Published online Sep 25, 2023. doi: 10.5501/wjv.v12.i4.221
Peer-review started: June 27, 2023
First decision: July 19, 2023
Revised: August 2, 2023
Accepted: August 21, 2023
Article in press: August 21, 2023
Published online: September 25, 2023
Processing time: 83 Days and 11.9 Hours
Alcohol-associated cirrhosis (AC) contributes to significant liver-related mortality in the United States. It is known to cause immune dysfunction and coagulation abnormalities. Patients with comorbid conditions like AC are at risk of worse clinical outcomes from coronavirus disease 2019 (COVID-19). The specific association between AC and COVID-19 mortality remains inconclusive, given the lack of robust clinical evi
To study the predictors of mortality and the outcomes of AC in patients hospitalized with COVID-19 in the United States.
We conducted a retrospective cohort study using the National Inpatient Sample (NIS) database 2020. Patients were identified with primary COVID-19 hospitalizations based on an underlying diagnosis of AC. A matched com
A total of 1325 COVID-19 patients with AC were matched to 1135 patients without AC. There was no difference in median length of stay and hospital charges in COVID-19 patients with AC compared to non-AC (P > 0.05). There was an increased prevalence of septic shock (5.7% vs 4.1%), ventricular fibrillation/ventricular flutter (0.4% vs 0%), atrial fibrillation (13.2% vs 8.8%), atrial flutter (8.7% vs 4.4%), first-degree atrioventricular nodal block (0.8% vs 0%), upper extremity venous thromboembolism (1.5% vs 0%), and variceal bleeding (3.8% vs 0%) in the AC cohort compared to the non-AC cohort (P < 0.05). There was no difference in inpatient mortality in COVID-19 patients with non-AC compared to AC, with an odds ratio of 0.97 (95% confidence interval: 0.78-1.22, P = 0.85). Predictors of mortality included advanced age, cardiac arrhythmias, coagulopathy, protein-calorie malnutrition, fluid and electrolyte disorders, septic shock, and upper extremity venous thromboembolism.
AC does not increase mortality in patients hospitalized with COVID-19. There is an increased association between inpatient complications among COVID-19 patients with AC compared to non-AC.
Core Tip: High-risk comorbid conditions significantly increase the mortality linked to coronavirus disease 2019 (COVID-19). In this large National Inpatient Sample-based retrospective cohort study, we aimed to evaluate the specific clinical impact of alcohol-associated cirrhosis (AC) on patients hospitalized with COVID-19. We analyzed the patient outcomes based on comorbidities, mechanical ventilation, intensive care unit admission, and mortality predictors. Our findings show that AC does not increase mortality in patients hospitalized with COVID-19. Pertinently, there is an increased association between inpatient complications and COVID-19 patients with AC compared to non-AC. Predictors of mortality included advanced age, cardiac arrhythmias, coagulopathy, protein-calorie malnutrition, fluid and electrolyte disorders, septic shock, and upper extremity venous thromboembolism.
- Citation: Inayat F, Ali H, Patel P, Dhillon R, Afzal A, Rehman AU, Afzal MS, Zulfiqar L, Nawaz G, Goraya MHN, Subramanium S, Agrawal S, Satapathy SK. Association between alcohol-associated cirrhosis and inpatient complications among COVID-19 patients: A propensity-matched analysis from the United States. World J Virol 2023; 12(4): 221-232
- URL: https://www.wjgnet.com/2220-3249/full/v12/i4/221.htm
- DOI: https://dx.doi.org/10.5501/wjv.v12.i4.221
The global pandemic of coronavirus disease 2019 (COVID-19) has profoundly affected patients with pre-existing comorbi
Patients with cirrhosis often develop immunological perturbations, leading to systemic inflammation and immune deficiency[8]. This immune dysfunction could potentially make it more difficult for cirrhotics to fight COVID-19. Nume
Based on available clinical epidemiologic studies, there is a limited understanding of the etiologic basis of the clinical outcomes of cirrhosis in patients with COVID-19. However, several studies have investigated this subject. For example, in a single-center retrospective analysis of a cohort of patients hospitalized for COVID-19 with a prevailing alcoholic CLD, liver cirrhosis was linked to a fourfold increase in 30-d mortality[15]. A Korean study suggested that people with under
Our objective is to assess the impact of AC on outcomes and mortality in patients hospitalized with COVID-19 using a large, multicenter database. We also seek to identify mortality predictors in COVID-19 patients with AC. To our knowle
This retrospective cohort study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines[18]. We utilized the recently released National Inpatient Sample (NIS) database 2020. It is designed by the Agency for Healthcare Research and Quality[19]. NIS is the largest inpatient database in the United States healthcare system[19]. The design of this database enables the calculation of national estimates using sampling weights and a 20% stratified sample of hospitals[19]. Detailed information on the design of NIS and sampling methods is available at https://www.hcup-us.ahrq.gov. NIS 2020 utilized the International Classification of Diseases (ICD) 10 coding system to store and report data. We identified hospitalizations with a primary diagnosis (DX1) of COVID-19 using the “U07.1” ICD-10 code, which was introduced in March 2020[20]. Hospitalizations were excluded if the patient age was < 18 years, individuals were transferred, and/or COVID-19 was listed as a secondary diagnosis. Furthermore, patients were excluded if there was any history of cirrhosis due to nonalcoholic and other causes (viral, autoimmune, or non-specified), hepatocellular carcinoma, malignant neoplasm, end-stage renal disease requiring dialysis, quadriplegia, lymphoma, renal transplant, or liver transplant, as these were deemed high-risk conditions that could confound our analysis.
Primary outcomes included median length of stay, median inpatient charges, and in-hospital mortality. Secondary out
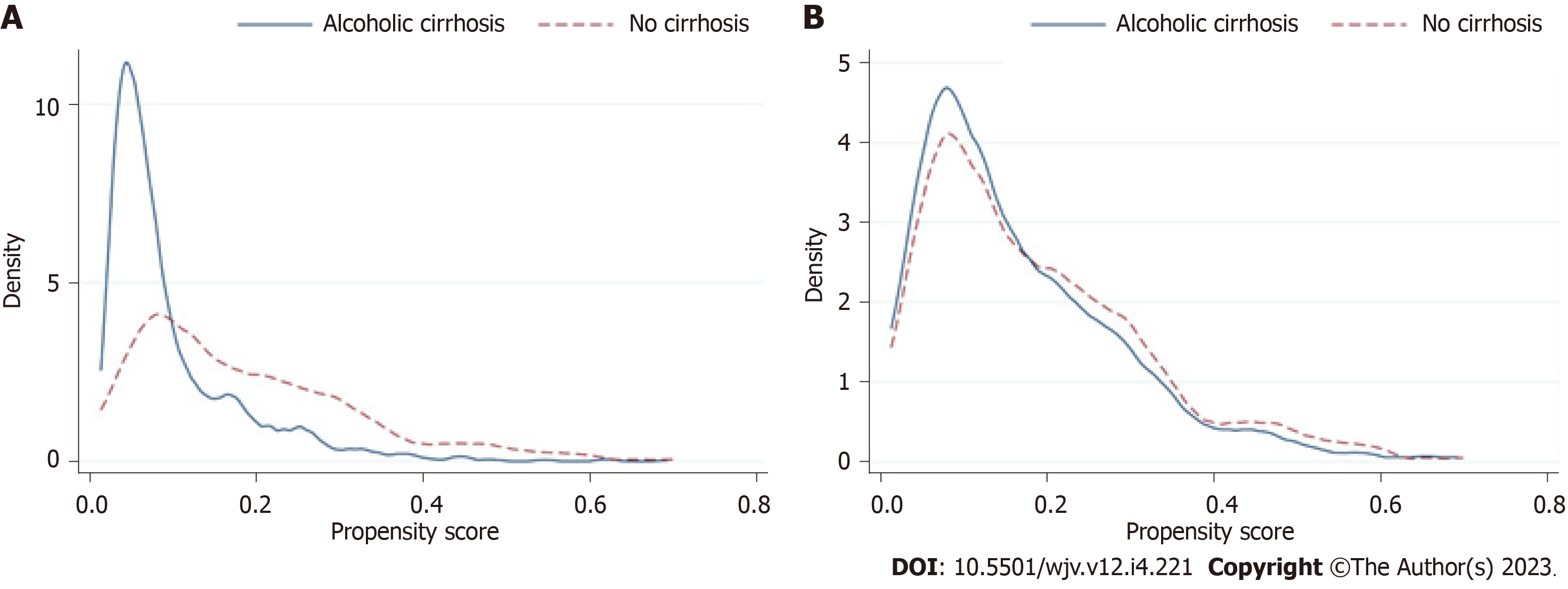
Statistical analysis was performed using Statistical Software for Data Science (StataCorp LLC, College Station, TX, United States) version 16.0. Two cohorts were created based on the presence or absence of a secondary diagnosis of AC using ICD-10 codes employed in the published literature[21]. We developed matched cohorts using propensity score matching (PSM) to minimize the effect of comorbid imbalances between comparison cohorts. Each case was assigned a propensity score using a multivariable logistic regression that included baseline sociodemographic characteristics (age, sex, race, socioeconomic status, and Elixhauser comorbidities). Propensity scores between the two cohorts were matched in a 1:N fashion using the nearest-neighbor method within 0.01 standard deviations of the calculated score[22]. The covariate balance was then visualized using the two-way plot (Figure 1). A two-sample Wilcoxon rank-sum (Mann-Whitney) test was utilized for continuous variables. The Chi-square test was used to compare categorical variables. The significance threshold was set at P < 0.05. For logistic regression, hierarchical models were designed using any unbalanced variables in PSM (race). The positive mortality predictors were then used to build a final multivariate model. Only significant posi
The NIS is a de-identified hospital-level, third-party database. The privacy of patients, clinicians, and medical centers is protected by its design. Patient consent was waived as the hospitalization data were stripped of any patient identifiers. The approval of the institutional review board (IRB) was not required for this study.
A total of 738010 primary COVID-19 hospitalizations fulfilled the selection criteria and were included in the study. A total of 1325 hospitalizations with AC were matched to 1135 without AC using nearest-neighbor matching (Table 1). The age group 50-64 years had a higher prevalence in the AC cohort than the non-AC cohort (P < 0.001). The median age was 60 years, with an interquartile range (IQR) of 54-67 in the AC cohort and 61 years (IQR: 54-67) in the non-AC cohort. There was no disparity based on gender (P = 0.31). Hispanics (33.2% vs 26.0%) and Native Americans (4.9% vs 4.8%) had a higher prevalence in the AC cohort compared to the non-AC cohort (P < 0.001). There was a higher prevalence of an Elixhauser Comorbidity Index (ECI) score ≥ 3 (98.9% vs 2.2%) in the AC group compared to the non-AC group (P < 0.001). Mortality was significantly higher in hospitalizations with non-AC compared to AC (15.0% vs 14.7%, P = 0.024). Disposition was more likely to be against medical advice if there was a secondary diagnosis of AC compared to non-AC (3.8% vs 3.5%, P = 0.024). There was no difference in intensive care unit (ICU)-level care (10.6% each, P = 1.00). There was no significant difference in median length of stay or median hospital charges between the AC and non-AC groups (P > 0.05).
| Factor | Alcoholic cirrhosis | No alcoholic cirrhosis (before matching) | P value | No alcoholic cirrhosis (after matching) | P value |
| Total hospitalizations | 1325 | 736685 | 1135 | ||
| Age groups (yr) | < 0.001 | < 0.001 | |||
| 18-34 | 30 (2.3) | 36860 (5.0) | 60 (5.3) | ||
| 34-49 | 195 (14.7) | 105475 (14.3) | 205 (18.1) | ||
| 50-64 | 625 (47.2) | 213325 (29.0) | 435 (38.3) | ||
| 65-79 | 375 (28.3) | 242015 (32.9) | 350 (30.8) | ||
| ≥ 80 | 100 (7.5) | 139010 (18.9) | 85 (7.5) | ||
| Gender | < 0.001 | 0.31 | |||
| Male | 930 (70.2) | 380300 (51.6) | 775 (68.3) | ||
| Female | 395 (29.8) | 356385 (48.4) | 360 (31.7) | ||
| Race | < 0.001 | < 0.001 | |||
| White | 580 (43.8) | 388810 (52.8) | 515 (45.4) | ||
| Black | 145 (10.9) | 129635 (17.6) | 180 (15.9) | ||
| Hispanic | 440 (33.2) | 156285 (21.2) | 295 (26.0) | ||
| Asian | 25 (1.9) | 25065 (3.4) | 35 (3.1) | ||
| Native American | 65 (4.9) | 6500 (0.9) | 55 (4.8) | ||
| Other | 70 (5.3) | 30390 (4.1) | 55 (4.8) | ||
| Elixhauser Comorbidity Index score | < 0.001 | < 0.001 | |||
| 0 | 0 (0.0) | 44530 (6.0) | 25 (2.2) | ||
| 1 | 0 (0.0) | 98560 (13.4) | 70 (6.2) | ||
| 2 | 15 (1.1) | 141150 (19.2) | 1040 (91.6) | ||
| ≥ 3 | 1310 (98.9) | 452445 (61.4) | 25 (2.2) | ||
| Region of hospital | < 0.001 | 0.008 | |||
| Northeast | 280 (21.1) | 133450 (18.1) | 245 (21.6) | ||
| Midwest | 250 (18.9) | 168480 (22.9) | 275 (24.2) | ||
| South | 430 (32.5) | 309115 (42.0) | 335 (29.5) | ||
| West | 365 (27.5) | 125640 (17.1) | 280 (24.7) | ||
| Location/teaching status of hospital | < 0.001 | 0.009 | |||
| Rural | 65 (4.9) | 88910 (12.1) | 75 (6.6) | ||
| Urban nonteaching | 180 (13.6) | 147560 (20.0) | 115 (10.1) | ||
| Urban teaching | 1080 (81.5) | 500215 (67.9) | 945 (83.3) | ||
| Primary payer | < 0.001 | 0.030 | |||
| Medicare | 570 (43.0) | 369155 (50.1) | 455 (40.1) | ||
| Medicaid | 420 (31.7) | 93610 (12.7) | 385 (33.9) | ||
| Private | 250 (18.9) | 243440 (33.0) | 245 (21.6) | ||
| Other | 85 (6.4) | 30480 (4.1) | 50 (4.4) | ||
| Median household income national quartile for patient ZIP code | 0.074 | 0.31 | |||
| 1st (0-25th) | 480 (36.2) | 249205 (33.8) | 445 (39.2) | ||
| 2nd (26th-50th) | 335 (25.3) | 203325 (27.6) | 280 (24.7) | ||
| 3rd (51st-75th) | 310 (23.4) | 163695 (22.2) | 235 (20.7) | ||
| 4th (76th-100th) | 200 (15.1) | 120460 (16.4) | 175 (15.4) | ||
| Disposition of patient | < 0.001 | 0.024 | |||
| Discharged to home or self-care (routine discharge) | 685 (51.7) | 445390 (60.5) | 555 (48.9) | ||
| Transfer to short-term hospital | 40 (3.0) | 21910 (3.0) | 20 (1.8) | ||
| Transfer other: Skilled nursing facility, intermediate care facility, or another type of facility | 235 (17.7) | 98350 (13.4) | 205 (18.1) | ||
| Home health care | 120 (9.1) | 99700 (13.5) | 145 (12.8) | ||
| Against medical advice | 50 (3.8) | 7535 (1.0) | 40 (3.5) | ||
| Died during hospitalization | 195 (14.7) | 63625 (8.6) | 170 (15.0) | ||
| Day of admission | 0.43 | 0.27 | |||
| Weekday | 990 (74.7) | 543410 (73.8) | 870 (76.7) | ||
| Weekend | 335 (25.3) | 193275 (26.2) | 265 (23.3) | ||
| Mechanical ventilation | 130 (9.8) | 43865 (6.0) | < 0.001 | 140 (12.3) | 0.046 |
| ICU admission | 140 (10.6) | 43120 (5.9) | < 0.001 | 120 (10.6) | 1.00 |
| Vasopressor requirement | 30 (2.3) | 9345 (1.3) | < 0.001 | 30 (2.6) | 0.54 |
| Age in years at admission, median (IQR) | 60.0 (54.0, 67.0) | 65.0 (53.0, 77.0) | < 0.001 | 61.0 (51.0, 72.0) | 0.65 |
| Length of stay in days, median (IQR) | 5.0 (3.0, 9.0) | 5.0 (3.0, 8.0) | 0.016 | 6.0 (3.0, 11.0) | 0.1 |
| Total hospital charges in USD, median (IQR) | 44739.0 (24963.0, 80405.0) | 39061.0 (22215.0, 71177.0) | < 0.001 | 49862.0 (25884.0, 90286.0) | 0.098 |
There was an increased prevalence of septic shock (5.7% vs 4.1%), ventricular fibrillation/ventricular flutter (0.4% vs 0%), atrial fibrillation (13.2% vs 8.8%), atrial flutter (8.7% vs 4.4%), first-degree atrioventricular nodal block (0.8% vs 0%), upper extremity venous thromboembolism (VTE) (1.5% vs 0%), variceal bleeding (3.8% vs 0%), and pulmonary hyper
| Variables | Alcoholic cirrhosis | No alcoholic cirrhosis (before matching) | P value | No alcoholic cirrhosis (after matching) | P value |
| n | 1325 | 736685 | 1135 | ||
| Asthma exacerbation | 5 (0.4) | 13170 (1.8) | < 0.001 | 5 (0.4) | 0.81 |
| ARDS | 80 (6.0) | 31970 (4.3) | 0.002 | 100 (8.8) | 0.008 |
| Type I respiratory failure | 545 (41.1) | 379975 (51.6) | < 0.001 | 475 (41.9) | 0.72 |
| Type II respiratory failure | 15 (1.1) | 7640 (1.0) | 0.73 | 15 (1.3) | 0.67 |
| Bacterial pneumonia | 10 (0.8) | 6005 (0.8) | 0.81 | 10 (0.9) | 0.73 |
| Klebsiella pneumonia | 0 (0.0) | 1425 (0.2) | 0.11 | 0 (0.0) | - |
| Streptococcus pneumonia | 0 (0.0) | 805 (0.1) | 0.23 | 0 (0.0) | - |
| Staphylococcus pneumonia | 5 (0.4) | 3850 (0.5) | 0.46 | 10 (0.9) | 0.11 |
| Hemophilus pneumonia | 5 (0.4) | 300 (< 1) | < 0.001 | 0 (0.0) | - |
| Anosmia | 0 (0.0) | 1240 (0.2) | 0.14 | 0 (0.0) | - |
| Hemorrhagic CVA | 0 (0.0) | 615 (0.1) | 0.29 | 0 (0.0) | - |
| Ischemic CVA | 0 (0.0) | 475 (0.1) | 0.36 | 0 (0.0) | - |
| Dysgeusia | 0 (0.0) | 695 (0.1) | 0.26 | 0 (0.0) | - |
| Diarrhea | 80 (6.0) | 44110 (6.0) | 0.94 | 65 (5.7) | 0.74 |
| Septic shock | 75 (5.7) | 21780 (3.0) | < 0.001 | 47 (4.1) | 0.007 |
| SVT | 30 (2.3) | 9375 (1.3) | 0.001 | 25 (2.2) | 0.92 |
| VT | 20 (1.5) | 10065 (1.4) | 0.65 | 40 (3.5) | 0.001 |
| Vfib/Vflutter | 5 (0.4) | 1315 (0.2) | 0.087 | 0 (0.0) | 0.038 |
| Afib | 175 (13.2) | 77340 (10.5) | 0.001 | 100 (8.8) | < 0.001 |
| Aflutter | 115 (8.7) | 47410 (6.4) | < 0.001 | 50 (4.4) | < 0.001 |
| First-degree AV nodal block | 10 (0.8) | 3250 (0.4) | 0.086 | 0 (0.0) | 0.003 |
| Second-degree AV nodal block | 0 (0.0) | 1515 (0.2) | 0.098 | 0 (0.0) | - |
| Complete AV nodal block | 0 (0.0) | 1375 (0.2) | 0.12 | 10 (0.9) | < 0.001 |
| ECMO | 0 (0.0) | 120 (< 1) | 0.64 | 0 (0.0) | - |
| Total acute VTE | 50 (3.8) | 26120 (3.5) | 0.65 | 75 (6.6) | 0.001 |
| Portal venous thrombosis | 5 (0.4) | 190 (< 1) | < 0.001 | 15 (1.3) | 0.009 |
| Budd Chiari | 0 (0.0) | 35 (< 1) | 0.80 | 0 (0.0) | - |
| Upper extremity VTE | 20 (1.5) | 1960 (0.3) | < 0.001 | 0 (0.0) | < 0.001 |
| Lower extremity VTE | 10 (0.8) | 8050 (1.1) | 0.24 | 10 (0.9) | 0.73 |
| Other VTE | 10 (0.8) | 1285 (0.2) | < 0.001 | 5 (0.4) | 0.32 |
| Pulmonary embolism | 20 (1.5) | 18055 (2.5) | 0.027 | 50 (4.4) | < 0.001 |
| Variceal bleeding | 50 (3.8) | 100 (< 1) | < 0.001 | 0 (0.0) | < 0.001 |
| Hepatorenal syndrome | 5 (0.4) | 140 (< 1) | < 0.001 | 15 (1.3) | 0.009 |
| Hyponatremia | 325 (24.5) | 123080 (16.7) | < 0.001 | 285 (25.1) | 0.74 |
| Pulmonary hypertension | 280 (21.1) | 1210 (0.2) | < 0.001 | 75 (6.6) | < 0.001 |
| Spontaneous bacterial peritonitis | 15 (1.1) | 35 (< 1) | < 0.001 | 5 (0.4) | 0.057 |
| Acute liver failure | 4 (0.34) | 303 (0.45) | < 0.001 | 4 (0.30) | 0.82 |
| New HD | 2 (0.15) | 750 (0.11) | < 0.001 | 0 (0) | 0.1 |
On multivariate regression, significant predictors of mortality for the matched cohort included cardiac arrhythmias (OR = 2.34, 95%CI: 1.38-3.97, P = 0.002), coagulopathy (OR = 1.87, 95%CI: 1.28-2.73, P = 0.001), protein-calorie malnutri
| Variables | Odds ratios | P value |
| Alcohol-associated cirrhosis | 0.82 (0.64-1.05) | 0.12 |
| Cardiac arrhythmias | 2.34 (1.38-3.97) | 0.002 |
| Coagulopathy | 1.87 (1.28-2.73) | 0.001 |
| Protein-calorie malnutrition | 5.96 (3.67-9.68) | < 0.001 |
| Fluid and electrolyte disorders | 1.56 (1.05-2.32) | 0.027 |
| Septic shock | 18.77 (10.02-35.13) | < 0.001 |
| Atrial fibrillation | 2.01 (1.11-3.63) | 0.020 |
| Spontaneous bacterial peritonitis | 4.28 (0.91-20.1) | 0.065 |
| Upper extremity venous thromboembolism | 11.38 (3.65-35.46) | < 0.001 |
| Increasing age | 1.06 (1.04-1.07) | < 0.001 |
This national population-based study evaluated hospitalized COVID-19 patients with and without AC according to age, gender, and race to identify high-risk individuals. Our findings indicate that AC does not significantly increase mortality among patients hospitalized with COVID-19. However, it is associated with a higher prevalence of inpatient complications, particularly in certain demographic groups and people with higher ECI scores. Interestingly, despite these complications, there was no significant difference in the need for ICU-level care, length of stay, or hospital charges be
Alcohol consumption increased during the COVID-19 pandemic due to isolation and social distancing protocols[23]. A study conducted in the United States analyzed changes in adult alcohol consumption during the pandemic[24]. It repor
In our analysis, the mortality rates were comparable in both the AC and non-AC cohorts. One potential explanation could be that during the COVID-19 pandemic, only the most critically ill cirrhotic patients were admitted, rendering the presence of infection negligible in impacting the clinical outcome. Moreover, comorbidities among AC patients with higher ECI scores could affect the mortality rate. This might have resulted in similar ICU admission rates in both AC and non-AC cohorts. Intriguingly, our data showed that more non-AC patients received mechanical ventilation than AC patients. This is noteworthy because it may indicate an attempt to avoid mechanical ventilation, which is commonly regarded as a predictor of death in cirrhosis[10]. Shalimar et al[27] showed in their study that the need for mechanical ven
Cirrhosis-associated immune dysfunction is characterized by systemic inflammation and impaired immunocompetence[28]. The dysregulated complement factors, immunoglobulins, and acute-phase proteins may correlate with cirr
Patients with cirrhosis often develop portal hypertension. It frequently leads to upregulation of angiotensin-converting enzyme 2 (ACE-2) to counteract this major complication of cirrhosis[32]. This makes patients with AC more susceptible to COVID-19 infection, as ACE-2 is the critical functional receptor for SARS-CoV-2[33,34]. Therefore, it could possibly be associated with inpatient complications and a poor prognosis. During the pandemic, hospital stays for patients with cirrhosis were shorter, and more of these patients were discharged to go home compared to the pre-COVID era. Similarly, we observed a higher proportion of AC patients discharged home than non-AC patients in our study. This trend reflects the drive to conserve inpatient resources and promote home isolation unless the patient requires urgent medical treatment. However, it might have increased the risk of early post-hospital discharge mortality in patients with decompensated cirrhosis during the COVID-19 period[35]. A United States national cohort study using data from the Veterans Health Administration reported a decrease in hospitalizations of cirrhotic patients during the pandemic from January to April 2020[36]. Contrarily, in line with the increase in alcohol consumption during the early phase of the pandemic, we might foresee a rise in long-term morbidity and mortality related to alcohol-associated liver disease[37]. Therefore, AC hospitalizations may increase in the future, requiring high-level hepatology care and follow-up.
In patients with cirrhosis, the risk of developing VTE is significantly increased due to the reduced ability to synthesize anticoagulation factors[38]. In line with these findings, we also noted a higher prevalence of VTE in the AC cohort. In addition, the presence of VTE was identified as a mortality predictor. Therefore, cirrhotic patients should undergo a case-by-case consideration of thromboprophylaxis for deep vein thrombosis[39]. Another significant variable observed in our data was the higher prevalence of atrial fibrillation among the AC cohort. A nationwide study conducted in Korea demonstrated a 46% increased risk of developing atrial fibrillation in cirrhotic patients compared to the non-cirrhotic control group[40]. Furthermore, with COVID-19 in these patients, there is a higher chance of observing electrocardiographic abnormalities. In a systematic review, a fourfold higher risk of death was reported among COVID-19 patients with atrial fibrillation[41]. This finding overlaps with our data, where atrial fibrillation was also identified as a mortality predictor. Upon multivariate analysis, we also identified cardiac arrhythmias and coagulopathy as mortality predictors for the AC cohort. Therefore, cirrhotic patients must be carefully monitored before being discharged home to prevent such serious complications. Notably, the clinical management of cirrhotics with COVID-19 is complicated because most pharmacological agents are metabolized by cytochrome P450 monooxygenases in the liver[42]. Therefore, patients with COVID-19 may have a higher risk of developing hepatotoxicity in the presence of CLD due to drug-drug interactions[42]. This might have contributed to the overall ICU admission rate reported in the AC cohort in our study.
Recent research revealed several associations between SARS-CoV-2 infection and medical conditions that may lead to liver dysfunction[43]. Moreover, studies have demonstrated that there is an increased risk of decompensation and mor
Our retrospective study is one of the largest to evaluate the specific clinical impact of AC on COVID-19 hospitalizations. We analyzed the patient outcomes based on comorbidities, mechanical ventilation, ICU admission, and mortality predictors. Given the critical nature of the disease association, a multidisciplinary approach is required to manage hospitalized COVID-19 patients with AC. This study will provide invaluable information with regard to identifying high-risk patients and monitoring the factors associated with the rising prevalence of AC. One of the major strengths of this study is the detailed comparison between COVID-19 patients with and without AC. This allows for a better understanding of the variables associated with mortality among the two cohorts.
We acknowledge certain limitations to our study. The ICD-10 coding system may present inaccurate data when utilizing a large database like NIS, which may potentially skew the analysis. In addition, the NIS data might only be representative of those hospitals participating in the Healthcare Cost and Utilization Project[45]. The information regarding severity of the disease or treatment is not provided in the NIS database. The COVID-19 waves varied from state to state within the United States. Hence, some areas were more heavily impacted than others. Upon analysis of geographical regions in our data, urban teaching hospitals had a greater number of patients with AC. A nationwide study of hospitalized patients in the United States identified urban hospitals as being associated with a greater risk of infection in cirrhotic patients[46]. These infections included sepsis, pneumonia, and spontaneous bacterial peritonitis[46]. Therefore, the mortality rate might be higher, especially in urban hospitals. Moreover, it can be difficult to diagnose chronic non-AC as COVID-19 may lead to abnormalities in liver function testing in hospitalized patients[47,48].
This study has thoroughly analyzed the influence of AC on COVID-19 hospitalizations. Our results indicate that the presence of AC does not significantly impact mortality in COVID-19 patients, warranting further evaluation in a larger cohort. Interestingly, despite the higher ECI scores among the AC cohort, the length of stay and ICU admission rates were comparatively similar across the non-AC cohort. Advanced age was found to be a predictor of death in patients with AC, along with other variables like cardiac arrhythmias, coagulopathy, protein-calorie malnutrition, fluid and electrolyte disorders, septic shock, and upper extremity VTE. Due to the multifactorial nature of hepatic injury in COVID-19, further research will be required to evaluate effective pharmacological treatments in COVID-19 patients with AC. Despite the fact that COVID-19 transmission has slowed down, identifying high-risk groups early on is important. It will make it more convenient for hospitalized COVID-19 patients with AC to receive a tailored medical treatment that could improve their prognosis.
Patients with chronic liver disease (CLD) may be at risk of adverse outcomes following coronavirus disease 2019 (COVID-19). Initial findings from Wuhan indicated potential liver injury in COVID-19 patients. With the growing role of obesity and alcohol, understanding the specific implications of alcohol-associated cirrhosis (AC) for patients with COVID-19 is of paramount clinical importance for prognostication and appropriate therapeutic strategy.
Cirrhosis is typically associated with immune system impairments, which might hinder the ability of patients to combat COVID-19. While several studies have indicated an increased risk of COVID-19 severity in cirrhotic patients, the specific impact of AC on inpatient outcomes remains incompletely defined.
This study primarily aims to assess the impact of AC on inpatient outcomes and mortality rates in patients hospitalized with COVID-19 compared to those without AC. Furthermore, we intend to identify predictors of mortality within the COVID-19 patient cohort with AC.
A retrospective cohort study was conducted using the National Inpatient Sample database 2020, focusing on hospitalizations with a primary diagnosis of COVID-19. Two cohorts were established based on the presence or absence of AC. Pro
Of the 738010 patients hospitalized with COVID-19 that fulfilled the selection criteria, 1325 with AC were compared to 1135 without AC. It was found that AC did not significantly increase mortality in COVID-19 patients. However, it was linked to a higher prevalence of inpatient complications, especially in certain demographics and those with higher Elix
AC does not increase mortality rates in the context of COVID-19 hospitalizations. However, it is associated with a heigh
This research provides valuable insights into the implications of AC for COVID-19 patients. It prompts clinicians to conduct future research and delve deeper into understanding the exact mechanisms leading to these complications in COVID-19 patients with AC. Exploring preventive measures or pharmacological treatment adjustments for this vulne
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: American College of Gastroenterology; American Gastroenterological Association.
Specialty type: Virology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Muro M, Spain; Nasa P, United Arab Emirates S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | World Health Organization. WHO Coronavirus (COVID-19) Dashboard. [cited 21 June 2023]. Available from: https://www.covid19.who.int. |
| 2. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12977] [Article Influence: 2595.4] [Reference Citation Analysis (1)] |
| 3. | Qi RB, Wu ZH. Association between COVID-19 and chronic liver disease: Mechanism, diagnosis, damage, and treatment. World J Virol. 2023;12:22-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Gao X, Lv F, He X, Zhao Y, Liu Y, Zu J, Henry L, Wang J, Yeo YH, Ji F, Nguyen MH. Impact of the COVID-19 pandemic on liver disease-related mortality rates in the United States. J Hepatol. 2023;78:16-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 5. | Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2650-2666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 805] [Cited by in RCA: 720] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 6. | Huang DQ, Terrault NA, Tacke F, Gluud LL, Arrese M, Bugianesi E, Loomba R. Global epidemiology of cirrhosis - aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol. 2023;20:388-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 377] [Article Influence: 188.5] [Reference Citation Analysis (0)] |
| 7. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 384] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 8. | Albillos A, Martin-Mateos R, Van der Merwe S, Wiest R, Jalan R, Álvarez-Mon M. Cirrhosis-associated immune dysfunction. Nat Rev Gastroenterol Hepatol. 2022;19:112-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 230] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 9. | Gaspar R, Castelo Branco C, Macedo G. Liver and COVID-19: From care of patients with liver diseases to liver injury. World J Hepatol. 2021;13:1367-1377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 10. | Nagarajan R, Krishnamoorthy Y, Rajaa S, Hariharan VS. COVID-19 Severity and Mortality Among Chronic Liver Disease Patients: A Systematic Review and Meta-Analysis. Prev Chronic Dis. 2022;19:E53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 11. | Kapuria D, Gangu K, Chourasia P, Boba A, Nguyen A, Ryu M, Peicher M, Flores M, Chela HK, Daglilar ES, Sheikh AB, Shekhar R. COVID-19 Alcoholic Cirrhosis and Non-Alcoholic Steatohepatitis Cirrhosis Outcomes among Hospitalized Patients in the United States: Insight from National Inpatient Sample Database. Trop Med Infect Dis. 2022;7:421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, Shaw J, Pearson M, Chew M, Fagan A, de la Rosa Rodriguez R, Worthington J, Olofson A, Weir V, Trisolini C, Dwyer S, Reddy KR. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70:531-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 176] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 13. | Ge J, Pletcher MJ, Lai JC; N3C Consortium. Outcomes of SARS-CoV-2 Infection in Patients With Chronic Liver Disease and Cirrhosis: A National COVID Cohort Collaborative Study. Gastroenterology. 2021;161:1487-1501.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 14. | Moon AM, Webb GJ, Aloman C, Armstrong MJ, Cargill T, Dhanasekaran R, Genescà J, Gill US, James TW, Jones PD, Marshall A, Mells G, Perumalswami PV, Qi X, Su F, Ufere NN, Barnes E, Barritt AS, Marjot T. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J Hepatol. 2020;73:705-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 15. | Grgurevic I, Lucijanić M, Pastrovic F, Barisic Jaman M, Tjesic Drinkovic I, Zelenika M, Milosevic M, Medic B, Kardum D, Bokun T, Luksic I, Piskac Zivkovic N, Keres T, Grabovac V, Persec J, Barsic B. Short-term outcomes of patients with chronic liver disease hospitalised with COVID-19. Intern Med J. 2022;52:1891-1899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Yoo HW, Jin HY, Yon DK, Effenberger M, Shin YH, Kim SY, Yang JM, Kim MS, Koyanagi A, Jacob L, Smith L, Yoo IK, Shin JI, Lee SW. Non-alcoholic Fatty Liver Disease and COVID-19 Susceptibility and Outcomes: a Korean Nationwide Cohort. J Korean Med Sci. 2021;36:e291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 17. | Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, Perumalswami P, Roytman M, Li M, Vogel AS, Catana AM, Wegermann K, Carr RM, Aloman C, Chen VL, Rabiee A, Sadowski B, Nguyen V, Dunn W, Chavin KD, Zhou K, Lizaola-Mayo B, Moghe A, Debes J, Lee TH, Branch AD, Viveiros K, Chan W, Chascsa DM, Kwo P, Dhanasekaran R. Predictors of Outcomes of COVID-19 in Patients With Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2021;19:1469-1479.e19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 18. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3667] [Cited by in RCA: 6890] [Article Influence: 626.4] [Reference Citation Analysis (0)] |
| 19. | Nationwide Inpatient Sample. Rockville, MD: Agency for Healthcare Research and Quality. [cited 25 June 2023]. Available from: http://www.hcup-us.ahrq.gov/nisoverview.jsp. |
| 20. | Clausen S, Stahlman S, Cost A. Early use of ICD-10-CM code "U07.1, COVID-19" to identify 2019 novel coronavirus cases in Military Health System administrative data. MSMR. 2020;27:55-59. [PubMed] |
| 21. | Patel P, Ali H, Inayat F, Pamarthy R, Giammarino A, Ilyas F, Smith-Martinez LA, Satapathy SK. Racial and gender-based disparities and trends in common psychiatric conditions in liver cirrhosis hospitalizations: A ten-year United States study. World J Hepatol. 2023;15:289-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 22. | Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083-3107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3915] [Cited by in RCA: 4440] [Article Influence: 277.5] [Reference Citation Analysis (1)] |
| 23. | Huang DQ, Mathurin P, Cortez-Pinto H, Loomba R. Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol. 2023;20:37-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 265] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 24. | Pollard MS, Tucker JS, Green HD Jr. Changes in Adult Alcohol Use and Consequences During the COVID-19 Pandemic in the US. JAMA Netw Open. 2020;3:e2022942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 586] [Article Influence: 117.2] [Reference Citation Analysis (0)] |
| 25. | Kim IH, Kisseleva T, Brenner DA. Aging and liver disease. Curr Opin Gastroenterol. 2015;31:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 308] [Article Influence: 30.8] [Reference Citation Analysis (2)] |
| 26. | Krishnan A, Prichett L, Liu Y, Ting PS, Alqahtani SA, Kim AK, Ma M, Hamilton JP, Woreta TA, Chen PH. Risk of Severe Illness and Risk Factors of Outcomes of COVID-19 in Hospitalized Patients with Chronic Liver Disease in a Major U. S. Hospital Network. Can J Gastroenterol Hepatol. 2022;2022:8407990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Shalimar AE, Elhence A, Vaishnav M, Kumar R, Pathak P, Soni KD, Aggarwal R, Soneja M, Jorwal P, Kumar A, Khanna P, Singh AK, Biswas A, Nischal N, Dar L, Choudhary A, Rangarajan K, Mohan A, Acharya P, Nayak B, Gunjan D, Saraya A, Mahapatra S, Makharia G, Trikha A, Garg P. Poor outcomes in patients with cirrhosis and Corona Virus Disease-19. Indian J Gastroenterol. 2020;39:285-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Simbrunner B, Hartl L, Jachs M, Bauer DJM, Scheiner B, Hofer BS, Stättermayer AF, Marculescu R, Trauner M, Mandorfer M, Reiberger T. Dysregulated biomarkers of innate and adaptive immunity predict infections and disease progression in cirrhosis. JHEP Rep. 2023;5:100712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 29. | Bolarín JM, Pérez-Cárceles MD, Hernández Del Rincón JP, Luna A, Minguela A, Muro M, Legaz I. Causes of Death and Survival in Alcoholic Cirrhosis Patients Undergoing Liver Transplantation: Influence of the Patient's Clinical Variables and Transplant Outcome Complications. Diagnostics (Basel). 2021;11:968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Guerra Veloz MF, Cordero Ruiz P, Ríos-Villegas MJ, Del Pino Bellido P, Bravo-Ferrer J, Galvés Cordero R, Cadena Herrera ML, Vías Parrado C, Bellido Muñoz F, Vega Rodríguez F, Caunedo Álvarez Á, Rodríguez-Baño J, Carmona Soria I. Liver manifestations in COVID-19 and the influence of pre-existing liver disease in the course of the infection. Rev Esp Enferm Dig. 2021;113:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Schütte A, Ciesek S, Wedemeyer H, Lange CM. Influenza virus infection as precipitating event of acute-on-chronic liver failure. J Hepatol. 2019;70:797-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 32. | Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 281] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 33. | Gao F, Zheng KI, Fan YC, Targher G, Byrne CD, Zheng MH. ACE2: A Linkage for the Interplay Between COVID-19 and Decompensated Cirrhosis. Am J Gastroenterol. 2020;115:1544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Hanafy AS, Abd-Elsalam S. Challenges in COVID-19 drug treatment in patients with advanced liver diseases: A hepatology perspective. World J Gastroenterol. 2020;26:7272-7286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Gananandan K, Phillips A, Chikhlia A, Old H, Sim SJY, Thakur N, Hussain I, Kazankov K, Mookerjee RP. Negative impact of the pandemic on hospital admissions, morbidity and early mortality for acute cirrhosis decompensation. BMJ Open Gastroenterol. 2023;10:e001071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 36. | Mahmud N, Hubbard RA, Kaplan DE, Serper M. Declining Cirrhosis Hospitalizations in the Wake of the COVID-19 Pandemic: A National Cohort Study. Gastroenterology. 2020;159:1134-1136.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 37. | Julien J, Ayer T, Tapper EB, Barbosa C, Dowd WN, Chhatwal J. Effect of increased alcohol consumption during COVID-19 pandemic on alcohol-associated liver disease: A modeling study. Hepatology. 2022;75:1480-1490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 38. | Wu H, Nguyen GC. Liver cirrhosis is associated with venous thromboembolism among hospitalized patients in a nationwide US study. Clin Gastroenterol Hepatol. 2010;8:800-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 39. | Roberts LN, Hernandez-Gea V, Magnusson M, Stanworth S, Thachil J, Tripodi A, Lisman T. Thromboprophylaxis for venous thromboembolism prevention in hospitalized patients with cirrhosis: Guidance from the SSC of the ISTH. J Thromb Haemost. 2022;20:2237-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 40. | Lee H, Choi EK, Rhee TM, Lee SR, Lim WH, Kang SH, Han KD, Cha MJ, Oh S. Cirrhosis is a risk factor for atrial fibrillation: A nationwide, population-based study. Liver Int. 2017;37:1660-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 41. | Romiti GF, Corica B, Lip GYH, Proietti M. Prevalence and Impact of Atrial Fibrillation in Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. J Clin Med. 2021;10:2490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 42. | Wang G, Xiao B, Deng J, Gong L, Li Y, Li J, Zhong Y. The Role of Cytochrome P450 Enzymes in COVID-19 Pathogenesis and Therapy. Front Pharmacol. 2022;13:791922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 43. | Nasa P, Juneja D, Jain R, Nasa R. COVID-19 and hemolysis, elevated liver enzymes and thrombocytopenia syndrome in pregnant women - association or causation? World J Virol. 2022;11:310-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 44. | Nasa P, Alexander G. COVID-19 and the liver: What do we know so far? World J Hepatol. 2021;13:522-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Healthcare Cost and Utilization Project. Rockville, MD: Agency for Healthcare Research and Quality. [cited 25 June 2023]. Available from: https://www.hcup-us.ahrq.gov. |
| 46. | Singal AK, Salameh H, Kamath PS. Prevalence and in-hospital mortality trends of infections among patients with cirrhosis: a nationwide study of hospitalised patients in the United States. Aliment Pharmacol Ther. 2014;40:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 47. | Saleem S, Ishtiaq R, Inayat F, Aziz M, Bleibel W. Gastrointestinal and Liver Manifestations in COVID-19 Population. Middle East J Dig Dis. 2021;13:281-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 48. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |