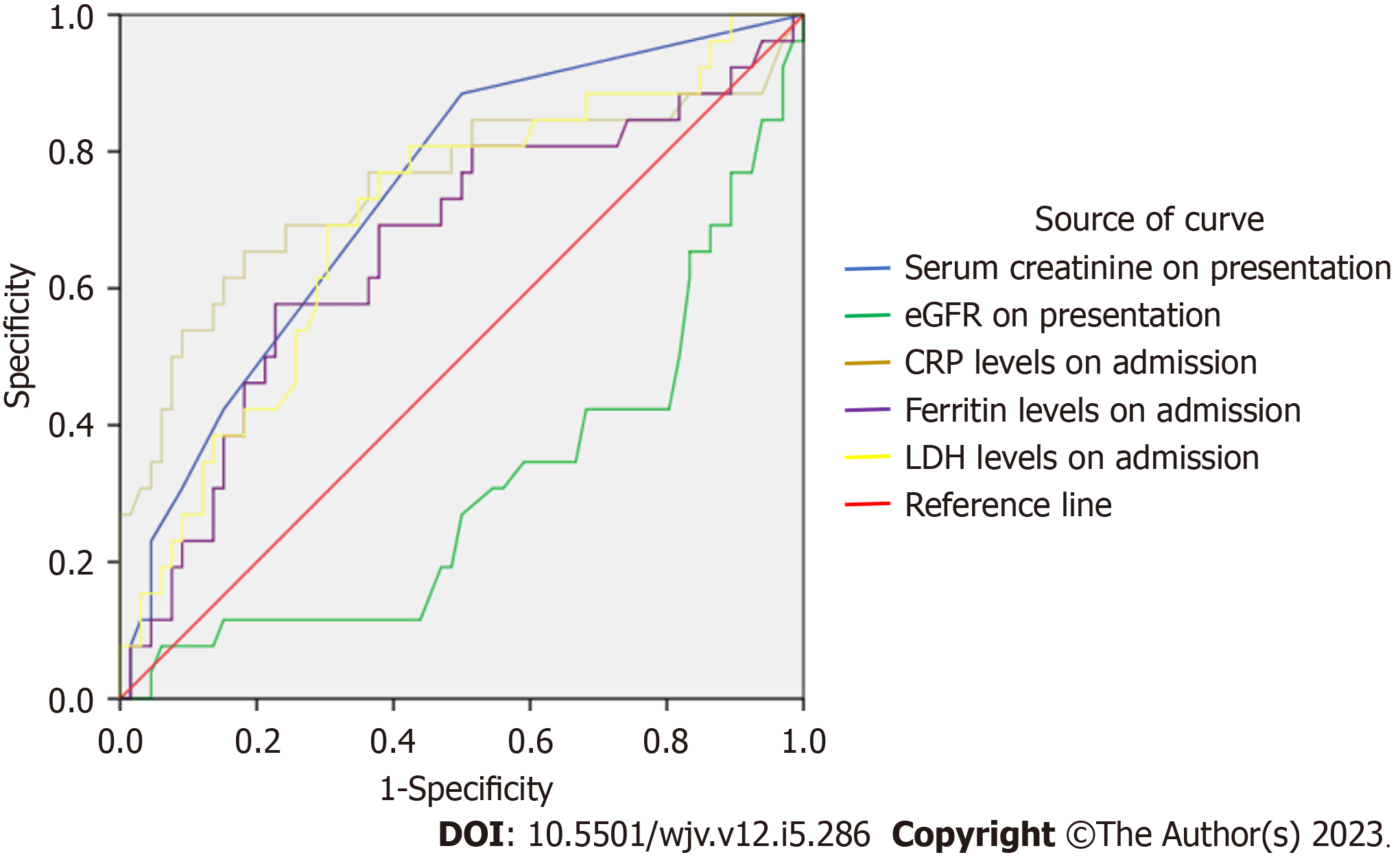
The AUROC for NIMV was as follows: For CRP, AUROC 0.663 (95%CI: 0.527-0.799, P = 0.015) that yielded a sensitivity of 69.2% and specificity of 53%; for ferritin, AUROC 0.667 (CI: 0.555-0.778, P = 0.013) with a sensitivity of 80.8% and specificity of 53%; and for D-dimer, AUROC 0.740 (CI: 0.62-0.86, P = 0.004) with a sensitivity of 80.8% and specificity of 50% (Figure 2, Table 7).
| Variables on admission | AUC | P value | 95% confidence interval | Sensitivity (%) | Specificity (%) | |
| Lower limit | Upper limit | |||||
| CRP level | 0.663 | 0.015 | 0.527 | 0.799 | 69.2 | 53 |
| Ferritin level | 0.667 | 0.013 | 0.555 | 0.778 | 80.8 | 53 |
| LDH level | 0.573 | 0.280 | 0.445 | 0.700 | 61.5 | 55 |
| D-dimer level | 0.740 | 0.0004 | 0.620 | 0.860 | 80.8 | 50 |
This study is unique in assessing the utility of inflammatory markers, such as CRP, ferritin, LDH, and D-dimer in predicting the need for non-invasive as well as IMV in COVID-19 disease in patients with CKD. We observed that a higher proportion of COVID-19 patients with CKD who had elevated inflammatory marker levels ultimately required MV. The average inflammatory marker levels in all 3 groups (MV, NIMV and no MV) were high. Elevated levels of inflammatory markers were highly predictive of the need for IMV with corresponding AUROC of 0.747, 0.658, 0.699, and 0.751 for CRP, ferritin, LDH, and D-dimer, respectively. Although not all markers were predictive of the need for NIMV, CRP, ferritin, and D-dimer were predictive, with corresponding AUROCs of 0.663, 0.667, and 0.74, respectively. Although the pathophysiology explaining elevated LDH levels in patients requiring IMV but not amongst patients requiring NIMV is not explicitly clear, we hypothesize that this could be secondary to the LDH cutoff that was used to define levels as elevated. LDH enzyme plays a prominent role in active metabolism and levels are elevated with minor abnormalities such as tissue hypoxia and lysis necessitating a higher cutoff to detect significantly elevated LDH levels[8]. The results of our study reinforced the predictive value of CRP, ferritin, and D-dimer in patients with COVID-19 and underlying stages IIIb-V CKD. Among patients with CKD alone, studies have shown baseline elevated inflammatory marker levels, due to a chronic inflammatory milieu and decreased renal clearance of these inflammatory markers[7]. Our study highlighted the positive correlation of these markers with invasive as well as NIMV in COVID-19 patients with stages IIIb-V CKD; the high sensitivity of these markers demonstrated by the AUROC signifies their predictive potential.
In our study, the demographic variables were similar to the previous studies[4,5]. Male sex was associated with an increased risk of the need for invasive and NIMV. Sex may influence the severity of SARS-CoV-2 as the X-chromosome contains a higher density of immune-related genes and immunoregulatory elements related to innate and adaptive immunity[9]. There was an equal distribution of the need for MV in the presence of associated comorbidities, such as hypertension, diabetes mellitus, chronic liver disease, chronic obstructive pulmonary disease, coronary artery disease, and congestive heart failure. We noticed a significantly increased steroid administration rate in the NIMV group compared to the no MV group. One possible explanation for this finding could be the greater severity of the disease although there is no clear evidence to demonstrate the same
Biomarkers are a clinical reflection of the underlying disease process and help us assess the disease activity. This was frequently employed in COVID-19 disease with studies showing a correlation between elevated inflammatory marker levels and severe COVID-19 disease[5,6]. Although markers such as IL-6 were initially explored, they are cost-prohibitive and thus unsuitable for routine monitoring in COVID-19 patients[4]. This led to research on more routine biomarkers, including CRP, ferritin, LDH, and D-dimer, which have been shown to correlate well with the severity of COVID-19 disease[10]. Despite the use of different values of CRP to define elevation in multiple studies, such as Koozi et al[11] > 1000mg/L, Ryoo et al[12] > 140mg/L, and Liu et al[13] > 41.8 mg/L, there was a uniformly observed greater risk of severe COVID-19 disease[11-14].
Inflammatory markers are used for risk stratification and prognostication in several infectious diseases and ma
The inflammatory markers are renally cleared, and hence reduced kidney function is associated with elevated levels of serum inflammatory markers. In addition, CKD is associated with chronic inflammation. Studies have demonstrated an elevation of CRP levels in patients with CKD and a negative correlation between CRP levels and glomerular filtration rate (GFR). There is evidence that inflammation, as measured by CRP level, increases with declining renal function in CKD patients[22-24]. A study by Keller et al[25] showed that in patients with initial stages of CKD and with end stage renal disease, the levels of CRP, fibrinogen, D-dimer, coagulation factor VII, factor VIII were increased, either due to increased production vs decreased clearance. CKD stages IIIb-V was selected since there was a significant increase in mortality rate amongst patients with CKD IIIb-V[26].
In our study, the mean CRP levels at admission in COVID-19 patients with stages IIIb-V CKD requiring IMV were remarkably higher than those who did not require MV (160.19 vs 67.02, P = 0.001). This finding likely reflects the impact of acute, severe COVID-19-related illness on the existing chronic inflammation in CKD, and concomitant reduced renal clearance of inflammatory markers. We found CRP, ferritin, LDH, and D-dimer to be good predictors of IMV and CRP, ferritin, and D-dimer to be good predictors of NIMV. Regardless of the negative correlation of inflammatory biomarkers with GFR in CKD, our study validated their high sensitivity in predicting COVID-19 prognosis in this specific population.
Limitations: One of the limitations of our study includes a small study population. We also did not include patients who had a history of renal transplantation, in order to minimize the influence of immunosuppressive medications in our study population. Another limiting factor includes the absence of information about baseline inflammatory marker levels in the setting of their underlying CKD. There are multiple factors that influence inflammatory marker levels, such as age, body mass index, sex, use of nicotine, blood pressure, and liver injury[20]. We did not study more specific markers such as IL-6, IL-1β, and IL-8, which are more sensitive but are cost-prohibitive in the real-world setting. We did not study the inter
Future implications: Further prospective studies are needed to establish the correlation between the levels of inflammatory markers and the need for MV in COVID-19 patients with CKD. Validation of these inflammatory biomarkers is key in establishing their use as predictive indices. With the clinical utility of these inflammatory markers being described, it is imperative to study the impact of different disease processes on these inflammatory markers before employing them as clinical tools to guide the diagnosis and management of acute COVID-19 infection.
Our study explored the efficacy and predictive ability of inflammatory markers in detecting the risk of respiratory failure and the subsequent need for invasive and NIMV among COVID-19 patients with pre-existing CKD. We demonstrated that inflammatory markers, including CRP, ferritin, and D-dimer are useful predictive indicators of invasive and non-invasive MV in COVID-19 patients with stages IIIb-V CKD. The AUROC demonstrates good sensitivity for CRP levels in predicting the need for MV in the general population as well as in patients with stages IIIb-V CKD. This could be ex
Inflammatory markers have been validated in multiple studies to help predict the severity of disease and the need for mechanical ventilation (MV). Studies have shown baseline elevation in these same inflammatory markers in patients with chronic kidney disease (CKD) alone, due to a chronic inflammatory milieu in CKD and reduced renal clearance of these inflammatory markers. The clinical utility of these inflammatory markers to predict the need for MV among patients with coronavirus disease 2019 (COVID-19) and underlying CKD is unclear.
The use of biomarkers has been progressively increasing since the COVID-19 pandemic and the need for establishing the utility of these biomarkers in the presence of multiple comorbidities becomes essential to establish their clinical utility. Hence there is utmost need for this study to assess use of C-reactive protein level in assessing MV risk in CKD patients.
Since an increased level of inflammatory markers were observed in patients with chronic kidney disease, especially amongst those with stages IIIb-V, we planned to assess the utility of inflammatory biomarkers by evaluating the rate of MV and the levels of inflammatory biomarkers in stages IIIb-V chronic kidney disease patients who are diagnosed with COVID-19.
In order to analyze the association between inflammatory marker levels and rate of MV, we did a single-center retro
A total of 290 patients were admitted between the study period of December 2019 to January, 2022 and amongst them 118 met the inclusion criteria. When we compared the rates of IMV, the group with IMV patients had a greater level of inflammatory markers. We also found a similar result when we compared the inflammatory marker levels amongst NIMV patients.
Our results showed that elevated inflammatory marker levels were still associated with an increased rate of IMV and NIMV even amongst stage IIIb-V CKD patients with COVID-19 disease, thereby demonstrating the clinical utility of these biomarkers in assessing disease severity despite their baseline elevated levels observed in CKD patients.
Validation of these inflammatory biomarkers is key in establishing their use as predictive indices. With the clinical utility of these inflammatory markers being described, it is imperative to study the impact of different disease processes on these inflammatory markers before employing them as clinical tools to guide the diagnosis and management of acute COVID-19 infection.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Virology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He YF, China; Kelleni MT, Egypt; Wang MK, China S-Editor: Qu XL L-Editor: A P-Editor: Xu ZH
| 1. | Lipworth B, Chan R, Lipworth S, RuiWen Kuo C. Weathering the Cytokine Storm in Susceptible Patients with Severe SARS-CoV-2 Infection. J Allergy Clin Immunol Pract. 2020;8:1798-1801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | Muthyala A, Sasidharan S, John KJ, Lal A, Mishra AK. Utility of cardiac bioenzymes in predicting cardiovascular outcomes in SARS-CoV-2. World J Virol. 2022;11:375-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 3. | Chidambaram V, Shanmugavel Geetha H, Kumar A, Majella MG, Sivakumar RK, Voruganti D, Mehta JL, Karakousis PC. Association of Lipid Levels With COVID-19 Infection, Disease Severity and Mortality: A Systematic Review and Meta-Analysis. Front Cardiovasc Med. 2022;9:862999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, von Bergwelt-Baildon M, Klein M, Weinberger T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146:128-136.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 696] [Cited by in RCA: 713] [Article Influence: 142.6] [Reference Citation Analysis (0)] |
| 5. | Li W, Lin F, Dai M, Chen L, Han D, Cui Y, Pan P. Early predictors for mechanical ventilation in COVID-19 patients. Ther Adv Respir Dis. 2020;14:1753466620963017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Payán-Pernía S, Gómez Pérez L, Remacha Sevilla ÁF, Sierra Gil J, Novelli Canales S. Absolute Lymphocytes, Ferritin, C-Reactive Protein, and Lactate Dehydrogenase Predict Early Invasive Ventilation in Patients With COVID-19. Lab Med. 2021;52:141-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Khan AA, Allemailem KS, Alhumaydhi FA, Gowder SJT, Rahmani AH. The Biochemical and Clinical Perspectives of Lactate Dehydrogenase: An Enzyme of Active Metabolism. Endocr Metab Immune Disord Drug Targets. 2020;20:855-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 8. | Muslimovic A, Rasic S, Tulumovic D, Hasanspahic S, Rebic D. Inflammatory Markers and Procoagulants in Chronic Renal Disease Stages 1-4. Med Arch. 2015;69:307-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Schurz H, Salie M, Tromp G, Hoal EG, Kinnear CJ, Möller M. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum Genomics. 2019;13:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 250] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 10. | Malik P, Patel U, Mehta D, Patel N, Kelkar R, Akrmah M, Gabrilove JL, Sacks H. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med. 2021;26:107-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 376] [Cited by in RCA: 383] [Article Influence: 95.8] [Reference Citation Analysis (0)] |
| 11. | Koozi H, Lengquist M, Frigyesi A. C-reactive protein as a prognostic factor in intensive care admissions for sepsis: A Swedish multicenter study. J Crit Care. 2020;56:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Ryoo SM, Han KS, Ahn S, Shin TG, Hwang SY, Chung SP, Hwang YJ, Park YS, Jo YH, Chang HL, Suh GJ, You KM, Kang GH, Choi SH, Lim TH, Kim WY; Korean Shock Society (KoSS) Investigators. The usefulness of C-reactive protein and procalcitonin to predict prognosis in septic shock patients: A multicenter prospective registry-based observational study. Sci Rep. 2019;9:6579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, Li B, Song X, Zhou X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 742] [Cited by in RCA: 706] [Article Influence: 141.2] [Reference Citation Analysis (0)] |
| 14. | Geetha HS, Singh G, Sekar A, Gogtay M, Singh Y, Abraham GM, Trivedi N. Hyperglycemia in COVID-19 infection without diabetes mellitus: Association with inflammatory markers. World J Clin Cases. 2023;11:1287-1298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Wang C, He W, Yuan Y, Zhang Y, Li K, Zou R, Liao Y, Liu W, Yang Z, Zuo D, Qiu J, Zheng Y, Li B. Comparison of the prognostic value of inflammation-based scores in early recurrent hepatocellular carcinoma after hepatectomy. Liver Int. 2020;40:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Yamamoto M, Kobayashi T, Kuroda S, Hamaoka M, Okimoto S, Honmyo N, Yamaguchi M, Ohdan H. Verification of inflammation-based prognostic marker as a prognostic indicator in hepatocellular carcinoma. Ann Gastroenterol Surg. 2019;3:667-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1356] [Cited by in RCA: 1805] [Article Influence: 361.0] [Reference Citation Analysis (0)] |
| 18. | Vabret N, Samstein R, Fernandez N, Merad M; Sinai Immunology Review Project; Trainees; Faculty. Advancing scientific knowledge in times of pandemics. Nat Rev Immunol. 2020;20:338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol. 2020;92:2409-2411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 20. | Ghahramani S, Tabrizi R, Lankarani KB, Kashani SMA, Rezaei S, Zeidi N, Akbari M, Heydari ST, Akbari H, Nowrouzi-Sohrabi P, Ahmadizar F. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur J Med Res. 2020;25:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 194] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 21. | Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol. 2018;9:754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 899] [Cited by in RCA: 1711] [Article Influence: 244.4] [Reference Citation Analysis (0)] |
| 22. | Annuk M, Soveri I, Zilmer M, Lind L, Hulthe J, Fellström B. Endothelial function, CRP and oxidative stress in chronic kidney disease. J Nephrol. 2005;18:721-726. [PubMed] |
| 23. | Jalal D, Chonchol M, Etgen T, Sander D. C-reactive protein as a predictor of cardiovascular events in elderly patients with chronic kidney disease. J Nephrol. 2012;25:719-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Baravkar PN, Bavikar JS, Asegaonkar SB, Bavikar SS, Bardapurkar JS, Thorat A. Study of serum uric acid and C-reactive protein levels in patients with chronic renal disease. Int J Biol Med Res. 2013;4:2758-2761. |
| 25. | Keller C, Katz R, Sarnak MJ, Fried LF, Kestenbaum B, Cushman M, Shlipak MG; CHS study. Inflammatory biomarkers and decline in kidney function in the elderly: the Cardiovascular Health Study. Nephrol Dial Transplant. 2010;25:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Ang YG, Heng BH, Saxena N, Liew STA, Chong PN. Annual all-cause mortality rate for patients with diabetic kidney disease in Singapore. J Clin Transl Endocrinol. 2016;4:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | John KJ, Mishra AK, Ramasamy C, George AA, Selvaraj V, Lal A. Heart failure in COVID-19 patients: Critical care experience. World J Virol. 2022;11:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | John K, Mishra AK, Nayar J, Mehawej J, Lal A. Coronavirus disease 2019 and mechanical circulatory support devices: a comprehensive review. Monaldi Arch Chest Dis. 2022;93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 29. | Ramasamy C, Mishra AK, John KJ, Lal A. Clinical considerations for critically ill COVID-19 cancer patients: A systematic review. World J Clin Cases. 2021;9:8441-8452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |