Published online Jun 25, 2023. doi: 10.5501/wjv.v12.i3.193
Peer-review started: December 29, 2022
First decision: April 13, 2023
Revised: April 28, 2023
Accepted: May 24, 2023
Article in press: May 24, 2023
Published online: June 25, 2023
Processing time: 174 Days and 6.2 Hours
The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been a major challenge to be faced in recent years. While adults suffered the highest morbidity and mortality rates of coronavirus disease 2019, children were thought to be exclusively asymptomatic or to present with mild conditions. However, around April 2020, there was an outbreak of a new clinical syndrome related to SARS-CoV-2 in children - multisystemic inflammatory syndrome in children (MIS-C) - which comprises a severe and uncon-trolled hyperinflammatory response with multiorgan involvement. The Centers for Disease Control and Prevention considers a suspected case of MIS-C an individual aged < 21 years presenting with fever, high inflammatory markers levels, and evidence of clinically severe illness, with multisystem (> 2) organ involvement, no alternative plausible diagnoses, and positive for recent SARS-CoV-2 infection. Despite its severity, there are no definitive disease management guidelines for this condition. Conversely, the complex pathogenesis of MIS-C is still not completely understood, although it seems to rely upon immune dysregulation. Hence, in this study, we aim to bring together current evidence regarding the pathogenic mechanisms of MIS-C, clinical picture and management, in order to provide insights for clinical practice and implications for future research directions.
Core Tip: Multisystem inflammatory syndrome in children (MIS-C) comprises a severe and out-of-control inflammatory response with multiorgan dysfunction following severe acute respiratory syndrome coronavirus 2 infection. Despite its severity, there are no definitive disease management guidelines for this condition. Conversely, the complex pathogenesis of MIS-C is still not completely understood, although it seems to rely upon immune dysregulation. Hence, in this study, we aim to bring together current evidence regarding the pathogenic mechanisms of MIS-C, clinical picture and management, in order to provide insights for clinical practice and implications for future research directions.
- Citation: Silva Luz M, Lemos FFB, Rocha Pinheiro SL, Marques HS, de Oliveira Silva LG, Calmon MS, da Costa Evangelista K, Freire de Melo F. Pediatric multisystem inflammatory syndrome associated with COVID-19: Insights in pathogenesis and clinical management. World J Virol 2023; 12(3): 193-203
- URL: https://www.wjgnet.com/2220-3249/full/v12/i3/193.htm
- DOI: https://dx.doi.org/10.5501/wjv.v12.i3.193
Coronavirus disease 2019 (COVID-19) pandemic has been a major challenge to be faced in recent years. With variable clinical manifestations, the disease mainly affects older people with comorbidities, whereas children were not initially considered a risk group for its severe form[1]. However, even though COVID-19 in children is generally milder when compared to adults, it was noticed that a small part of infected children are subject to a post-infectious severe condition presenting with multiorgan dysfunction and systemic inflammation, thus called multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19[2,3]. Several aspects of this syndrome are still unclear, given its recent discovery. Initially, the heterogeneous clinical picture of MIS-C was attributed to conditions such as Kawasaki disease (KD) or toxic shock syndrome, due to the existing similarities. However, as COVID-19 pandemic progressed, there was an urge to recognize MIS-C as a new clinical condition that needs to be thoroughly understood[4].
Epidemiological data, although not very robust, suggest that the incidence of MIS-C is higher in African, Afro-Caribbean and Hispanic countries, while the incidence in East Asian countries is lower[5]. Furthermore, factors such as age, viral load and chronic comorbidities are considered risk factors for severe manifestations of COVID-19 and therefore may be associated with the development of MIS-C[6-8].
MIS-C usually develops weeks after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and, even though its pathophysiological mechanisms are still unclear, studies point to an exacerbated immune response that leads to a state of hyperinflammation, i.e., an imbalance between host pro-inflammatory and anti-inflammatory mechanisms with the potential to affect multiple organs[9]. Regarding its presentation, MIS-C associated with COVID-19 has a broad clinical spectrum in which fever is the main symptom. In addition, patients may also manifest gastrointestinal disorders, such as diarrhea and vomiting, abdominal pain, shock and/or hypotension, respiratory symptoms such as cough and dyspnea, as well as cardiac and neurological symptoms[10].
There are differences between the main definitions of MIS-C made by the Royal College of Pediatrics and Child Health (RCPCH), Center for Disease Control and Prevention (CDC) and the World Health Organization (WHO). However, all three institutions agree on the presence of fever, laboratory findings indicative of active inflammation, multisystem organ involvement without a plausible underlying diagnosis, as well as proven infection by COVID-19 or recent exposure to a case of COVID-19[11]. In this sense, a well-established definition is essential for early diagnosis and exclusion of the main differential diagnoses.
Considering that MIS-C is a serious condition with numerous possibilities of complications and scarce scientific evidence on the subject, standardizing a therapeutic line to be followed has been a challenge[5]. The main guidelines advise that the treatment should be done individually, aiming to control hyperinflammation and recover organic function[12]. Some medications such as intravenous immunoglobulin (IVIG) and other anti-inflammatory and immunosuppressive therapies are considered the mainstay of treatment for MIS-C. In addition, other drugs such as steroids and immunobiologicals and aspirin have also been widely used with reservations[13]. This minireview aims to bring together current evidence regarding the pathogenic mechanisms of MIS-C, clinical picture and management, in order to provide insights for clinical practice and implications for future research directions.
The pathogenesis of MIS-C is a complex event that has not yet been fully elucidated. To date, three hypotheses that may be associated with the development of the syndrome are highlighted: (1) SARS-CoV-2 spike protein superantigenic profile[14,15]; (2) Chronic response to viral antigen exposure[16]; or (3) Production of autoantibodies in response to the viral infection[17]. These may provide a subset for further understanding about the immunopathological features of MIS-C, which, in turn, apparently rely on autoreactivity, cytokine storm, and immune dysregulation[18]. Thus, we hereby discuss, in detail, the main findings regarding the immunological issues associated with MIS-C.
A major factor that maintains the hyperinflammatory state of MIS-C is immune dysregulation[19]. Currently, several manuscripts have demonstrated the occurrence of disorders related to innate and adaptive immunity and to cytokine response[20-22]. In this regard, Huang et al[20] exploit some of these aspects. Apparently, classic monocytes and type 2 dendritic cells were found to be downregulated in MIS-C, while type 1 DCs were greatly activated. Interestingly, some findings also suggest that this syndrome presents with impaired antigen presentation, given the low levels of HLA-DR and CD86 in monocytes and dendritic cells[23]. Altogether, these data support a possible role for antigenic cross-presentation as one of the possible mechanisms of MIS-C, since this process is strongly related to the action of CLEC9A, a major marker of type 1 DCs[20]. Moreover, elevated expression of alarmin-related S100A genes is also documented[19]. These molecules are closely related to the triggering of inflammatory mechanisms in the innate immune response, via Toll-like receptors (TLRs), and play a role in apoptosis and organ damage[24].
As for T cells, Ramaswamy et al[19] describe an increased expression of perforins, granzyme A and H on natural killer cells and CD8+ T cells. These cytotoxic molecules greatly increase tissue damage characteristic of MIS-C. Also, skewed T cell receptor (TCR) repertoire with TRBV11-2 expansion, found in severe MIS-C patients may indicate exposure to a superantigen (SAg) or be related to the presence of autoreactive T cells, while γδ and CD4+CCR7+ T cells activation is highlighted by elevated HLA-DR expression[23]. On the other hand, B-cells show an intriguing pattern in MIS-C, characterized by increased numbers of plasmablasts. However, their role in the pathophysiology of the syndrome requires further investigation[25]. Additionally, MIS-C patients apparently present interferon-gamma (IFN-γ) response dysregulation, which is characterized by exacerbated production of CXCL9 in response to IFN-γ[22]. Of note, excessive production of this chemokine is related to increased disease severity[26].
Emerging evidence has highlighted MIS-C as a state of out-of-control release of cytokines - referred to as “cytokine storm” - and immune cell hyperactivation[27]. In fact, high levels of interleukin-1β (IL-1β), IL-6, IL-8, IL-10, IL-17, and IFN-γ, with raised C-reactive protein (CRP) and ferritin, were reported in the acute phase of MIS-C[23]. Carter et al[23] also demonstrated that MIS-C patients show raised fibrinogen levels, raised D-dimer, and low platelet count, which suggests a possible interplay between cytokine storm/hyperinflammation and pro-coagulant state development. This crosstalk has also been extensively explored in the pathophysiology of severe acute COVID-19[28-30]. Since the up-regulated cytokines IL-1β, IL-6, and IL-8 are critically involved in pro-inflammatory-induced coagulation in other conditions, their role in MIS-C-related hypercoagulable state should be further investigated[31-34].
Except for IL-1β and IL-17 elevations, these results are ratified by Diorio et al[35]. In that study, the authors report that the sum of IL-10 and tumor necrosis factor (TNF)-α levels was hypothesized to differentiate between MIS-C and severe COVID-19 patients [mean (95% confidence interval); severe: 30.06 (9.54-50.6) vs MIS-C: 82.25 (32.5-132.0), P = 0.036]. The significant and paradoxical elevation of IL-10 - a potent anti-inflammatory cytokine with immunoregulatory functions[36] - also differs from the cytokine profile seen in KD[37]. There were also noted increases in markers of endothelial dysfunction in both MIS-C and severe COVID-19 with elevated sC5b-9 concentrations. At last, univariate linear regression modeling revealed that sC5b-9 levels correlated in a statistically significant manner with IL-6, IL-8, and TNF-α[35].
Although Diorio et al[35] demonstrate that sC5b-9 was significantly elevated in patients with severe COVID-19 in comparison with those with minimal COVID-19, the observed elevated levels in MIS-C patients did not reach statistical significance. However, a subsequent study by Syrimi et al[21] revealed that complement protein C9 and C5b-9 were significantly increased in MIS-C patients at the acute stage of the disease. C5b-9 is a marker of the formation of the membrane attack complex, the final common pathway of complement activation[38]. The hyperactivation of complement components including C5a in sera and C5b-9 correlates with the out-of-control release of pro-inflammatory cytokines including TNF-α, IL-1, and IL-6[39]. These authors also demonstrated that MIS-C patients had significantly increased levels of the chemokine’s monocyte chemoattractant protein 1 (MCP-1/CCL2) and interferon gamma-induced protein 10 (IP-10/CXCL10). Higher levels of IL-6, IL-18, and IL-10 were also observed[21]. On the other hand, in contrast with previous findings, no difference was observed in the levels of IL-1β, IL-8 (CXCL8), IL-17A, IFN-α2, IFN-γ, and TNF-α in MIS-C patients in comparison with the healthy donor controls.
Cytokine profiling also identified elevated signatures of inflammation (IL-18 and IL-6), lymphocytic and myeloid chemotaxis and activation (CCL3, CCL4, and CDCP1), and mucosal immune dysregulation (IL-17A, CCL20, and CCL28) in another report[40]. Gruber et al[40] suggested that elevations in unique chemokines (CXCL5, CXCL11, CXCL1, and CXCL6) and cytokines (including IL-17A, CD40, and IL-6) appear to distinguish MIS-C patients from pediatric COVID-19 patients. In another study, IL-6, IL-17A, and CXCL10 were also found to contribute the most to the cytokine storm. In contrast, the main negative contributors were adenosine deaminase, stem cell factor, and TWEAK[17] - a suppressor of production of IFN-γ and IL-12, that attenuates the innate response and its transition to adaptive Th1 immunity[41]. However, despite being raised in MIS-C patients, IL-17A seems to drive Kawasaki- but not MIS-C-associated hyperinflammation[17].
At last, more recent studies reiterate that a robust cytokine release might explain the severity and outcome of MIS-C. Abo-Haded et al[42] recently reported that mild MIS-C patients present higher levels of IFN-α, IFN-γ, IL-6, IL-8, IL-10, and CXCL10 in comparison with the control group. Conversely, there was an extremely significant difference regarding all measured cytokines when comparing both study groups (mild and severe) (P < 0.0001)[42]. Severe MIS-C patients showed higher levels of IFN-α, IL-1β, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and HMGB1 (P < 0.0001), alongside less significant increases (P < 0.05) in IL-8, TNF, and GM-CSF. These findings suggest that the cytokine profile and its serum levels may be determining factors for the clinical outcome of patients with MIS-C.
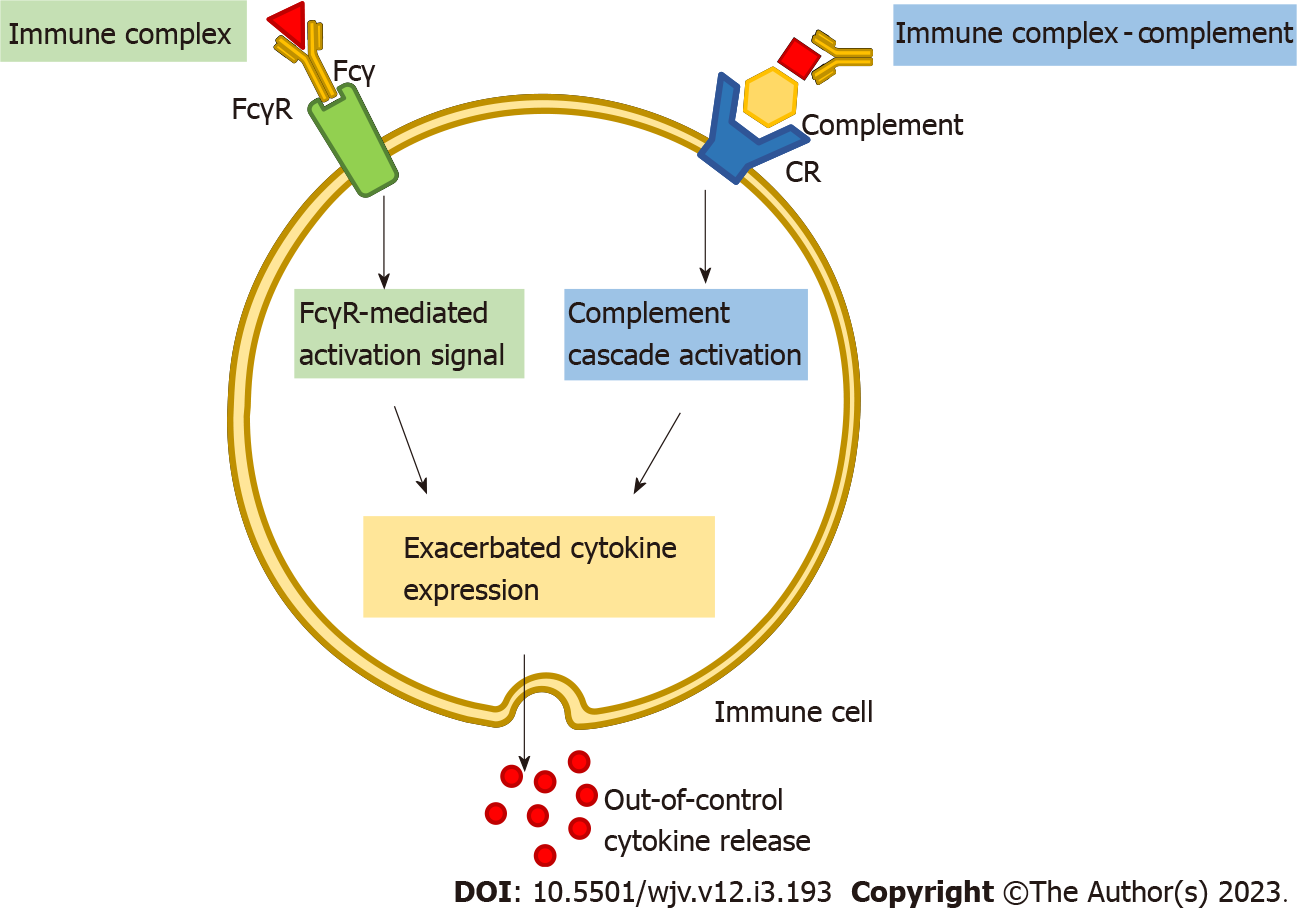
Despite these findings, the molecular features of the MIS-C-related cytokine storm have not been completely understood. One potential explanation for the out-of-control release of cytokines seems to be an antibody-dependent enhancement (ADE) mechanism[43,44]. In ADE - also called immune enhancement - pre-existing non-neutralizing or sub-neutralizing antibodies (nNAbs) bind to virus-derived antigens[43,44]. The resulting immunocomplexes may further interact with the immune cell’s membrane harboring the immunoglobulin G (IgG) Fcγ receptor (FcγR) through the Fc domain of the immunoglobulin, thereby activating the immunoreceptor tyrosine-based activation motifs of these receptors[45-47]. When triggered, downstream signaling pathways induce the release of a variety of cytokines and immune cell recruitment[48,49]. The engagement of complement receptors could also represent a possible ADE-related mechanism. Once recognized by complement receptors, immunocomplexes bonded to complement-derived molecules could also trigger cytokine release and immune activation[49] (Figure 1). On the other hand, ADE may also promote SARS-CoV-2 epitope intake into the host cell cytoplasm[50]. nNAbs-antigen immunocomplexes interaction with FcγR facilitates the internalization of the virus into host cells by endocytosis[48]. At last, upon internalization, endocytic TLRs can then recognize these viral particles and also induce immune cell activation and recruitment[50].
In contrast, other studies have highlighted that MIS-C cytokine storm could also be attributed to a SAg-induced immune response. SAgs are highly potent immunostimulatory molecules that induce T cell activation through binding to specific β chains of TCRs at their variable domain in a complementarity-determining region 3-independent (CDR3-independent) manner[51,52]. This activation requires simultaneous interaction of the SAg with the Vβ domain of the TCR and HLA class II molecules on the surface of an antigen-presenting cell[53]. The specificity of SAg attachment to different TCR Vβ chains results in Vβ skewing, whereby the frequency of T cells responding to SAg exposure exceeds that of conventional peptide antigens[54].
It has been recently demonstrated that an insertion unique to SARS-CoV-2 spike glycoprotein exhibits a SAg-like sequence motif that possesses a high affinity for binding TCR, interacting closely with both the α- and β-chains variable domains’ complementarity-determining regions[14,55]. In this sense, Porritt et al[15] showed that MIS-C patients present a profound expansion of TCRβ variable gene 11-2 (TRBV11-2), with up to 24% of clonal T cell space occupied by TRBV11-2 T cells. The author also found a significant correlation between TRBV11-2 usage and TNF-α, IFN-γ, IL-6, and IL-10 levels[14]. Therefore, it seems that the TRBV11-2 expansion and possible activation may be one of the main features in the MIS-C-related out-of-control cytokine release. These findings were ratified by Moreews et al[56], that observed a specific expansion of activated T cells expressing the Vβ21.3 T cell receptor β chain variable region in both CD4 and CD8 subsets, and this was also associated with the observed cytokine storm. Although multiple cytokine storm-induction mechanisms have been proposed, further research is needed to build a more reliable model of MIS-C-related immunopathophysiology.
The rapid resolution of disease following high-dose IVIG administration has raised the hypothesis that MIS-C could be an autoantibody-mediated condition[57]. IVIG is a usual approach to triggering inhibitory FcγR, thus exerting a multitude of immunomodulatory properties[58]. Upon FcγR engagement, IVIG may thereby preclude Th1 and Th17 differentiation, enhance CD4+FoxP3+ regulatory T cells expansion, and inhibit autoantibody release by B-cells, for example[59-61].
Based on this, Gruber et al[40] tested the hypothesis that SARS-CoV-2 infection leads to a secondary autoreactive humoral response. These authors’ differential autoantibody analysis returned 189 peptide candidates for IgG autoantigens and 108 IgA autoantigens. More interestingly, the majority of these antigens present enrichment in organ systems that play a central role in the pathophysiology of MIS-C. Possible autoantigens include peptides expressed in the gastrointestinal tract (MUC15, TSPAN13, and SH3BP1), the endothelial and cardiac tissue (P2RX4, ECE1, and MMP14), and, notably, in immune cells (CD244, IL-1A, IFNGR2, IL-6R, and LAMP1)[40]. Moreover, the analysis of MIS-C plasma also revealed well-known disease-associated autoantibodies - namely anti-La, and anti-Jo-1[40]. Similarly, Consiglio et al[17] found that 26 Gene Ontology (GO)-terms were enriched in MIS-C samples. Among these, there were autoantigen peptides involved in lymphocyte activation processes, immune cell signalling, and structural proteins in the heart and blood vessels[17]. In turn, Porritt et al[61] identified a number of IgG-target tissue-specific antigens from the gastrointestinal and cardiovascular tracts, skeletal muscle, and brain tissues. On the other hand, Burbelo et al[62] have failed to detect autoantibodies against the majority (16/18) of the most relevant autoantigens raised by previous studies in MIS-C patients who did not receive IVIG. These data have highlighted that the first-line IVIG therapy may be a confounding factor in autoantibody measurements in MIS-C[62]. Therefore, these results suggest that secondary autoreactive humoral response may play a pivotal role in MIS-C pathobiology; however, further studies, taking into account possible confounding factors, are necessary to elucidate this matter.
Regarding the clinical manifestations of MIS-C, we relied on recent meta-analyses to identify the most prevalent symptoms of the syndrome. Overall, fever was the most predominant reported symptom, being present in 82.4% to 100% of total cases and with median duration of approximately 5 d, followed by gastrointestinal symptoms including diarrhea, abdominal pain, and vomiting (82%-85.2%)[10,63-66]. Respiratory symptoms have also been reported (25%-50.3%), such as cough, dyspnea, and sore throat, although not as prevalent as expected, which diverges from the classical COVID-19 manifestations, especially in comparison to adults[63,64]. Thus, a greater vulnerability for children to develop gas-trointestinal symptoms is apparent, which highlights the need to consider the possibility of MIS-C in infants with non-respiratory manifestations along with a hyperinflammatory profile.
In addition, cutaneous manifestations similar to KD are reported, especially, polymorphic maculopapular exanthema (63.7%), and non-purulent conjunctivitis (56%)[65]. Furthermore, neurological and cardiac involvement are also outlined and represent a relevant aggravating factor. In this sense, neurological symptoms are commonly expressed as headaches, in most cases, irritability and lethargy[10,64,66]. On the other hand, cardiocirculatory manifestations are more frequently observed, present in approximately 80% of cases, with a broad spectrum that includes from tachycardia (76.7%), hypotension (77%) and shock (52%-68.1%) to myocarditis (41.4%), aneurysms (10.3%) and mild dilatation of the coronary artery (11.6%)[63,64,66].
Consistent with the syndromic manifestations of MIS-C, the laboratory findings demonstrate an expected hyperinflammatory state. The most important laboratory findings demonstrate increased levels of inflammatory markers, such as CRP, ferritin and IL-6[63,66], along with increased erythrocyte sedimentation rate and procalcitonin[66,67]. Moreover, complete blood count often reveals leukocytosis, with increased neutrophil levels[67]. Lymphocytopenia is also a common finding, along with anemia and normal or reduced platelet count[63,64,66]. Regarding the coagulation panel, D-dimer and fibrinogen showed a considerable increase[64,65,67].
As for markers of myocardial injury, such as troponin, B-type natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP), evidence points to a frequent elevation, being current factors of concern in the management of children with MIS-C[63,64,67]. Troponin levels > 32 pg/mL are considered a major predictor of myocardial involvement in MIS-C[68]. Similarly, elevated levels of NT-proBNP are associated with myocardial dysfunction and severe MIS-C, being considered a useful biomarker for early identification of impaired cardiac function[69].
Currently, MIS-C diagnosis is conducted through criteria established by important health institutions on characteristics such as age, clinical picture, inflammatory markers, and molecular or serological COVID-19 testing[70]. Of note, three distinct case definitions stand out in the diagnosis of MIS-C: That of the RCPCH[71], the WHO[72] and the CDC[73].
Overall, when faced with a suspected case of MIS-C, specific laboratory findings should be measured to assist the diagnosis[74]. In this regard, Gottlieb et al[75] points out the major parameters necessary for the evaluation of these cases, such as: Complete blood cell count; electrolytes; renal and liver function; inflammatory markers (CRP, D-dimer, albumin and coagulation panel); BNP; tests for SARS-CoV-2, such as polymerase chain reaction (PCR) and/or serologies; as well as troponin, ferritin, fibrinogen and procalcitonin.
In this context, the available case definitions serve as a guide for clinical practice, as they synthesize the main findings related to the diagnosis of MIS-C. According to the RCPCH, MIS-C is characterized by persistent fever > 38.5 °C, inflammation and single or multi-organ dysfunction, along with additional features such as abdominal pain, conjunctivitis, lymphadenopathy and rash and exclusion of any other microbial cause[71]. On the other hand, WHO case-definition of MIS-C includes individuals aged 0-19 years with fever ≥ 3 d, at least 2 concomitant systemic symptoms, elevated markers of inflammation, evidence of COVID-19 or contact with contaminated patients and no apparent other etiology for the inflammation[72]. Ultimately, CDC diagnosis of MIS-C includes: Individuals under 21 years of age presenting with fever ≥ 38.0 °C for ≥ 24 h; laboratory evidence of inflammation, presence of severe multisystemic disease; no other plausible alternative diagnosis; presence of reverse transcription PCR (RT-PCR), serology, or antigen test positive for COVID-19 currently or recently, or exposure to the virus within 4 wk prior to symptom onset[73]. Table 1 summarizes the major differences between these case definitions. Among these case definitions, Hoste et al[63] demonstrate a greater accuracy of the WHO MIS-C definition, comprising 97% of cases, followed by the CDC (62%) and lastly, the RCPCH.
| Criteria | RCPCH[71] | WHO[72] | CDC[73] |
| Age | ND | < 21 | < 19 |
| Clinical | Persistent fever > 38.5 °C. Evidence of single or multi-organ dysfunction with additional features1 | Fever ≥ 3 d; two of the following: (1) Mucocutaneous inflammation signs; (2) Hypotension or shock; (3) Cardiac issues2; (4) Evidence of coagulopathy; and (5) Acute gastrointestinal problems | Fever ≥ 38.0 °C for ≥ 24 h; multisystemic disease involving multiple (≥ 2) organs (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, or neurological). Requirement of hospitalization |
| Inflammatory markers | Neutrophilia, elevated CRP and lymphopenia | Elevated markers (such as ESR, CRP or procalcitonin) | Elevated CRP, ESR, fibrinogen, procalcitonin, d-dimer, ferritin, neutrophils, LDH, or IL-6 or reduced albumin and/or lymphocytes |
| COVID-19 test | Positive or negative SARS-CoV-2 RT-PCR test | Evidence of COVID-19 (via RT-PCR, antigen test, or serology) or probable contact with patients contaminated with SARS-CoV-2 | RT-PCR, serology or antigen test positive for COVID-19 (currently or in recent period) or exposure to the virus within 4 wk prior to symptom onset |
| Exclusion factors | Exclusion of any other microbial cause | No other obvious etiology for the inflammation | Absence of other plausible alternative diagnoses |
Treatment for patients with MIS-C relies on continuous monitoring of inflammatory markers to assess the progression of the condition[67]. Given the multisystemic involvement of MIS-C, its management should be performed with multidisciplinary team support, such as pediatric cardiologist, rheumatologist, and infectologist, and may require the assistance of other specialties, such as intensive care, neurology, nephrology, or gastroenterology[18]. Management of the condition follows a supportive approach and an immunomodulatory perspective. Thus, supportive care is crucial, including res-piratory and circulatory support, ranging from intubation, intravenous fluids and inotropic support to extracorporeal membrane oxygenation[76]. In addition to supportive measures, immunomodulatory therapy with IVIG and steroids is established as first-line treatment for children with MIS-C, with a recommended dose of 2 g/kg IVIG[5,77]. In this regard, the American College of Rheumatology (ACR) prescribes IVIG alone in patients without shock or organ-threatening diseases, whereas the combination of IVIG combined with glucocorticoids in patients presenting with these conditions is recommended[5]. Furthermore, in patients in whom IVIG and corticosteroid therapy is not successful, the ACR recommends treatment with anakinra or infliximab at high doses[5].
Some studies have compared the efficacy of treatment with combined IVIG and methylprednisolone vs IVIG alone[78,79]. It is noted that the combined regimen is associated with significantly lower risk of poor outcome, such as treatment failure, acute myocardial dysfunction, need for hemodynamic support or second-line treatments. Furthermore, it is also established that in patients at high risk of thrombosis, anticoagulation therapy may be required[5], and that children with shock should be treated according to established guidelines for pediatric septic shock[80]. Given the complexity of management, children presenting with MIS-C-related symptoms should be quickly transferred to a pediatric intensive care center once stabilized[75].
In this study, we have brought together the current evidence regarding the pathogenic mechanisms of MIS-C, clinical picture and disease management. Emerging evidence has highlighted this condition as a state of out-of-control release of cytokines, immune cell recruitment and activation, and production of auto-reactive peptides. However, there is still much to be clarified regarding the mechanisms behind the induction of this response profile. Treatment for patients with MIS-C currently relies on continuous monitoring of inflammatory markers to assess the progression of the condition and IVIG administration. However, despite MIS-C severity, there are still no definitive disease management guidelines for this condition.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Virology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fabbri N, Italy S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12976] [Article Influence: 2595.2] [Reference Citation Analysis (1)] |
| 2. | Simon Junior H, Sakano TMS, Rodrigues RM, Eisencraft AP, Carvalho VEL, Schvartsman C, Reis AGADC. Multisystem inflammatory syndrome associated with COVID-19 from the pediatric emergency physician's point of view. J Pediatr (Rio J). 2021;97:140-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Zimmermann P, Curtis N. Coronavirus Infections in Children Including COVID-19: An Overview of the Epidemiology, Clinical Features, Diagnosis, Treatment and Prevention Options in Children. Pediatr Infect Dis J. 2020;39:355-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 714] [Cited by in RCA: 695] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 4. | Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395:1741-1743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 336] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 5. | Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, Behrens EM, Kernan KF, Schulert GS, Seo P, Son MBF, Tremoulet AH, VanderPluym C, Yeung RSM, Mudano AS, Turner AS, Karp DR, Mehta JJ. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 3. Arthritis Rheumatol. 2022;74:e1-e20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 164] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 6. | Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020;145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2328] [Cited by in RCA: 2412] [Article Influence: 482.4] [Reference Citation Analysis (0)] |
| 7. | Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu JM, Peiris M, Poon LLM, Zhang W. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656-657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1225] [Cited by in RCA: 1122] [Article Influence: 224.4] [Reference Citation Analysis (0)] |
| 8. | Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2672] [Cited by in RCA: 2509] [Article Influence: 501.8] [Reference Citation Analysis (2)] |
| 9. | Alunno A, Carubbi F, Rodríguez-Carrio J. Storm, typhoon, cyclone or hurricane in patients with COVID-19? Beware of the same storm that has a different origin. RMD Open. 2020;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Santos MO, Gonçalves LC, Silva PAN, Moreira ALE, Ito CRM, Peixoto FAO, Wastowski IJ, Carneiro LC, Avelino MAG. Multisystem inflammatory syndrome (MIS-C): a systematic review and meta-analysis of clinical characteristics, treatment, and outcomes. J Pediatr (Rio J). 2022;98:338-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 11. | Sperotto F, Friedman KG, Son MBF, VanderPluym CJ, Newburger JW, Dionne A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr. 2021;180:307-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 248] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 12. | Algarni AS, Alamri NM, Khayat NZ, Alabdali RA, Alsubhi RS, Alghamdi SH. Clinical practice guidelines in multisystem inflammatory syndrome (MIS-C) related to COVID-19: a critical review and recommendations. World J Pediatr. 2022;18:83-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Emeksiz S, Çelikel Acar B, Kibar AE, Özkaya Parlakay A, Perk O, Bayhan Gİ, Cinel G, Özbek N, Azılı MN, Çelikel E, Akça H, Dibek Mısırlıoğlu E, Bayrakçı US, Çetin İİ, Neşe Çıtak Kurt A, Boyraz M, Hızlı Ş, Şenel E. Algorithm for the diagnosis and management of the multisystem inflammatory syndrome in children associated with COVID-19. Int J Clin Pract. 2021;75:e14471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Cheng MH, Zhang S, Porritt RA, Noval Rivas M, Paschold L, Willscher E, Binder M, Arditi M, Bahar I. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc Natl Acad Sci U S A. 2020;117:25254-25262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 235] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 15. | Porritt RA, Paschold L, Rivas MN, Cheng MH, Yonker LM, Chandnani H, Lopez M, Simnica D, Schultheiß C, Santiskulvong C, Van Eyk J, McCormick JK, Fasano A, Bahar I, Binder M, Arditi M. HLA class I-associated expansion of TRBV11-2 T cells in multisystem inflammatory syndrome in children. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 140] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 16. | Mazer MB, Bulut Y, Brodsky NN, Lam FW, Sturgill JL, Miles SM, Shein SL, Carroll CL, Remy KE; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network and BLOODNET Immunology Section. Multisystem Inflammatory Syndrome in Children: Host Immunologic Responses. Pediatr Crit Care Med. 2022;23:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Consiglio CR, Cotugno N, Sardh F, Pou C, Amodio D, Rodriguez L, Tan Z, Zicari S, Ruggiero A, Pascucci GR, Santilli V, Campbell T, Bryceson Y, Eriksson D, Wang J, Marchesi A, Lakshmikanth T, Campana A, Villani A, Rossi P; CACTUS Study Team, Landegren N, Palma P, Brodin P. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell. 2020;183:968-981.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 679] [Cited by in RCA: 655] [Article Influence: 131.0] [Reference Citation Analysis (0)] |
| 18. | Tiwari V, Daniel AA. Multisystem Inflammatory Syndrome in Children: A Year in Review. Eur J Rheumatol. 2022;9:167-175. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Ramaswamy A, Brodsky NN, Sumida TS, Comi M, Asashima H, Hoehn KB, Li N, Liu Y, Shah A, Ravindra NG, Bishai J, Khan A, Lau W, Sellers B, Bansal N, Guerrerio P, Unterman A, Habet V, Rice AJ, Catanzaro J, Chandnani H, Lopez M, Kaminski N, Dela Cruz CS, Tsang JS, Wang Z, Yan X, Kleinstein SH, van Dijk D, Pierce RW, Hafler DA, Lucas CL. Immune dysregulation and autoreactivity correlate with disease severity in SARS-CoV-2-associated multisystem inflammatory syndrome in children. Immunity. 2021;54:1083-1095.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 177] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 20. | Huang JJ, Gaines SB, Amezcua ML, Lubell TR, Dayan PS, Dale M, Boneparth AD, Hicar MD, Winchester R, Gorelik M. Upregulation of type 1 conventional dendritic cells implicates antigen cross-presentation in multisystem inflammatory syndrome. J Allergy Clin Immunol. 2022;149:912-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Syrimi E, Fennell E, Richter A, Vrljicak P, Stark R, Ott S, Murray PG, Al-Abadi E, Chikermane A, Dawson P, Hackett S, Jyothish D, Kanthimathinathan HK, Monaghan S, Nagakumar P, Scholefield BR, Welch S, Khan N, Faustini S, Davies K, Zelek WM, Kearns P, Taylor GS. The immune landscape of SARS-CoV-2-associated Multisystem Inflammatory Syndrome in Children (MIS-C) from acute disease to recovery. iScience. 2021;24:103215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Diorio C, Shraim R, Vella LA, Giles JR, Baxter AE, Oldridge DA, Canna SW, Henrickson SE, McNerney KO, Balamuth F, Burudpakdee C, Lee J, Leng T, Farrel A, Lambert MP, Sullivan KE, Wherry EJ, Teachey DT, Bassiri H, Behrens EM. Proteomic profiling of MIS-C patients indicates heterogeneity relating to interferon gamma dysregulation and vascular endothelial dysfunction. Nat Commun. 2021;12:7222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 23. | Carter MJ, Fish M, Jennings A, Doores KJ, Wellman P, Seow J, Acors S, Graham C, Timms E, Kenny J, Neil S, Malim MH, Tibby SM, Shankar-Hari M. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. 2020;26:1701-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 24. | Gonzalez LL, Garrie K, Turner MD. Role of S100 proteins in health and disease. Biochim Biophys Acta Mol Cell Res. 2020;1867:118677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 25. | Sacco K, Castagnoli R, Vakkilainen S, Liu C, Delmonte OM, Oguz C, Kaplan IM, Alehashemi S, Burbelo PD, Bhuyan F, de Jesus AA, Dobbs K, Rosen LB, Cheng A, Shaw E, Vakkilainen MS, Pala F, Lack J, Zhang Y, Fink DL, Oikonomou V, Snow AL, Dalgard CL, Chen J, Sellers BA, Montealegre Sanchez GA, Barron K, Rey-Jurado E, Vial C, Poli MC, Licari A, Montagna D, Marseglia GL, Licciardi F, Ramenghi U, Discepolo V, Lo Vecchio A, Guarino A, Eisenstein EM, Imberti L, Sottini A, Biondi A, Mató S, Gerstbacher D, Truong M, Stack MA, Magliocco M, Bosticardo M, Kawai T, Danielson JJ, Hulett T, Askenazi M, Hu S; NIAID Immune Response to COVID Group; Chile MIS-C Group; Pavia Pediatric COVID-19 Group, Cohen JI, Su HC, Kuhns DB, Lionakis MS, Snyder TM, Holland SM, Goldbach-Mansky R, Tsang JS, Notarangelo LD. Immunopathological signatures in multisystem inflammatory syndrome in children and pediatric COVID-19. Nat Med. 2022;28:1050-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 165] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 26. | Rodriguez-Smith JJ, Verweyen EL, Clay GM, Esteban YM, de Loizaga SR, Baker EJ, Do T, Dhakal S, Lang SM, Grom AA, Grier D, Schulert GS. Inflammatory biomarkers in COVID-19-associated multisystem inflammatory syndrome in children, Kawasaki disease, and macrophage activation syndrome: a cohort study. Lancet Rheumatol. 2021;3:e574-e584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 27. | Sharma C, Ganigara M, Galeotti C, Burns J, Berganza FM, Hayes DA, Singh-Grewal D, Bharath S, Sajjan S, Bayry J. Multisystem inflammatory syndrome in children and Kawasaki disease: a critical comparison. Nat Rev Rheumatol. 2021;17:731-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 159] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 28. | Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8:e46-e47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 789] [Cited by in RCA: 880] [Article Influence: 176.0] [Reference Citation Analysis (0)] |
| 29. | Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, Leaf DE, Kuter DJ, Rosovsky RP. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 926] [Article Influence: 185.2] [Reference Citation Analysis (0)] |
| 30. | Savla SR, Prabhavalkar KS, Bhatt LK. Cytokine storm associated coagulation complications in COVID-19 patients: Pathogenesis and Management. Expert Rev Anti Infect Ther. 2021;19:1397-1413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 31. | Bester J, Pretorius E. Effects of IL-1β, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci Rep. 2016;6:32188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 239] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 32. | Yang H, Ko HJ, Yang JY, Kim JJ, Seo SU, Park SG, Choi SS, Seong JK, Kweon MN. Interleukin-1 promotes coagulation, which is necessary for protective immunity in the lung against Streptococcus pneumoniae infection. J Infect Dis. 2013;207:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Stouthard JM, Levi M, Hack CE, Veenhof CH, Romijn HA, Sauerwein HP, van der Poll T. Interleukin-6 stimulates coagulation, not fibrinolysis, in humans. Thromb Haemost. 1996;76:738-742. [PubMed] |
| 34. | Montes-Worboys A, Rodriguez-Portal JA, Arellano-Orden E, Digón-Pereiras J, Rodriguez-Panadero F. Interleukin-8 activates coagulation and correlates with survival after talc pleurodesis. Eur Respir J. 2010;35:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Diorio C, Henrickson SE, Vella LA, McNerney KO, Chase J, Burudpakdee C, Lee JH, Jasen C, Balamuth F, Barrett DM, Banwell BL, Bernt KM, Blatz AM, Chiotos K, Fisher BT, Fitzgerald JC, Gerber JS, Gollomp K, Gray C, Grupp SA, Harris RM, Kilbaugh TJ, John ARO, Lambert M, Liebling EJ, Paessler ME, Petrosa W, Phillips C, Reilly AF, Romberg ND, Seif A, Sesok-Pizzini DA, Sullivan KE, Vardaro J, Behrens EM, Teachey DT, Bassiri H. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest. 2020;130:5967-5975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 316] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 36. | Sabat R, Grütz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21:331-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 780] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 37. | Rowley AH, Shulman ST. Pathogenesis and management of Kawasaki disease. Expert Rev Anti Infect Ther. 2010;8:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Morgan BP. The membrane attack complex as an inflammatory trigger. Immunobiology. 2016;221:747-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 39. | Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I. COVID-19 cytokine storm: The anger of inflammation. Cytokine. 2020;133:155151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 323] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 40. | Gruber CN, Patel RS, Trachtman R, Lepow L, Amanat F, Krammer F, Wilson KM, Onel K, Geanon D, Tuballes K, Patel M, Mouskas K, O'Donnell T, Merritt E, Simons NW, Barcessat V, Del Valle DM, Udondem S, Kang G, Gangadharan S, Ofori-Amanfo G, Laserson U, Rahman A, Kim-Schulze S, Charney AW, Gnjatic S, Gelb BD, Merad M, Bogunovic D. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell. 2020;183:982-995.e14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 422] [Cited by in RCA: 432] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 41. | Maecker H, Varfolomeev E, Kischkel F, Lawrence D, LeBlanc H, Lee W, Hurst S, Danilenko D, Li J, Filvaroff E, Yang B, Daniel D, Ashkenazi A. TWEAK attenuates the transition from innate to adaptive immunity. Cell. 2005;123:931-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 42. | Abo-Haded HM, Alshengeti AM, Alawfi AD, Khoshhal SQ, Al-Harbi KM, Allugmani MD, El-Agamy DS. Cytokine Profiling among Children with Multisystem Inflammatory Syndrome versus Simple COVID-19 Infection: A Study from Northwest Saudi Arabia. Biology (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 43. | Rothan HA, Byrareddy SN. The potential threat of multisystem inflammatory syndrome in children during the COVID-19 pandemic. Pediatr Allergy Immunol. 2021;32:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 44. | Panaro S, Cattalini M. The Spectrum of Manifestations of Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV2) Infection in Children: What We Can Learn From Multisystem Inflammatory Syndrome in Children (MIS-C). Front Med (Lausanne). 2021;8:747190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Maemura T, Kuroda M, Armbrust T, Yamayoshi S, Halfmann PJ, Kawaoka Y. Antibody-Dependent Enhancement of SARS-CoV-2 Infection Is Mediated by the IgG Receptors FcγRIIA and FcγRIIIA but Does Not Contribute to Aberrant Cytokine Production by Macrophages. mBio. 2021;12:e0198721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 46. | Ajmeriya S, Kumar A, Karmakar S, Rana S, Singh H. Neutralizing Antibodies and Antibody-Dependent Enhancement in COVID-19: A Perspective. J Indian Inst Sci. 2022;102:671-687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 47. | Zhang A, Stacey HD, D'Agostino MR, Tugg Y, Marzok A, Miller MS. Beyond neutralization: Fc-dependent antibody effector functions in SARS-CoV-2 infection. Nat Rev Immunol. 2022;1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 110] [Reference Citation Analysis (0)] |
| 48. | Kundu A, Maji S, Kumar S, Bhattacharya S, Chakraborty P, Sarkar J. Clinical aspects and presumed etiology of multisystem inflammatory syndrome in children (MIS-C): A review. Clin Epidemiol Glob Health. 2022;14:100966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 49. | Zanella I, Degli Antoni M, Marchese V, Castelli F, Quiros-Roldan E. Non-neutralizing antibodies: Deleterious or propitious during SARS-CoV-2 infection? Int Immunopharmacol. 2022;110:108943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Okuya K, Hattori T, Saito T, Takadate Y, Sasaki M, Furuyama W, Marzi A, Ohiro Y, Konno S, Takada A. Multiple Routes of Antibody-Dependent Enhancement of SARS-CoV-2 Infection. Microbiol Spectr. 2022;10:e0155321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 51. | Malchiodi EL, Eisenstein E, Fields BA, Ohlendorf DH, Schlievert PM, Karjalainen K, Mariuzza RA. Superantigen binding to a T cell receptor beta chain of known three-dimensional structure. J Exp Med. 1995;182:1833-1845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Currier JR, Deulofeut H, Barron KS, Kehn PJ, Robinson MA. Mitogens, superantigens, and nominal antigens elicit distinctive patterns of TCRB CDR3 diversity. Hum Immunol. 1996;48:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Li H, Llera A, Malchiodi EL, Mariuzza RA. The structural basis of T cell activation by superantigens. Annu Rev Immunol. 1999;17:435-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 238] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 54. | Nur-ur Rahman AK, Bonsor DA, Herfst CA, Pollard F, Peirce M, Wyatt AW, Kasper KJ, Madrenas J, Sundberg EJ, McCormick JK. The T cell receptor beta-chain second complementarity determining region loop (CDR2beta governs T cell activation and Vbeta specificity by bacterial superantigens. J Biol Chem. 2011;286:4871-4881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Cheng MH, Zhang S, Porritt RA, Arditi M, Bahar I. An insertion unique to SARS-CoV-2 exhibits superantigenic character strengthened by recent mutations. bioRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 56. | Moreews M, Le Gouge K, Khaldi-Plassart S, Pescarmona R, Mathieu AL, Malcus C, Djebali S, Bellomo A, Dauwalder O, Perret M, Villard M, Chopin E, Rouvet I, Vandenesh F, Dupieux C, Pouyau R, Teyssedre S, Guerder M, Louazon T, Moulin-Zinsch A, Duperril M, Patural H, Giovannini-Chami L, Portefaix A, Kassai B, Venet F, Monneret G, Lombard C, Flodrops H, De Guillebon JM, Bajolle F, Launay V, Bastard P, Zhang SY, Dubois V, Thaunat O, Richard JC, Mezidi M, Allatif O, Saker K, Dreux M, Abel L, Casanova JL, Marvel J, Trouillet-Assant S, Klatzmann D, Walzer T, Mariotti-Ferrandiz E, Javouhey E, Belot A. Polyclonal expansion of TCR Vbeta 21.3(+) CD4(+) and CD8(+) T cells is a hallmark of Multisystem Inflammatory Syndrome in Children. Sci Immunol. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 57. | Licciardi F, Baldini L, Dellepiane M, Covizzi C, Mogni R, Pruccoli G, Orsi C, Rabbone I, Parodi E, Mignone F, Montin D. MIS-C Treatment: Is IVIG Always Necessary? Front Pediatr. 2021;9:753123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Li S, Jin T, Zhang HL, Yu H, Meng F, Concha Quezada H, Zhu J. Circulating Th17, Th22, and Th1 cells are elevated in the Guillain-Barré syndrome and downregulated by IVIg treatments. Mediators Inflamm. 2014;2014:740947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 59. | Maddur MS, Trinath J, Rabin M, Bolgert F, Guy M, Vallat JM, Magy L, Balaji KN, Kaveri SV, Bayry J. Intravenous immunoglobulin-mediated expansion of regulatory T cells in autoimmune patients is associated with increased prostaglandin E2 levels in the circulation. Cell Mol Immunol. 2015;12:650-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 60. | Berger M, McCallus DE, Lin CS. Rapid and reversible responses to IVIG in autoimmune neuromuscular diseases suggest mechanisms of action involving competition with functionally important autoantibodies. J Peripher Nerv Syst. 2013;18:275-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 61. | Porritt RA, Binek A, Paschold L, Rivas MN, McArdle A, Yonker LM, Alter G, Chandnani HK, Lopez M, Fasano A, Van Eyk JE, Binder M, Arditi M. The autoimmune signature of hyperinflammatory multisystem inflammatory syndrome in children. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 62. | Burbelo PD, Gordon SM, Waldman M, Edison JD, Little DJ, Stitt RS, Bailey WT, Hughes JB, Olson SW. Autoantibodies are present before the clinical diagnosis of systemic sclerosis. PLoS One. 2019;14:e0214202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 63. | Hoste L, Van Paemel R, Haerynck F. Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur J Pediatr. 2021;180:2019-2034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 271] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 64. | Ruvinsky S, Voto C, Roel M, Fustiñana A, Veliz N, Brizuela M, Rodriguez S, Ulloa-Gutierrez R, Bardach A. Multisystem Inflammatory Syndrome Temporally Related to COVID-19 in Children From Latin America and the Caribbean Region: A Systematic Review With a Meta-Analysis of Data From Regional Surveillance Systems. Front Pediatr. 2022;10:881765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Toraih EA, Hussein MH, Elshazli RM, Kline A, Munshi R, Sultana N, Taghavi S, Killackey M, Duchesne J, Fawzy MS, Kandil E. Multisystem inflammatory syndrome in pediatric COVID-19 patients: a meta-analysis. World J Pediatr. 2021;17:141-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 66. | Yasuhara J, Watanabe K, Takagi H, Sumitomo N, Kuno T. COVID-19 and multisystem inflammatory syndrome in children: A systematic review and meta-analysis. Pediatr Pulmonol. 2021;56:837-848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 67. | Zhao Y, Yin L, Patel J, Tang L, Huang Y. The inflammatory markers of multisystem inflammatory syndrome in children (MIS-C) and adolescents associated with COVID-19: A meta-analysis. J Med Virol. 2021;93:4358-4369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 68. | Kostik MM, Bregel LV, Avrusin IS, Efremova OS, Belozerov KE, Dondurei EA, Kornishina TL, Isupova EA, Abramova NN, Felker EY, Masalova VV, Santimov AV, Kozlov YA, Barakin AO, Snegireva LS, Konstantinova J, Vilnits AA, Bekhtereva MK, Argunova VM, Matyunova AE, Sleptsova PA, Burtseva TE, Shprakh VV, Boyko TV, Kalashnikova OV, Chasnyk VG. Heart Involvement in Multisystem Inflammatory Syndrome, Associated With COVID-19 in Children: The Retrospective Multicenter Cohort Data. Front Pediatr. 2022;10:829420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 69. | Rodriguez-Gonzalez M, Castellano-Martinez A. Age-adjusted NT-proBNP could help in the early identification and follow-up of children at risk for severe multisystem inflammatory syndrome associated with COVID-19 (MIS-C). World J Clin Cases. 2022;10:10435-10450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Vogel TP, Top KA, Karatzios C, Hilmers DC, Tapia LI, Moceri P, Giovannini-Chami L, Wood N, Chandler RE, Klein NP, Schlaudecker EP, Poli MC, Muscal E, Munoz FM. Multisystem inflammatory syndrome in children and adults (MIS-C/A): Case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39:3037-3049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 167] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 71. | Royal College of Paediatric and Child Health. Paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS) - guidance for clinicians. [cited 15 November 2022]. Available from: https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance. |
| 72. | World Health Organization. Multisystem inflammatory syndrome in children and adolescents with COVID-19. 2020. [cited 15 November 2022]. Available from: https://apps.who.int/iris/bitstream/handle/10665/332095/WHO-2019-nCoV-Sci_Brief-Multisystem_Syndrome_Children-2020.1-eng.pdf. |
| 73. | Centers for Disease Control and Prevention. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). [cited 15 November 2022]. Available from: https://www.cdc.gov/mis/mis-c.html. |
| 74. | Waseem M, Shariff MA, Tay ET, Mortel D, Savadkar S, Lee H, Kondamudi N, Liang T. Multisystem Inflammatory Syndrome in Children. J Emerg Med. 2022;62:28-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 75. | Gottlieb M, Bridwell R, Ravera J, Long B. Multisystem inflammatory syndrome in children with COVID-19. Am J Emerg Med. 2021;49:148-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 76. | Porta KR, Zammit C. Multisystem inflammatory syndrome in children. JAAPA. 2022;35:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 77. | Waseem M, Shariff MA, Lim CA, Nunez J, Narayanan N, Patel K, Tay ET. Multisystem Inflammatory Syndrome in Children. West J Emerg Med. 2022;23:505-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 78. | Ouldali N, Toubiana J, Antona D, Javouhey E, Madhi F, Lorrot M, Léger PL, Galeotti C, Claude C, Wiedemann A, Lachaume N, Ovaert C, Dumortier M, Kahn JE, Mandelcwajg A, Percheron L, Biot B, Bordet J, Girardin ML, Yang DD, Grimaud M, Oualha M, Allali S, Bajolle F, Beyler C, Meinzer U, Levy M, Paulet AM, Levy C, Cohen R, Belot A, Angoulvant F; French Covid-19 Paediatric Inflammation Consortium. Association of Intravenous Immunoglobulins Plus Methylprednisolone vs Immunoglobulins Alone With Course of Fever in Multisystem Inflammatory Syndrome in Children. JAMA. 2021;325:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 246] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 79. | Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, Arakawa H, Kato T, Hara T, Hamaoka K, Ogawa S, Miura M, Nomura Y, Fuse S, Ichida F, Seki M, Fukazawa R, Ogawa C, Furuno K, Tokunaga H, Takatsuki S, Hara S, Morikawa A; RAISE study group investigators. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet. 2012;379:1613-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 451] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 80. | Weiss SL, Peters MJ, Alhazzani W, Agus MSD, Flori HR, Inwald DP, Nadel S, Schlapbach LJ, Tasker RC, Argent AC, Brierley J, Carcillo J, Carrol ED, Carroll CL, Cheifetz IM, Choong K, Cies JJ, Cruz AT, De Luca D, Deep A, Faust SN, De Oliveira CF, Hall MW, Ishimine P, Javouhey E, Joosten KFM, Joshi P, Karam O, Kneyber MCJ, Lemson J, MacLaren G, Mehta NM, Møller MH, Newth CJL, Nguyen TC, Nishisaki A, Nunnally ME, Parker MM, Paul RM, Randolph AG, Ranjit S, Romer LH, Scott HF, Tume LN, Verger JT, Williams EA, Wolf J, Wong HR, Zimmerman JJ, Kissoon N, Tissieres P. Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children. Pediatr Crit Care Med. 2020;21:e52-e106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 596] [Article Influence: 119.2] [Reference Citation Analysis (0)] |